1. Introduction
Sepsis is a life-threatening condition characterized by a dysregulated inflammatory response to infection, leading to organ dysfunction and high mortality [
1]. The pathophysiology of bacterial sepsis is based on an exaggerated hyperinflammatory response triggered by endotoxin (the principal component of the outer membrane of Gram-negative bacteria) with subsequent uncontrolled release of pro- and anti-inflammatory cytokines. Studies show that higher endotoxin and cytokine concentrations in the systemic circulation are responsible for higher mortality [
2,
3,
4]. Therefore, extracorporeal techniques aimed at lowering the concentration of circulating inflammatory mediators may provide supportive care to maintain critical organ function and potentially improve the outcome of a septic patient. However, there are still many uncertainties about the most appropriate extracorporeal blood purification method, modality, initiation time and duration of the therapy.
A novel blood purification device Oxiris® hemofilter (GAMBRO Industries, Baxter) is made of membrane with three different layers that allow the combination of renal support, endotoxin and cytokine removal, and local anticoagulation properties via heparin grafting in one device. The bulk of the hemofilter originated from negatively charged acrylonitrile and sodium methallylsulfonate copolymer called AN69 membrane, which provides both the removal of solutes and positively charged cytokines via convention and adsorption. The positively charged polyethyleneimine (PEI) layer enhances the hemocompatibility of the AN69 membrane by neutralizing the negative charges, and at the same time enables the adsorption of negatively charged endotoxins. Oxiris® has demonstrated effective adsorption of endotoxin and cytokines
in vitro [
5]. In addition, it has shown promising results in hemodynamic stabilization, reduction of lactate levels, endotoxin and cytokines and a positive impact on mortality in patients with sepsis-associated acute kidney injury [
6,
7,
8].
While the Oxiris® membrane is designed as a hemofilter and is traditionally used during continuous renal replacement therapy (CRRT), its properties allow it to be used as a stand-alone modality for hemoperfusion to reduce the concentration of cytokines and endotoxin. In this context, the Oxiris® membrane should be connected to the renal replacement machine (PrisMax or Prismaflex system) to perform hemoperfusion and the modality “slow continuous ultrafiltration” without fluid withdrawal should be set up in the prescription settings, as stated in the official instructions for use of the distributor (revised version 2023-07-01). However, only a few studies are known to date that use Oxiris® as a pure hemoperfusion device without renal function replacement [
9,
10].
Oxiris® is essentially a hemofilter but has adsorption capacities that allow non-selective adsorption of endotoxin and cytokines. This poses a challenge for nomenclature, as Oxiris® is not a conventional hemofilter, but also does not meet the definition of a classical hemoadsorbent. Therefore, we will refer to the procedure as hemoperfusion in the following text, emphasizing the main reason for initiating the treatment - the removal of inflammatory mediators.
In our case series, we report the clinical changes during hemoperfusion with the Oxiris® in adult patients with septic shock refractory to conventional treatment. Our study shows a different view of early application of Oxiris® hemadsorption membrane in patients with bacterial refractory septic shock before the hyperinflammatory response leads to loss of renal function. The novel approach in hemoperfusion modality used in our treatment protocol, with a zero total effluent flow rate and systemic heparin anticoagulation (thus excluding convective clearance) allowed a precise evaluation of the immune-adsorptive capability of the Oxiris® membrane in reducing cytokines and endotoxin in vivo.
2. Materials and Methods
2.1. Description of the Study Population
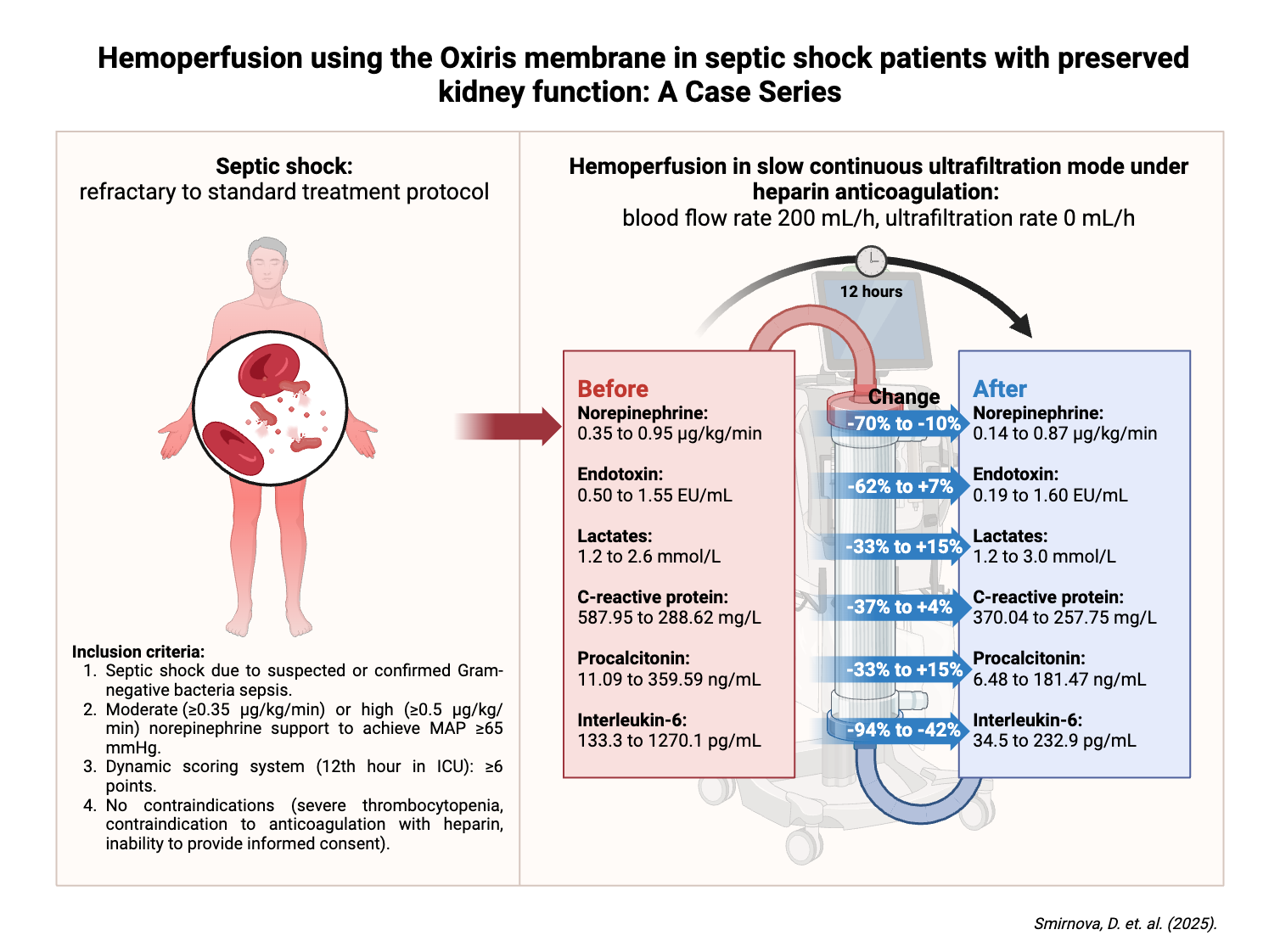
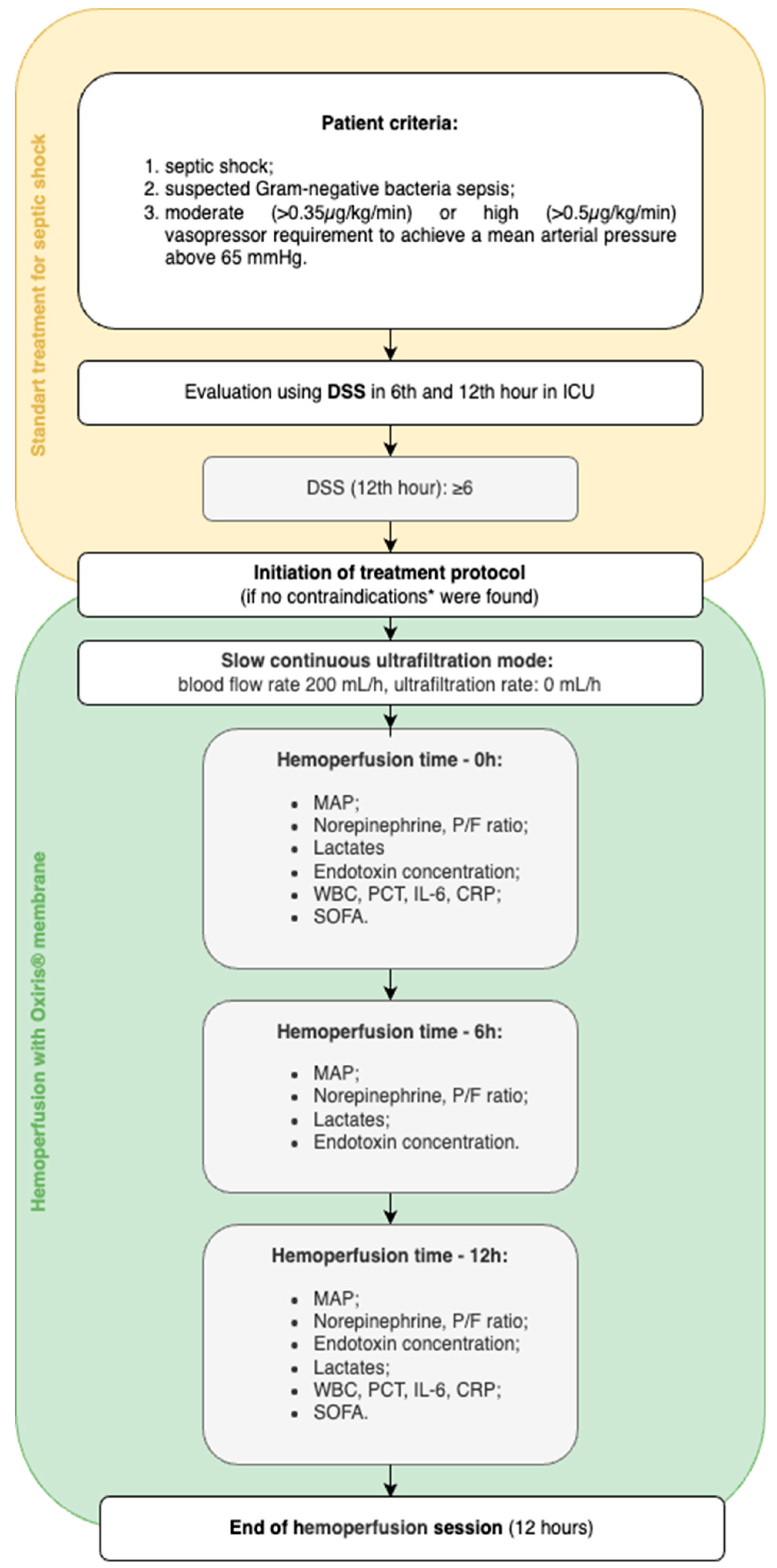
In this case series three adult patients with refractory septic shock were admitted to Intensive Care Unit (ICU) in the tertiary care center in Pauls Stradiņš Clinical University Hospital in Riga, Latvia and received hemoperfusion with Oxiris® membrane. The decision to initiate hemoperfusion was made if patients met the following criteria (
Figure 1): septic shock (based on the Third International Consensus definition for Sepsis and Septic shock (Sepsis-3) [
1]) with moderate (≥0.35 µg/kg/min) or high (≥0.50 µg/kg/min) norepinephrine requirement to achieve a mean arterial pressure above 65 mmHg, suspected Gram-negative bacteria with elevated inflammatory markers, and worsening clinical course despite standard treatment for septic shock according to the Surviving Sepsis Campaign guidelines [
11]. Worsening of the clinical course was defined as an increase in Dynamic Score System (DSS) points during the first 12 hours of ICU admission, reaching at least 6 points. Patients in whom any of the following contraindications to treatment were suspected – severe thrombocytopenia, contraindication to anticoagulation with heparin or inability to provide informed consent - were excluded from further analysis and were provided with the standard treatment for severe septic shock.
2.2. Description of the Treatment Protocol and Technical Considerations for Hemoperfusion
All patients admitted to the ICU received the standard protocol for the treatment of septic shock: broad-spectrum antibiotics covering both gram-negative and positive flora, sufficient fluid resuscitation (30 mL/kg of balanced crystalloid for the first three hours and subsequent fluid according to clinical need), vasopressor support, and surgery to control the source of infection if required by the primary diagnosis. The dynamic changes in vasopressor and lactate levels in the first twelve hours after ICU admission were evaluated, and if the pathologic changes were scored 6 or higher on the DSS, a situation refractory to standard therapy were defined and the patients were eligible for Oxiris® hemoperfusion (
Figure 1).
Under ultrasound guidance, a 13 French central venous catheter was placed in the right internal jugular vein and the Oxiris set was connected to the PrisMax continuous renal replacement therapy (CRRT) system to perform a hemoperfusion session. Systemic heparinization was chosen as an anticoagulation strategy and the following parameters were prescribed: blood flow rate of 200 mL/hour; replacement flow rate, dialysate flow rate and net ultrafiltration rate of 0 mL/hour (referred to as "zero effluent dose" in our study). For this purpose, the technical modality “slow continuous ultrafiltration” was set on the CRRT machine, as recommended in the manufacturer's instructions for use. Since hemoperfusion therapy was initiated early in septic shock patients with preserved diuresis, net ultrafiltration was not included in the treatment protocol. Although, intensivists were permitted to remove fluid when clinically indicated. The duration of each hemoperfusion session was 12 hours, after which it was discontinued. All included patients received at least one treatment session. One of the patients received a second 12-hour hemoperfusion session. The decision to repeat the hemoperfusion session was based on the clinical course of septic shock in the relevant case - clinical condition was improving (reduction in norepinephrine support and lactate levels) during and after the first session seemingly as a positive effect of hemoperfusion but failed to achieve a satisfactory state still needing high norepinephrine support (≥0.5 µg/kg/min) and preserving high inflammatory markers.
2.3. Data Collection and Analysis
The data collection included information on patients’ data (age, gender, body mass index (BMI)), the main diagnosis prompting ICU admission and pre-existing comorbidities, source control interventions and bacterial growth in the blood. The data required for the DSS score were collected and the DSS score was determined twice within the first twelve hours after admission to the intensive care unit. Alterations in infection markers (white blood cells (WBC), procalcitonin (PCT), interleukin-6 (IL-6), C-reactive protein (CRP), lactates (Lac)) and severity, as determined by Sequential Organ Failure Assessment (SOFA) scores, were analyzed before and after hemoperfusion. The amount of norepinephrine (NE), ratio of partial pressure of oxygen in arterial blood to the fraction of inspiratory oxygen concentration (P/F) and vital signs were analyzed during the hemoperfusion.
In addition, lactate levels and endotoxin concentration were measured immediately before (0 h) and 6 and 12 hours after the start of the hemoperfusion with Oxiris®. Endotoxin concentration was performed using enzyme-linked immunosorbent assay (ELISA) for the quantitative detection of gram-negative endotoxin in human samples [
Appendix A]. Blood samples for endotoxin concentrations measurement were collected from extracorporeal circuit arterial line into a heparinized vial coated with ethylene-tetra-acetic acid (EDTA), immediately centrifuged and plasma were stored at -80°C for up to one month until analyses were performed in the Riga Stradiņš University Scientific Laboratory of Biochemistry.
The rate of complications possibly related to hemoperfusion was also documented.
Informal consent was obtained from all included patients or from a person with decision-making responsibility prior to data collection (including information about the methods used, data protection measures, conditions of participation, the possibility to refuse the use of their data and the further use of their data).
2.4. Statistical Analysis
Descriptive statistics for participant characteristics and blood test results were reported. The analysis focused on absolute and relative changes in key clinical and biochemical parameters to evaluate the effects of hemoperfusion session. The primary outcomes were the reduction in norepinephrine support, improvements in hemodynamic stability (MAP, respiratory stability (P/F ratio) and changes in biomarkers (WBC, CRP, PCT, IL-6, Lac and endotoxin concentration) after hemoperfusion.
Absolute differences before and after hemoperfusion protocol in norepinephrine support, MAP, P/F ratio, biomarkers were calculated as [Absolute change] = [Post-treatment value] - [Pre-treatment value]. Percentage changes (%) for each parameter were calculated as [Relative change] = ([Post-treatment value] - [Pre-treatment value]) / Pre-treatment value x 100.
Given the nature of the study as a case series with only three patients, statistical significance was not assessed. Instead, the analysis focused on clinically important changes in key hemodynamic and biochemical parameters.
3. Case Presentation and Description
In our case series we are presenting three cases of refractory septic shock in which the previously mentioned treatment protocol was initiated. All of these patients survived until the hospital discharge for further rehabilitation and did not require further lung or kidney support. A short overview of the cases described is shown in
Table 1.
3.1. Clinical Case 1 (Patient A)
A 61-year-old male was admitted to the hospital with complaints of shortness of breath, radiating pain from the left side of thorax to the left hand. These symptoms had progressively worsened over the previous three days. Blood analysis revealed high levels of inflammatory markers. A computed tomography scan of the chest confirmed right-sided pleurisy, and the patient was transferred to the pulmonology department. Under direct ultrasonographic guidance, a surgical drain was inserted, and pleural fluid culture was obtained for bacteriological analysis.
Empirical treatment with amoxicillin/clavulanic acid was initiated, but this was later switched to ampicillin/sulbactam and colistin when blood cultures identified Acinetobacter baumannii. On the 11th intrahospital day, the patient experienced first-time generalized seizures and increasing shortness of breath with desaturation; consequently, oxygen support via a high-flow nasal cannula was started. The following day, a video-assisted thoracoscopy was performed. Despite these interventions, respiratory failure and hemodynamic instability progressed and the patient was transferred to the ICU for initiation of invasive mechanical lung ventilation and continuation of hemodynamic support.
During the first six hours in the ICU, the patient scored 4 points on the DSS. By the 12th hour, the DSS had increased to 6, and the SOFA score was 10. The haemodynamic instability had progressed and norepinephrine infusion had been increased to 0.35 µg/kg/min.
As the patient met the inclusion criteria, the baseline concentration of endotoxin and inflammatory markers (WBC 15.3 x10
9/L, CRP 587.95 mg/L, PCT 11.09 ng/mL, IL-6 398.6 pg/mL, Lac 1.2 mmol/L) in the blood were determined and hemoperfusion with the Oxiris® based on the previously mentioned parameters was initiated. After 12 hours of hemoperfusion, stabilization of the general condition was observed (
Table 2,
Table 3): endotoxin concentrations decreased to 0.19 EU/mL, inflammatory markers improved (WBC 11.8 x10
9/L, CRP 369.39 mg/L, PCT 6.48 ng/mL, IL-6 232.9 pg/mL, Lac 1.3 mmol/L), catecholamines support had been reduced to norepinephrine infusion rate of 0.14 µg/kg/min with MAP 81 mmHg, SOFA score decreased to 9 and respiratory function slightly improved (P/F ratio 228.0). No complications related to hemoperfusion were observed during the treatment and the patient had preserved spontaneous diuresis of 2 liters - stage I acute kidney injury according to Kidney Disease: Improving Global Outcomes (KDIGO 2012) classification [13]. Norepinephrine support gradually decreased until discontinued completely on the 6th day in ICU.
After twenty-two days in the ICU the patient was transferred back to the pulmonology department and 11 days later was completely weaned off the ventilator and subsequently discharged.
3.2. Clinical Case 2 (Patient B)
A 53-year-old female was transferred from a municipal secondary care hospital with bilateral hydropneumothorax and a suspected diagnosis of esophageal perforation and mediastinitis. The patient had previously been diagnosed with phenylketonuria and severe mental disability. Computed tomography with oral contrast revealed a defect in the frontal wall of the lower third of the esophagus, with spreading of contrast medium into the pleural space.
The following day, esophageal stenting and video-assisted thoracoscopy with surgical drainage were performed and afterwards the patient was transferred to the ICU with septic shock for haemodynamic and respiratory support and continuation of antibacterial therapy with piperacillin/tazobactam. Initial blood bacteriology results were sterile, however intraoperatively taken pleural fluid analysis was positive for Streptococcus anginosus. In the first 6 hours in ICU the DSS score was 4 points. At 12 hours the DSS had increased to 6 points, SOFA score was 7, norepinephrine infusion had been increased to 0.5 μg/kg/min with MAP 72 mmHg and P/F ratio was 387.5.
Hemoperfusion session was started, baseline endotoxin concentration and inflammatory markers were determined (
Table 2,
Table 3) - 1.55 EU/mL, WBC 10.2 x10
9/L, PCT 22.17 ng/mL, IL-6 162.2 pg/mL, CRP 411.55 mg/L, Lac 1.8 mmol/L.
At the end of hemoperfusion, the patient had stabilized (
Table 2,
Table 3) (SOFA score 5). Norepinephrine support was reduced to 0.43 μg/kg/min with MAP 81 mmHg, P/F ratio increased to 428, endotoxin concentration slightly decreased to 1.34 EU/mL. Inflammatory markers also improved: WBC 8.1 x10
9/L, CRP 370.04 mg/L, PCT 15.68 ng/mL, IL-6 34.5 pg/mL. No hemoperfusion-related complications were observed and spontaneous diuresis during the procedure was 1.5 L.
On the fourth day of hospitalization, the patient was successfully weaned off mechanical ventilation. The following day, vasopressor support was discontinued, and the patient was transferred to the pulmonology department for further treatment. The patient was discharged 29 days later.
3.3. Clinical Case 3 (Patient C)
A 73-year-old female was admitted to the hospital with a primary complaint of constipation that had persisted for the previous three days. Her past medical history included a laparoscopic cholecystectomy three years earlier, chronic autoimmune thyroiditis, non-Hodgkin’s lymphoma, a spontaneous intracerebral hemorrhage, diverticulosis, and dementia.
Upon admission, diagnostic imaging revealed small intestinal ileus, gastric stasis, acute bilateral lobar (aspiration) pneumonia, and an incarcerated hiatal hernia. Given the critical findings, an urgent surgical intervention was performed, which included endotracheal intubation, herniotomy, small intestine resection, and entero-entero anastomosis.
During the perioperative period, the patient’s condition deteriorated, leading to septic shock and necessitating transfer to the ICU for further treatment. Bacterial cultures taken from the tracheal aspirate on admission were positive for methicillin-susceptible
Staphylococcus aureus (MSSA) and
Escherichia coli, and treatment with piperacillin-tazobactam was continued. Over the following 12 hours in the ICU, the patient’s clinical condition worsened: the Dynamic Score System (DSS) rose from 6 points at the 6th ICU hour to 7 points, haemodynamic instability and respiratory support requirements progressed (norepinephrine infusion rate reached 0.97 μg/kg/min with MAP 73 mmHg, P/F ratio was 268.0). Consequently, the protocol was initiated. Baseline endotoxin concentration was 1.50 EU/mL, inflammatory markers were determined as follows: WBC 9.4 x10
9/L, CRP 288.62 mg/L, PCT 359.59 ng/mL, IL-6 1270.1 pg/mL, Lac 2.6 mmol/L. After 12 hours, hemoperfusion was discontinued as per protocol. By the end of this session (
Table 2,
Table 3), patient’s overall clinical status had slightly improved (SOFA score 9), norepinephrine support had decreased to 0.87 µg/kg/min (MAP 94 mmHg), notable improvements could be seen in lung functionality - P/F ratio 332.0. Most inflammatory markers decreased significantly - WBC 14.0 x10
9/L, CRP 302.12 mg/L, PCT 181.47 ng/mL, IL-6 76.8 pg/mL, Lac 3.0 mmol/L.
As the inflammatory markers remained at increasingly high levels for the next 12 hours (WBC 14.0 x10
9/L, CRP 301.75 mg/L, PCT 122.11 ng/mL, IL-6 133.3 pg/mL, Lac 2.1 mmol/L) and overall, no noticeable further clinical improvement was observed (P/F ratio 201, SOFA score 10, DSS increased from 5 to 6 compared to the 6th hour after the first hemoperfusion session) except for a reduction of norepinephrine infusion of 0.5 μg/kg/min (MAP 95 mmHg). It was decided to initiate a hemoperfusion session for the second time in this patient (
Table 2,
Table 3). After this session, hemodynamic parameters improved further (norepinephrine infusion was reduced to 0.15 µg/kg/min with MAP 92 mmHg) together with respiratory parameters (P/F ratio was 363). Overall immune response and sepsis biomarkers showed a downward trend (WBC 10.1 x10
9/L, CRP 257.75 mg/L, PCT 68.76 ng/mL, IL-6 69.5 pg/mL, Lac 2.3 mmol/L), with a SOFA score of 9.
No complications related to hemoperfusion were observed during any of the treatment sessions and the patient had preserved spontaneous diuresis of 0.4 liters (classified as stage I acute kidney injury per the KDIGO 2012 criteria [13]) and 4.4 liters respectively. Vasopressor support ended on the 5th day of hospitalization, and the patient was weaned off mechanical ventilation on the 8th day. Two days later she was transferred to the pulmonology department and subsequently transferred back to the municipal secondary care hospital on day 28th.
4. Discussion
4.1. Summary of Main Findings
In this case series, we report on a new treatment approach for hemoperfusion therapy with Oxiris® membrane in patients with refractory septic shock before hyperinflammation leads to loss of renal function. Hemoperfusion was performed in patients with residual spontaneous diuresis, where the main key being the reduction of inflammatory mediators.
We found that 12 hours of early initiated hemoperfusion with Oxiris® was associated with a markable reduction in inflammatory mediators such as IL-6, inflammatory markers (such as CRP, PCT and WBC) and an improvement in hemodynamic status (related to reduction in norepinephrine support). In addition, an improvement in organ function score (SOFA) was also observed compared to pre- and post-treatment values. No complications possibly related to hemoperfusion (severe reduction of thrombocytes, electrolytes levels depletion, central catheter infections or hemothorax) were documented. All patients did not require additional renal replacement therapy during treatment and were discharged from ICU after a mean of 12 days without the need for hemodynamic, renal or pulmonary support.
The baseline endotoxin concentration was significantly increased in all patients, averaging 1.18 EU/mL, and two patients (Patient A and Patient B) showed a reduction in endotoxin levels after a 12-hours treatment session (by 62.0% and 13.6%, respectively). The Patient C remained high endotoxin concentration that reflected on a relatively high norepinephrine support after the first hemoperfusion session and required a second treatment session. Although significant clinical and laboratory improvement was observed during the hemoperfusion session in patient C (related to the reduction in norepinephrine support and inflammatory markers, and improvement in respiratory status), the post-treatment endotoxin concentration remained the same as the pre-treatment concentration. This finding appears to be related to an overproduction of endotoxin rather than the inability of the membrane to adsorb endotoxin through the positively charged PEI layer. It is known that many factors besides hemoadsorption contribute to endotoxin load in the blood compartment, as endotoxin concentration is the result of an imbalance between endotoxin production and elimination [14]. Endotoxin production depends mainly on the bacterial load, the characteristics of the bacterial species and the bacterial degradation caused by the immune system or antibacterial therapy with subsequent release of endotoxin into the bloodstream. Furthermore, endotoxin production in the in vivo situation is not a single pulse, but rather the constant seeding from bacterial colonies. This could theoretically explain our finding of an increased endotoxin concentration at the 6-hour time point in the taken blood samples of the Patient C. The elimination of endotoxin is mainly related to endotoxin degradation by the host's immune system, spontaneous degradation of endotoxin and adsorption of endotoxin by a suitable hemadsorption device, such as the PEI layer in the case of Oxiris®.
4.2. Reflection to Previous Studies
Although several types of extracorporeal blood purification devices are in clinical use to remove excess endotoxin, Oxiris® is the only device designed to remove both cytokines and endotoxin from circulation. In addition, its cost is relatively low compared to other direct endotoxin hemoadsorbents [15], it is easy to use with continuous renal replacement therapy machines, and its endotoxin removal capacity is similarly effective [
5]. Our decision to start hemoperfusion with the Oxiris® membrane was driven by the availability of the hemoperfusion devices for endotoxin removal in our clinical center.
Oxiris® is traditionally used as a hemofilter in renal replacement therapy and, according to a recently published meta-analysis [16; 17], could provide beneficial effects on the clinical course of patients with septic shock. However, the timing of the initiation of Oxiris® treatment remains controversial. In the absence of strict guidelines, the clinical use of Oxiris® depends on the decision at institutional level. According to the Asia-Pacific expert consensus published in 2021, Oxiris® can be considered in patients with sepsis also before acute kidney injury (AKI) develops, based on several key clinical factors such as severe hemodynamic instability, microcirculatory and organ dysfunction [18]. Once patients meet the indication for treatment, CRRT with Oxiris should be initiated as soon as possible.
While Oxiris® is designed as a hemofilter, its chemical characteristics allow use of its immuno-adsorptive properties. In the context of hemoperfusion, SCUF technical modality with no fluid removal prescription is recommended. However, only a few studies are known to date that use Oxiris® as a hemoperfusion device. In a recent SIRAKI02 randomized controlled trial investigating the ability of Oxiris® to reduce the incidence of AKI in patients undergoing non-emergent cardiac surgery, the Oxiris® membrane was connected to the extracorporeal circuit of the cardiopulmonary bypass in SCUF modality with an ultrafiltration rate of 0 mL/h and a blood flow of 200-300 mL/min [
10]. In addition to the primary endpoint results, significant reductions in tumor necrosis factor α and interleukin 8 plasma concentrations during the cardiopulmonary bypass were found in the Oxiris® group compared to the control group. Another group of researchers from Germany recently published an experimental animal study in which the clinical efficacy of Oxiris® in reducing cytokines after endotoxin infusions was investigated using the pumpless extracorporeal hemadsorption technique [
9]. No differences were observed between the Oxiris® and control groups in terms of cytokine reduction during the six-hour therapy. However, since the study methodology required a single infusion of the endotoxin with subsequent spontaneous release of interleukins, the endotoxin load might be insufficient to achieve an imbalance between production and removal of inflammatory mediators. Therefore, the lack of difference in the concentration of interleukins after 6 hours of hemadsorption between the two groups may be due to adequate spontaneous degradation of mediators by the host immune system rather than immunoadsorption of the membrane.
In our hemoperfusion treatment protocol the chosen technical settings on CRRT machine (SCUF modality with zero effluent dose) and anticoagulation strategy offered the possibility to exclude the effect of elimination of inflammatory markers by convection and thus to evaluate the exact immuno-absorptive capacity of the membrane. This provided new insights to better understand the hemadsorption properties of the Oxiris® membrane in vivo situations. The decision for a relatively short treatment duration (twelve hours) was made to reduce the risk of a membrane saturation phenomenon, i.e., the time during which the membrane has exhausted its own capacity to absorb cytokines and endotoxins. Although the saturation limit of the Oxiris® is still under discussion, it is recommended to elective change prior to 12 hours to achieve the most optimal immunoadsorption [19; 20].
Finally, the modality chosen in our treatment protocol might have a lower cost compared to continuous veno-venous hemofiltration (CVVH) with Oxiris® with similar efficacy during the first 12 hours of treatment, as reported in our previous research [21] (unpublished data), mainly because there is no additional cost for replacement fluid. In this prospective, randomized study, the novel hemoperfusion modality was compared with the conventional CVVH modality. Both groups showed a significant reduction in vasopressor support in the early phase of treatment. And the group of patients treated with isolated hemoperfusion showed a significant reduction in endotoxin compared to the conventional group, although the basal endotoxin concentration was higher.
4.3. Strength and Limitation
Our study provides novel insights into the exact immunoadsorption properties of the Oxiris® membrane. Another strength of our study is the detection of endotoxin concentration and the implementation of a new diagnostic approach as a Dynamic Score System to dynamically assess the severity of the clinical course in patients with septic shock. We believe that this dynamic assessment of refractory septic shock could help clinicians decide on the optimal time to initiate hemoperfusion. However, there are some limitations that could be considered in further studies. First, the main limitation of this study is the relatively small number of participants. Second, the lack of a control group to clarify whether the decrease in endotoxin concentration is related to hemadsorption properties of the membrane or spontaneous degradation of endotoxin. Future studies should include a control group with a conventional treatment approach. Expanding the sample size and comparison with the control group would enhance the validity of the study. Third, we used ELISA assays instead of the Endotoxin activity assay (EAA) in our case studies. Therefore, the endotoxin concentration results were shifted in time, limiting the comprehensive real-time understanding of the treatment effect. The use of rapid qualitative tests to detect endotoxins, such as the EAA, would simplify the inclusion criteria and initiation of the treatment protocol. However, this test is not widely used in our region. Fourth, our treatment protocol utilized a hemoperfusion modality with a zero total effluent flow rate under systemic heparinization (technically set as SCUF modality in the CRRT machine), excluding both diffusive and convective clearance as well as fluid removal. While this approach enables the evaluation of the pure immunoadsorption properties of the Oxiris® membrane, our study does not quantify the impact of convective clearance on the cytokine removal efficiency of the AN69 layer. Finally, in our study, antibiotic clearance was not monitored during the hemoperfusion session. This raises concern about the unintended removal of antimicrobials, which could potentially compromise treatment efficacy. Future studies should account for this effect and implement appropriate strategies to optimize antibiotic dosing and ensure optimal patient outcomes.
5. Conclusions
In conclusion, early hemoperfusion with the Oxiris® membrane is a safe option in managing patients with septic shock who do not respond to conventional therapy. It provides significant clearance of inflammatory mediators such as interleukin-6 and endotoxin. This reduction appears to be associated with improved hemodynamics, as evidenced by a decreased need for norepinephrine to maintain mean arterial pressure, and preservation of organ function. This makes it a promising and potent treatment option for patients with refractory septic shock. However, further studies are required to compare the new treatment modality with a larger sample size and a conventional treatment group before this treatment approach can be implemented in routine clinical practice.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1: Research methodology; Table S1: Patient characteristics and hospitalization data; Table S2: Immune response, sepsis biomarkers and SOFA dynamics changes before and after hemoperfusion; Table S3: Haemodynamic, respiratory and endotoxin, lactate changes before, during and after hemoperfusion.
Author Contributions
Conceptualization, D.S. and V.L.; methodology, D.S., R.S. and O.S.; validation, O.S., G.V. and A.S.; investigation, D.S., A.S. and A.L..; resources, D.S. R.S.; data curation, D.S., R.S. and M.K.; writing—original draft preparation, D.S., R.S.; writing—review and editing, D.S., M.K. and G.V.; visualization, R.S., D.S..; supervision, O.S., V.L. and G.V. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by Riga Stradiņš University for PhD studentbD.S., grant number Nr. 6-DN-20/2/2025”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Riga Stradiņš University Ethical Committee (approval number 2-PĒK-4/429/2022; date of approval September 29 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article’s material; further inquiries can be directed to the corresponding author(s).
Acknowledgments
We would like to acknowledge all healthcare staff involved in the treatment of patients and data collection in the Intensive Care Unit of Pauls Stradiņš Clinical University hospital. Graphical abstract was created in BioRender. Serzans, R. (2025)
https://BioRender.com/b76a574.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| The following abbreviations are used in this manuscript |
. |
| AKI |
acute kidney injury |
| BMI |
body mass index |
| CRP |
C-reactive protein |
| CRRT |
continuous renal replacement therapy |
| CVVH |
continuous veno-venous hemofiltration |
| DSS |
Dynamic Score System |
| EAA |
endotoxin activity assay |
| EDTA |
ethylene-tetra-acetic acid |
| ELISA |
enzyme-linked immunosorbent assay |
| ET |
endotoxin |
| ICU |
intensive care unit |
| IL-6 |
interleukin-6 |
| Lac |
lactates |
| MAP |
mean arterial pressure |
| MSSA |
methicillin-susceptible Staphylococcus aureus
|
| NE |
norepinephrine |
| OD |
optical density |
| P/F |
ratio of partial pressure of oxygen in arterial blood to the fraction of inspiratory oxygen concentration |
| PCT |
procalcitonin |
| PEI |
polyethyleneimine |
| SCUF |
slow continuous ultrafiltration |
| SOFA |
sequential organ failure assessment |
| WBC |
white blood cells |
Appendix A
Blood samples for endotoxin concentration measurement were collected from extracorporeal circuit arterial line into a heparinized vial coated with EDTA and immediately centrifuged for 15 minutes at room temperature. Plasma samples were stored at -80°C for up to one month until analyses were performed in the Riga Stradiņš University Scientific Laboratory of Biochemistry.
Endotoxin concentration was measured using a quantitative colorimetric enzyme-linked immunosorbent assay (ELISA) kit. This kit is specifically designed for detecting Gram-negative endotoxin in plasma samples, with a detection range of 100–2500 pg/mL (equivalent to 1–25 EU/mL) and a sensitivity of ≤0.005 EU/mL. The plasma samples were brought to room temperature (18-25°C) without additional heating and diluted 1:10 in endotoxin-free diluent before performing the assay. The assay procedure, including steps such as the addition of detection reagents, TMB substrate, stop solution, incubation times and temperature settings, was performed strictly according to the manufacturer’s instructions (available at: LSBio ELISA Kit Instructions
https://www.lsbio.com/elisakits/manualpdf/ls-f15272.pdf). The optical density (OD) of each diluted sample and control was measured immediately using a microplate reader set to 450 nm. The measured OD values were then converted to endotoxin concentrations (EU/mL). The final endotoxin concentrations (reported in this study) in the plasma samples were calculated using the following formula:
[Endotoxin concentration in the plasma sample] = [Determined concentration in the diluted plasma sample] × [dilution factor (11)]
References
- Singer, M.; Deutschman, C. S.; Seymour, C. W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G. R.; Chiche, J. D.; Coopersmith, C. M.; Hotchkiss, R. S.; Levy, M. M.; Marshall, J. C.; Martin, G. S.; Opal, S. M.; Rubenfeld, G. D.; van der Poll, T.; Vincent, J. L.; Angus, D. C. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA, 2016, 315(8), 801–810. [CrossRef]
- Payen, D.; Dupuis, C.; Deckert, V.; Pais de Barros, J. P.; Rérole, A. L.; Lukaszewicz, A. C.; Coudroy, R.; Robert, R.; Lagrost, L. Endotoxin mass concentration in plasma is associated with mortality in a multicentric cohort of peritonitis-induced shock. Front. Med., 2021, 8, 749405. [CrossRef]
- Seymour, C. W.; Kennedy, J. N.; Wang, S.; Chang, C. H.; Elliott, C. F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; Huang, D. T.; Kellum, J. A.; Mi, Q.; Opal, S. M.; Talisa, V.; van der Poll, T.; Visweswaran, S.; Vodovotz, Y.; Weiss, J. C.; Yealy, D. M.; Angus, D. C. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA, 2019, 321(20), 2003–2017. [CrossRef]
- Hurley, J. C. Endotoxemia and Gram-negative bacteremia as predictors of outcome in sepsis: A meta-analysis using ROC curves. J. Endotoxin Res., 2003, 9(5), 271–279. [CrossRef]
- Malard, B.; Lambert, C.; Kellum, J. A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp., 2018, 6(1), 12. [CrossRef]
- Siew, L. Y.; Lee, Z. Y.; Yunos, N. M.; Atan, R.; Cove, M. E.; Lumlertgul, N.; Srisawat, N.; Hasan, M. S. Outcomes of extracorporeal blood purification with oXiris® membrane in critically ill patients: A systematic review and meta-analysis. J. Crit. Care, 2024, 83, 154844. [CrossRef]
- Broman, M. E.; Hansson, F.; Vincent, J. L.; Bodelsson, M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS One, 2019, 14(8), e0220444. [CrossRef]
- Wang, G.; He, Y.; Guo, Q.; Zhao, Y.; He, J.; Chen, Y.; Chen, W.; Zhou, Y.; Peng, Z.; Deng, K.; Guan, J.; Xie, W.; Chang, P.; Liu, Z. Continuous renal replacement therapy with the adsorptive oXiris filter may be associated with lower 28-day mortality in sepsis: A systematic review and meta-analysis. Crit. Care (Lond. Engl.), 2023, 27(1), 275. [CrossRef]
- Kalenka, A.; Arens, P.; Müllenbach, R. M.; Weigand, M. A.; Brune, M.; Fiedler-Kalenka, M. O. Effects of Oxiris® therapy on cytokine elimination after a LPS infusion—An experimental animal study. Int. J. Mol. Sci., 2024, 25(17), 9283. [CrossRef]
- Pérez-Fernández, X.; Ulsamer, A.; Cámara-Rosell, M.; Sbraga, F.; Boza-Hernández, E.; Moret-Ruíz, E.; Plata-Menchaca, E.; Santiago-Bautista, D.; Boronat-García, P.; Gumucio-Sanguino, V.; Peñafiel-Muñoz, J.; Camacho-Pérez, M.; Betbesé-Roig, A.; Forni, L.; Campos-Gómez, A.; Sabater-Riera, J.; SIRAKI02 Study Group. Extracorporeal blood purification and acute kidney injury in cardiac surgery: The SIRAKI02 randomized clinical trial. JAMA, 2024, 332(17), 1446–1454. [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C. M.; French, C.; Machado, F. R.; McIntyre, L.; Ostermann, M.; Prescott, H. C.; Schorr, C.; Simpson, S.; Wiersinga, W. J.; Alshamsi, F.; Angus, D. C.; Arabi, Y.; Azevedo, L.; Beale, R.; Beilman, G.; Belley-Cote, E.; … Levy, M. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med., 2021, 47(11), 1181–1247. [CrossRef]
- Kogelmann, K.; Hübner, T.; Schwameis, F.; Drüner, M.; Scheller, M.; Jarczak, D. First evaluation of a new dynamic scoring system intended to support prescription of adjuvant CytoSorb hemoadsorption therapy in patients with septic shock. J. Clin. Med., 2021, 10, 2939. [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl. 2012; 2:1–138.
- Broman, M. E.; Bodelsson, M. Analysis of endotoxin adsorption in two Swedish patients with septic shock. Blood Purif., 2019, 47(Suppl 3), 1–3. [CrossRef]
- Kim, H. S.; Chung, Y. J.; Lee, G. R.; Kim, E. Y. The clinical efficacy and suitable implementation of two extracorporeal blood purification therapies: AN69-oXiris versus PMX-HP. Front. Med., 2024, 11, 1344893. [CrossRef]
- Wang, G.; He, Y.; Guo, Q.; Zhao, Y.; He, J.; Chen, Y.; Chen, W.; Zhou, Y.; Peng, Z.; Deng, K.; Guan, J.; Xie, W.; Chang, P.; Liu, Z. Continuous renal replacement therapy with the adsorptive oXiris filter may be associated with lower 28-day mortality in sepsis: A systematic review and meta-analysis. Crit. Care (Lond. Engl.), 2023, 27(1), 275. [CrossRef]
- Siew, L. Y.; Lee, Z. Y.; Yunos, N. M.; Atan, R.; Cove, M. E.; Lumlertgul, N.; Srisawat, N.; Hasan, M. S. Outcomes of extracorporeal blood purification with oXiris® membrane in critically ill patients: A systematic review and meta-analysis. J. Crit. Care, 2024, 83, 154844. [CrossRef]
- Zhang, L.; Cove, M.; Nguyen, B. G.; Lumlertgul, N.; Ganesh, K.; Chan, A.; Bui, G. T. H.; Guo, C.; Li, J.; Liu, S.; Peng, M.; Foong, K. W.; Zhang, J.; Wang, M.; Goldstein, J.; Harenski, K. Adsorptive hemofiltration for sepsis management: Expert recommendations based on the Asia Pacific experience. Chin. Med. J., 2021, 134(18), 2258–2260. [CrossRef]
- De Vriese, A. S.; Colardyn, F. A.; Philippé, J. J.; Vanholder, R. C.; De Sutter, J. H.; Lameire, N. H. Cytokine removal during continuous hemofiltration in septic patients. J. Am. Soc. Nephrol., 1999, 10(4), 846–853. [CrossRef]
- Wong, E. T.; Ong, V.; Remani, D.; Wong, W. K.; Haroon, S.; Lau, T.; Nyeo, H. Q.; Mukhopadhyay, A.; Tan, B. H.; Chua, H. R. Filter life and safety of heparin-grafted membrane for continuous renal replacement therapy - A randomized controlled trial. Semin. Dial., 2021, 34(4), 300–308. [CrossRef]
- Pretkalniņš, D.N.; Smirnova, D.; Klibus, M.; Šķesters, A.; Freijs, Ģ.; Liguts, V.; Sabeļņikovs, O. Clinical Efficacy of the Oxiris® Membrane in Patients with Refractory Septic Shock: Comparison Between Isolated Hemadsorption and Continuous Veno-Venous Hemofiltration Modalities. In Proceedings of the RSU Research Week 2025, Rīga Stradiņš University, Riga, Latvia, 26–28 March 2025. Available online: https://rw2025.rsu.lv.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).