1. Introduction
Spodoptera frugiperda (J. E. Smith), Lepidoptera, Noctuidae, also called fall armyworm (FAW), has become a major invasive pest worldwide in recent years.FAW was officially reported to invaded China in January 2019 [
1], It has progressively spread to southern provinces and is advancing northward in China, which poses a significant threat to the grain production and agricultural development, even threatening the nation's food security.
The native home of FAW is under attack from numerous predators (Coleoptera,Hymenoptera,Dermaptera and Hemiptera,) [
2,
3,
4]. Except for the reports of a few of studies [
5,
6,
7], little is known about the impact of natural enemies in reducing FAW populations in agricultural systems. Since its invasion of China, Using pesticides for emergency control has increased and reduced susceptibility to several insecticides used for decades in its native range.Consequently, developing local natural enemies resources for ecological management is a vital strategy for the sustainable prevention and control of
S. frugiperda. During the period from May to October in 2021,we discovered a specific type of carabidae larva in Luoning County, Luoyang City, Henan Province. This discovery was made during the field study of digging natural enemy resources of
S. frugiperda. Subsequent to feeding these carabidae larvae to adult in the laboratory, we observed that the dorsal side of both male and female mature insects was dark with a coppery sheen interspersed between spots. The insects had rectangular elytra, adorned with four rows of golden coarse spots on each. Based on the characteristics mentioned in Liang et al., 2000, these carabidae larvae were identified as
Chlaenius bioculatus Kirby[
8].
C. bioculatus, belonging to the Carabidae family within the Coleoptera order, has a broad distribution across several regions, including Heilongjiang, Liaoning, Inner Mongolia Autonomous Region, Ningxia Hui Autonomous Region, Gansu, Hebei, Shanxi, Shandong, Jiangsu, Anhui, Zhejiang, Hubei, Jiangxi, Fujian, Sichuan, and Yunnan.This study aims to provide a scientific basis for the conservation, development, and utilization of this natural predator in managing S. frugiperda. Here, we established an experimental predator carabidae population in laboratory.The predator population was further confirmed by morphological and molecular method.The predation behaviors and capabilities of the 1-3 instar larvae, as well as male and female adults of C. chinensis, towards the larvae, pupae, and adults of S. frugiperda were elucidated, and their predation preferences were further evaluated.
2. Results
2.1. Morphological and Molecular Identification
2.1.1. Morphological Identification
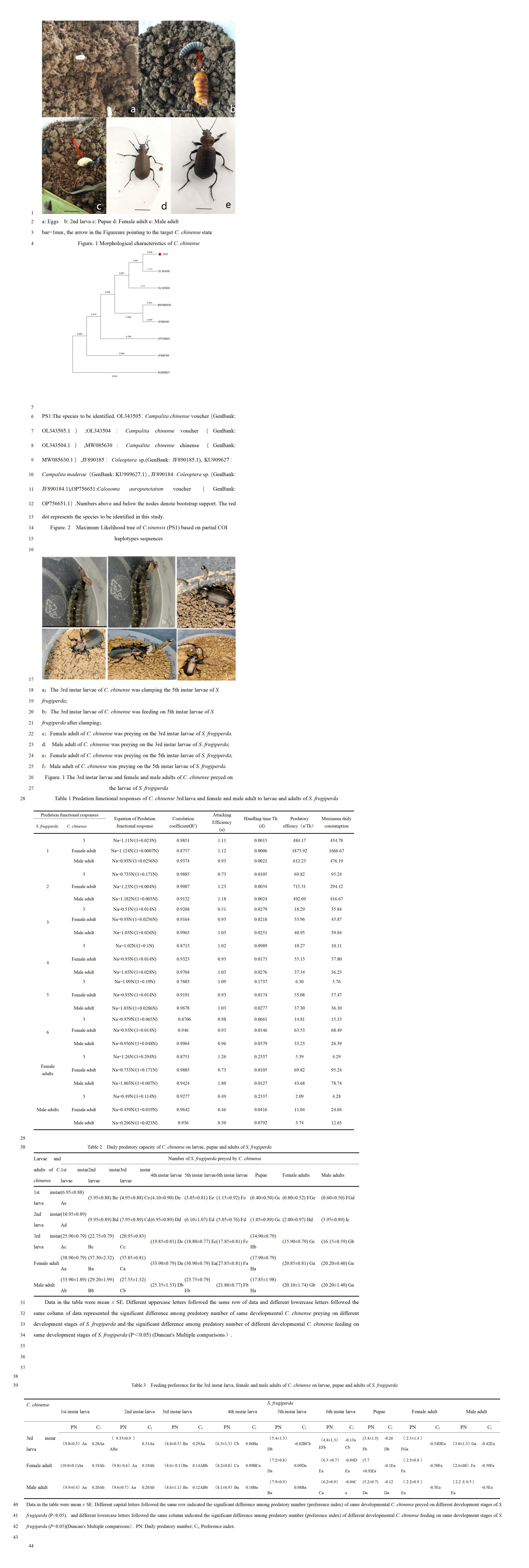
C. chinense female adults laid an average of 50.2 ± 18.5 eggs per female and typically spawn 2.35 ± 6.2 times during the breeding season. The eggs were deposited individually and found approximately 7.75 ± 3.24 cm beneath the soil surface. Freshly laid eggs appeared milky white, transitioning to light yellow after one day, and eventually dark brown (
Figure 1a).
The larvae of
C. chinense were slender, elongated, and black, exhibiting agility and liveliness, with a tendency to be active at night while remaining concealed during the day. They underwent three instars throughout their larval stage. The older, mature larvae first constructed a pupal chamber before entering pupae (
Figure 1b,c).
C. chinense typically exhibited a bronze coloration with a prominent metallic sheen. Small, irregularly arranged particles could be observed between the rows of the elytra, measuring approximately 1 cm in length. For male adults, the first 1 to 3 tarsal segments of the foreleg and the first tarsal segment of the mid-leg were slightly widened, and there was a hairy pad on the abdomen. In contrast, female adults were lacking these characteristics in males (
Figure 1.d,e).
2.1.2. Molecular Identification
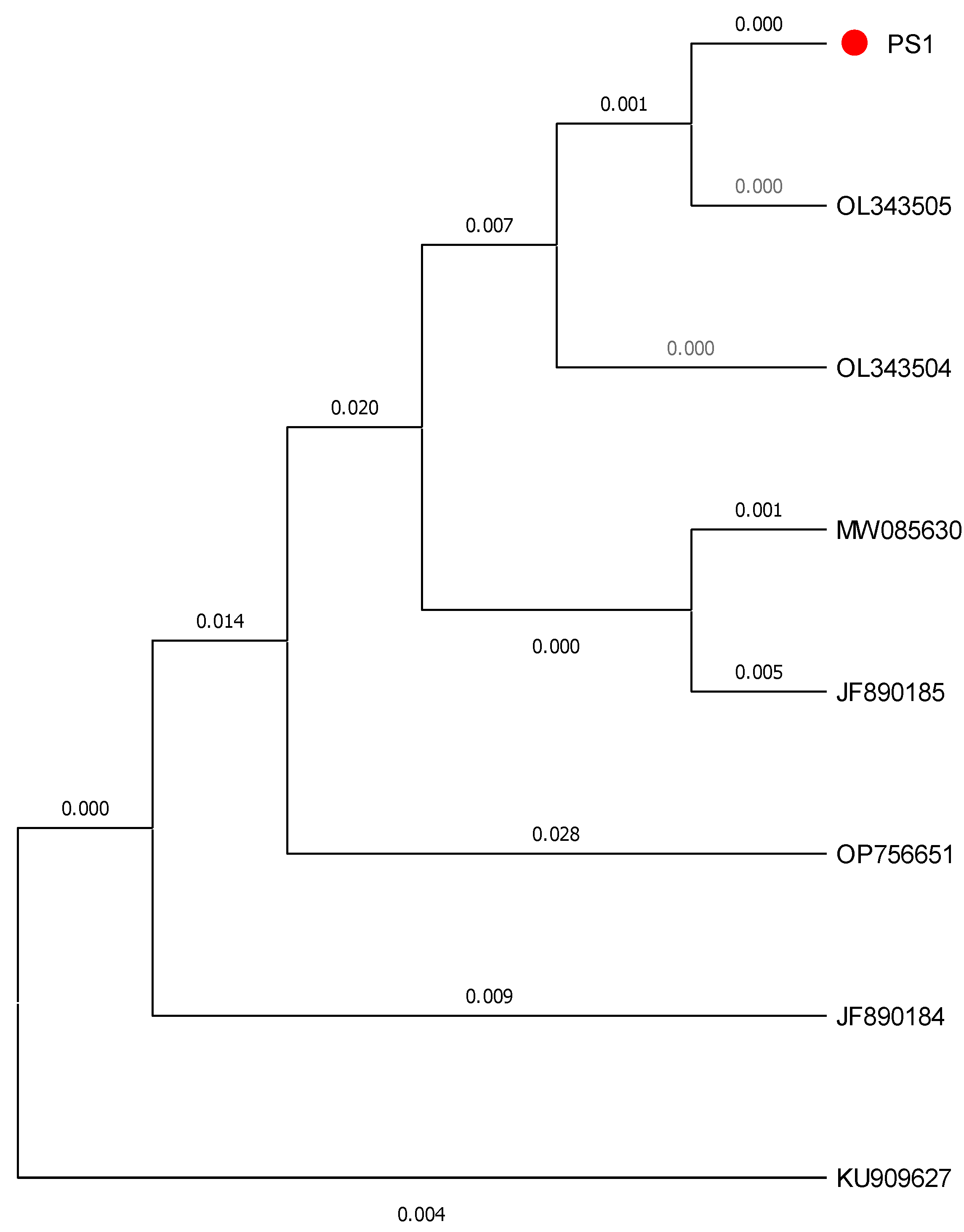
Target samples were obtained by PCR amplification. The length of mitochondrial CO I sequence was 700 bp.The sequence was blasted with GenBank and BOLD database, and the similarity with
C.sinensis COI (GenBank: OL343503.1) sequence was 100 %. Based on the analysis results of Clustal W multiple sequence alignment (
Figure 2). To assess branch support in our ML trees we used non-parametric bootstrapping with heuristic searches of 1,000 replications. Target samples and
C. chinense is clustered into a single branch, which is obviously separated from other related species. It can be determined that the target sample was
C. chinense.
2.2. Predatory Functional Response of 3rd Larva, Female and Male Adults of C. sinensis to the First to Sixth Instar Larvae of S. frugiperda
2.2.1. The Predation Behavior of C. sinensis Against S. frugiperda
Laboratory predation experiments revealed that the 1st to 3rd instar larvae, as well as both male and female adults of
C. sinensis, exhibited significant predation capabilities on the 1st to 6th instar larvae, pupae, and adults of
S. frugiperda (
Figure 2). The 1st to 3rd instar larvae of
C. chinensis were capable of preying on
S. frugiperda larvae across multiple instars and could burrow into the soil to search preys. The total predation process comprised four steps: searching, tempting, attacking, biting and eating. During the predation,
C. sinensis utilized the upper jaws to grasp
S. frugiperda larvae. For the 1st to 3rd instar
S. frugiperda larvae, they consumed the entire larva before moving on to other targets. While for 4th to 5th instar preys,
C. sinensis used their appendages and mouthparts to immobilize the larvae until the prey stopped struggling. Then
C. sinensis punctured the epidermis to extract body fluids, gradually feeding on the entire body of
S. frugiperda.
C. sinensis usually consumed the soft part of the prey’s body, leaving only the hard remnants.There were no significant behavioral differences between larvae and adults. Remarkably, if hungry,1st to 3rd instar larvae of
C. chinensis could fully consume the entire body of 6th instar
S. frugiperda larvae, which were much larger than themselves, before preying on additional targets.
During the predation experiments, both larvae and adults of C. sinensis could emit unpleasant white secretions. If satiated, they often only extracted a small amount of body fluid from the prey larvae after gnawing the cuticle, subsequently abandoning the prey to search for other preys. Notably, 1st to 2nd instar C. sinensis larvae might occasionally fall prey to 5th to 6th instar S. frugiperda larvae.
2.2.2. Predatory Functional Response of 3rd Instar Larva and Female and Male Adults of C. sinensis to the First to Sixth Instar Larvae of S. frugiperda
A type II functional response was shown by 3rd instar larva, female and male adults of
C. sinensis to the first to sixth instar larvae of
S. frugiperda and it was fitted to the Holling’s disc equation (
Table 1). With the prey density increased, the consumption by
C. sinensis also rose progressively. Once the
S. frugiperda larvae reached a specific density, the predation rate stabilized.
Among 3rd instar larva, female and male adult of C. sinensis.The female adult exhibited the highest daily consumption, with a maximum of 1666.67 recorded during the first larval stage of S. frugiperda. Followed by male adults and 3rd instar larva.C. sinensis consumed more prey as the instar of S. frugiperda larvae decreased.The attack rate and handling time also showed with the attack rate increased and handling time first increased and then decreased.
Figure 1.
The 3rd instar larvae and female and male adults of C. chinense preyed on the larvae of S. frugiperda. a:The 3rd instar larvae of C. chinense was clamping the 5th instar larvae of S. frugiperda; b:The 3rd instar larvae of C. chinense was feeding on 5th instar larvae of S. frugiperda after clamping;c:Female adult of C. chinense was preying on the 3rd instar larvae of S. frugiperda. d: Male adult of C. chinense was preying on the 3rd instar larvae of S. frugiperda; e:Female adult of C. chinense was preying on the 5th instar larvae of S. frugiperda; f:Male adult of C. chinense was preying on the 5th instar larvae of S. frugiperda.
Figure 1.
The 3rd instar larvae and female and male adults of C. chinense preyed on the larvae of S. frugiperda. a:The 3rd instar larvae of C. chinense was clamping the 5th instar larvae of S. frugiperda; b:The 3rd instar larvae of C. chinense was feeding on 5th instar larvae of S. frugiperda after clamping;c:Female adult of C. chinense was preying on the 3rd instar larvae of S. frugiperda. d: Male adult of C. chinense was preying on the 3rd instar larvae of S. frugiperda; e:Female adult of C. chinense was preying on the 5th instar larvae of S. frugiperda; f:Male adult of C. chinense was preying on the 5th instar larvae of S. frugiperda.
2.3. Predatory Ability of C. sinensis to Larvae, Pupae and Adults of S. frugiperda
The results indicated that 1st to 3rd instar larvae, along with male and female adults of
C. sinensis, were capable of preying on larvae, pupae, and both sexes of
S. frugiperda. The predation rates of
S. frugiperda larvae at various developmental stages consumed by
C. sinensis of the same age diminished as the instar stage of
S. frugiperda increased. Among various developmental stages of the predators, female
C. sinensis adults exhibited the highest predatory capacity, consuming an average of (38.90±0.79) first instar
S. frugiperda larvae daily, which was 2.17 times greater than their consumption of sixth instar larvae (17.90±0.79). Male adults of
C. sinensis followed closely, with a daily predation number of 33.90±1.89 on first instar larvae. Additionally, first instar larva of
C. sinensis also demonstrated a particular predation ability, with an average daily consumption of 6.95±0.88 first instar
S. frugiperda larvae and 0.40±0.50 of
S. frugiperda pupae. In conclusion,
C. sinensis displayed effective predation on both
S. frugiperda larvae and adults, particularly preferred on prey on younger larvae of
S. frugiperda (
Table 2).
Furthermore, the predation by
C. sinensis at various instar stages on
S. frugiperda larvae of the same instar increased with the developmental stage. For
S. frugiperda larvae from the 1st to 3rd instar, female
C. sinensis adults had the highest predation efficiency, followed by male adults and then 3rd instar larvae. However, there were no significant differences observed in the predation rates on 4th to 6th instar larvae or on male and female adults of
S. frugiperda when considering male and female adults of
C. sinensis and 3rd instar larvae (
Table 2).
2.4. Predatory Selectivity of the 3rd Instar Larvae, Male and Female Adults of C. sinensis Against S. frugiperda
Among the mixed preys of different instar larvae, pupae, female adults and male adults of
S. frugiperda, the 3rd instar larvae, female adults and male adults of
C.chinensis could prey on all developmental stages of
S. frugiperda, and could even consumed on hard puparium. However,
C. chinensis preferred young larvae, with the highest predation amount observed in female adults on the 1st instar larvae of
S. frugiperda, averaging 10.0 ± 0.1 individuals per day. The predation quantity among adults was lower than that of larvae, and the 3rd instar larvae consumed the fewest female adults of S. frugiperda, with an average of 2.5 ± 1.4 individuals per day. Throughout all stages,
C. chinensis showed a positive preference for 1-4 instar larvae of
S. frugiperda and a negative preference for 5-6 instar larvae, as well as male and female adults of
S. frugiperda (
Table 3).
3. Discussions
The life cycle of
C. chinense typically spans around one year in Henan Province, China. Upon collecting young larvae in the field, professionals faced challenges in making accurate identifications due to the absence of distinct hardened diagnostic features. Generally, laboratory rearing was necessary until the larvae mature into adults, a process that could extend from several months to a year, thus prolonging the identification period. To mitigate this situation, our study employed DNA barcoding technology, utilizing the mitochondrial CO I gene as a molecular marker to identify the collected carabids. This approach significantly reduced both the time and cost associated with identification. Combined with morphological characteristics, the identification results were not only accurate but also objective and straightforward[
9].
An invasive insect species often lacks effective natural predators in its new environment, making it susceptible to outbreaks that can severely impact agricultural production. Over the past five years,
S.
frugiperda has rapidly spread across most regions of China. The use of local natural enemies to control the fall armyworm has become a crucial topic.
C. sinensis is one of the voracious predators.Also,the predatory earwig
Doru luteipes Scudder was considered for augmentative release in maize as a natural enemy[
10]. Releasing one pair of
D. luteipes per corn could control the fall armyworm population and increase maize production by 7%[
11] .These predatory natural enemies played a significant role in regulating the population of
S. frugiperda in the natural environment.
Predicting the impact of local predators on invasive species is important for prioritizing control interventions.Functional response experiments, which examine the consumption of local predators in relation to prey density,were a useful method for assessing the potential strength of novel predator-prey relationships. However, such experiments were often conducted without consideration of different developmental stage of predator to reduce invasion risk. Here, we performed the functional responses of 3rd, male and female of C. sinensis, a generalist predator, feeding on the global invader (S. frugiperda) to conduct whether the 3rd and two sexes have similar potential for impact. We also examined potential correlates of predation behaviour by measuring prey choice.3rd larva and two sexes of predator displayed a Type II hyperbolic functional response, which can disturb S. frugiperda populations at low prey densities. However, 3rd larva, males and females exhibited some differences in foraging behaviour. Females had slightly lower attack rates, which were not linked to sex differences in movement, and slightly longer handling times, which were not linked to sex differences in prey choice. These small, non-significant differences nevertheless changed into significantly greater functional response ratios, which were used to predict the ecological impact of invasive species, for males than females. There was significant difference in the proportion of preys consumed between males and females, but females have lower handling time. Taken together, these results and stage-level modelling suggest that trying to evaluate the potential impact of C. sinensis on S. frugiperda populations by sampling only one of stages of predator could result in wrong estimation, even in populations that have male-biased sex-ratios. Consumer stage of predator might generally be an important characteristic to consider when using functional response experiments to estimate the control effect of new invasive species, especially those with marked migratory characteristics that affect foraging.
In our study,the predator
C. sinensis exhibited a type II functional response when feeding on all life stages of
S. frugiperda. The number of
S. frugiperda consumed increases with increasing
C. sinensis density until prey consumption reached saturation.In another report on predatory actions against
S. frugiperda, Tian et al. (2021)[
12] also observed this type of functional response with the dermapteran
Labidura riparia Pallas controlling the same prey.This functional response type was perfect for biological control because predators can detect and attack their preys at low densities[
13] .
Our study demonstrated that
C. sinensis exhibited effective predation on
S. frugiperda , with both its larvae and adults could consume different insect stages of
S. frugiperda. This study indicated that
C. sinensis can lead to the immediate death of young
S. frugiperda larvae, pupae and adults.Outperforming parasitic natural enemies, whose victims often remained movement and continued feeding for a period after post-parasitism.
C. sinensis had also been documented to prey on various pests, including
Mythimna separata,
Spodoptera litura,
Agrotis ypsilon,
A. segetum,
A. tokionis,
Dolerus tritici and
Pieris rapae larvae [
14,
15]. Therefore,
C. sinensis not only serves as a natural enemy against the invasive
S. frugiperda but also plays a role in controlling other common pest species. Although all the stages of
C. sinensis could control
S. frugiperda in the laboratory, further study is needed to determine whether the damage they may cause to maize.
This study revealed that as the instar stage of
S. frugiperda larvae progressed, the population of predators targeting the 3rd instar larvae of
C. chinensis diminished steadily, alongside an increase in the duration of prey treatment. In instances of approaching satiation, when the predator breached the prey's epidermis, it tended to extract only a minimal quantity of body fluid from the prey larvae, subsequently abandoning it to seek out other larvae for continued predation. While hunting
S. frugiperda larvae, both adult and larval
C. chinensis exhibited defensive behaviors by spraying an unpleasant white secretion when confronted with strong prey that exceeded their own body size, before proceeding to prey upon other preys. This behavior indicated that they, like many Coleoptera insects, were not deterred by larger individuals.Chemical defense had emerged as the primary means of protection for themselves. The chemical substances produced were generally stored in specialized defense glands, and when provoked, these carabids choose to attack by releasing the substances rather than fleeing.The same phenomenon had also been observed in the predation of armyworms and
Helicoverpa armigera (Hübner, 1808) by
Labidura riparia Pallas, which is a normal defensive response[
16].
4. Materials and Methods
4.1. Predators and Preys
The beetle larvae of proposed C. bioculatus and the 5th instar larvae of S. frugiperda were collected from the corn field (34.43 N, 111.66 E) in Wangyao Village, Luoning County, Henan Province, China in June 2021. Both the predators and preys were reared under the conditions of 28 ± 0.5 °C, with a relative humidity of 70 ± 5%, and a 16L:8D photoperiod.The larvae of S. frugiperda were reared for over 3 generations with artificial diet.The larvae and adults of C. bioculatus were feed on larvae of S. frugiperda over 2 generations.Before the predation experiment, the 1-3 instar larvae and the male and female adults of C.chinensis were subjected to a 24-hour starvation.
4.2. Morphological and Molecular Identification for C. bioculatus
The eggs, larvae, pupae,female adults and male adults of
C. bioculatus were observed using the 3D Microscope Osmic Micro 3DM-HD202WF made by Aos Micro Optical Instrument Co., Ltd.Shenzheng,China.Morphological characteristics of eggs, larvae, pupae and adults according to Yu described in 1982 [
17] .
The genomic DNA was extracted from 300 eggs,one individual each from 1-3 instar larvae,pupae,female adult and male adult of the F1 generation post-adult emergence, respectively. The target samples were rinsed with distilled water, ground with liquid nitrogen, and deposited in a 1.5 mL centrifuge tube. The genomic DNA was prepared using the Trans Direct Animal Tissue PCR Kit (Beijing Full Jin Sheng Technology Co., Ltd.), following the manufacturer's instructions.Briefly, 40 μL of AD1 buffer and 10 μL of AD2 buffer were mixed in the centrifuge tube, the samples were thoroughly ground using a grinding rod, left to stand at room temperature for 10 minutes, then 40 μL of AD3 buffer was added and stored at -20°C for PCR use.
From the COI Gene region of the mitochondrial DNA, approximately 700 base pairs(bp) were successfully amplified using a combination of two designed primers:
PCR reaction system was 50 μL: DNA template 2 μL, dNTPs (2.5 mmol · μL − 1) 4 μL, upstream and downstream primers (20 μM) 2 μL, 10 × PCR buffer. (containing Mg2 +) 5 μL, Taq DNA polymerase (5 U · μL − 1) 0.25 μL, ddH2O 34.75 μL. PCR reaction conditions: 94 °C 1 min; 94 °C 30 s, 50 °C 30 s, 72 °C 1 min, 30 cycles; 72 °C for 8 min. The amplified product was detected as a single bright object by electrophoresis. The amplified products were purified and sequenced by Sangon Biotech (Shanghai) Co., Ltd.
4.3. Predatory Functional Responses of 3rd Instar Larvae, Male and Female Adults of C. sinensis to 1st-6th Instar Larvae and Adults of S. frugiperda
The experiment was conducted in a culture dish with a diameter of 15 cm and a height of 2.5 cm. The predation densities for different instar of S. frugiperda larvae were determined based on the density designed as follows: for 1st instar larvae, densities were established at 60, 120, 180, 240, and 300 individuals per dish; for 2nd instar larvae, densities were set at 40, 60, 80, 100, and 120 larvae per dish; for 3rd instar larvae, the densities were 5, 10, 20, 40, and 60 larvae per dish; for 4th instar larvae, the densities were 10, 20, 30, 40, and 50 larvae per dish; for 5th instar larvae, they were set at 5, 10, 15, 20, and 25 larvae per dish; and for 6th instar larvae, the densities were 1, 3, 5, 7 and 9 individuals per dish. Each treatment was implemented with a control group, repeated 20 times, and the natural mortality rate was documented. After a period of 24 hours, the number of surviving larvae at different densities were observed and recorded.The artificial diet were provided in a petri dish for feeding the S. frugiperda larvae. Each treatment included the corresponding density of S. frugiperda as a blank control, and survival individuals were assessed after 24 hours. All the experiment conditions remained consistent throughout.
4.4. Predation Capacity of C. sinensis on the 1st to 6th Instar Larvae, Pupae, Male and Female Adults of S. frugiperda
In laboratory conditions, first to third instar larvae of C. sinensis, together with male and female adults that emerged and completed feeding on the same day, were each placed individually in transparent plastic cages measuring 300 mL (120 mm × 90 mm × 43 mm).To mimic ecological feeding conditions, the cages were filled with 3 cm of moist sand. Based on the results from the preliminary experiment, on the second day, various developmental stages of S. frugiperda (larvae, pupae, and adults) were introduced into each cage. After initial laboratory trials, 50 individuals of S. frugiperda, including 1-6 larvae, pupae, and both male and female adults, together with one individual of C. sinensis at different stages, were added to each cage. Additionally, artificial diet were provided as food for S. frugiperda larvae. Adults were provided with a wick-shaped honeypot containing 5% honey solution for nourishment. Each treatment encompassed a control group in which C. sinensis was keeping without any prey, only provided with defatted cotton balls soaked in sterilized distilled water to sustain the predator. The control group's prey was not exposed to C. sinensis, enabling the assessment of natural mortality rates for both predators and prey. The treatments were repeated 20 times, the number of preys and cannibalism were counted.
The predation behavior of S. frugiperda on C. chinensis was monitored using a video microscope (Osmic Micro 3DM-HD202WF, Shenzhen Osmic Micro Optical Instrument Co., Ltd.).After a 24-hour period, the counts of natural deaths and predation of S. frugiperda, as well as the natural deaths and self-mutilation of C. chinensis under various treatments were documented. The corrected predation rate for each prey was calculated as follows: (Numbers of S. frugiperda death in the treatment group minus numbers of S. frugiperda death in the control group).
4.5. Prey Choice by 3rd Instar Larvae, Male Adults, and Female Adults of C. sinensis Among Larvae, Pupae and Adults of S. frugiperda
Based on preliminary experiment about predation capabilities that the 3rd instar larvaes and both male and female adults of C. chinensis exhibited strong predation abilities, making them ideal candidates for the release of natural enemies in corn field conditions. Consequently, the predation preferences of these 3rd instar larvaes and adult C. chinensis towards the larvae, pupae, and adults of S. frugiperda were assessed. The selected 3rd instar larvae and female and adults were subjected to a period of starvation for 24 hours in an cage with the same specifications as previously mentioned above. Following this preliminary experiment, a mixture of 10 larvae (including those fed with artificial diet), pupae, and both male and female adults (with a honeypot containing 5% honey nutrient solution for adults) from various instars of S. frugiperda were placed in a rectangular insect cage (dimensions: 250 mm × 180 mm × 100 mm) and filled into the starved C. chinensis, with one individual per cage. The larvae were maintained in an artificial climate chamber at a temperature of (27.2 ± 0.5) °C, with a light cycle of 16 hours of light followed by 8 hours of darkness, and a relative humidity of (80±5)%. From 8:00 to 12:00 the following day, the quantity of S. frugiperda consumed by the C. chinensis of various life stages in each cage was monitored and recorded, with each treatment repeated 20 times. The control treatments were consistent with section 4.3 above. The predation preference of C. chinensis for different developmental stages of S. frugiperda was determined using the preference index value Ci, calculated as follows: Ci = (Qi - Fi) / (Qi + Fi), where Ci represents the predator's preference index for the prey, Qi is the proportion of predators targeting the prey of “i”, and Fi indicates the proportion of the prey of “i” in the environment. In this context, Ni denotes the number of the prey of “i” in the environment, and Nai is the number of predators consuming the prey of “i”, leading to Fi = Ni /∑Ni and Qi = Nai /∑Nai. A positive preference for the first prey is indicated by 0 < Ci < 1, while a negative preference is represented by -1 < Ci < 0.
4.6. Statistics and Analysis
The functional response model, developed by Rogers in 1972[
21], was then used to describe how the consumption of
C. chinense changed with the availability of
S. frugiperda. The functional response was described by the equation: Na = aNTr / (1+aThN), while the search effect was represented by S = a / (1 + aThN). In these equations, Na denoted the number of each instar larva of
S. frugiperda, N represented the density of each instar larva, “a” indicated the predator's instantaneous attack rate on the prey, Tr indicated the total duration of the predation test, which lasted for 24 hours, Th is the time taken by
C. chinensis to consume a single
S. frugiperda larva, and S stands for the search effect [
22]. Initially, the data were processed using Excel software, an ANOVA was performed followed by a pair-wise comparison of the mean consumption at the different treatments to determine significant differences conducted with R-4.4.2 software.
5. Summary and Conclusions
In this study, the predation capacity of the third instar larvae and both sexes of adults of C. sinensis to the first to six instar larvae, pupae, male and female adults of S. frugiperda were elucidated.The predation preference for different developmental stage of S. frugiperda were further clarified. Our results showed that the whole stages of C. sinensis could prey on S. frugiperda at various stages, even to adults of S. frugiperda, especially prefer to young larvae, which possess strong predatory capacities.
Among them, female adults of C. sinensis had the strongest predatory capacity against S. frugiperda 1st instar larvae, 1st instar larvae of C. sinensis also showed control ability to S. frugiperda, The predation ability of C. sinensis larvae increased with instar, especially to 1st - 3rd instar S. frugiperda larvae.C. sinensis female adults have the strongest predation ability, followed by male adults, and then the 3rd instar larvae of C. sinensis. However, C. sinensis showed a positive choice to S. frugiperda larvae from the 1st to 4th instar, while to the 5th to 6th instar exhibited a negative preference.
FAW, a well-known seriously agricultural pest in its native range,South and North America, and has become a vital invasive insect around the world in recent decade,mainly feeds on corn.C.sinensis served as significant natural enemies of agricultural pests in the maize field in Huang-Huai-Hai region.It preys on a large number of lepidopteran larvae and were the important natural enemies of pests such as armyworms and fall armyworm. The ability of Calosoma to control armyworms was well documented[23].The predatory capacity of C. sinensis is regarded ideal, as the daily predation capacity can exceed 28 of 6th instar larvae of S. frugiperda per day. This suggested that C. sinensis had the great potential to be used as a biocontrol agent for this greedy pests-S. frugiperda.However, on the other hand, C. sinensis might also become a dangerous pests to Bombyx mori Linnaeus if they appeared in the silk industry. The fall armyworm, originating from the Americas, is now "settling down" in China.The management of this species would be developed considering food resources of non-crop habitats and the utilization of shelter for sustaining natural enemies like C. sinensis based on open field experiments in the further[24].
In the agricultural ecosystem, protecting and utilizing these natural enemies is one of the key strategies for the comprehensive prevention and control of S. frugiperda. Integrated pest management, rather than reliance on a single tactic, is the best way to suppress S. frugiperda population.
Author Contributions
C.T., X. Y. and H. F.: conceptualization. C.T., J.H. , A.D., J.Z., and G.L.,: experiment. C.T.: data processing and graphing. C.T., X. Y., and H. F., : writing the original draft. X. Y., and H. F.,: review and editing. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Key R&D Programs of China (Grant No.2021YFD1400701), Independent Innovation Project of Henan Academy of Agricultural Sciences(Grant No.2025ZC52),Scientific and technological breakthrough foundation of Henan Province (Grant No.232103810016,222102240057),National Industrial Technology System (CARS-27), Joint Fund of Henan Province (235101610045).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Yongkang You, Ziqi Zhang and Mengzhen Cao for their help in the experiment. We also thanked Mehran Ali (University of Agricultural, Peshawar, Pakistan) for his help in polishing the English writing.
References
- Jiang,Y. Y.; Liu, J.;Zhu. X.M. Analysis of occurrence dynamics and future trend of S. frugiperda invasion in China. China Plant Protection 2019, 39, 33–35. [Google Scholar]
- Ashley, T.R. Classification and distribution of fall armyworm parasites. Fla. Entomol. 1979, 62, 114–23. [Google Scholar] [CrossRef]
- Gardner, W.A.; Fuxa, JR. Pathogens for the suppression of the fall armyworm. Fla. Entomol. 1980, 63, 439–47. [Google Scholar] [CrossRef]
- Molina-Ochoa, J.; Carpenter, JE.; Heinrichs, E.A.; Foster, JE. Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean Basin: an inventory. Fla. Entomol. 2003, 86, 254–89. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder., C.; Baudron., F.; Chinwada., P.; Midega., C.; Schaffner., U.; Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag 2019, 243, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Varella, A.C.; Menezes-Netto. A.C.; Alonso, J.D.D.;Caixeta, D.F.; Peterson, R.K.D.; Fernandes,O.A.;Mortality dynamics of Spodoptera frugiperda (Lepidoptera: Noctuidae) immatures in maize. PLOS ONE 2015, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, P. Natural enemies of insects 2000, 22, 160–167.
- Yu, P. Studies on the Genus Asterina in China. Entomotaxonomia 1982, 4, 317–322. [Google Scholar]
- Pasini, A.; Parra, J.R.P.; Lopes, J,M. Artificial diet for rearing Doru luteipes (Scudder) (Dermaptera: Forficulidae), a predator of the fall armyworm, Spodoptera frugiperda ( J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2007, 36, 308–11. [Google Scholar] [CrossRef] [PubMed]
- Cruz, I.; Valicente, F.H. Manejo da lagarta-do-cartucho, Spodoptera frugiperda, em milho, usandoo predador Doru luteipes e baculovirus. In Relatorio Tecnico Anual CNPMS.
- Tian, C.; Cao, H.; Zhang, J.; Liu, X.; Cai, T.; Li, G.; Huang, J.; Feng, H. Behavioral and Functional Responses of Labidura riparia Pallas Preying on Spodoptera frugiperda. Chinese Journal of Biological Control 2021, 37, 1160–1165. [Google Scholar] [CrossRef]
- Nikos, P.; Hayden, M.; Argyro, F.; Dionysios, P.; Michael, B.; Christopher, D. Adaptive experimental design produces superior and more efficient estimates of predator functional response. PLoS One 2023, 18, e0288445. [Google Scholar] [CrossRef]
- Liang, X.S. Exploration on the function and utilization of Calosoma chinenese Kirby. Natural Enemies of Insects 1980, 4, 52–56. [Google Scholar]
- Holliday, N.J. Age and sex-related variation in defensive secretions of adult chlaenius cordicollis and evidence for their role in sexual communication. Chemoecology. 2016, 26, 107–119. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, J.; Xu, C.; Li, G.; Huang, J.; Liu, Yi.; Wang, Gensong. ; Feng, H.;Yin, X.; Feng, H.Predadtion capacity of Labidura riparia Pallas against Helicoverpa armigera (Hübner). Plant Protetion. 2023, 49, 157–163. [Google Scholar] [CrossRef]
- Zhang, H. Preliminary study of natural enemies of armyworms-Calosoma maderae Kirby. Journal of Applied Entomology 1964, 2, 60–62. [Google Scholar]
- Takuma, N.; Keiko, S. An Extension of the Kimura Two-Parameter Model to the Natural Evolutionary Process. J Mol Evol. 2019, 87, 60–67. [Google Scholar] [CrossRef]
- Matthew, M.; Mathieu, F. Differentiable phylogenetics via hyperbolic embeddings with Dodonaphy. Bioinform Adv. 2024, 4, vbae082. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol Biol Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D. Random search and insect population models. J Anim Ecol. 1972, 41, 369–383. [Google Scholar] [CrossRef]
- Wu, K,J. ; Sheng, C.F.; Gong, P.Y. Equation of predator functional response and estimation of the parameters in it. Entomological Knowledge 2004, 41, 267–269. [Google Scholar]
- Cariou, M.; Henri, H.; Martinez, S.; Duret, L.; Charlat, S. How consistent is RAD-seq divergence with DNA-barcode based clustering in insects? Mol Ecol Resour. 2020, 20, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).