1. Introduction
A transient ischemic attack (TIA) is a medical emergency characterized by a temporary episode of neurological dysfunction caused by localized ischemia in the brain, spinal cord, or retina, without resulting in acute infarction or tissue damage. Most TIAs last less than an hour, often just a few minutes [
1].
The definition of TIA has evolved from being time-based, focusing on symptom resolution within 24 hours, to emphasizing tissue involvement detected through advanced imaging techniques such as MRI-DWI.
The European stroke organization (ESO) defines a transient ischemic attack (TIA) as transient neurological symptoms, likely due to focal cerebral or ocular ischemia, which last less than 24 hours [
2].
Patients with TIA are clinically unstable and require prompt evaluation to reduce the risk of stroke. Accurately identifying which patients are at the highest and lowest risk of stroke in the critical days and weeks following a TIA is essential for implementing effective secondary prevention measures. Incorporating MRI-DWI and neurovascular imaging, together with tools such as the ABCD2 score, enhances the prediction of ischemic stroke following a TIA.
MRI-DWI plays a pivotal role in the early evaluation of TIA by detecting acute ischemic lesions in approximately 34% of cases [
3]. DWI-positive findings are associated with a higher risk of early stroke recurrence, enabling more precise risk stratification and informing targeted secondary prevention strategies. This imaging modality enhances diagnostic accuracy, distinguishing true ischemic events from mimics, and supports timely, evidence-based interventions to reduce recurrent stroke risk and improve outcomes.
HrDWI has further advanced the understanding and diagnosis of TIA. Unlike the traditional time-based definition, hrDWI enables the detection of ischemic lesions in a substantial proportion of patients, often exceeding previously reported rates. These findings are frequently associated with higher clinical severity, as reflected by increased National Institutes of Health Stroke Scale (NIHSS) and ABCD2 scores and are often linked to perfusion deficits and vascular occlusion. By providing a more sensitive method for detecting ischemic brain injury, hrDWI supports the transition toward a tissue-based definition of TIA, offering critical implications for risk stratification and the development of targeted secondary prevention strategies.
The usefulness of MRI in patients presenting with transient or minor neurological symptoms remains an area of ongoing investigation. Our objective was to assess the proportion of participants with these symptoms who exhibited MRI evidence of acute ischemia, across varying clinical probabilities of TIA or minor stroke. While previous studies have explored TIA diagnosis and DWI positivity, this study focuses on a cohort in Cluj, Romania, providing insights into the application of tissue-based definitions of TIA in outpatient settings.
In Romania, access to MRI machines is limited, with approximately 336 machines available nationwide, of which only 56 are in the public sector. This restricted availability means that advanced imaging modalities like MRI-DWI are primarily accessible in larger cities, creating disparities in diagnostic capabilities for TIA patients across the country. Moreover, there are no strict national guidelines for imaging in TIA cases, which further impacts the consistency and frequency of imaging investigations for these patients. These factors significantly influence the generalizability of the study’s findings, as the use of DWI for TIA diagnosis remains uncommon in routine clinical practice in many parts of Romania. Consequently, this study highlights the importance of addressing these disparities to improve diagnostic precision and patient outcomes in resource-limited settings.
2. Materials and Methods
2.1. Study Design
This was a retrospective observational study aimed at assessing the prevalence of ischemic lesions on DWI in patients with a clinical diagnosis of TIA without infarction on CT scan among adults in Cluj, Romania.
2.2. Study Population and Sample
The study targeted adults aged 18–90 years residing in Cluj County, Romania, who underwent MRI at CMT Transilvania. Inclusion criteria were a clinical diagnosis of TIA by a neurologist and completion of a DWI MRI scan within the first week after the TIA episode. All patients with a clinical diagnosis of TIA who underwent MRI at CMT Transilvania were assessed for eligibility for the study. Data cleaning was performed to ensure completeness and accuracy, with missing values excluded where necessary. Potential confounders, such as MRI timing, comorbidities, and medication use, were acknowledged but not adjusted due to the small sample size. The sample size was determined by the number of eligible patients during the study period, reflecting its exploratory nature.
The reclassification of cases from TIA to stroke did not involve follow-up or additional counseling for participants, as this was a retrospective study. However, all data were anonymized, and no interventions were made based on these findings, ensuring ethical handling of the results while acknowledging their clinical relevance.
2.3. Data Collection
Data collection occurred between May 2023 and June 2024. Participants completed a structured questionnaire that gathered demographic information and relevant medical history. Additionally, DWI MRI results were reviewed to confirm the presence of ischemic lesions. DWI images were analyzed by a neuroradiologist with expertise in stroke imaging, focusing on lesion size, location, and correlation with clinical presentation.
2.4. Statistical Analysis
Descriptive statistics, including means, standard deviations, and frequencies, were used to summarize demographic and clinical data. These analyses aimed to characterize the sample and identify the prevalence of ischemic lesions on DWI among the study participants. We primarily analyzed our data using RStudio (version 4.4.2) and Microsoft Office.
2.5. Ethical Considerations:
Written informed consent was obtained from all participants. Confidentiality was maintained by anonymizing all participant data and storing it on secure, password-protected computers. The study protocol adhered to ethical guidelines and obtained approval from the relevant ethics committee.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Prevalence of Ischemic Lesions
Ischemic lesions were present in 26.92%7 ( 95% CI: 11.6%–47.8%) of patients, with trends observed in older patients, males, and those with hypertension. This indicates that approximately one in four patients in the cohort had evidence of ischemic lesions.
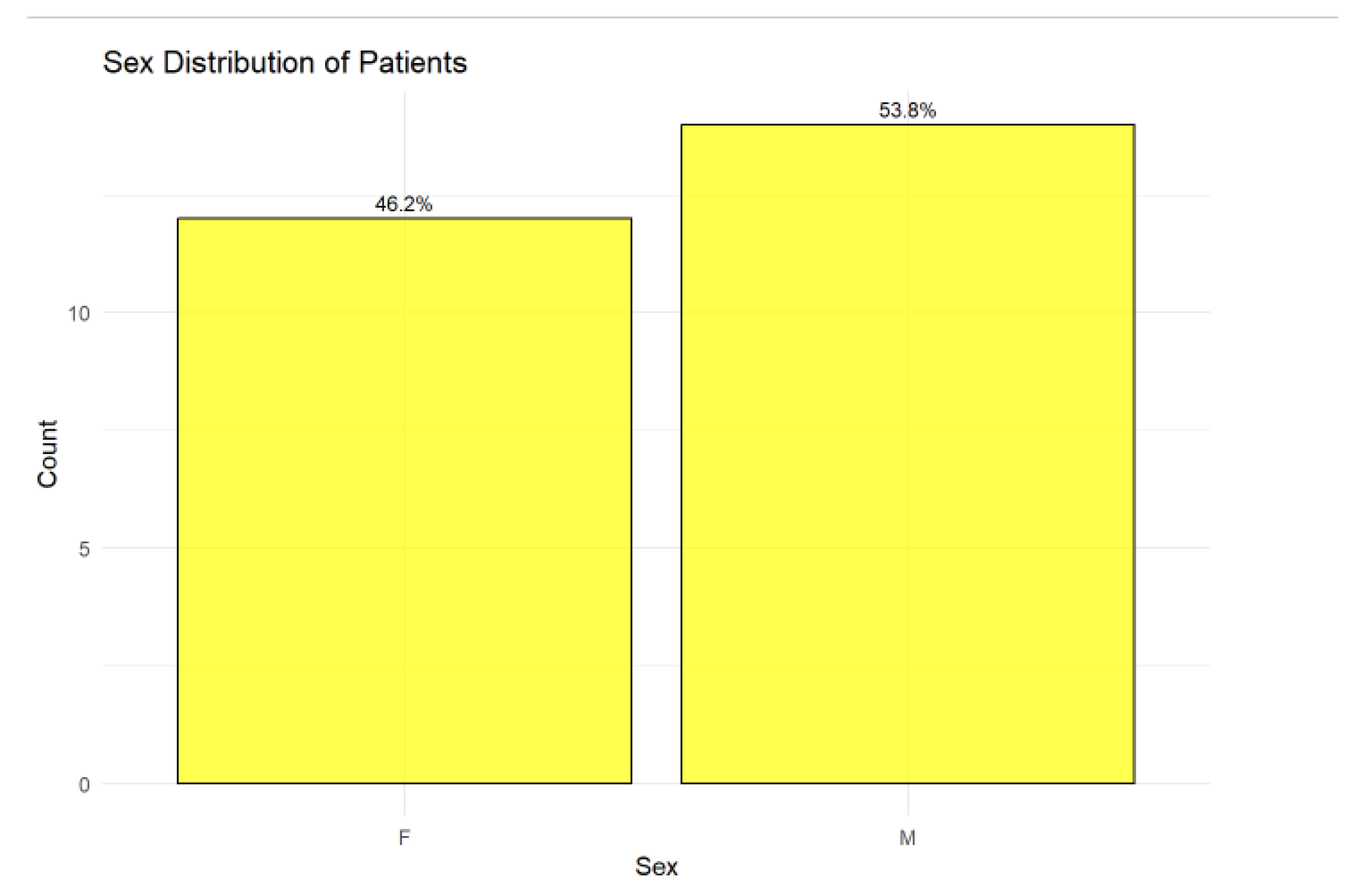
3.2. Sex Distribution
The cohort comprised 26 patients, of whom 46.15% (n = 12) were female and 53.85% (n = 14) were male. The slightly higher proportion of male patients suggests a modest male predominance in the studied population as in
Figure 1.
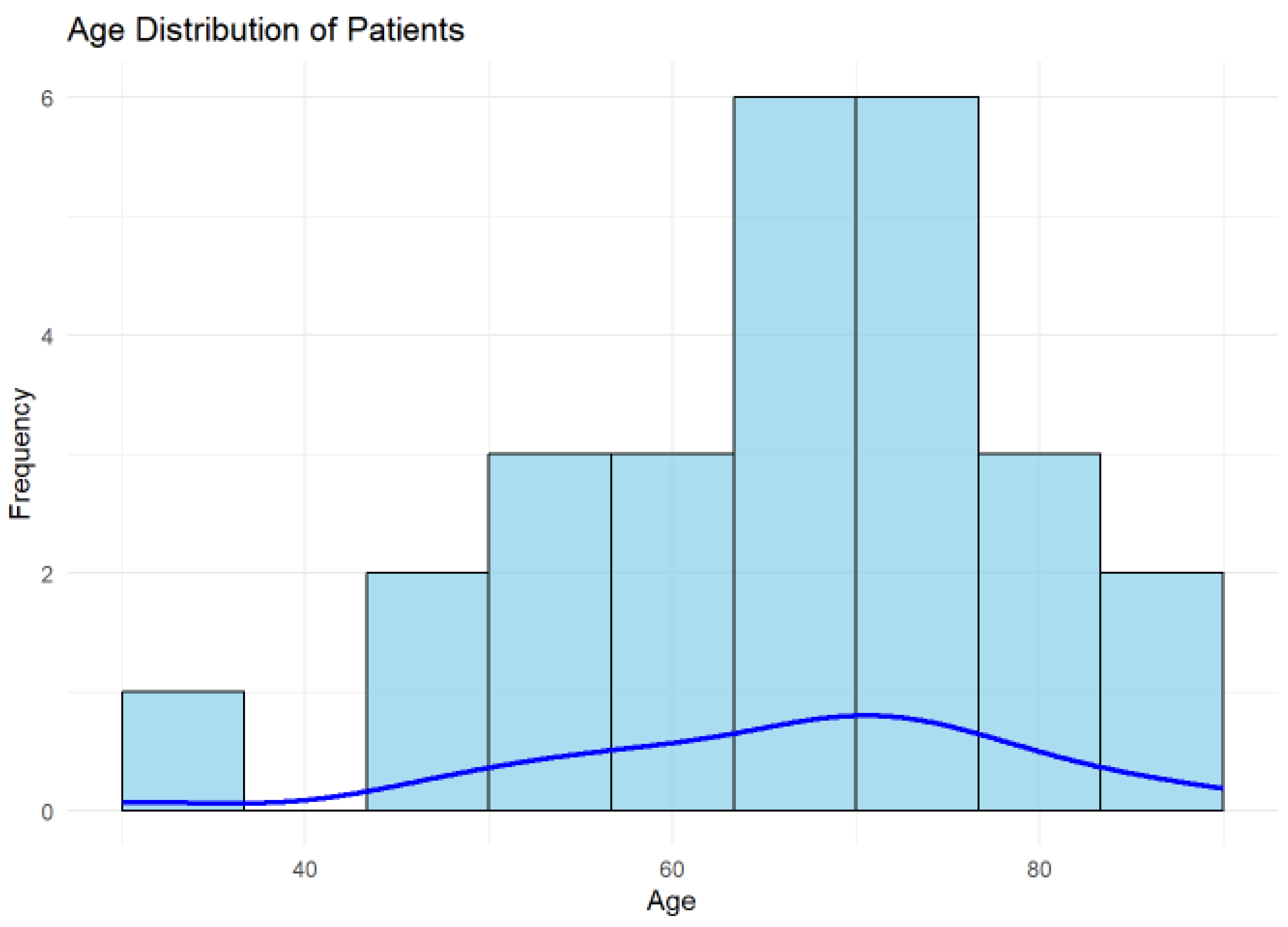
3.3. Age Distribution
The mean age of the cohort was 66.19 years (SD = 13.49 years), with a median age of 68.5 years, as shown in
Figure 2. The interquartile range (IQR) was 16 years, reflecting a moderate spread in the ages of the studied population. These findings indicate that the cohort predominantly included older adults.
The histogram illustrates the distribution of patient ages in the cohort, with the blue density curve providing a smoothed estimate of the age distribution. The highest frequency of patients lies between 60 and 70 years, indicating this is the most common age group in the cohort.
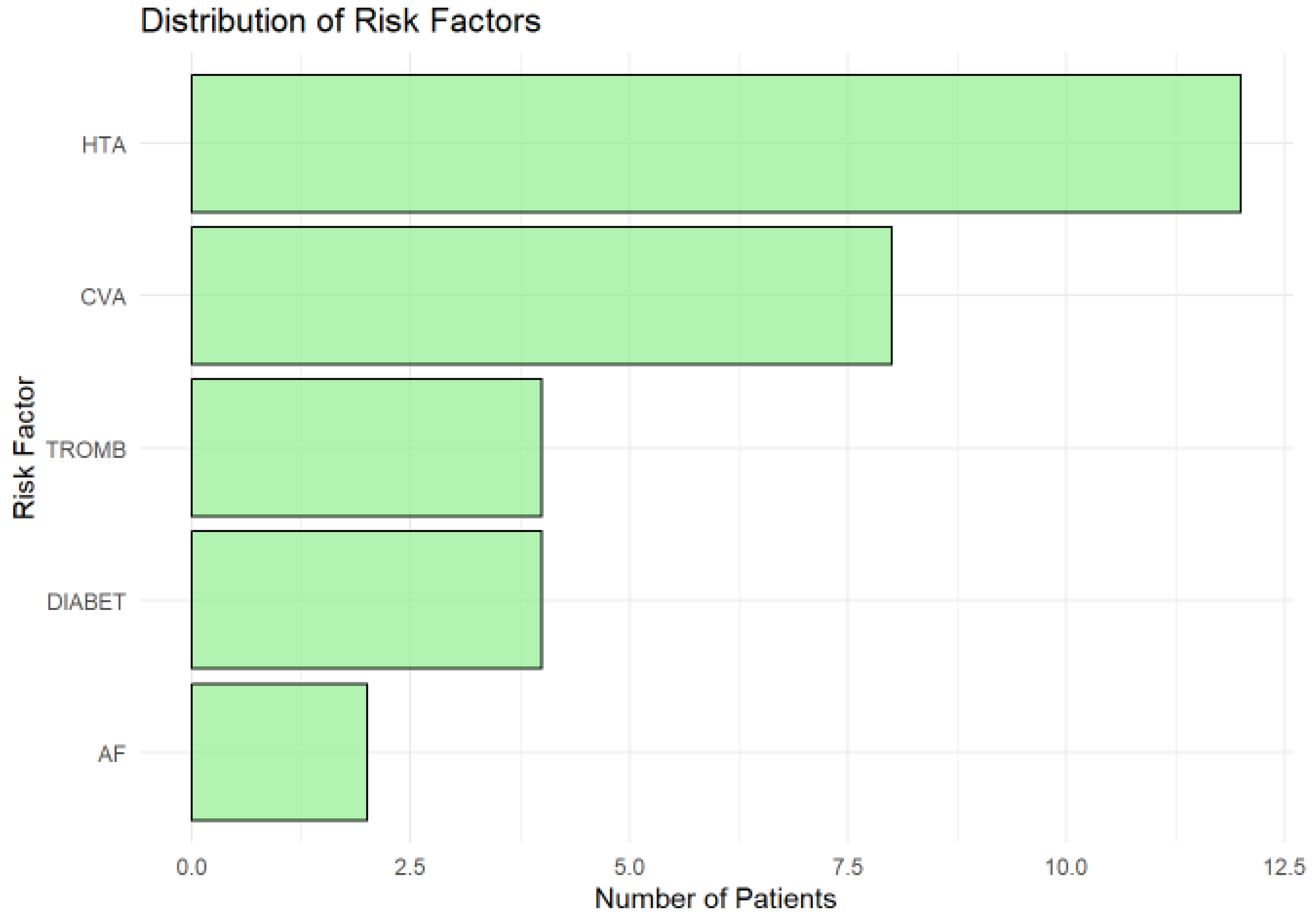
3.4. Distribution of Risk Factors
The distribution of risk factors (
Figure 4.) in the cohort highlights hypertension (HTA) as the most prevalent, affecting approximately 12 patients, underscoring its role as a major modifiable risk factor for ischemic lesions and cerebrovascular diseases. A history of cerebrovascular accident (CVA) was the second most common risk factor, present in around 9 patients, reflecting the vulnerability of the cohort to recurrent ischemic events and the need for robust secondary prevention strategies.
Thrombophilia (TROMB) and diabetes (DIABET) were moderately prevalent, affecting about 4–5 patients each, highlighting the importance of addressing coagulation and metabolic risks in this population. Atrial fibrillation (AF), although less common (observed in fewer than 3 patients), remains clinically significant due to its strong association with cardioembolic strokes. Its low frequency in this cohort may reflect underdiagnosis or cohort-specific characteristics.
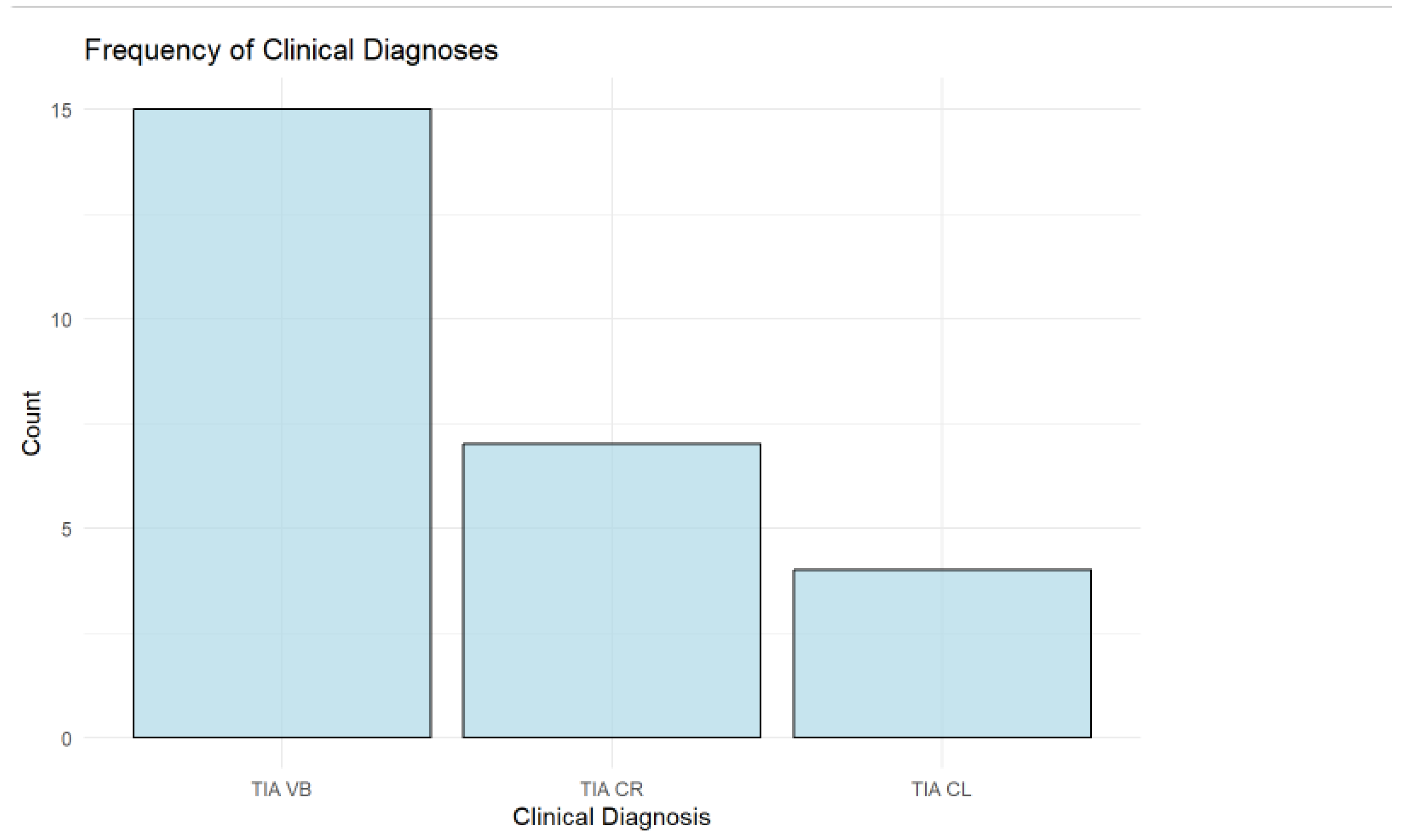
3.5. Frequency of Clinical Diagnoses
The analysis of clinical diagnoses shows that the vertebrobasilar (TIA VB) type is the most common, accounting for 57.69% (n = 15) of the cohort. This is followed by right carotid (TIA CR) cases, comprising 26.92% (n = 7), while left carotid (TIA CL) cases are the least frequent at 15.38% (n = 4) as shown in
Figure 5.
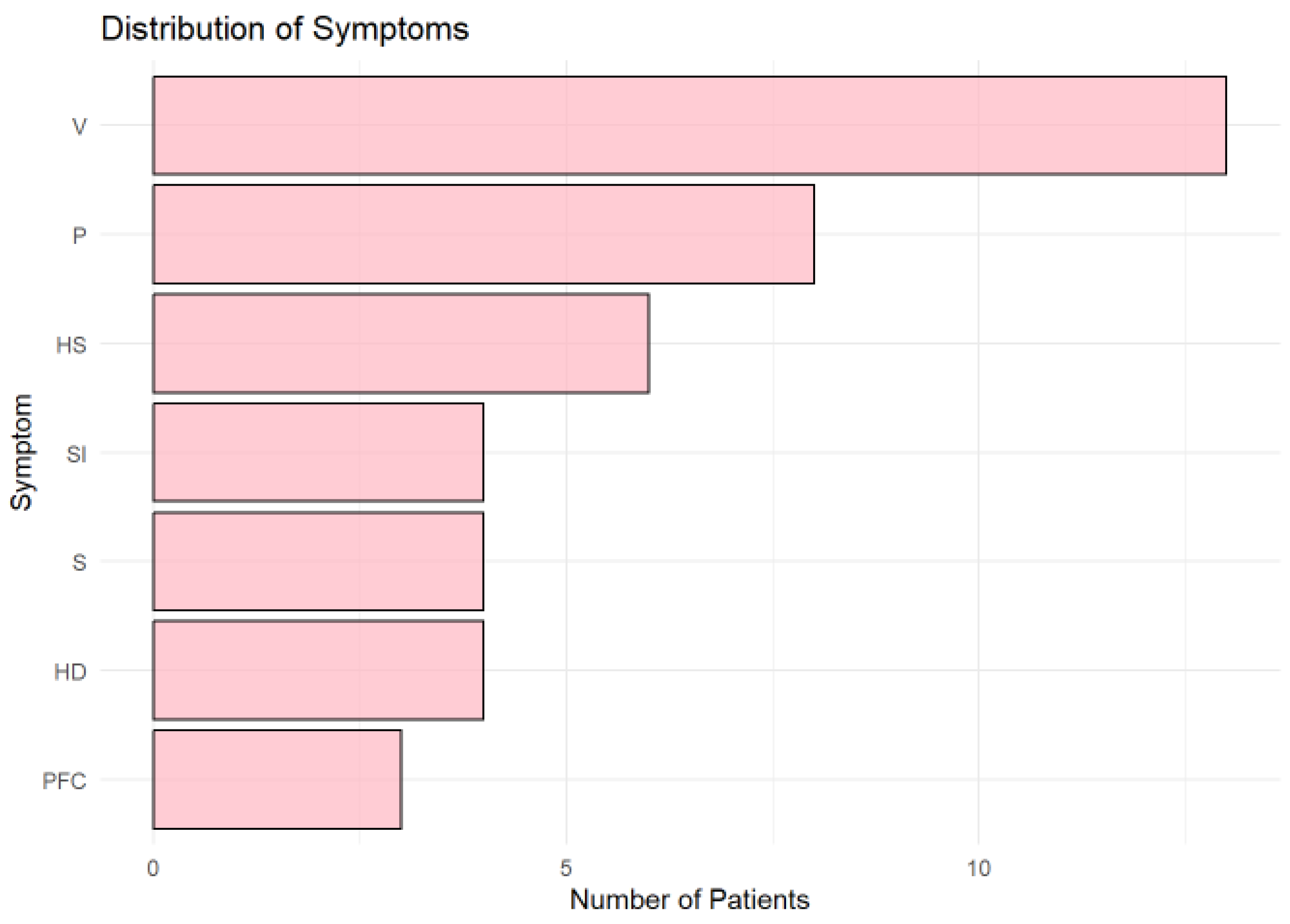
3.6. Distribution of Symptoms
Vertigo (V) was the most frequently reported symptom (
Figure 6.), observed in more than 10 patients, followed by paresthesia (P) and left-sided hemiparesis (HS). Other symptoms, including speech impairment (SI), sight impairment (S), and right-sided hemiparesis (HD), were moderately represented. Central facial palsy (PFC) was the least common symptom.
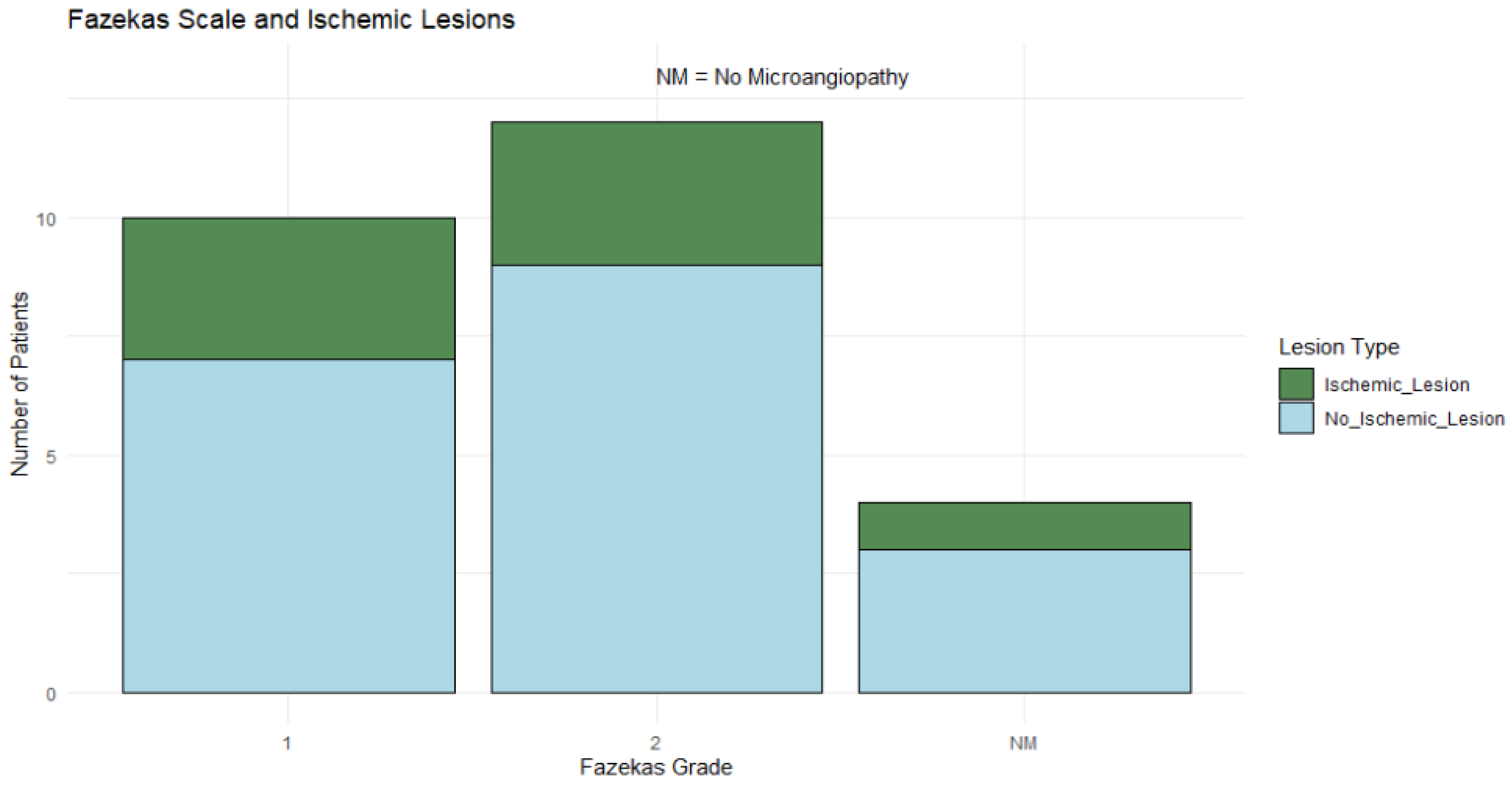
3.7. Fazekas Scale Distribution and Association with Ischemic Lesions
Fazekas grade 2 was the most common, observed in 46.15% (n = 12) of patients, followed by grade 1 (38.46%, n = 10), while 15.38% (n = 4) of patients showed no microangiopathy. Ischemic lesions were observed across all Fazekas grades, though no definitive pattern emerged in the distribution as shown in
Figure 7.
The distribution indicates that most patients in the cohort are older adults, which is consistent with the higher prevalence of ischemic lesions and related conditions like TIA in older populations.
Younger patients (30–40 years) may represent outliers or individuals with unique risk factors or atypical presentations, warranting further investigation.
The age and risk factor distributions underscore the importance of targeting hypertension and vascular risk factors in this population.
Fisher's Exact Test was applied to evaluate the association between Fazekas scale grades and the presence of ischemic lesions, given the categorical nature of these variables and the limited sample size, but the test did not yield statistically significant findings.
4. Discussion
The rate of DWI positivity observed in our study (27% of TIAs) is consistent with previously reported ranges of 12% to 67%. By providing a more sensitive method for detecting ischemic brain injury, DWI supports the transition toward a tissue-based definition of TIA, offering critical implications for risk stratification and the development of targeted secondary prevention strategies. This study explores the prevalence of DWI-detected lesions in TIA and their clinical relevance.
All patients underwent DWI imaging using 1.5T or 3T MRI systems with a slice thickness of 5 mm. Standard-resolution DWI was used for all patients, which may have limited sensitivity for detecting smaller ischemic lesions compared to high-resolution sequences. This limitation could have impacted the detection of subtle lesions and should be considered when interpreting the findings.
Transitioning to a tissue-based definition of TIA, using DWI, offers valuable prognostic insight. Notably, all acute DWI lesions detected in this analysis were included, regardless of their alignment with the patients’ clinical symptoms. In clinical practice, symptoms may not always align with tissue localization due to due to unknown factors. Including all acute DWI lesions ensures comprehensive analysis and avoids overlooking atypical presentations. Future studies could categorize lesions as "clinically relevant" versus "incidental" to refine the reclassification of TIA versus minor stroke.
Existing literature indicates that TIA patients with acute ischemic changes on DWI imaging face an increased 90-day stroke risk, possibly due to a heightened vulnerability of brain tissue to infarction [
4]. In our cohort, individuals with DWI-positive lesions were more frequently male, aged 60–80 years, and demonstrated a trend toward a higher prevalence of hypertension (HTA).
The strengths of this study include TIA diagnoses confirmed by a neurologist and thorough radiologic evaluation of the imaging results. However, there are limitations that warrant consideration. Previous studies have shown that TIA diagnoses based solely on clinical notes often result in moderate agreement among stroke specialists, highlighting the subjectivity of diagnostic thresholds. The type of scale used also influences ratings, particularly for "unlikely TIA" cases, where substantial disagreement may impact patient management. These findings emphasize the need for standardized criteria to improve consistency [
5].
The relatively small sample size and the predominantly Caucasian population limit the generalizability of the findings to more diverse populations. The small sample size, based on the number of eligible patients during the study period, limits the precision and generalizability of the fidndings. Additionally, some DWI-negative TIA cases might have had non-vascular etiologies for their symptoms. Furthermore, the interval between the qualifying event and DWI scanning (up to 7 days) might have influenced lesion detection due to factors such as transient DWI lesion reversal or clinically silent new lesions [
6]. This limitation is consistent with other studies, where delays in imaging have similarly affected the ability to detect acute ischemic lesions.
We cannot be certain that these findings are fully applicable to hyperacute settings, such as the emergency department, as our study primarily focused on a predominantly outpatient cohort. The time window of up to 7 days after symptom onset may have influenced the detection of DWI-positive lesions. Immediate imaging in the emergency department could identify more acute ischemic lesions, while delayed imaging might miss some findings. Conversely, delayed imaging could also detect new, clinically silent lesions that develop after the initial event, potentially overestimating the prevalence of DWI positivity. These factors highlight the need for further studies in hyperacute settings to better understand the timing of optimal imaging for TIA patients.
The cohort consisted of participants presenting with transient or minor neurological symptoms from both emergency and outpatient settings. Clinicians at various levels of training provided a differential diagnosis, with TIA or stroke considered the most likely diagnosis prior to conducting a 1.5 or 3T brain MRI within 7 days of symptom onset. MRI evidence of acute ischemia was determined based on two independent readings of the MRI scans.
The predominance of vertebrobasilar TIA (57.69%) aligns with the observation that vertigo was the most frequently reported symptom in this cohort. This association highlights the involvement of the posterior circulation, as the vertebrobasilar territory supplies critical structures such as the brainstem and vestibular system. Ischemia in this region commonly manifests as vertigo due to transient dysfunction of the vestibular nuclei or their pathways.
Distribution factors findings emphasize the need for a multifaceted approach to risk management, with a primary focus on hypertension control and secondary prevention in patients with prior cerebrovascular events. Addressing metabolic, coagulation, and cardiac rhythm disorders remains critical for reducing the overall burden of ischemic lesions.
Identifying DWI-positive lesions in TIA cases underscores the need for more aggressive secondary prevention, such as early initiation or intensification of antithrombotic therapy. These findings support routine DWI imaging within 72 hours to 1 week of suspected TIA to improve risk stratification and guide management.
5. Conclusions
This study emphasizes the role of MRI-DWI in redefining TIA cases as minor strokes, with 27% showing ischemic lesions. The findings support tissue-based definitions to enhance risk stratification and guide prevention. However, limited MRI access and the lack of standardized imaging guidelines in Romania pose challenges. Larger studies are needed to validate and expand these findings.
Author Contributions
Conceptualization, E.M. and R. C.; methodology, I. S. F. and K. A. A. ; software, K. A. A.; validation, A. B. AND A.-S. F., ; formal analysis, K. A. A. ; investigation, E.M.; resources, A.B.; data curation, I. S. F., A-S F.; writing—original draft preparation, K.A.A. ; writing—review and editing, R.C. and E.M. ; visualization, E.M. ; supervision, R.C.; project administration, K.A.A.; funding acquisition, K.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Centrul Medical Transilvania (protocol code PO-MG-03, approved on 06.11.2023). Ethical approval covered all procedures, including participant recruitment, data collection, and analysis of MRI-DWI findings.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Given the retrospective nature of your study and potential ethical restrictions on patient data: "The data presented in this study are not publicly available due to ethical and privacy considerations but may be available on reasonable request from the corresponding author, subject to Institutional Review Board approval.
Acknowledgments
This paper was supported by the project POC-A.1-A.1.1.1-A-2015– " RESEARCH AND DEVELOPMENT CENTER FOR THE DIAGNOSIS AND TREATMENT OF CEREBRIAL VASCULAR ACCIDENTS" SMIS Code 121574, a project co-funded through the Operational Program Competitiveness 2014-2020. This work was made possible by the generous support of Centrul Medical Transilvania Company. Additionally, we mention the assistance of ChatGPT in providing editorial support to enhance the clarity and coherence of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| TIA |
Transient Ischemic Attack |
| MRI |
Magnetic Resonance Imaging |
| DWI |
Diffusion-Weighted Imaging |
| hrDWI |
High-resolution diffusion-weighted imaging |
| MRI-DWI |
Magnetic Resonance Imaging with Diffusion-Weighted Imaging |
| CT |
Computed Tomography |
| |
|
| |
|
References
- Panuganti, K.K.; Tadi, P.L.F. Transient Ischemic Attack. In: StatPearls [Internet] [Updated 2023 Jul 17] [Internet]. Treasure Island (FL): StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459143/? 4591. [Google Scholar]
- Fonseca, A.C.; Merwick, Á.; Dennis, M.; Ferrari, J.; Ferro, J.M.; Kelly, P.; et al. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur. Stroke J. 2021, 6, CLXIII. [Google Scholar] [CrossRef] [PubMed]
- Brazzelli, M.; Chappell, F.M.; Miranda, H.; Shuler, K.; Dennis, M.; Sandercock, P.A.G.; et al. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann Neurol. 2014, 75, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Coutts, S.B.; Modi, J.; Patel, S.K.; Demchuk, A.M.; Goyal, M.; Hill, M.D. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: Results of the prospective CATCH study. Stroke 2012, 43, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.; Mlynash, M.; Lee, K.; Caulfield, A.F.; Wolford, C.; Kemp, S.; et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke 2010, 41, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, F.G.; Vermeer, S.E.; Gõraj, B.M.; Koudstaal, P.J.; Richard, E.; De Leeuw, F.E.; et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol. 2015, 78, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).