Submitted:
06 February 2025
Posted:
06 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Ethics Approval Statement
2.2. Patients
2.3. Sample Size Calculation
2.4. Gamma Knife Treatment
2.5. MRI
- 3D-T1w magnetization-prepared rapid acquisition (MPRAGE) sequence: gradient echo, TR/TE/TI 6.8/3.2/900ms, flip angle 8°, measured voxel size 0.6*0.6*1.0 mm, before and after intravenous injection of contrast medium.
- T2w sequence: TR/TE 3693.8/80ms, 150 transversal slices, thickness 1mm, matrix 512×512.
- Fluid-attenuated inversion recovery (FLAIR) sequence: TR/TE/TI 11,000/120/2800ms, 90 transversal slices, thickness 2mm, matrix 512×512.
2.6. Postprocessing
2.7. Follow-up
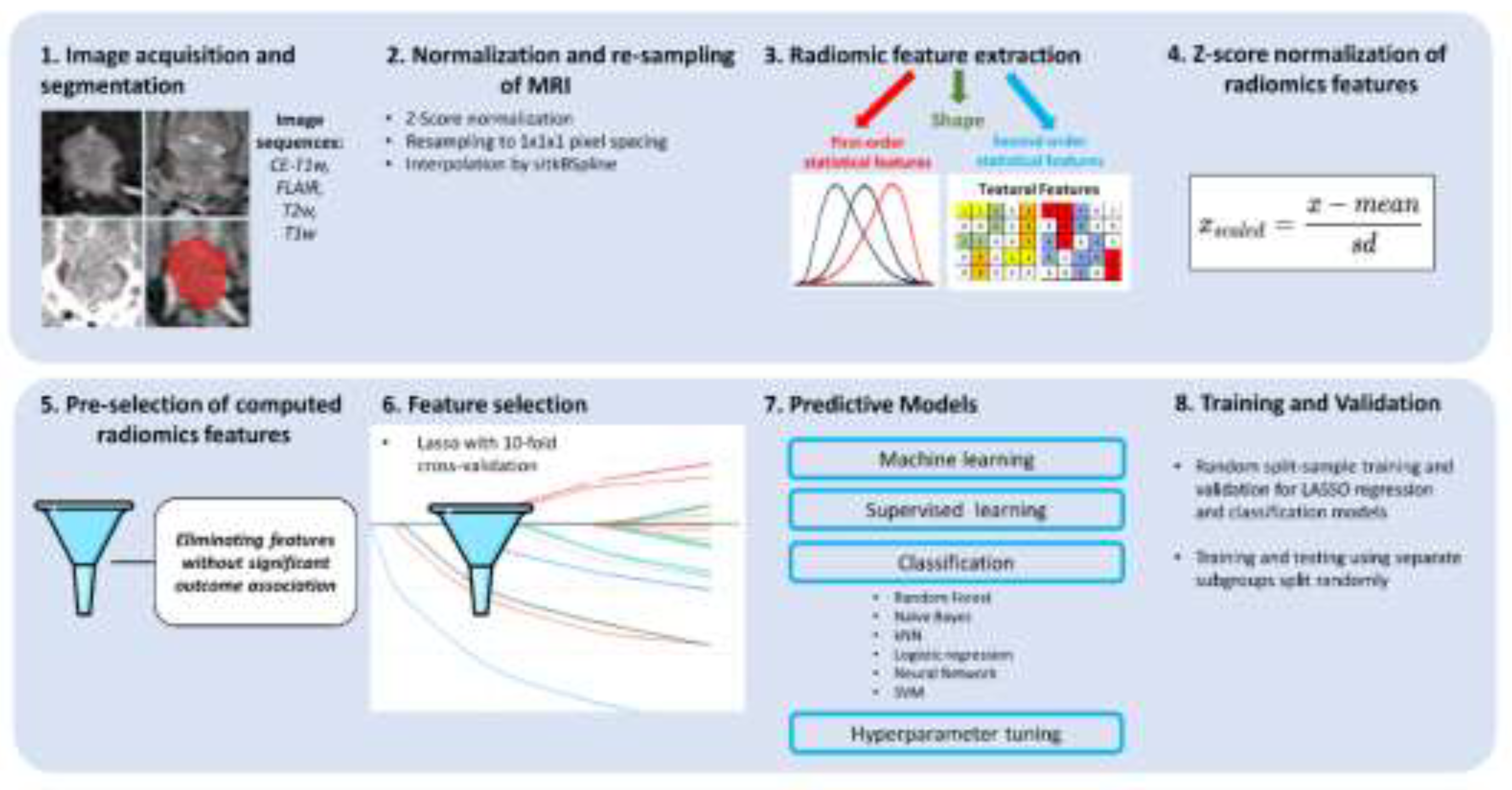
2.8. Feature Extraction
2.9. Model Selection
2.10. Evaluation of Predictive Performance
2.11. Validation
3. Results
3.1. Patients’ Characteristics
3.2. Experimental Design
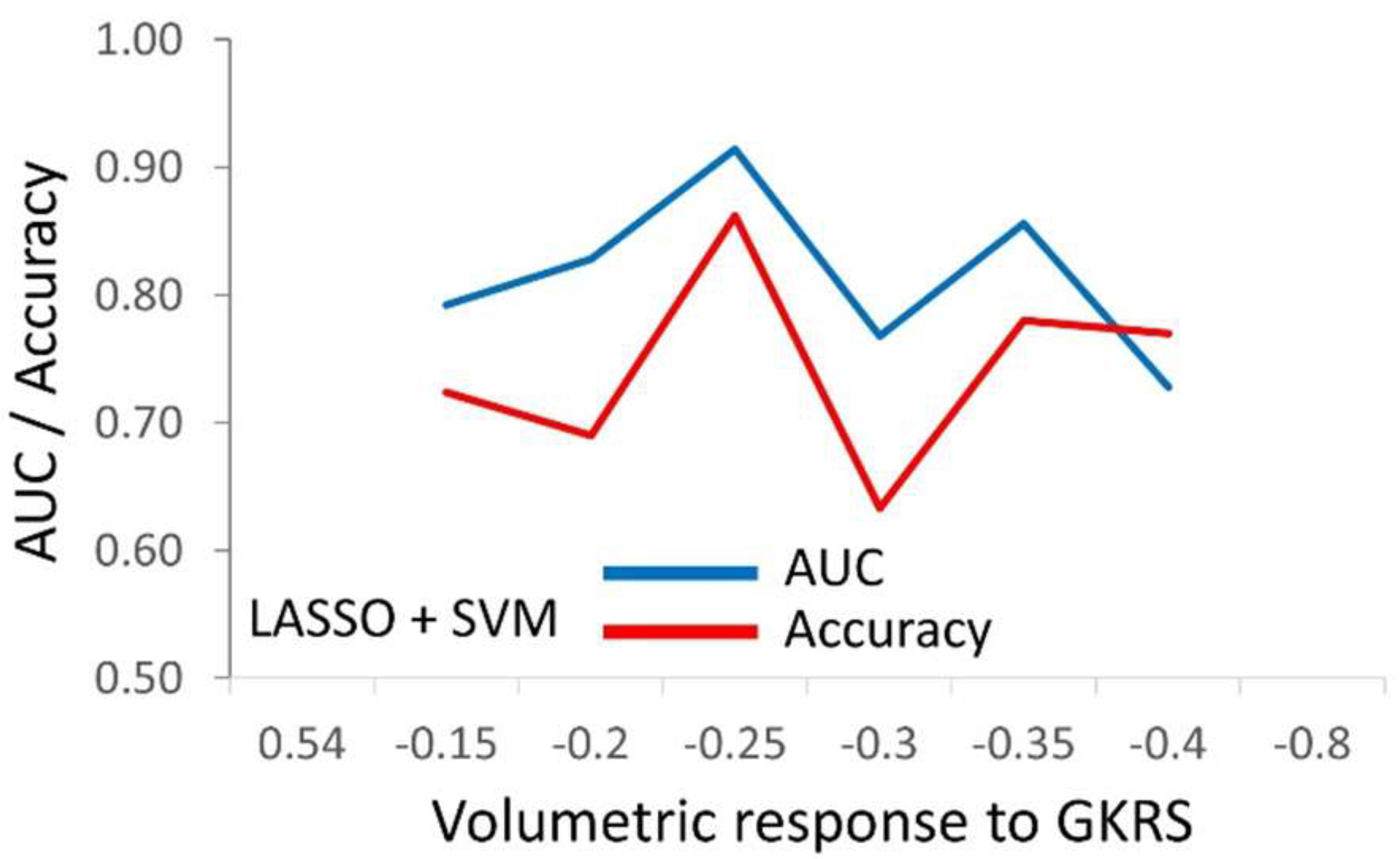
3.3. The Predictive Models for PA Response to Radiosurgery
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Blinded for review
Disclosures
Code and Data Availability
Acknowledgments/Funding Sources
Author Contributions
References
- Trifiletti, D.M.; Dutta, S.W.; Lee, C.C.; Sheehan, J.P. Pituitary Tumor Radiosurgery. Prog Neurol Surg. 2019, 34, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Sahgal, A.; Rubens, M.; De Salles, A.; Fariselli, L.; Pollock, B.E.; Levivier, M.; Ma, L.; Paddick, I.; Regis, J.; Sheehan, J.; Yomo, S.; Suh, J.H. Stereotactic radiosurgery for non-functioning pituitary adenomas: meta-analysis and International Stereotactic Radiosurgery Society practice opinion. Neuro Oncol. 2020, 22, 318–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehrer, E.J.; Kowalchuk, R.O.; Trifiletti, D.M.; Sheehan, J.P. The Role of Stereotactic Radiosurgery for Functioning and Nonfunctioning Pituitary Adenomas. Neurol India. 2023, 71 (Supplement), S133–S139. [Google Scholar] [CrossRef] [PubMed]
- Dayawansa, S.; Abbas, S.O.; Mantziaris, G.; Dumot, C.; Donahue, J.H.; Sheehan, J.P. Volumetric Assessment of Nonfunctional Pituitary Adenoma Treated With Stereotactic Radiosurgery: An Assessment of Long-Term Response. Neurosurgery. 2023. [Google Scholar] [CrossRef] [PubMed]
- Pomeraniec, I.J.; Xu, Z.; Lee, C.C.; Yang, H.C.; Chytka, T.; Liscak, R.; Martinez-Alvarez, R.; Martinez-Moreno, N.; Attuati, L.; Picozzi, P.; Kondziolka, D.; Mureb, M.; Bernstein, K.; Mathieu, D.; Maillet, M.; Ogino, A.; Long, H.; Kano, H.; Lunsford, L.D.; Zacharia, B.E.; Mau, C.; Tuanquin, L.C.; Cifarelli, C.; Arsanious, D.; Hack, J.; Warnick, R.E.; Strickland, B.A.; Zada, G.; Chang, E.L.; Speckter, H.; Patel, S.; Ding, D.; Sheehan, D.; Sheehan, K.; Kvint, S.; Buch, L.Y.; Haber, A.R.; Shteinhart, J.; Vance, M.L.; Sheehan, J.P. Dose to neuroanatomical structures surrounding pituitary adenomas and the effect of stereotactic radiosurgery on neuroendocrine function: an international multicenter study. J Neurosurg. 2021, 136, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mantziaris, G.; Pikis, S.; Chytka, T.; Liščák, R.; Sheehan, K.; Sheehan, D.; Peker, S.; Samanci, Y.; Bindal, S.K.; Niranjan, A.; Lunsford, L.D.; Kaur, R.; Madan, R.; Tripathi, M.; Pangal, D.J.; Strickland, B.A.; Zada, G.; Langlois, A.M.; Mathieu, D.; Warnick, R.E.; Patel, S.; Minier, Z.; Speckter, H.; Xu, Z.; Kormath Anand, R.; Sheehan, J.P. Adjuvant versus on-progression Gamma Knife radiosurgery for residual nonfunctioning pituitary adenomas: a matched-cohort analysis. J Neurosurg. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Speckter H, Radulovic M, Trivodaliev K, Vranes V, Joaquin J, Hernandez W, Mota A, Bido J, Hernandez G, Rivera D, Suazo L, Valenzuela S, Stoeter P. MRI radiomics in the prediction of the volumetric response in meningiomas after gamma knife radiosurgery. J Neurooncol. 2022, 159, 281–291. [CrossRef] [PubMed]
- Djuričić, G.J.; Ahammer, H.; Rajković, S.; Kovač, J.D.; Milošević, Z.; Sopta, J.P.; Radulovic, M. Directionally Sensitive Fractal Radiomics Compatible With Irregularly Shaped Magnetic Resonance Tumor Regions of Interest: Association With Osteosarcoma Chemoresistance. J Magn Reson Imaging. 2023, 57, 248–258. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J. The linear quadratic model: usage, interpretation and challenges. Phys Med Biol 2018, 64, 01TR01. [Google Scholar] [CrossRef]
- Speckter, H.; Santana, J.; Miches, I.; Hernandez, G.; Bido, J.; Rivera, D.; Suazo, L.; Valenzuela, S.; Garcia, J.; Stoeter, P. Assessment of the alpha/beta ratio of the optic pathway to adjust hypofractionated stereotactic radiosurgery regimens for perioptic lesions. J Radiat Oncol 2019, 8, 279–289. [Google Scholar] [CrossRef]
- Speckter, H.; Hernandez, G.; Bido, J.; Rivera, D.; Suazo, L.; Valenzuela, S.; Santana, J.; Hernandez, W.; Moreno, L.; Peralta, I.; Paulino, J.; Stoeter, P. Assessment of the alpha/beta Ratios of Pituitary Adenomas and Craniopharyngiomas. Presented at the International Stereotactic Radiosurgery Society (ISRS) meeting Milan, Italy, June 2022.
- Speckter, H.; Santana, J.; Lara, G.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Stoeter, P. Assessment Of The Alpha/Beta Ratios Of Pituitary Adenomas And Craniopharyngiomas For The Quantification Of Single Fraction Equivalent Dose Benefits From Hypofractionated Radiosurgery. Int J Radiat Oncol Biol Phys. [CrossRef]
- JJM van Griethuysen, J.J.M.; A Fedorov, C. Parmar, A.; Hosny, N. Aucoin, V. Narayan, R.H.H. Beets-Tan, J.C. Fillion-Robin, S. Pieper, H.J.W.L. Aerts. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 7, e104–e107. [Google Scholar]
- Paddick, I. A simple scoring ratio to index the conformity of radiosurgical treatment plans: technical note. J Neurosurg 2000, 93 (Suppl 3), 219–222. [Google Scholar] [CrossRef] [PubMed]
- Speckter, H.; Palque-Santos, S.; Mota-Gonzalez, R.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Gonzalez-Curi, M.; Stoeter, P. Can Apparent Diffusion Coefficient (ADC) maps replace Diffusion Tensor Imaging (DTI) maps to predict the volumetric response of meningiomas to Gamma Knife Radiosurgery? J Neurooncol. 2023, 161, 547–554. [Google Scholar] [CrossRef] [PubMed]
- B. Efron. Bootstrap Methods: Another Look at the Jackknife. The Annals of Statistics 1979, 7, 1–26.
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; Raizer, J.; Schiff, D.; Soffietti, R.; Tonn, J.C.; Vogelbaum, M.; Weber, D.; Reardon, D.A.; Wen, P.Y. Proposed response assessment and endpoints for meningioma clinical trials: report from the response assessment in neuro-oncology working group. Neuro-Oncology 2019, 21, 26–36. [Google Scholar] [CrossRef]
- Imber, B.S.; Lin, A.L.; Zhang, Z.; Keshavamurthy, K.N.; Deipolyi, A.R.; Beal, K.; Cohen, M.A.; Tabar, V.; DeAngelis, L.M.; Geer, E.B.; Yang, T.J.; Young, R.J. Comparison of Radiographic Approaches to Assess Treatment Response in Pituitary Adenomas: Is RECIST or RANO Good Enough? J Endocr Soc. 2019, 3, 1693–1706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Speckter, H.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Miches, I.; Oviedo, J.; Gonzalez, C.; Stoeter, P. Pretreatment texture analysis of routine MR images and shape analysis of the diffusion tensor for prediction of volumetric response after radiosurgery for meningioma. J Neurosurg 2018, 129, 31–37. [Google Scholar] [CrossRef]
- Speckter, H.; Santana, J.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Oviedo, J.; Gonzalez, C.F.; Stoeter, P. Texture Analysis of Standard Magnetic Resonance Images to Predict Response to Gamma Knife Radiosurgery in Vestibular Schwannomas. World Neurosurg. 2019, 132, e228–e234. [Google Scholar] [CrossRef] [PubMed]
- Langenhuizen, P.; Sebregts, S.H.P.; Zinger, S.; Leenstra, S.; Verheul, J.B.; de With, P.H.N. Prediction of transient tumor enlargement using MRI tumor texture after radiosurgery on vestibular schwannoma. Med Phys 2020, 47, 1692–1701. [Google Scholar] [CrossRef]
- George-Jones, N.A.; Wang, K.; Wang, J.; Hunter, J.B. Prediction of Vestibular Schwannoma Enlargement After Radiosurgery Using Tumor Shape and MRI Texture Features. Otol Neurotol. 2021, 42, e348–e354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Geng, D.; Yu, T.; Xia, W.; She, D.; Liu, L.; Yin, B. Prognostic value of pretreatment MRI texture features in breast cancer brain metastasis treated with Gamma Knife radiosurgery. Acta Radiol. 2021, 62, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Park JH, Choi BS, Han JH, Kim CY, Cho J, Bae YJ, Sunwoo L, Kim JH. MRI Texture Analysis for the Prediction of Stereotactic Radiosurgery Outcomes in Brain Metastases from Lung Cancer. J Clin Med. 2021, 10, 237. [CrossRef] [PubMed] [PubMed Central]
- Langenhuizen, P.; Zinger, S.; Leenstra, S.; Kunst, H.P.M.; Mulder, J.J.S.; Hanssens, P.E.J.; de With, P.H.N.; Verheul, J.B. Radiomics based prediction of long-term treatment response of vestibular schwannomas following stereotactic radiosurgery. Otol Neurotol 2020, 41, e1321–e1327. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Wu, C.C.; Lee, C.C.; Huang, H.E.; Lee, W.K.; Chung, W.Y.; Wu, H.M.; Guo, W.Y.; Wu, Y.T.; Lu, C.F. Prediction of pseudoprogression and long-term outcome of vestibular schwannoma after Gamma Knife radiosurgery based on preradiosurgical MR radiomics. Radiother Oncol 2021, 155, 123–130. [Google Scholar] [CrossRef]
- Mouraviev, A.; Detsky, J.; Sahgal, A.; Ruschin, M.; Lee, Y.K.; Karam, I.; Heyn, C.; Stanisz, G.J.; Martel, A.L. Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro Oncol 2020, 22, 797–805. [Google Scholar] [CrossRef]
- Liao, C.Y.; Lee, C.C.; Yang, H.C.; Chen, C.J.; Chung, W.Y.; Wu, H.M.; Guo, W.Y.; Liu, R.S.; Lu, C.F. Enhancement of radiosurgical treatment outcome prediction using MRI radiomics in patients with non-small cell lung cancer brain metastases. Cancers (Basel) 2021, 13, 4030. [Google Scholar] [CrossRef]
- Wang, H.; Xue, J.; Qu, T.; Bernstein, K.; Chen, T.; Barbee, D.; Silverman, J.S.; Kondziolka, D. Predicting local failure of brain metastases after stereotactic radiosurgery with radiomics on planning MR images and dose maps. Med Phys 2021, 48, 5522–5530. [Google Scholar] [CrossRef]
- Mulford, K.; Chen, C.; Dusenbery, K.; Yuan, J.; Hunt, M.A.; Chen, C.C.; Sperduto, P.; Watanabe, Y.; Wilke, C. A radiomics-based model for predicting local control of resected brain metastases receiving adjuvant, S. R.S. Clin Transl Radiat Oncol 2021, 29, 27–32. [Google Scholar] [CrossRef]
- Gao, D.; Meng, X.; Jin, H.; Liu, A.; Sun, S. Assessment of gamma knife radiosurgery for unruptured cerebral arteriovenous malformations based on multi-parameter radiomics of, M. R.I. Magn Reson Imaging 2022, 92, 251–259. [Google Scholar] [CrossRef]
- Meng, X.; Gao, D.; Jin, H.; Wang, K.; Bao, E.; Liu, A.; Li, Y.; Sun, S. Factors affecting volume reduction velocity for arteriovenous malformations after treatment with dose-stage stereotactic radiosurgery. Front Oncol 2021, 11, 769533. [Google Scholar] [CrossRef]
- Meng, X.; Gao, D.; He, H.; Sun, S.; Liu, A.; Jin, H.; Li, Y. A machine learning model predicts the outcome of SRS for residual arteriovenous malformations after partial embolization: a realworld clinical obstacle. World Neurosurg 2022, 163, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Chen TC, Zee CS, Miller CA, Weiss MH, Tang G, Chin L, et al: Magnetic resonance imaging and pathological correlates of meningiomas. Neurosurgery 1992, 31, 1015–1021.
- Suzuki Y, Sugimoto T, Shibuya M, Sugita K, Patel SJ: Meningiomas: correlation between MRI characteristics and operative findings including consistency. Acta Neurochir (Wien) 1994, 129, 39–46. [CrossRef] [PubMed]
- Maiuri F, Iaconetta G, de Divitiis O, Cirillo S, Di Salle F, De Caro ML: Intracranial meningiomas: correlations between MR imaging and histology. Eur J Radiol 1999, 31, 69–75. [CrossRef] [PubMed]
- Yao, A.; Rutland, J.W.; Verma, G.; Banihashemi, A.; Padormo, F.; Tsankova, N.M.; Delman, B.N.; Shrivastava, R.K.; Balchandani, P. Pituitary adenoma consistency: Direct correlation of ultrahigh field 7T MRI with histopathological analysis. Eur J Radiol. 2020, 126, 108931. [Google Scholar] [CrossRef] [PubMed]
- BSitthinamsuwan, I. Khampalikit, S. Nunta-aree, P. Srirabheebhat, T. Witthiwej, A. Nitising, Predictors of meningioma consistency: a study in 243 consecutive cases. Acta Neurochir. 2012, 154, 1383–1389. [Google Scholar]
- Yamaguchi, N.; Kawase, T.; Sagoh, M.; Ohira, T.; Shiga, H.; Toya, S. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging, Surg. Neurol. 1997, 48, 579–583. [Google Scholar]
- K.A. Smith, J.D. Leever, R.B. Chamoun, Predicting consistency of meningioma by magnetic resonance imaging. J. Neurol. Surg. B Skull Base 2015, 76, 225–229. [CrossRef]
- Černý, M.; Sedlák, V.; Lesáková, V.; Francůz, P.; Netuka, D. Methods of preoperative prediction of pituitary adenoma consistency: a systematic review. Neurosurg Rev. 2022, 46, 11. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wu, C.; Meng, M.; Liu, T.; Li, C.; Ma, J.; Qin, Z. Radiomic Features on Multiparametric MRI for Preoperative Evaluation of Pituitary Macroadenomas Consistency: Preliminary Findings. J Magn Reson Imaging. 2022, 55, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
| all PAs | functional PAs | nonfunctional PAs | ||||
| Patient and treatment characteristics | Value | Range | Value | Range | Value | Range |
| Number of patients | 81 | - | 29 [36%] | - | 52 [64%] | - |
| Age in years (mean, range) | 45.6 | (11.4 / 83.2) | 38.3 | (11.4 / 65.5) | 49.7 | (21.2 / 83.2) |
| Pre-SRS tumor volume in cm3 (mean, range) | 6.30 | (0.16 / 40.33) | 6.00 | (0.18 / 33.54) | 6.39 | (0.16 / 40.33) |
| previous RT, SRS | 0 | - | 0 | - | 0 | - |
| previous surgery | 69 [85%] | - | 25 [86%] | - | 44 [85%] | - |
| KPS before SRS (mean, range) | 83.3 | (60 / 100) | 82.7 | (60 / 100) | 83.8 | (60 / 100) |
| Single fraction SRS treatments | 49 [60%] | - | 13 [45%] | - | 36 [69%] | - |
| Hypofractionated SRS treatments | 32 [40%] | - | 16 [55%] | - | 16 [31%] | - |
| Number of Fractions (mean, range) | 2.21 | (1 / 5) | 2.83 | (1 / 5) | 1.86 | (1 / 4) |
| Gradient index (mean, range) | 2.83 | (2.42 / 3.60) | 2.82 | (2.48 / 3.48) | 2.83 | (2.42 / 3.60) |
| Coverage index (mean, range) | 96.7% | (91.0% / 100%) | 96.8% | (91.0% / 100%) | 96.6% | (91.0% / 100%) |
| Selectivity index (mean, range) | 67.8% | (18.0% /89.0%) | 63.6% | (18.0% / 85.0%) | 70.3% | (39.0% / 89.0%) |
| Paddick conformity index (mean, range) | 65.5% | (17.6% / 83.7%) | 61.5% | (17.6% / 80.8%) | 67.8% | (39.0% / 83.7%) |
| Margin physical Dose in Gy (mean, range) | 20.5 | (12 / 40) | 26.7 | (15 / 40) | 17.0 | (12 / 24) |
| Margin BED in Gy (mean, range) | 100.9 | (40.4 / 284.5) | 106.2 | (40.4 / 284.5) | 97.8 | (60.5 / 181.9) |
| Margin SFED in Gy (mean, range) | 16.2 | (11.1 / 35.0) | 19.6 | (11.8 / 35.0) | 14.2 | (11.1 / 20.0) |
| Treatment results | Value | Range | Value | Range | Value | Range |
| Follow up period in months (mean, range) | 40.4 | (6.7 / 105.5) | 38.6 | (6.7 / 85.1) | 41.4 | (7.2 / 105.5) |
| Complete response | 0 [0%] | - | 0 [0%] | - | 0 [0%] | - |
| Partial response (PR, decrease by ≥30%) | 60 [74.1%] | - | 27 [93%] | - | 33 [63.5%] | - |
| Stable disease (SD, neither PR, no PD) | 20 [24.7%] | - | 2 [7%] | - | 18 [34.6%] | - |
| Progressive disease (PD, increase by ≥20%) | 1 [1.2%] | - | 0 [0%] | - | 1 [1.9%] | - |
| Absolute volume change in cm3 (mean, range) | -2.80 | (-15.83 / 1.80) | -3.35 | (-15.00 / -0.06) | -2.49 | (-15.83 / 1.80) |
| Relative volume change (mean, range) | -45.7% | (-90.2% / 92.7%) | -55.4% | (-81.0% / -27.6%) | -40.2% | (-90.2% / 92.7%) |
| Volume change per month (mean, range) | -1.58% | (-9.84% / 1.75%) | -2.21% | (-9.84% / -0.57%) | -1.22% | (-4.83% / 1.75%) |
| Abbreviations: PA, pituitary adenoma; SRS, stereotactic radiosurgery; RT, radiotherapy; KPS, Karnofsky Performance Status; BED, biologically effective dose; SFED, single fraction equivalent dose | ||||||
| Test folds (10) | ||
| Model | R2 | Selected l |
| CP | 0.272 | 0.0092 |
| T1w | 0.464 | 0.0086 |
| CE-T1w | 0.281 | 0.0130 |
| T1w+CE-T1w | 0.502 | 0.0144 |
| CP+T1w+CE-T1w | 0.584 | 0.0138 |
| T2w | 0.665 | 0.0115 |
| FLAIR | 0.312 | 0.0149 |
| Clinical parameters | ||||
| T-test in the entire cohort | ||||
| Featurea | T-statistic | P-value | Mean±SD | Mean Difference±SD |
| Age | -3.38 | 0.001 | 45.5±14.2 | 10.6 ± 3.0 |
| Fraction number | 2.62 | 0.011 | 2.2±1.6 | -0.80 ± 0.32 |
| Dose per fraction | -0.06 | 0.953 | 13.1±7.2 | -0.26 ± 1.4 |
| Accumulated dose | 4.99 | 0.000 | 20.6±6.7 | -5.7 ± 1.2 |
| BED | 0.368 | 0.714 | 100.6±44.6 | -4.7 ± 9.1 |
| SFED | 2.588 | 0.012 | 16.2±5.0 | -2.5 ± 0.93 |
| Coverage | 0.711 | 0.479 | 96.7±2.0 | -0.32 ± 0.47 |
| Selectivity | 0.008 | 0.994 | 67.8±11.6 | 0.37 ± 2.7 |
| PCI | 0.096 | 0.924 | 0.66±0.11 | 0.001 ± 0.027 |
| BOT | 0.641 | 0.523 | 40.9±24.6 | -4.9 ± 5.2 |
| Chi-square test in the entire cohort | ||||
|
Pearson Chi-square |
P-value | Gamma | P-value | |
| Functionality b | 0.42 | 0.001 | 0.81 | <0.001 |
| Modelsc | ||||
| AUC and Accuracy in the test folds (8)d | ||||
| Model | AUC | Accuracy | True positives | True negatives |
| CP | 0.846 ± 0.046 | 0.800 ± 0.049 | 0.542 ± 0.123 | 0.770 ± 0.049 |
| T1w* | 0.924 ± 0.022 | 0.859 ± 0.054 | 0.823 ± 0.049 | 0.850 ± 0.035 |
| CE-T1w | 0.759 ± 0.076 | 0.724 ± 0.091 | 0.614 ± 0.049 | 0.830 ± 0.113 |
| T1w + CE-T1w* | 0.899 ± 0.054 | 0.859 ± 0.062 | 0.810 ± 0.131 | 0.873 ± 0.089 |
| CP + T1w + CE-T1w* | 0.909 ± 0.016 | 0.854 ± 0.024 | 0.845 ± 0.051 | 0.866 ± 0.101 |
| Averages of prognostic evaluators ± SD | ||||
| Train folds (8) | Test folds (8) | |||
| Classifier | AUC | Accuracy | AUC | Accuracy |
| Random Forest | 0.941±0.033 | 0.867±0.060 | 0.846 ± 0.048 | 0.773 ± 0.066 |
| Naive Bayes | 0.896±0.052 | 0.804±0.086 | 0.795 ± 0.073 | 0.704 ± 0.088 |
| kNN | 0.962±0.028 | 0.892±0.049 | 0.845 ± 0.056 | 0.790 ± 0.028 |
| Logistic Regression | 0.991±0.015 | 0.950±0.042 | 0.877 ± 0.031 | 0.815 ± 0.064 |
| Neural Network | 0.990±0.020 | 0.957±0.042 | 0.878 ± 0.033 | 0.824 ± 0.065 |
| SVM | 0.977±0.017 | 0.927±0.045 | 0.889 ± 0.043 | 0.820 ± 0.045 |
| Feature | B | 95% CI | P | |
| Age | 1.192 | -18.8 | 122.6 | 0.015 |
| Accumulated dose | -1.020 | -204.3 | 13.4 | 0.084 |
| Orig_firstorder_Entropy_T1w | -5.144 | -989.7 | -3.30 | 0.002 |
| Log_gldm_Smalldepemph_T1w | 1.775 | -84.4 | 417.5 | 0.026 |
| Log_glcm_Id_T1w | -2.936 | -601.5 | 8.9 | 0.010 |
| Lbp2D_gldm_lgdepLowGraylevemph_T1w | -0.784 | -147.4 | 400.8 | 0.296 |
| Logarithm_glcm_JointEnergy_CE-T1w | 0.681 | -33.3 | 111.3 | 0.040 |
| Lbp2D_glrlm_LongRunLowGraylevemph_T1w | 0.955 | -218.6 | 228.8 | 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





