1. Introduction
Over the last twenty years, much research has been done in the areas of root exudates. Plants, as sessile organisms, utilize root exudates to respond to and mediate their surrounding soil environment known as the rhizosphere. Up to 21% of carbon allocated to the roots are exuded into the surrounding environment (Upadhyay et al., 2022). Prior research has demonstrated that the allocation of root carbon is highly intertwined with the surrounding water and nutrient cycles (Canarini et al., 2019). Root Exudation is mostly a passive process, but active mechanisms have been recently linked to exudate secretion (Badri & Vivanco, 2009). Efforts to quantify and qualify exudates have been challenging due to the destruction of roots during exudate collection and contamination by other organisms in the rhizosphere. Thus far, the organic and inorganic compounds secreted by plant roots into the surrounding rhizosphere have primarily been studied in so-called “model” plants. Plants such as arabidopsis, along with crops crucial to monoculture systems including maize (Hu et al., 2018), soybean (Sugiyama, 2019), and rice (Matsushima et al., 2021) have been the central focus of studies inside and outside the United States. This has left a gap in the literature regarding crops in Controlled Environment Agriculture systems. Controlled Environment Agriculture (CEA) is a fast-growing industry which saw at least $900 million in sales in the United States alone in 2022 (USDA Census of Agriculture, 2022). It has been hailed as a keystone to sustainable production in a rapidly industrializing world. The combination of quick production time and high planting density has allowed it to flourish among the indoor fruit and vegetable production industry. When CEA crops have been studied, their focus has been in combating disease and response to stress, rather than strictly qualitative or quantitative analysis of the mechanisms at play. This lack of focus on qualitative analysis has left the scientific community without a database of root exudates that would otherwise be found for crops such as maize, soybean, and rice. Controlled Environment Systems ranging from simple greenhouses to highly advanced, closed loop, nutrient film technique hydroponic systems have yet to be studied with regard to the mechanisms and role of exudates in these respective systems. There is evidence from traditional, soil agriculture which demonstrates a key role for root exudates to aid in crop production (Upadhyay et al., 2022). A role may exist in CEA systems for exudates, particularly with regard to allelopathy, yield increase, and carbon fixation. In synthesizing this review, we have attempted to compile a list of the common root exudates secreted by CEA crops, along with their common functions in the plant and surrounding rhizosphere. The first section of this review describes the amino acids, organic acids, and sugars commonly exuded by CEA crops. It explores their functions, and possible relationships that exudates can have with microbiota. The second section focuses on case studies with regards to specific crops. It encompasses water stress conditions, substrate relationsships, and allelopathy– all situations which provide novel benefits to CEA crop production. The review concludes with an overview of relationships and major findings, and ponders the future directions for root exudate research in CEA crops.
2. Compounds, Functions, and Microbial Interactions
Root exudates can be broadly separated into two categories: Low Molecular Weight Compounds (LMWC) and High Molecular Weight Compounds (HMWC) (Vives-Peris et al., 2019; Upadhyay et al., 2022). The former is made up of ions including H+, various inorganic acids, and all twenty amino acids. Various carbohydrates and sugars including Glucose, Fructose, Galactose, and Sucrose can be present as well. Phenolics or Phenocarboxylic acids, and various organic acids including acetic acid, citric acid, caffeic acid, and malic acid round out the Low Molecular Weight Compounds. Vegetable and fruit crop exudates in CEA systems primarily consist of three compounds. These are amino acids, organic acids, and sugars (Canarini et al., 2019; Vives-Peris et al., 2020; Vančura & Hovadik, 1965).
Amino Acids
While roots of vegetable and fruit crops can and do exude all twenty amino acids, the most common are found to be α-Alanine, β-alanine, arginine, asparagine, cystine/cysteine, glutamine, glycine, histidine, lysine, methionine, phenylalanine, proline, and serine (Vančura & Hovadik, 1965; Trovato et al., 2021). Each serves a function when exuded from the roots, as well as in general plant metabolism. Amino acids are categorized as “Low Molecular Weight Compounds” (Canarini et al., 2019). They can be categorized into subgroups based upon the location of structural functional groups. These subgroups include “alpha, beta, and gamma” designations (Trovato et al., 2021). Roots exudates are dominated by the “alpha” amino acid subgroup. These alpha amino acids are made up of a central carbon atom that is bonded to the amine group as well as a carboxylic group (Trovato et al., 2021). Beta and Gamma subgroups have a differing structure and make up various peptides and compounds such as Butyric Acid (gamma) or β-alanines. While nearly all of the aforementioned amino acids have a role in protein or enzyme synthesis, several of them have other specialized roles as well. α-Alanine’s aid in the process of photosynthesis, while β-alanines are built up in higher concentrations in response to biotic and abiotic stressors (Parthasarathy et al., 2019).
Biotic stressors include predators and microbial attacks. Abiotic stressors include temperature fluctuations, toxic metal accumulation, anoxic conditions, and drought conditions (Vescio et al., 2021). Arginine is involved in nitrogen preservation and recycling, and helps the plant adapt to abiotic stressors (Yang & Gao, 2007). It accumulates in the roots during temperature drops. Asparagine is a non-essential amino acid synthesized in the reproductive tissue of plants. It serves to facilitate nitrogen transport in developing tissues (Sieciechowicz et al., 1988). Cysteine is synthesized to break down sulfur found in the soil (Romero et al., 2014). Sulfur is an important component of enzymatic activity and nitrogen metabolism in plant growth (Xiao et al., 2023). Glycine and glutamine have a role in iron chelation and chlorophyll synthesis respectively (Kan et al., 2015). As well, nitrates and ammonium can be converted into glutamines to make them available for plant use, increasing photosynthetic capacity (Kan et al., 2015). Histidine, lysine, and phenylalanine have roles throughout the plant in signaling responses to environmental stressors (See Figure 2a) (Lopez & Mohiuddin, 2023). Methionine acts as a precursor to ethylene, the hormone which cross-communicates with Auxin to mitigate plant growth and regulate the stress response (Trovato et al., 2021). Proline aids in recovery from plant stress by protecting RUBISCO and stabilizing the electron transport chain (Hayat et al., 2012). Serine is a key amino acid in maintaining homeostasis. It helps in signaling against environmental and abiotic stressors and threats (Ros et al., 2014). More recently, it has been found that serine also aids in the synthesis of other amino acids and phospholipids, marking its utility throughout the entire plant (Ros et al., 2014). In summary, amino acids most commonly exuded by vegetable and fruit crops in CEA systems can be further divided into three main functions (see
Table 1). First, there are those that signal against environmental stressors. Second, those compounds which help to mediate or alleviate the environmental stressors. Third, are the compounds which facilitate nutrient uptake or transport from the rhizosphere and surrounding soil.
Organic Acids
Vegetable and fruit crops in CEA systems primarily exude four organic acids into the surrounding rhizosphere. These are Citric Acid, Tartaric Acid, Lactic Acid, and Aspartic Acid (Kasukawa & Miyazawa, 2020). Citric acid secretions appear to contribute to increases in dissolved organic carbon content. Furthermore, they are a key component of the sulfur and iron redox reaction cycles occurring in the soil. Increased exudation of Citric acid is significantly linked to increases in iron and sulfide concentrations—both of which are necessary for photosynthetic processes (Xiao et al., 2023). Over time, Citric acid exudation can also lower soil pH (Xiao et al., 2023). Tartaric acid, which occurs in most fruits, works in conjunction with Citric acid. It can help with phytoextraction(Khan et al., 2022)—that is the removal of contaminants in the ground where they are then concentrated in shoot tissues. Research by Khan et al., (2022) showed that in conjunction with Citric acid, Tartaric acid enhanced the removal of lead from the environment in turnip crops. Tartaric acid soil amendments have yet to be studied in hydroponic fruit and vegetable crops, and other CEA systems. Lactic acid secretion appears to attract an array of beneficial microbes known as Lactic acid bacteria, or LAB (Murindangabo et al., 2023). These gram positive, facultative aerobes are extremely common across a vast array of environments including the human microbiome as well. In plants, they have several benefits. First, studies indicate that they can serve as a biocontrol agent. This biocontrol translates across bacteria, fungi, insects, and predators (Murindangabo et al., 2023). Increased LAB concentrations led to decreased pest populations through a release of secondary metabolites. Furthermore, these LAB can degrade various polysaccharides, and increase the availability of other nutrients. Plants grown in conjunction with LAB show significant increases in biomass as well as root and shoot length (Lamont et al., 2017). With these increases in bioavailability, LAB has a stabilizing effect on soil structure, and increases water holding capacity (Lamont et al., 2017). Aspartic acid supports a number of functions which are critical to plant survival. Most notably in general metabolism, it serves as a basic amino acid that along with nitrogen and carbon, serves as a backbone of dozens of metabolic processes (Lei et al., 2022). As part of the Krebs cycle, aspartic acid helps to synthesize several of the organic acids in the process. Aspartic acid also aids in the creation of other amino acids, nucleotides, and sugars. As part of the rhizosphere when exuded by plant roots, it helps to assimilate inorganic nitrogen into plant available forms. Under environmental stress, plants can secrete or build up their Aspartic acid content to mitigate drought, salt, heavy metal or even heat (Lei et al., 2022). Aspartic acid remains a critical component of the plant life cycle.
Sugars
Sugars exuded by vegetable crops in CEA systems can alter the composition of the rhizosphere (Lopes et al., 2022). They are a critical part of a substance developed in the rhizosphere known as “Mucilage.” Mucilage is a sticky substance comprised of exuded sugars which provides not only a suitable environment for soil dwelling microbes, but also a source of nutrients (Pettolino et al., 2012; Walker et al., 2003). As of recently, a majority of the studies involving exuded sugars have been restricted to large scale monoculture crops such as Maize or Soybean. Therefore, the functions and mechanisms involved with exuded sugars in CEA crops are speculative as of yet.
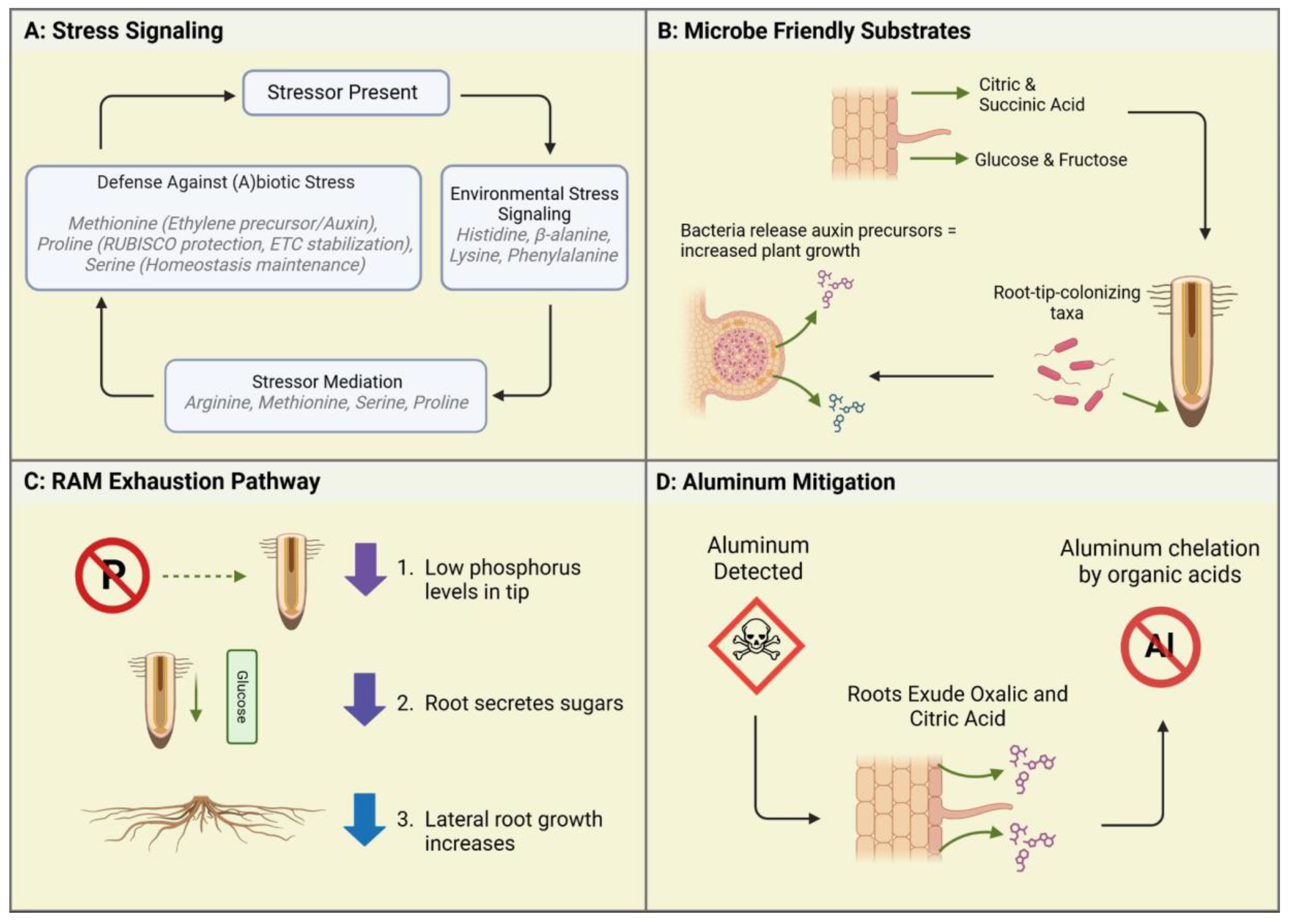
The most common sugars exuded by CEA crops are Xylose, Fructose, and Glucose (Kamilova et al., 2006). Xylose is a monosaccharide that is synthesized through the breakdown of hemicellulose—one of the components of plant cell walls (Harper & Bar-Peled, 2002). Xylose was found to be the primary sugar exuded by seeds and seedlings. The sugar composition of exudates changes with age (Vives-Peris et al., 2020). The sugar exudates of mature plants are composed of fructose and glucose, both monosaccharides, which often bond to each other to create sucrose. They are involved in a plethora of metabolic functions, perhaps most notably cellular respiration. Notably, there is a close connection between root morphology, phosphorus, and exuded sugars (Canarini et al., 2019). Compared to other nutrients, the Root Apical Meristem (RAM) is more sensitive to the abundance (or lack thereof) of phosphorus. When the levels of phosphorus at the root tip are detected as “low,” the plant first sends out organic acids, particularly citric acid, which activate a mechanism known as “RAM exhaustion.” RAM Exhaustion is a process by which the meristematic growth is halted, and sugars are sent to the root tip (Kamilova et al., 2006). When RAM Exhaustion begins, lateral root growth dominates the root architecture (See
Figure 2c). While the exact mechanism is unknown, it can be supposed that the sugars sent to the RAM aid in the building of these lateral roots (Kamilova et al., 2006).
Microbial Interactions
The microbial world of fungi, bacteria, algae, and various protists is nearly innumerable. Plants communicate via their roots with bacteria through the production of certain chemicals and the sensing of the response afterward (Ryan et al., 2016). This call and response can be initiated and well regulated by the plant via its root exudates (Bais et al., 2006). The root colonizing nutrient-fixing bacteria which live in nodules is perhaps one of the best-known plant-microbe interactions. It is a symbiotic relationship as well. To attract the rhizobacteria, legume species will secrete secondary metabolites known as flavonoids, signal to the bacteria, as well as triggers root system transformation—helping create the nodules which the rhizobia will inhabit (Micallef et al., 2009). The common species include Azospirillum, Bacillus, and Klebsiella. Nitrogen fixation is not the only skill of some of these rhizobacteria. Phosphate solubilizing bacteria, or PSB, species of Pseudomonas, Rhizobium, Enterobacter, are just a few of the species which can be signaled by plants under nutrient stress (Lei et al., 2023). Rock and mineralized phosphates are free to be broken down into simpler forms by these bacteria.
Though bacteria are the most efficient in this process, some fungi can be involved as well. Aspergillus and Penicillium are among these species. Rounding out the macronutrients, there are specialized exudations, namely gibberellins, abscisic acid, and ethylene which attract bacteria that break down Potassium (Meena et al. 2014). Again, the organic acids are the most common secretions which attract these (Xu et al., 2021). Potassium is typically bound up in rocks like muscovite, mica, and illite, which can be broken down potassium solubilizing bacteria (KSB). These usually aerobic species include Pseudomonas, B. edaphicus, and B. mucilaginosus. Much of these species have yet to be studied in CEA systems. Their potential impacts remain a topic of future study, with expected differences between soil based, and hydroponic based systems. The growing world of symbiotic microbe-plant interactions promises the possibility of exciting soil additives and soil fertility treatments, utilizing these unique properties in the future (Lei et al., 2023; Upadhyay et al. 2022.; Hu et al., 2018).
3. Changing Environments, Alters Exudates
Controlled environment agriculture can be conducted in a number of ways, with the primary distinction being soil-based systems vs water-based systems. Soil based systems include high tunnels, greenhouses, hoop houses, and growth chambers which utilize soil or soil substitute. Water-based systems are typically hydroponic systems such as ebb and flow, nutrient film technique, or deep water culture. These systems utilize an inert media such as rockwool, LECA clay pebbles, or styrofoam and supplement nutrition through fertigation. Differing systems lead to differing compositions of root exudates in controlled environment agriculture (see
Figure 1). This is primarily due to four main factors including the substrate itself, biotic and abiotic stressors, nutrient availability, and microbial presence. Varying combinations of the aforementioned factors can lead to exudation that enhances plant growth or detracts from it.
Substrates play a key role in determining the composition of exudates through their ability to serve as a habitat for microorganisms as well as influence nutrient availability. Productive soil systems have a balance of high surface area, adequate water holding capacity, and high nutrient availability (see
Figure 1a). Exudates in these systems tend to be a blend of the aforementioned amino acids, organic acids, and sugars (Badri & Vivanco, 2009). The amino and organic acids can aid in nutrient uptake, while sugars can be utilized to attract beneficial bacteria (Dannehl et al., 2015). Water-based systems have inert media that have large surface areas for beneficial microorganisms, providing no nutrients, where water holding capacity is not a concern as many of these systems continuously flow (see
Figure 1b). In these systems, the substrate does not promote a balanced mixture of the three categories of exudates, instead it promotes those which can attract beneficial microorganisms (Hal et al., 2007, Kamilova et al., 2006).
Abiotic stressors are another differing factor which influences the composition of exudates in these systems. Water-based systems can have salinity, pH, mineral content, and importantly water availability, regulated daily or even hourly depending on the sophistication of the equipment (Vescio et al., 2021). Plants in soil based systems, which often cannot regulate these factors, must rely on exudates to aid them in their survival (Parthasarathy et al., 2019, Upadhyay et al., 2022). Thus under ideal conditions, the plants in the water-based system are likely to not exude amino acids which are a part of abiotic stress condition responses (
Figure 1b).
Nutrient availability in soil systems is typically determined by the organic matter content, the soil structure, and the amount of fertilization. Some soil based systems such as coconut coir and perlite systems can sidestep this through fertigation, but the majority of outdoor CEA relies on the aforementioned factors to bring nutrients to the crops. Exudates in such systems include the “Nutrient Uptake Facilitator” branch of amino acids including Asparagine, Cysteine, Glycine and α-Alanine (Trovato et al., 2021). Balanced water-based systems are less likely to have high concentrations of these exudates as their nutrient availability is high from consistent fertigation efforts (Lei et al., 2023).
Microorganisms are a driving factor in the differentiation of exudates in soil vs water-based systems. Studies delving into the microbial populations that exist in water-based CEA systems are limited. These systems can be sterile or nonsterile, and utilize substrates which may create surface area for microbiota or not. Section three will explore a case study from the limited literature regarding hydroponic microorganisms. The literature of soil-based systems has broadened significantly since the discovery of nitrifying root-colonizing bacteria (Canarini et al., 2019). Along with rhizobacteria, various beneficial fungi have been identified as well (Bais et al., 2006). As explained in section one, plants exude sugars in order to attract these bacteria. In water-based systems, these sugars may simply flow into a sump tank and bypass the ability to attract microorganisms completely (
Figure 1). Despite this, the literature reveals that even in higher flow hydroponic systems, plants will continue to exude sugars (Ryan et al., 2016).
Figure 1.
Comparison of Flows of root exudates and their functions in soil-based and hydroponic-based Controlled Environment Agriculture (CEA) systems.(A) Soil-Based System: Root exudates, such as amino acids (e.g., proline, serine, cysteine, glycine) and sugars (e.g., xylose, glucose), contribute to microbial recruitment, nutrient absorption, and stress mitigation. These functions remain largely unchanged in soil systems, where exudates facilitate interactions with fungi and bacteria, enhancing nutrient availability and environmental resilience. (B) Hydroponic-Based System: While root exudates in hydroponic systems contain similar compounds, their function is altered due to the absence of soil. Microbial recruitment is limited, and the impact of exudates on rhizosphere composition, root morphology, and nutrient uptake remains uncertain. Hydroponic systems exhibit reduced exudation of nutrient uptake facilitators due to the controlled, low-stress environment. Further research is needed to understand how these differences affect plant growth and system sustainability.
Figure 1.
Comparison of Flows of root exudates and their functions in soil-based and hydroponic-based Controlled Environment Agriculture (CEA) systems.(A) Soil-Based System: Root exudates, such as amino acids (e.g., proline, serine, cysteine, glycine) and sugars (e.g., xylose, glucose), contribute to microbial recruitment, nutrient absorption, and stress mitigation. These functions remain largely unchanged in soil systems, where exudates facilitate interactions with fungi and bacteria, enhancing nutrient availability and environmental resilience. (B) Hydroponic-Based System: While root exudates in hydroponic systems contain similar compounds, their function is altered due to the absence of soil. Microbial recruitment is limited, and the impact of exudates on rhizosphere composition, root morphology, and nutrient uptake remains uncertain. Hydroponic systems exhibit reduced exudation of nutrient uptake facilitators due to the controlled, low-stress environment. Further research is needed to understand how these differences affect plant growth and system sustainability.
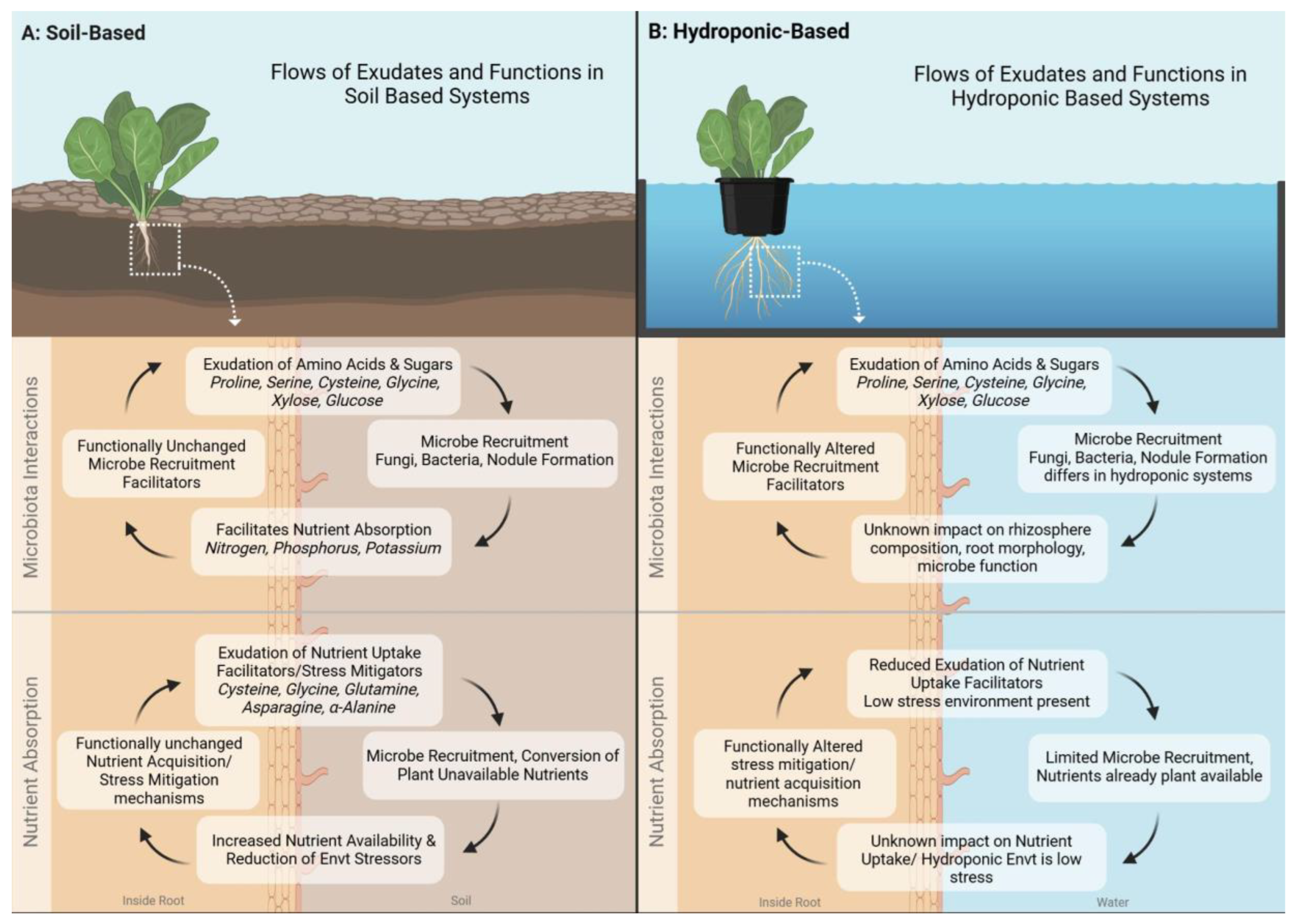
Figure 2.
Case studies illustrating root exudate functions in response to environmental conditions within Controlled Environment Agriculture (CEA). (A) Stress Signaling: When a stressor is present, plants initiate environmental stress signaling through the exudation of specific amino acids. In response, stressor mediation occurs via compounds like arginine, methionine, serine, and proline, which help regulate plant stress responses. Finally, plants deploy defense mechanisms against abiotic and biotic stressors by exuding compounds such as methionine, proline, and serine, contributing to overall stress resilience. (B) Microbe-Friendly Substrates: Root exudates, including organic acids and sugars, facilitate beneficial microbial colonization. Microbes respond by releasing auxin precursors, enhancing plant growth and recruiting root-tip-associated microbial taxa. This process is particularly relevant in hydroponic and soilless systems, where microbial recruitment differs from traditional soil environments. (C) RAM Exhaustion Pathway: Under phosphorus deficiency, plants alter root growth dynamics by exuding sugars at the root tip. This signaling mechanism suppresses primary root elongation while stimulating lateral root proliferation, improving phosphorus acquisition efficiency under nutrient-limited conditions. (D) Aluminum Mitigation: When aluminum toxicity is detected, roots exude organic acids to chelate aluminum ions. This detoxification mechanism prevents aluminum from interfering with root growth, highlighting an essential plant survival strategy in acidic or contaminated environments.
Figure 2.
Case studies illustrating root exudate functions in response to environmental conditions within Controlled Environment Agriculture (CEA). (A) Stress Signaling: When a stressor is present, plants initiate environmental stress signaling through the exudation of specific amino acids. In response, stressor mediation occurs via compounds like arginine, methionine, serine, and proline, which help regulate plant stress responses. Finally, plants deploy defense mechanisms against abiotic and biotic stressors by exuding compounds such as methionine, proline, and serine, contributing to overall stress resilience. (B) Microbe-Friendly Substrates: Root exudates, including organic acids and sugars, facilitate beneficial microbial colonization. Microbes respond by releasing auxin precursors, enhancing plant growth and recruiting root-tip-associated microbial taxa. This process is particularly relevant in hydroponic and soilless systems, where microbial recruitment differs from traditional soil environments. (C) RAM Exhaustion Pathway: Under phosphorus deficiency, plants alter root growth dynamics by exuding sugars at the root tip. This signaling mechanism suppresses primary root elongation while stimulating lateral root proliferation, improving phosphorus acquisition efficiency under nutrient-limited conditions. (D) Aluminum Mitigation: When aluminum toxicity is detected, roots exude organic acids to chelate aluminum ions. This detoxification mechanism prevents aluminum from interfering with root growth, highlighting an essential plant survival strategy in acidic or contaminated environments.
4. Case Studies
Having introduced a theoretical perspective on the common compounds exuded in vegetable and fruit crops, it may be helpful to delve into case studies to better understand specific impacts and implications of certain exudates and controlled environmental conditions. This section will explore four case studies which introduce differing environmental conditions and explores their impacts on exudate secretion, makeup, and plant impact. For a complete list of case studies referenced below, as well as others pertinent to CEA systems see
Table 2.
Organic Acid Content in Water Stress Conditions
In controlled environment production, seedlings are grown separately from the main production area and are transplanted into the main growth and finishing area when they are still small. During this process, seedlings often become stressed and can be damaged (Kim et al., 2018). To alleviate this, it has been proposed to dose seedlings with a treatment of organic acids. Previous studies (Miyazawa, 2016; Kasukawa & Miyazawa, 2020) have established that treatments of organic acids, particularly citric acid, were effective in alleviating drought stress in leafy vegetables. Miyazawa (2016) proposed that Citric acid uptake through the roots acted as a signaling mechanism to leafy vegetables to increase antioxidant enzymatic activity. In turn, this should reduce oxidative damage stemming from water-related stress. To measure the potential damage mitigation effects of dosing seedlings, Kasukawa & Miyazawa (2020) grew cabbage and lettuce seedlings in hydroponics and subjected them to simulated drought stress. A measurement of enzymatic activity was conducted focusing on the catalase and ascorbate peroxidase enzymes– both of which are involved in oxidative activities. Samples that were subjected to drought conditions had increased concentrations of various organic acids, including Citric, Tartaric, Lactic, and Aspartic acid. As Citric acid was not the only organic acid found to be exuded in these treatments, the authors concluded there was not sufficient evidence to state that organic acids absorbed through the roots could act as signaling molecules which reduced oxidative stress. Notably, this study was conducted in a hydroponics system. Studies done in soil-based production systems have found either differing results, or results which reveal a possible interaction between the roots, exudates, and soil microbiome (Gargallo-Garriga et al., 2018). Kasukawa & Miyazawa (2020) note this before going on to explain that there may be a role for exogenous applications, particularly foliar applications, of organic acids to reduce drought stress. There is certainly room for further analysis in this area, specifically in hybrid systems with microbial friendly hydroponic media where results may be different.
Microbiota friendly substrates
As noted in the previous section, studies done under soil conditions appear to have differing results from inert, inorganic, neutral media. In hydroponics, this often means water, styrofoam, or glass beads as substrates. In an effort to create a hybridized system with a media that can be colonized by beneficial microbiota, researchers have used spun-rock: often characterized as rockwool or stonewool and commonly used in hydroponic and aquaponic systems (Dannehl et al., 2015). Spun-rock media, though still inert and neutral, has increased surface area and dry areas which can allow various species of bacteria to colonize and exhibit beneficial effects normally observed in soil (Kamilova et al., 2006). In a joint Dutch/Russian study, stonewool (Spun-rock) substrate was used in a hydroponic system where tomato, cucumber, and sweet peppers were grown. Their plants exuded the major organic acids, citric and succinic acid, along with major sugars glucose and fructose (Kamilova et al., 2006). These exudates prompted microbial colonization substantiated through the detection of increased concentrations of advantageous “root-tip-colonizing” genera
Pseudomonas (Kamilova et al., 2006). These bacteria rely on the organic acid exudates, primarily citric acid, as a carbon source and in turn synthesize plant growth signaling molecules, notably auxin precursors, which help to stimulate plant growth (see
Figure 2b). This effect was particularly noted in the two fruit crops: tomatoes and sweet peppers. While other organic acids varied in their concentration depending on plant age, citric acid content stayed relatively high for all ages of the plant. Malic acid, which is important in juvenile stages for promoting the production of chlorophyll in smaller plants, decreased as the plants reached more mature stages. The opposite was true with regards to succinic acid– an important part of the citric acid cycle. In soil, where results of hydroponic studies have been reaffirmed, succinic acid has also been shown to be secreted by the roots in response to colonization by root-tip and rhizospheric bacteria. The next section will examine how these bacteria and secondary metabolites can contribute to disease control, particularly in fruit crops.
Countering Contamination
Aluminum contamination has been a noted concern in Controlled Environment Production in urban areas. Sites with soil that has been previously contaminated due to waste production runoff, spillage, or other contaminating events may pose an issue to not only food safety but also plant production. However, some plants have been known to develop Aluminum mitigation responses, particularly in their underground root structures (Ofoe et al., 2022). One such response is the efflux of various organic acids (see
Figure 2d) (Chandra & Keshavkant, 2021). Yang et al. (2005), measured the efflux rate of organic acids of Spinach (
Spinacia oleracea) under exposure to aluminum to identify a detection and response mechanism. Young spinach plants grown under hydroponic conditions were exposed to a solution of 0.5 mM CaCl
2 containing varying concentrations of Aluminum Chloride. After six hours of exposure, the exudates were collected and analyzed. The organic acid Oxalate was noted to efflux constantly for six hours, but increased in concert with greater Aluminum concentrations. In the root tip, where aluminum can more easily penetrate, the efflux was 5x that of the rest of the root system. To further investigate whether this response was Aluminum-specific, the researchers attempted to trigger the Oxalate efflux utilizing phosphorus, and with a cation lanthanum. Results demonstrated Oxalate efflux was not affected by phosphorus indicating that spinach has aluminum-specific resistance mechanisms (Yang et al., 2004).The understanding and application of such mechanisms is critical for urban CEA production in soils which may have mild aluminum contamination, or areas which may be growing produce that is not suitable for human consumption (Chandra & Keshavkant, 2021). As CEA expands into (peri)urban landscapes, finding plants with mechanisms that can make them suitable for production in non-optimal areas is a key challenge that must be addressed.
Allelopathic potential of root exudates
Allelopathic compounds constitute a multitude of low-weight molecular compounds which are exuded from plants during various stages of secondary metabolism (Hao et al., 2007). First described in the 1930’s, they range in toxicity, concentration, effect, and composition (Cheng & Xu, 2013). As of late, they have begun to be studied as a part of monocropping systems due to their ability to suppress diseases that typically invade continuous cropping systems (Sheldon et al., 2021, Cheema, Khaliq & Saeed, 2004). With the increasing role of controlled environment agriculture, allelopathic potential has become a prescient issue, particularly among hydroponically grown fruit and vegetable crops. Studies conducted by Hao et al. (2010; 2007) measured the impacts the allelopathic exudates produced by mature hydroponically grown watermelon on watermelon and lettuce seedlings. Watermelon can be prone to autotoxicity, and disease susceptibility– particularly in continuously cropped systems. Utilizing a continuous root exudate tapping system (see Fig. 1 CRETS, Hao et al. 2007) the researchers were able to measure the exuded allelopathic compounds over the course of one-hundred days. Results indicated that all tissues in the mature watermelon plants contained and exuded allelopathic compounds, and that these compounds had inhibitory effects particularly on the radicle elongation of watermelon and lettuce seedlings (Hao et al., 2007).. However, as this study only observed the growth of other plants, it could not conclude on the potential for allelochemicals to mitigate crop diseases. For that, we turn to our next case, which measured the capabilities for allelochemicals to combat common watermelon diseases.
Allelopathic disease mitigation
Fusarium wilt is a common disease in watermelon crops caused by the fungi Fusarium oxysporum. It is a pathogen which decimates watermelon yields, particularly in continuous cropping systems (Rahman et al., 2021). Early research indicates an interaction between root exudates of watermelon and Fusarium. To qualify this interaction, and compare it to known allelopathic compounds, Hao et al. (2010) grew watermelon and rice hydroponically and measured the impact of their exudates on spore germination and sporulation of Fusarium. Rice has been previously demonstrated to have inhibitory effects on the growth of Fusarium spore germination and sporulation. Watermelon root exudates were found to significantly increase the growth of Fusarium while rice crops had anti-fungal properties which significantly limited the growth of the fungus. Thus, an intercropping system of rice with watermelon could successfully mitigate fungal growth in hydroponic systems and in turn reduce disease pressure, leading to increased watermelon yields (Hao et al., 2010). This research demonstrates that the disease mitigation properties of root exudates differ from crop to crop– particularly among fruit crops as will be discussed in the next example.
Pest mitigation responses differ significantly between CEA crop species– especially those that involve fruit development. Tomato crops are susceptible to a number of pests, with nematodes being a consistent source of pest pressure (Pulavarty et al., 2020). Yang et al. (2016) grew tomato crops in a greenhouse and inoculated the roots with Meloidogyne incognita, a common nematode to tomato production. The tomato root exudates were found to suppress the egg hatching capabilities of M. incognita. Furthermore, exudates increased the mortality of juvenile M. incognita hatchlings. In response to inoculation, phenolic compounds, known to have allelopathic effects, increased significantly. The set of secreted compounds appeared to have three functions. Dibutyl phthalate served to repel M. incognita, thereby preventing eggs from being laid. 2,6-Di-tert-butyl-p-cresol and dimethyl phthalate suppressed eggs from hatching. L-ascorbyl 2,6-dipalmate was correlated with increased juvenile mortality once the eggs hatched. The four compounds worked together to mitigate nematodal infection (Yang et al., 2016). The researchers did note that other studies show that certain compounds work to attract nematodes and other pests rather than repel them. Based upon prior literature, it can be postulated that these mechanisms have most likely been developed to attract beneficial microbiota such as rhizobia, but have the inadvertent effect of attracting certain pests instead (Cao et al., 2016). Thus, it becomes clear that the allelopathic effects of root exudates are only beginning to be understood, and have the potential to change cropping systems as researchers develop a better understanding of the network and cross-talk of root exudates and allelochemicals.
5. Discussion
While much headway has been made over the past two decades in understanding the composition and role of root exudates in controlled production systems, there are still several avenues of further exploration in CEA root exudates. For many years, root exudate research was focused on industrially produced field crop species such as maize, wheat, and soybean. As research began to branch out in the early 2010’s, studies continued to be focused on select crops. The limited number of studies outside of a few select CEA crops, namely tomato, lettuce, and pepper, along with an indoor production sector wide focus on strictly hydroponic production has left a sizable knowledge gap to be filled. Notably, studies regarding production in large scale fields are few and far between. An exhaustive search of the literature revealed just under twenty studies (see
Table 2) with direct implications in CEA root exudate crop production. Aforementioned studies in ecological niches or less cultivated species could create a database connecting crop species to specific exudates, and their applications. In conjunction, the exogenous application of specific exudates, such as organic acids or sugars has yet to be studied in depth. Furthermore, the ability for these applications to recruit microorganisms in both field conditions, as well as in hydroponic production systems remains relatively unexplored. Increases in yield, disease resistance, soil restoration, land reclamation, and more are just a few possibilities that future studies could yield in this sector. One final note is that as climate change continues to alter environmental conditions, it would be prudent to study alterations in CEA crop exudates under anticipated conditions including high heat, increased carbon dioxide concentrations, and limited water availability. With a world population projected to continue growing at a rapid pace, along with the industrialization of several major countries in the global south, global CEA production will continue to grow as well. More efficient utilization of resources can be achieved through expanding our database of root exudates, their functions, pathways, and impacts on plants and production systems.
Author Contributions
DC-S and CV conceptualized the manuscript. DC-S conducted the literature review and wrote the original draft. CV provided mentorship, contributed to writing and editing, and guided the overall direction of the manuscript. Both authors reviewed and approved the final version and agree to be accountable for its content.
Funding
This work is supported by the Hatch Capacity Program, project award no AWD13727, from the U.S. Department of Agriculture’s National Institute of Food and Agriculture.
Data Availability Statement
The datasets analyzed for this study are included within the article. Further inquiries can be directed to the corresponding author, at camilo.villouta@uri.edu
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant, Cell & Environment 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annual Review of Plant Biology 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- BioRender. Figure 2. Case Studies in Functions. 2025a. [Google Scholar]
- BioRender. Figure 1. Comparison of Exudate Functions by System. 2025b. [Google Scholar]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Frontiers in Plant Science 2019, 10. [Google Scholar]
- Cao, Z.-Q.; Fan, T.-F.; Bi, Y.-M.; Tian, G.-L.; Zhang, L.-S. Potassium deficiency and root exudates reduce root growth and increase Fusarium oxysporum growth and disease incidence in continuously cultivated strawberry. New Zealand Journal of Crop and Horticultural Science 2016, 44, 58–68. [Google Scholar] [CrossRef]
- Chandra, J.; Keshavkant, S. Mechanisms underlying the phytotoxicity and genotoxicity of aluminum and their alleviation strategies: A review. Chemosphere 2021, 278, 130384. [Google Scholar] [CrossRef]
- Dannehl, D.; Suhl, J.; Ulrichs, C.; Schmidt, U. Evaluation of substitutes for rock wool as growing substrate for hydroponic tomato production. Journal of Applied Botany and Food Quality 2015, 88, 6877. [Google Scholar] [CrossRef]
- Dhungana, I.; Kantar, M.B.; Nguyen, N.H. Root exudate composition from different plant species influences the growth of rhizosphere bacteria. Rhizosphere 2023, 25, 100645. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci Rep 2018, 8, 12696. [Google Scholar] [CrossRef]
- Hao, W.; Ren, L.; Ran, W.; Shen, Q. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Hao, Z.P.; Wang, Q.; Christie, P.; Li, X.L. Allelopathic potential of watermelon tissues and root exudates. Scientia Horticulturae 2007, 112, 315–320. [Google Scholar] [CrossRef]
- Harper, A.D.; Bar-Peled, M. Biosynthesis of UDP-Xylose. Cloning and Characterization of a Novel Arabidopsis Gene Family, UXS, Encoding Soluble and Putative Membrane-Bound UDP-Glucuronic Acid Decarboxylase Isoforms. Plant Physiology 2002, 130, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal Behav 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Verheust, Y.; Bonarrigo, G.; Van Hulle, S. Closed hydroponic systems: operational parameters, root exudates occurrence and related water treatment. Rev Environ Sci Biotechnol 2017, 16, 59–79. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; Schlaeppi, K.; Erb, M. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun 2018, 9, 2738. [Google Scholar] [CrossRef]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Azarova, T.; Makarova, N.; Lugtenberg, B. Organic Acids, Sugars, and l-Tryptophane in Exudates of Vegetables Growing on Stonewool and Their Effects on Activities of Rhizosphere Bacteria. MPMI 2006, 19, 250–256. [Google Scholar] [CrossRef]
- Kan, C.-C.; Chung, T.-Y.; Juo, Y.-A.; Hsieh, M.-H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genomics 2015, 16, 731. [Google Scholar] [CrossRef]
- Kasukawa, N.; Miyazawa, K. Examining Organic Acid Root Exudate Content and Function for Leafy Vegetables Under Water-Stressed Conditions. Journal of Horticultural Research 2020, 28, 83–90. [Google Scholar] [CrossRef]
- Kawasaki, A.; Okada, S.; Zhang, C.; Delhaize, E.; Mathesius, U.; Richardson, A.E.; Watt, M.; Gilliham, M.; Ryan, P.R. A sterile hydroponic system for characterising root exudates from specific root types and whole-root systems of large crop plants. Plant Methods 2018, 14, 114. [Google Scholar] [CrossRef]
- Khan, I.; Iqbal, M.; Raza, S.H.; Anwar, S.; Ashraf, M.; Shafiq, F. Tartaric acid soil-amendment increases phytoextraction potential through root to shoot transfer of lead in turnip. Chemosphere 2022, 296, 134055. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biology and Biochemistry 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, B.Y.; Lee, H.J. Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce (Lactuca sativa L.). Scientia Horticulturae 2006, 110, 119–128. [Google Scholar] [CrossRef]
- Lei, S.; Rossi, S.; Huang, B. Metabolic and Physiological Regulation of Aspartic Acid-Mediated Enhancement of Heat Stress Tolerance in Perennial Ryegrass. Plants 2022, 11, 199. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.D.; Wang, P.; Futrell, S.L.; Schachtman, D.P. n.d. Sugars and Jasmonic Acid Concentration in Root Exudates Affect Maize Rhizosphere Bacterial Communities. Appl Environ Microbiol 88, e00971-22. [CrossRef] [PubMed]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids, in: StatPearls. StatPearls Publishing, Treasure Island (FL). 2023. [Google Scholar]
- Marschner, P.; Crowley, D.E.; Higashi, R.M. Root exudation and physiological status of a root-colonizing fluorescent pseudomonad in mycorrhizal and non-mycorrhizal pepper (Capsicum annuum L.). Plant and Soil 1997, 189, 11–20. [Google Scholar] [CrossRef]
- Matsushima, C.; Shenton, M.; Kitahara, A.; Wasaki, J.; Oikawa, A.; Cheng, W.; Ikeo, K.; Tawaraya, K. Multiple analysis of root exudates and microbiome in rice (Oryza sativa) under low P conditions. Arch Microbiol 2021, 203, 5599–5611. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiological Research 2014, 169, 337–347. [Google Scholar] [CrossRef]
- Micallef, S.A.; Channer, S.; Shiaris, M.P.; Colón-Carmona, A. Plant age and genotype impact the progression of bacterial community succession in the Arabidopsis rhizosphere. Plant Signal Behav 2009, 4, 777–780. [Google Scholar] [CrossRef]
- Miyazawa, K. Drought stress alleviation of cabbage seedlings by citric acid application. Acta Hortic 2016, 101–108. [Google Scholar] [CrossRef]
- Murindangabo, Y.T.; Kopecký, M.; Perná, K.; Nguyen, T.G.; Konvalina, P.; Kavková, M. Prominent use of lactic acid bacteria in soil-plant systems. Applied Soil Ecology 2023, 189, 104955. [Google Scholar] [CrossRef]
- Neumann, G.; Bott, S.; Ohler, M.; Mock, H.-P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, Lord. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The Synthesis and Role of β-Alanine in Plants. Frontiers in Plant Science 2019, 10. [Google Scholar] [CrossRef]
- Pramanik, M.H.R.; Nagai, M.; Asao, T.; Matsui, Y. Effects of Temperature and Photoperiod on Phytotoxic Root Exudates of Cucumber (Cucumis sativus) in Hydroponic Culture. J Chem Ecol 2000, 26, 1953–1967. [Google Scholar] [CrossRef]
- Pulavarty, A.; Horgan, K.; Kakouli-Duarte, T. Effect of an Alltech soil health product on entomopathogenic nematodes, root-knot nematodes and on the growth of tomato plants in the greenhouse. Journal of Nematology 2020, 52, 1–10. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Ahmad, K.; Bashir Kutawa, A.; Siddiqui, Y.; Saad, N.; Geok Hun, T.; Hata, E.M.; Hossain, M.I. Biology, Diversity, Detection and Management of Fusarium oxysporum f. sp. niveum Causing Vascular Wilt Disease of Watermelon (Citrullus lanatus): A Review. Agronomy 2021, 11, 1310. [Google Scholar] [CrossRef]
- Romero, L.C.; Aroca, M.Á.; Laureano-Marín, A.M.; Moreno, I.; García, I.; Gotor, C. Cysteine and Cysteine-Related Signaling Pathways in Arabidopsis thaliana. Molecular Plant 2014, 7, 264–276. [Google Scholar] [CrossRef]
- Ros, R.; Muñoz-Bertomeu, J.; Krueger, S. Serine in plants: biosynthesis, metabolism, and functions. Trends Plant Sci 2014, 19, 564–569. [Google Scholar] [CrossRef]
- Ryan, P.R.; Delhaize, E.; Watt, M.; Richardson, A.E. Plant roots: understanding structure and function in an ocean of complexity. Ann Bot 2016, 118, 555–559. [Google Scholar] [CrossRef]
- Sieciechowicz, K.A.; Joy, K.W.; Ireland, R.J. The metabolism of asparagine in plants. Phytochemistry 1988, 27, 663–671. [Google Scholar] [CrossRef]
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. Journal of Advanced Research, Special Issue on Plant Microbiome 2019, 19, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino Acids in Plants: Regulation and Functions in Development and Stress Defense. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef]
- Upadhyay, S.; Srivastava, A.; Rajput, V.; Chauhan, P.; Bhojiya, A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Frontiers in Microbiology 2022, 916488. [Google Scholar] [CrossRef] [PubMed]
- USDA National Agricultural Statistics Service, 2022 Census of Agriculture. Complete data available at www.nass.usda.gov/AgCensus., n.d.
- Vančura, V.; Hovadík, A. Root exudates of plants. Plant Soil 1965, 22, 21–32. [Google Scholar] [CrossRef]
- Vescio, R.; Caridi, R.; Laudani, F.; Palmeri, V.; Zappalà, L.; Badiani, M.; Sorgonà, A. Abiotic and Herbivory Combined Stress in Tomato: Additive, Synergic and Antagonistic Effects and Within-Plant Phenotypic Plasticity. Life 2022, 12, 1804. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root Exudation and Rhizosphere Biology. Plant Physiol 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Q.; Zhao, S.; Chen, D.; Gao, N.; Huang, M.; Ye, X. Citric acid secretion from rice roots contributes to reduction and immobilization of Cr(VI) by driving microbial sulfur and iron cycle in paddy soil. Science of The Total Environment 2023, 854, 158832. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, H.; Zhu, B.; Hussain, H.A.; Zhang, K.; Tian, X.; Duan, M.; Xie, X.; Wang, L. Potassium Improves Drought Stress Tolerance in Plants by Affecting Root Morphology, Root Exudates and Microbial Diversity. Metabolites 2021, 11, 131. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, B.; Zhang, X.; Zhang, Z.; Wu, Y.; Zhang, Y.; Lü, S.; Zou, Q.; Gao, Y.; Teng, L. Effects of Tomato Root Exudates on Meloidogyne incognita. PloS one 2016, 11, e0154675. [Google Scholar] [CrossRef]
- Yang, H.-Q.; Gao, H.-J. [Physiological function of arginine and its metabolites in plants]. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2007, 33, 1–8. [Google Scholar] [PubMed]
- Yang, J.L.; Zheng, S.J.; He, Y.F.; Matsumoto, H. Aluminium resistance requires resistance to acid stress: a case study with spinach that exudes oxalate rapidly when exposed to Al stress. Journal of Experimental Botany 2005, 56, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- ZhongQun, H.; Junan, Z.; HaoRu, T.; Zhi, H. Different Vegetables Crops in Response to Allelopathic of Hot Pepper Root Exudates. 2012. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







