Submitted:
06 February 2025
Posted:
07 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Entomopathogenic Bacterial Families That Affect Aphids
2.1. Bacillaceae
2.2. Enterobacteriaceae
2.3. Moraxellaceae
2.4. Xanthomonadaceae
2.5. Pseudomonadaceae
2.6. Streptomycetaceae
2.7. Neisseriaceae
2.8. Brucellaceae
2.9. Leuconostocaceae
3. Possible Reasons for the Limited Toxicity of BPP Against Aphids
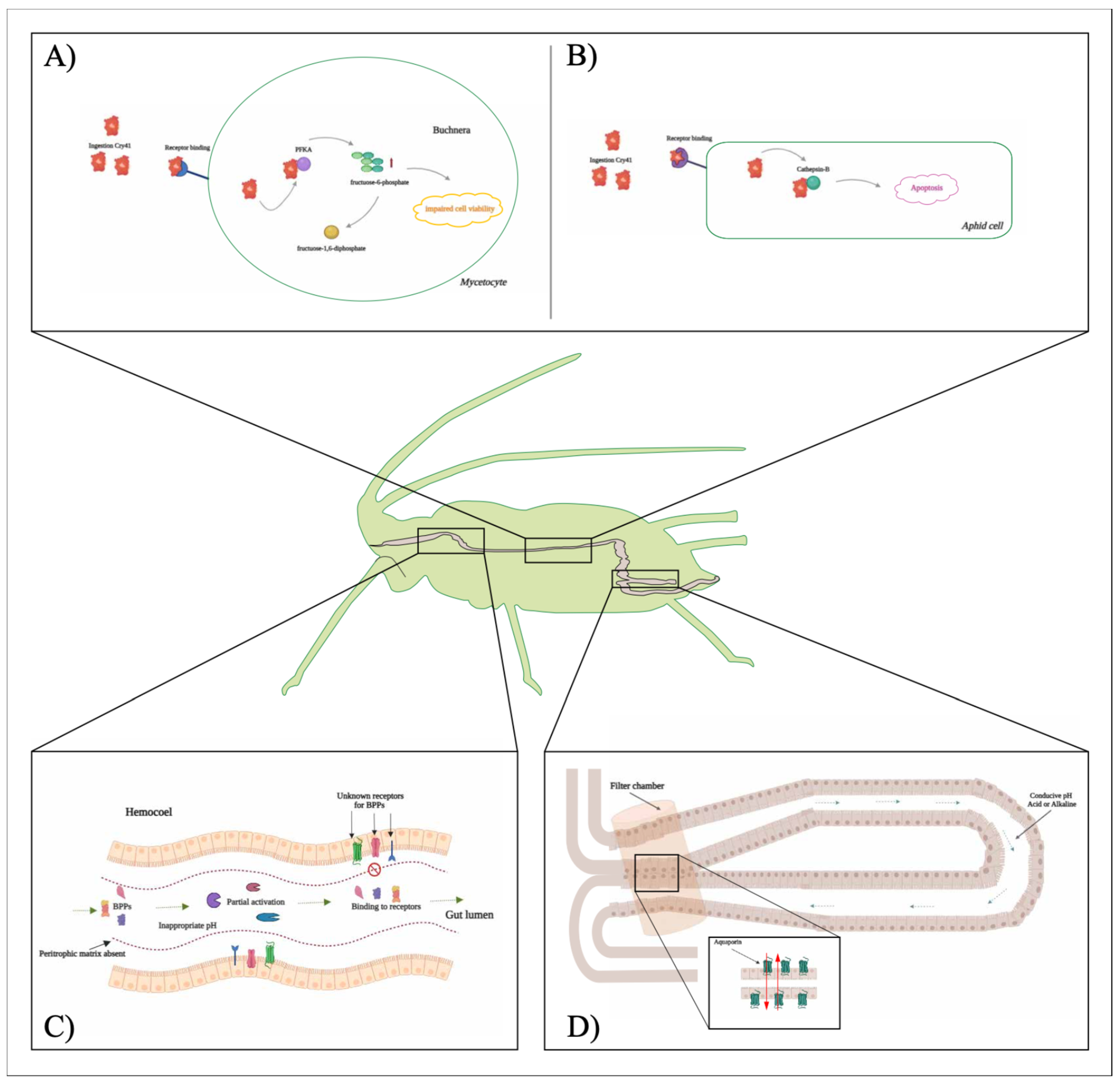
4. Mode of Action of Bacterial Pesticidal Proteins (BPPs) in Aphids
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Remaudière, G.; Remaudière, M. Catalogue of the worlds Aphididae (Homoptera Aphidoidea); INRA: París, Francia, 1997; p. 473. [Google Scholar]
- Emden, H.F. , Harrington, R. Aphids as crop pests; CAB International: Wallingford, Oxfordshire, UK, 2017; p. 714. [Google Scholar]
- Singh, B.U.; Padmaia, P.G.; Seetharama, N. Biology and management of the sugarcane aphid, Melanaphis sacchari (Zehntner) (Homoptera: Aphididae), in sorghum: a review. Crop Prot 2004, 23,739-755. [CrossRef]
- Bowling, R.D.; Brewer, M.J.; Kerns, D.L.; Gordy, J.; Seiter, N.; Elliott, N.; Buntin, G.D.; Way, M.O.; Royer, T.A.; Biles, S.; Maxson, E. Sugarcane aphid (Hemiptera: Aphididae): A new pest on sorghum in North America. J. Integr. Pest Manag, 2016; 7, 1–13. [Google Scholar] [CrossRef]
- Rodríguez, L.; _Terán, A. Melanaphis sacchari (Hemiptera: Aphididae): A new sorghum insect pest in Mexico. Southwest. Entomol 2015, 40, 433–434. [Google Scholar] [CrossRef]
- Field, L.M.; Devonshire, A.L.; Forde, B.G.; Molecular evidence that insecticide resistance in peach-potato aphids (Myzus persicae Sulz.) results from amplification of an esterase gene. Biochem J 1988, 251, 309-312. [CrossRef]
- Sarwar, M. The killer chemicals as controller of agriculture insect pests: The conventional insecticides. Int. J. Chem. Sci 2015, 1, 141–147. [Google Scholar]
- Vaňková, J.; Purrini, K. Natural epizooties caused by bacilli of the species Bacillus thuringiensis and Bacillus cereus. J. Appl. Entomol 1979, 88, 216–221. [Google Scholar] [CrossRef]
- Porcar,M.; Grenier, A.; Federici, B.; Rahbe, Y. Effects of Bacillus thuringiensis endotoxins on the Pea Aphid (Acyrthosiphon pisum). Appl. Environ. Microbiol 2009,75, 4897-4900. [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; de Escudero, I.R. , Caballero, P. Molecular and insecticidal characterization of a novel Cry-related protein from Bacillus thuringiensis toxic against Myzus persicae. Toxin 2014, 6, 3144–3156. [Google Scholar] [CrossRef]
- de Maagd, R.A.; Bravo, A.; Berry, C.; Crickmore, N.; Schnepf, H.E. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet 2003, 37, 409–433. [Google Scholar] [CrossRef]
- Alquisira, E.V.; Paredes, J.R.; Hernández, V. M.; Ramírez, J.A.; Peña, G. In vitro susceptibility of Varroa destructor and Apis mellifera to native strains of Bacillus thuringiensis. Apidologie 2014, 45, 707–718. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A. , Floris, I. Emerging entomopathogenic bacteria for insect pest management. Bull. Insectology 2013, 66, 181–186. [Google Scholar]
- Shi, Y.; Zhang, X.; Lou, K. Isolation, characterization, and insecticidal activity of an endophyte of drunken horse grass, Achnatherum inebrians. J. Insect Sci 2013, 13, 151. [Google Scholar] [CrossRef]
- Fisher, T.W.; Garczynski, S.F. Isolation, culture, preservation, and identification of entomopathogenic bacteria of the Bacilli. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Academic Press: New York, USA, 2012; pp. 75–79. [Google Scholar]
- Tsagou, V.; Lianou, A.; Lazarakis, D., Emmanouel, N.; Aggelis, G. Newly isolated bacterial strains belonging to Bacillaceae (Bacillus sp.) and Micrococcaceae accelerate death of the honey bee mite, Varroa destructor (V. jacobsoni), in laboratory assays. Biotechnol. Lett 2004, 26, 529-532. [CrossRef]
- Bulla, L.A.; Rhodes, R.A.; Julian, G.S. Bacteria as insect pathogens. Annu Rev Microbiol 1975, 29, 163–190. [Google Scholar] [CrossRef]
- Payne, J.R.; Cannon, R.J.C.. Use of Bacillus thuringiensis isolates for controlling pests in the family Aphididae. 1993. Patent NumberUS Patent 5, 262, 159.
- Lacey, L.A.; Grzywacz, D. Shapiro, D.I.; Frutos, R., Brownbridge, M. Goettel, M.S.Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 2015, 132,1-41. [CrossRef]
- Li, H.; Chougule, N.P.; Bonning, B.C. Interaction of the Bacillus thuringiensis delta endotoxins Cry1Ac and Cry3Aa with the gut of the pea aphid, Acyrthosiphon pisum (Harris). J. Invertebr. Pathol 2011, 107, 69–78. [Google Scholar] [CrossRef]
- Zhao, X.D.; Zhang, B.W.; Fu, L.J.; Li, Q.L.; Lin, Y.; Yu, X.Q. Possible Insecticidal Mechanism of Cry41-Related Toxin against Myzus persicae by Enhancing Cathepsin B Activity. J Agric Food Chem. 2020, 68, 4607–4615. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, B.W.; Lu, J.W.; Liao, J.A.; Zhu, Q.J.; Lin, Y.; Yu, X.Q. The mechanism of Cry41-related toxin against Myzus persicae based on its interaction with Buchnera-derived ATP-dependent 6-phosphofructokinase. Pest Manag Sci. 2023, 79, 1684–169. [Google Scholar] [CrossRef] [PubMed]
- Chougule, N.P.; Bonning, B.C. Toxins for transgenic resistance to hemipteran pests. Toxins 2012, 4, 405–429. [Google Scholar] [CrossRef]
- Mishra, R.; Arora, A.K.; Jiménez, J.; Dos Santos, C.; Banerjee, R.; Panneerselvam, S.; Bonning, B.C. Bacteria-derived pesticidal proteins active against hemipteran pests. J. Invertebr. Pathol 2022, 195, 107834. [Google Scholar] [CrossRef]
- Walters,F.S.; English, LH.. Toxicity of Bacillus thuringiensis δ-endotoxins toward the potato aphid in an artificial diet bioassay. Entomol. Exp. Appl 1995,77,211-216. [CrossRef]
- Gerardo, N.M.; Altincicek, B.; Anselme, C.; Atamian, H.; Barribeau, S.M.; de Vos, M.; Duncan, E.J.; Evans, J.D.; Gabaldón, T.; Ghanim, M.; Heddi, A.; Kaloshian, I.; Latorre, A.; Moya, A.; Nakabachi,A.; Parker, B.J.; Pérez, V.; Pignatelli, M.; Rahbé, Y.; Ramsey, J.S.; Spragg, C.J.; Tamames, J.; Tamarit, D.; Tamborindeguy, C.; Vincent, C.; Vilcinskas, A. Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol.2010,11,1-17. [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 2010, 55, 247–66. [Google Scholar] [CrossRef]
- Logan, N. A.; Vos, P. D. Bacillus. Bergey’s manual of systematics of archaea and bacteria, Whitman,W.B.,Wiley: Hoboken, New Jersey, 2015; pp.1-163. [CrossRef]
- Mandic,I.; Stefanic, P.; van Elsas, J. D. Ecology of Bacillaceae. Microbiol. Spectr 2015,3. [CrossRef]
- Lambert, B.; Peferoen, M. Insecticidal promise of Bacillus thuringiensis. Facts and mysteries about a successful biopesticide. BioScience 1992, 42, 112–122. [Google Scholar] [CrossRef]
- Pigott, CR.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef]
- Frankenhuyzen, K.V. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol 2009, 101, 1–16. [Google Scholar] [CrossRef]
- Malik, K.; Riazuddin, S. Immunoassay-based approach for detection of novel Bacillus thuringiensis-endotoxins, entomocidal to cotton aphids (Aphis gossypii) and whiteflies (Bemisia tabaci). Pak. J. Bot 2006, 38, 757–765. [Google Scholar]
- Monnerat, R.G.; Melatti, V.; Praça, L.; Martins, É.; Sujii, E.; Berry, C. , Selection of Bacillus thuringiensis strains toxic against cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). BioAssay 2010, 5, 2. [Google Scholar] [CrossRef]
- Alahyane, H.; Ouknin, M.; Alahyane, A.; Aboussaid, H.; Oufdou, K.; El Messoussi, S.; Mounir, A. : Majidi, L. Aphicidal activities of Moroccan Bacillus thuringiensis strains against cotton aphid (Aphis gossypii). Biointerface Res. Appl. Chem 2021, 12, 3348–3356. [Google Scholar] [CrossRef]
- Rajashekhar, M.; Kalia, V.K. Native Bt strains efficacy against cotton aphid Aphis gossypii Glover. J Pharmacogn Phytochem 2017, 6, 938–940. [Google Scholar]
- Ramasamy, A.; Suresh, M.; Mallesh, M.S.H. Toxicity evaluation of Aphidicidal crystalliferous toxins from Bacillus strains: a molecular study. Ann. Microbiol 2020, 70, 214. [Google Scholar] [CrossRef]
- Torres, M.C.; Arenas, I., Hernández, V.M., Suárez, R.; Peña, G. Characterization of Bacillus thuringiensis (Bacillaceae) strains pathogenic to Myzus persicae (Hemiptera: Aphididae). Fla. Entomol 2016, 99, 639-643. [CrossRef]
- López, G.; Alvarez, A.E.; Petroselli, G.; Erra,R.; Audisio, M.C. Aphicidal activity of Bacillus amyloliquefaciens strains in the peach-potato aphid (Myzus persicae). Microbiol Res. 2019,226, 41-47. [CrossRef]
- Sattar, S.; Biswas, P.K., Hossain, M.A.; Maiti, M.K.; Sen, S.K.; Basu, A.Search for vegetative insecticidal proteins (VIPs) from local isolates of Bacillus thuringiensis effective against lepidopteran and homopteran insect pests. Biopestic. Int. 2008, 1, 216-222. [CrossRef]
- Baazeem,A.; Alotaibi, S.S.; Khalaf, L.K.; Kumar, U.; Zaynab, M.; Alharthi, S.; Darwish, H.; Alghamdi, A.; Jat, S.K.; Al-Barty, A.; Albogami, B.; Noureldeen, A.; Ravindran, B. Identification and environment-friendly biocontrol potential of five different bacteria against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae). Front Microbiol.2022, 13,961349. [CrossRef]
- Yu, X.; Liu, T.; Liang, X.; Tang, C.; Zhu, J.; Wang, S., Li P. Rapid detection of vip1-type genes from Bacillus cereus and characterization of a novel vip binary toxin gene. FEMS Microbiol. Lett 2011,325, 30-36. [CrossRef]
- Sattar, S.; Maiti, M.K. Molecular characterization of a novel vegetative insecticidal protein from Bacillus thuringiensis effective against sap-sucking insect pest. Microbiol biotechn 2011, 21, 937–946. [Google Scholar] [CrossRef]
- Loth, K.; Costechareyre, D.; Effantin, G.; Rahbé, Y.; Condemine, G.; Landon, C.; da Silva, P. New Cyt-like δ-endotoxins from Dickeya dadantii: structure and aphicidal activity. Sci. Rep 2015, 5, 8791. [Google Scholar] [CrossRef]
- Paula, D.P.; Andow, D.A. Differential Cry toxin detection and effect on Brevicoryne brassicae and Myzus persicae feeding on artificial diet. Entomol. Exp. Appl 2016, 159, 54–60. [Google Scholar] [CrossRef]
- Rausch, M.A.; Chougule, N.P.; Deist, B.R.; Bonning, B.C. Modification of Cry4Aa toward improved toxin processing in the gut of the pea aphid, Acyrthosiphon pisum PloS One 2016, 11, e0155466. [CrossRef]
- Borman, E.K.; Stuart, C.A; Wheeler, K.M. Taxonomy of the family Enterobacteriaceae. J. Bacteriol 1944, 48, 351–367. [Google Scholar] [CrossRef]
- Jenkins,C.; Rentenaar, R.J.; Landraud, L.; Brisse, S. Enterobacteriaceae A2.In Infectious Diseases,4th ed., Elsevier: New York, 2017; 2 pp.1565–1578, e1562.
- Kang,E.; Crouse, A.; Chevallier, L.; Pontier, S.M.; Alzahrani, A.; Silué, N.; Campbell-Valois, F.X., Montagutelli, X.; Gruenheid, S.; Malo, D. Enterobacteria and host resitence to infection. Mamm Gaenome 2018, 29,558-576. [CrossRef]
- Harada, H.; Ishikawa, H. Experimental pathogenicity of Erwinia aphidicola to pea aphid, Acyrthosiphon pisum. Nat. Struct. Mol. Bio 1997, 43, 363–367. [Google Scholar] [CrossRef]
- Hashimoto, Y. Study of the bacteria pathogenic for aphids, isolation of bacteria and identification of insecticidal compound. Report of Hokkaido Prefectural Agricultural Experiment Stations 2002, 102, 1–48. [Google Scholar]
- Paliwal, D.; Hamilton, A.J. , Barrett, G.A.; Alberti, F.; van Emden, H.; Monteil, C.L.; Mauchline, T.H.; Nauen, R.; Wagstaff,C.; Bass, C.; Jackson, R.W. Identification of novel aphid-killing bacteria to protect plants. Microb Biotechnol 2022, 4, 1203–1220. [Google Scholar] [CrossRef]
- Stavrinides, J.; No, A.; Ochman, H. A single genetic locus in the phytopathogen Pantoea stewartii enables gut colonization and pathogenicity in an insect host. Environ. Microbiol 2010, 12, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Campillo,T.; Luna, E.; Portier, P; Fischer,L.; Saux, M.; Lapitan, N.; Tisserat, N.A.; Leach, J.E. Erwinia iniecta sp. nov., isolated from Russian wheat aphid (Diuraphis noxia). Int J Syst Evol Microbiol 2015, 65, 3625-3633. [CrossRef]
- Grenier, A.M.; Duport, G.; Pagès, S.; Condemine, G.; Rahbé, Y. The phytopathogen Dickeya dadantii (Erwinia chrysanthemi 3937) is a pathogen of the pea aphid. Appl. Environ. Microbiol 2006, 72, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Costechareyre, D.; Dridi, B.; Rahbé, Y.; Condemine, G. Cyt toxin expression reveals an inverse regulation of insect and plant virulence factors of Dickeya dadantii. Environ. Microbiol 2010, 12, 3290–3301. [Google Scholar] [CrossRef]
- Costechareyre, D.; Balmand, S.; Condemine, G.; Rahbé, Y. Dickeya dadantii, a plant pathogenic bacterium producing cyt-like entomotoxins, causes septicemia in the pea aphid Acyrthosiphon pisum. PLoS One 2012, 7, e30702. [Google Scholar] [CrossRef]
- Costechareyre, D.; Chich, J.F.; Strub, J.M.; Rahbé, Y.; Condemine, G. Transcriptome of Dickeya dadantii infecting Acyrthosiphon pisum reveals a strong defense against antimicrobial peptides. PloS One 2013, 8, e54118. [Google Scholar] [CrossRef]
- Renoz, F.; Noël, C.; Errachid, A.; Foray, V.; Hance, T. Infection dynamic of symbiotic bacteria in the pea aphid Acyrthosiphon pisum gut and host immune response at the early steps in the infection process PloS One 2015, 10, e0122099. [CrossRef]
- Wu, S.; Toews, M.D.; Cottrell, T.E.; Toxicity of Photorhabdus luminescens and Xenorhabdus bovienii bacterial metabolites to pecan aphids (Hemiptera: Aphididae) and the lady beetle Harmonia axyridis (Coleoptera:Coccinellidae)J. Invertebr. Pathol.2022,194,107806. [CrossRef]
- Altincicek, B.; Ter, B.; Laughton, A.M.; Udekwu, K.I.; Gerardo, N.M. Escherichia coli K-12 pathogenicity in the pea aphid, Acyrthosiphon pisum, reveals reduced antibacterial defense in aphids. Dev Comp Immunol 2011, 35, 1091–1097. [Google Scholar] [CrossRef]
- Teixeira, L.M.; Merquior, V.L.C. The Family Moraxellaceae. In The Prokaryotes: Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson,F.; Springer: Berlin, Germany, 2014, pp. 254-263. [CrossRef]
- LaSala, P.R.; Segal, J.; Han, F.S.; Tarrand, J.J.; Han, X.Y. First reported infections caused by three newly described genera in the family Xanthomonadaceae. J. Clin. Microbiol 2007, 45, 641–644. [Google Scholar] [CrossRef]
- An, S.Q.; Potnis, N.; Dow, M.; Vorhölter, F.J.; He, Y.Q.; Becker, A.;_ Teper, D.; Li, Y.; Wang, N.; Bleris, L.; Tang, J.L. Mechanistic insights into host adaptation, virulence and epidemiology of the phytopathogen Xanthomonas. FEMS Microbiol. Lett 2020, 44,1-32. [CrossRef]
- Palleroni,N.J. Introduction to the Family Pseudomonadaceae.In The Prokaryotes.;Starr, M.P.; Stolp, H.; Trüper.;H.G.; Balows, A.; Schlegel, H.G.; Springer: Berlin, Germany, 1981,pp. 655-665.
- Mnif, I.; Ghribi, D. Potential of bacterial derived biopesticides in pest management. Crop Prot 2015, 77, 52–64. [Google Scholar] [CrossRef]
- Barahona, E.; Navazo, A.; Martínez, F.; Zea, T.; Pérez, R.M.; Martín, M.; Rivilla, R. Pseudomonas fluorescens F113 mutant with enhanced competitive colonization ability and improved biocontrol activity against fungal root pathogens. Appl. Environ. Microbiol 2011, 77, 5412–5419. [Google Scholar] [CrossRef]
- Cronin, D., Moenne, Y., Fenton, A.; Dunne, C.; Dowling, D.N., O’gara, F. Role of 2, 4-diacetylphloroglucinol in the interactions of the biocontrol Pseudomonad strain F113 with the potato cyst nematode Globodera rostochiensis. Appl. Environ. Microbiol 1997, 63, 1357–136. [CrossRef]
- Villacieros, M.; Power, B.; Sánchez,M., Lloret, J., Oruezabal, R.I.; Martín, M.; Rivilla, R.. Colonization behaviour of Pseudomonas fluorescens and Sinorhizobium meliloti in the alfalfa (Medicago sativa) rhizosphere. Plant soil 2003, 251, 47-54. [CrossRef]
- Manjula, T.R.; Kannan, G.S.; Sivasubramanian, P. Field efficacy of Pseudomonas fluorescens against the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae) in Bt and non Bt cotton. Int J Curr Microbiol Appl Sci 2018, 6, 11–24.
- Paliwal, D.; Rabiey, M.; Mauchline, T.H.; Hassani-Pak, K.; Nauen, R.; Wagstaff, C.; Andrews, S.; Bass, C.; Jackson, R.W. Multiple toxins and a protease contribute to the aphid-killing ability of Pseudomonas fluorescens PpR24. Environ Microbiol 2024, 4, e16604. [Google Scholar] [CrossRef]
- Jansson, R.K.; Dybas, R.A. Avermectins: biochemical mode of action, biological activity and agricultural importance. In Insecticides with Novel Modes of Action-Mechanisms and Application.; Ishaaya, I.; Degheele,D., Springer: Berlin, Heidelberg, 1998; pp. 153-170.
- Fisher, M.H. , Mrozik, H. The chemistry and pharmacology of avermectins. Annu Rev Pharmacol Toxicol 1992, 32, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.C. Ivermectin and Abamectin, 1st ed.; Springer-Verlag: New York, USA, 1989; p. 363. [Google Scholar]
- Kim, S.K.; Kim, Y.C.; Lee, S.; Kim, J.C.; Yun, M.Y.; Kim, I.S. Insecticidal activity of rhamnolipid isolated from Pseudomonas sp. EP-3 against green peach aphid (Myzus persicae). J. Agric. Food Chem 2011, 59, 934-938. [CrossRef]
- Jang,J.Y.; Yang, S.Y.; Kim, Y.C.; Lee, C.W.; Park, M.S.; Kim, J.C.; Kim, I.S. Identification of orfamide A as an insecticidal metabolite produced by Pseudomonas protegens F6. J. Agric. Food Chem 2013,61, 6786-6791. [CrossRef]
- Xu, L.; Liang, K.; Duan, B. , Yu, M., Meng, W., Wang, Q., Yu, Q. A novel insecticidal peptide SLP1 produced by Streptomyces laindensis H008 against Lipaphis erysimi. Molecules 2016, 21, 1101. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lim, D.J.; Noh, M.Y.; Kim, J.C.; Kim, Y.C.; Kim, I.S. Characterization of biosurfactants as insecticidal metabolites produced by Bacillus subtilis Y9. Entomol. Res 2016, 47, 55–59. [Google Scholar] [CrossRef]
- Lim, D.J.; Yang, S.Y.; Noh, M.Y.; Lee, C.W.; Kim,J.C., Kim,I.S. Identification of lipopeptide xantholysins from Pseudomonas sp. DJ15 and their insecticidal activity against Myzus persicae. Entomol. Res 2017,47, 337-343. [CrossRef]
- López, G.; Alvarez, A.E.; Petroselli, G.; Erra,R.; Audisio, M.C. Aphicidal activity of Bacillus amyloliquefaciens strains in the peach-potato aphid (Myzus persicae). Microbiol Res. 2019,226, 41-47. [CrossRef]
- Rumyantsev, S.D.; Alekseev, V.Y.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Garafutdinov, R.R.; Maksimov, I.V.; Veselova, S.V. Additive Effect of the Composition of Endophytic Bacteria Bacillus subtilis on Systemic Resistance of Wheat against Greenbug Aphid Schizaphis graminum Due to Lipopeptides. Life (Basel) 2023, 13, 214. [Google Scholar] [CrossRef]
- Kämpfer, P.; Glaeser, S.P.; Parkes, L.; van Keulen, G.; Dyson. P. The Family Streptomycetaceae. In The Prokaryotes,; Rosenberg, E.; DeLong, E.F.; Lory, S., Stackebrandt, E.; Thompson, F., Springer: Berlin, Germany, 2014; pp. 889-1010.
- Procópio, R.E.; Silva, I.R.; Martins, M.K.; Azevedo, J.L.; Araújo, J.M. Antibiotics produced by Streptomyces. Braz J Infect Dis 2012, 466–471. [Google Scholar] [CrossRef]
- Aggarwal, N.; Thind, S.K.; Sharma, S. Role of secondary metabolites of actinomycetes in crop protection. In Plant Growth Promoting Actinobacteria, Subramaniam,G.; Arumugam, S.; Rajendran,V., Springer: Singapore, 2016; pp. 99-12.
- Bérdy, J. Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Shi,Y; Zhang, X.; Lou, K.. Isolation, characterization, and insecticidal activity of an endophyte of drunken horse grass, Achnatherum inebrians. J. Insect Sci 2013, 13, 151. [CrossRef]
- Chen,Y; Shafi, J.; Li, M.; Fu, D.; Ji, M. Insecticidal activity of endophytic actinomycetes isolated from Azadirachta indica against Myzus persicae. Arch. Biol. Sci 2017, 70, 349–357. [CrossRef]
- Dewhirst, F.E.; Paster, B.J.; Bright, PL. Chromobacterium, Eikenella, Kingella, Neisseria, Simonsiella, and Vitreoscilla species comprise a major branch of the beta group Proteobacteria by 16S ribosomal ribonucleic acid sequence comparison: transfer of Eikenella and Simonsiella to the family Neisseriaceae (emend.). Int J Syst Evol Microbiol 1989, 39, 258-266. [CrossRef]
- Martin, P.A.W.; Gundersen, D.; Blackburn, M.; Buyer, J. Chromobacterium subtsugae sp. a betaproteobacterium toxic to Colorado potato beetle and other insect pests. 2007,57,993-999. [CrossRef]
- Shapiro, D.I.; Cottrell, T.E.; Jackson, M.A.; Wood, B.W. Control of key pecan insect pests using biorational pesticides. J. Econ. Entomol. 2013, 106, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, L.; Satta, A. , Floris, I. Emerging entomopathogenic bacteria for insect pest management. Bull. Insectology 2013, 66, 181–186. [Google Scholar]
- Lacey, L.A.; Grzywacz, D. Shapiro, D.I.; Frutos, R., Brownbridge, M. Goettel, M.S.Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 2015, 132,1-4. [CrossRef]
- Blackburn, M.B.; Sparks, M.E.; Gundersen, D.E. The genome of the insecticidal Chromobacterium subtsugae PRAA4-1 and its comparison with that of Chromobacterium violaceum ATCC 12472. Genom Data 2016, 10, 1–3. [Google Scholar] [CrossRef]
- Rajendhran, J. Genomic insights into Brucella. Infect Genet Evol. 2021, 87. [Google Scholar] [CrossRef]
- Hemme, D.; Foucaud-Scheunemann,C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int. Dairy J 2004, 14, 467–494. [CrossRef]
- Hiebert,N; Kessel, T.; Skaljac,M.; Spohn, M.; Vilcinskas, A.; Lee, K.Z. The Gram-Positive Bacterium Leuconostoc pseudomesenteroides Shows Insecticidal Activity against Drosophilid and Aphid Pests. Insects 2020, 11, 471. [CrossRef]
- Rosell,R.C;Davidson, E.W.; Jancovich, J.K.; Hendrix, D.L.; Brown,J.Size Limitations in the Filter Chamber and Digestive Tract of Nymphal and Adult Bemisia tabaci Whiteflies (Hemiptera: Aleyrodidae), Ann. Entomol. Soc. Am 2003, 96,544–552.
- Shakesby, A.J.; Wallace, I.S.; Isaacs, H.V.; Pritchard, J.; Roberts, D.M.; Douglas, A.E. A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem Mol Biol. 2009, 39, 1–10. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yasuyuki, S.; Kazuhisa, M.; Hidetaka, H. GalNAc pretreatment inhibits trapping of Bacillus thuringiensis Cry1Ac on the peritrophic membrane of Bombyx mori. FEBS Letters 2004, 576, 331–335. [Google Scholar] [CrossRef]
- Terra, W.R.; Barroso, I. G.; Dias, R.O.; Ferreira, C. Molecular physiology of insect midgut. In Advances in insect physiology. Academic Press 2019, 56, 117–163. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Evolutionary trends of digestion and absorption in the major insect orders. Arthropod Struct. Dev 2020, 56, 100931. [Google Scholar] [CrossRef]
- Brandt, S.L.; Coudron, T. A.; Habibi, J.; Brown, G. R.: Ilagan,O. M.; Wagner, R.M.; Huesing, J. E. Interaction of two Bacillus thuringiensis δ-endotoxins with the digestive system of Lygus hesperus. Curr. Microbiol 2004, 48,1–9. [CrossRef]
- Cristofoletti, P.T.; Ribeiro, A. F.; Deraison, C.; Rahbé, Y.; Terra, W. R. Midgut adaptation and digestive enzyme distribution in a phloem feeding insect, the pea aphid Acyrthosiphon pisum. Insect Physiol 2003, 49, 11–24. [Google Scholar] [CrossRef]
- Zhao, X.D.; Zhang, B.W.; Fu, L.J.; Li, Q.L.; Lin, Y.; Yu, X.Q. Possible Insecticidal Mechanism of Cry41-Related Toxin against Myzus persicae by Enhancing Cathepsin B Activity. J Agric Food Chem. 2020, 68, 4607–4615. [Google Scholar] [CrossRef] [PubMed]
- Feng,H.; Edwards, N.; Anderson, C.; Althaus, M.; Duncan, R.; Hsu, Y. Trading amino acids at the aphid-Buchnera symbiotic interface.Proc Natl Acad Sci 2019,116,16003–16011. [CrossRef]
- Wilson,A.C.C.; Ashton, P.D.; Calevro, F.; Charles, H.; Colella, S.; Febvay, G. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol Biol. 2010,19, 249–258. [CrossRef]
| Target aphid | Protein | % Mortality | ng/mL (time) | Origin | Reference |
|---|---|---|---|---|---|
|
Potato aphid Macrosiphum euphorbiae |
Mixture CryI; CryIA(a), CryIA(b), CryIC and CryIF CryIIA CryIIIA CryIVD |
100 ± 0 93 ± 0 98 ± 4 93 ± 10 |
100 each Cry (4 days) 200 (4 days) 360 (4 days) 350 (4 days) |
Recombinant strains of Bt |
[25] |
|
Pea aphid Acyrthosiphon pisum |
CryIAb Cry3A Cry4A and Cry4B Cry11A CytIA |
35 60 100 100 Growth inhibition |
500 (5 days) 500 (6 days) 500 (4 days) 500 (3 days) 125 (7 days) |
Recombinant strains Bt subsp. israelensis |
[9] |
| Cotton aphid Aphis gossypii |
VipIAcI and Vip2Ae3 |
LC50 | 0.0875 (NR days) | Bacillus cereus | [42] |
| Pea aphid Acyrthosiphon pisum |
CryIAc Cry3Aa |
71 71 |
500 (7 days) | Bacillus thuringiensis | [20] |
|
Cotton aphid Aphis gossypii |
VipIAe and Vip2Ae |
LC50 |
0.576 (2 days) |
Bacillus thuringiensis |
[43] |
| Pea aphid Acyrthosiphon pisum |
Cyt2Aa CGAL1 CGAL3 CGAL4 CGSL1 CGSL4 |
LC50 LC50 LC50 LC50 LC50 LC50 |
150 ± 0.00 19.71 ± 5.74 9.55 ± 2.54 11.92 ± 1.99 28.74 ± 2.92 15.13 ± 0.23 |
Recombinant proteins |
[23] |
| Green peach aphid Myzus persicae |
Cyt2Aa CGAL1 CGAL3 CGAL4 CGSL1 CGSL4 |
LC50 LC50 LC50 LC50 LC50 LC50 |
150 ± 0.00 58.04 ± 2.08 42.68 ± 0.49 92.75 ± 2.54 ND ND |
Bacillus thuringiensis Recombinant proteins |
[23] |
| Green peach aphid Myzus persicae |
Cry-Related | LC50 |
32.7 (3 days) | Bacillus thuringiensis | [10] |
| Pea aphid Acyrthosiphon pisum |
CytIA CytC CytB CytA |
TL50 TL50 TL50 TL50 |
1000 (3.24 days) 1000 (10.1 days) 500 (5.1 days) 1000 (2.28 days) |
Dickeya dadantii |
[44] |
| Cotton aphid Aphis gossypii |
Cry1Ah Cry2Ab |
No mortality | >1000 | Bacillus thuringiensis | [21] |
| Cabbage aphid Brevicoryne brassicae Green peach aphid Myzus persicae |
Cry1Ac Cry1F Cry1Ac and Cry1F Cry1Ac Cry1F Cry1Ac and Cry1F |
Decreased the net population growth rate Decreased the net population growth rate |
20 (3 days) 20 (3 days) 20 (3 days) 20 (3 days) 20 (3 days) 20 (3 days) |
Bacillus sp |
[45] |
| Pea aphid Acyrthosiphon pisum |
Cry4Aa (trypsin activated) Cry4Aa 2A |
63.3 ± 24.5 51.1 ± 2.2 |
120 (2 days) 120 (2 days) |
Bacillus thuringiensis Modified toxin |
[46] |
| Green peach aphid Myzus persicae |
Cry1Cb2 |
LC50 |
6.58 (3 days) |
Bacillus sp. |
[38] |
| Target aphid | Molecule | % Mortality, or LC50-90 | μg/mL (time) | Origin | Reference |
|---|---|---|---|---|---|
| Pea aphid Acyrthosiphon pisum |
Avermectin B1 | LC90 | 0.4 ppm (72-96 h) | Streptomyces avermitilis | [72,73] |
|
Cotton aphid Aphis gossypii Black bean aphid Aphis fabae |
Avermectin B1 |
50% |
450 ppm (NM) |
Streptomyces avermitilis |
[74] |
|
Cotton aphid Aphis gossypii |
Viscosin |
90-99% |
200 ppm (6 days) |
Pseudomonas fluorescens |
[51] |
| Green peach aphid Myzus persicae |
Dirhamnolipid | 100% |
100 μg/mL (24 h) |
Pseudomonas sp. | [75] |
| Green peach aphid Myzus persicae |
Orfamide A | LC50 | 34.5 μg/mL (24 h) |
Pseudomonas protegens | [76] |
| Green peach aphid Myzus persicae |
Surfactin | LC50 | 35.82 μg/mL (24 h) |
Bacillus amyloliquefaciens | [42] |
| Mustard aphid Lipaphis erysimi | Peptide | 100% | 700 μg/mL (48 h) |
Streptomyces laindensis | [77] |
|
Green peach aphid Myzus persicae |
Surfactin isomers |
LC50 |
20.4 μg/mL 22.2 μg/mL 54.5 μg/mL (24 h) |
Bacillus subtilis |
[78] |
| Green peach aphid Myzus persicae |
Xantholysins A and B | LC50 | 13.4 μg/mL 24.6 μg/mL (24 h) |
Pseudomonas sp. | [79] |
| Green peach aphid Myzus persicae |
Kurstakins, Surfactins, Iturins, Fngycins |
No mortality |
ND |
Bacillus amyloliquefaciens strains CBMDDrag3, PGPBacCA2 |
[80] |
| Greenbug Aphid Schizaphis graminum |
Surfactin Iturin lipopeptides |
100% Using a mix of both strains |
112 μg/mL (5 days) |
Bacillus subtilis strains 26D and 11VM | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





