Introduction
Figure 1.
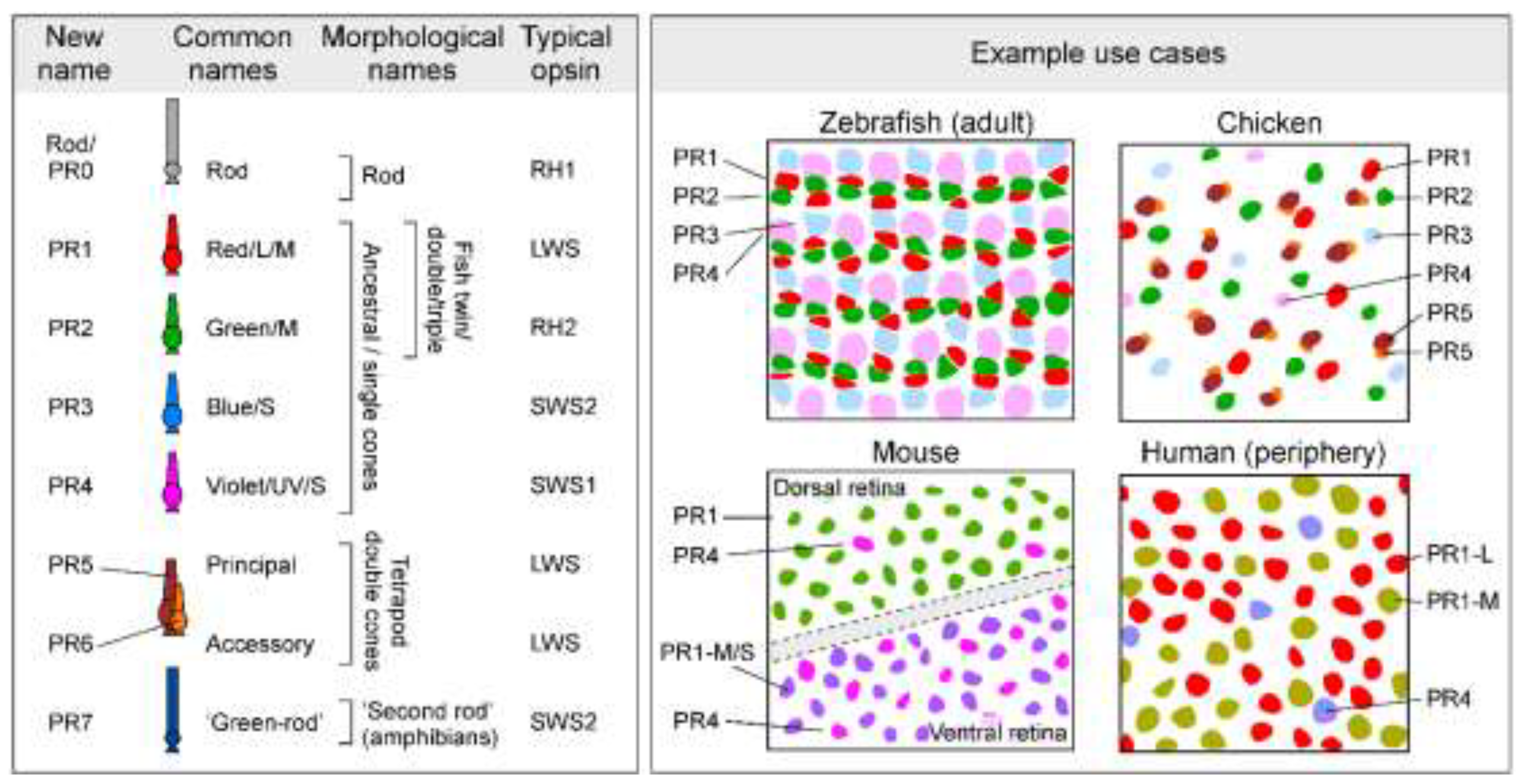
Summary of proposed naming system for vertebrate photoreceptors (left) and its relationship to prior systems [1–4]. Application illustrated in four well-studied species (right).
Figure 1.
Summary of proposed naming system for vertebrate photoreceptors (left) and its relationship to prior systems [1–4]. Application illustrated in four well-studied species (right).
The common ancestor of extant vertebrates likely possessed a photoreceptor system consisting of one rod and four cone types, based on the shared presence of these five cell types in present-day jawed and jawless vertebrates [5,6]. Accordingly, We begin our classification with these five types (
Figure 1). Following prior studies of other retinal cell classes, we propose a simple naming system in which the photoreceptor types are designated with two letters (‘PR’ for ‘photoreceptor’) followed by a number (
Figure 1). Thus, cones derived from the four ancestral single cone types will be designated PR1-PR4 (alternative: ‘Type 1-4 photoreceptors’), and rods will be PR0. Prior studies have shown that retinal cell types typically occur in regular spatial mosaics, in which cells are tiled in a non-random distribution with minimal spacing between neurons of the same type [7]. A prior study of cone photoreceptor distributions in chicken suggested that mosaic spacing is also a feature of photoreceptor types [8]. Accordingly, we propose mosaic spacing as a working definition of photoreceptor type, similar to the approach used for defining other retinal neuron types (e.g. Refs [9–11]). Under this definition, regional variation in photoreceptor morpholology or gene expression can occur in a single photoreceptor type, without needing to designate regional variants (e.g., foveal vs. peripheral cones in human) as distinct cell types. Our classification also includes the tetrapod double cone, which consists of principal (PR5) and accessory (PR6) members, and the so-called ‘green rod’ of amphibians (PR7). The two members of the double cone likely arose in the common ancestor of tetrapods, whereas the ‘green rod’ appears to have arisen early in the amphibian lineage [12]. According to this system, zebrafish retinas possess PR0-PR4 [13], while chicken retinas have PR0-PR6 [14]. Eutherian mammals, which include mice and humans, have PR0, PR1, and PR4 types [15] Depending on context, special notation may be required to distinguish unique subtypes of photoreceptors, such as human ‘green/M’ and ‘red/L’ cones, which differ only in the expression of paralogous LWS opsin genes. In this case, we propose the designations PR1-M and PR1-L, for ‘M’ and ‘L' cones, respectively. Additional photoreceptor types can readily be added to this system when they are discovered.
The Problem
Current naming systems for vertebrate ciliary photoreceptors represent a grab bag of species-specific schemes, typically based on a single functional, morphological, or molecular feature. For example, the cone types of the human eye are often referred to as red/L, green/M, and blue/S. This naming system references the wavelength of the cones’ maximal spectral sensitivity as imparted by the visual pigment [1,16] (opsin + 11-cis retinal chromophore), and oil droplet filtering [17], when present. Since the 1990’s [18–20] vertebrate visual photoreceptors have commonly been classified by the main opsin that they express: RH1 (rods) and LWS, RH2, SWS2, SWS1 (cones). These ancient gene sub-families were already present in the last common vertebrate ancestor [5], and exhibit remarkable evolutionary conservation, for example in the spectral ranges of the visual pigments, and, as we explain here, in the cell types in which they are expressed. Even so, naming cone types according to the opsin they express can be problematic. For example, human red and green cones differ by a single feature, namely which of two paralogues of the LWS opsin gene is expressed [15]. Human L and M cones derive from a single ancestral photoreceptor type, and homologs of this cell type are present in the eyes of most extant vertebrates [4]. Why, then, should this photoreceptor type not share a common designation across species?
In general, the names of photoreceptor types fall into two major groups: those that refer to spectral properties and/or the expressed type(s) of opsin (Box 1), and those that describe morphological properties, such as the shape of their outer segment, their size, or their pairing with other photoreceptor types (Box 2). However, ‘spectral’ (‘red/L’) and opsin (‘LWS’) terms are problematic because the properties that they reference are subject to routine variation within and between species, for example on account of evolutionary variation in peak spectral sensitivity4. Likewise, morphological terms such as ‘single’, ‘double’, ‘twin’, and ‘paired’ mean different things in different animals [3]: In teleost fishes, the latter three refer to coupled combinations of ancestral ‘red’ and ‘green’ cones [21], commonly found as part of mosaics of variable regularity [22,23]. In contrast, ‘tetrapod double cones’ [24] are molecularly [21,25], morphologically [8,26,27] and functionally17 distinct from the four ancestral single cones [28] and likely have a distinct evolutionary origin.
To overcome such naming confusions, current progress in life sciences requires cell-type definitions that encompass a wide spectrum of features [29–31] , ideally combining morphological [11], functional [32] , developmental [21,33] and molecular traits [34]. We also emphasize the importance of deeply conserved transcription factors as type-defining features in our classification.
The vertebrate retina, with its planar structure and regularly tiled architecture has long served as a central model system for cell type taxonomy. Retinal neurons form spatially repeating units [35] that anchor efforts to define types and their plausible subdivisions across animal brains. In some species such as the mouse, the catalogue of retinal neuron types is probably close to complete. This momentous achievement has been gradually unlocked by the use of transgenic tools [9], large-scale physiological recording techniques [32,36], EM-connectomics [10,11,37], and, perhaps most importantly single cell transcriptomics [10,11,37] which can assign a genome-wide molecular signature to anatomical and functional catalogues.
The bipolar cells of the mouse retina were amongst the first to be ‘solved’ in this way [37,38,41], and it has taken only a few more years for the tabulation of other retinal neuron types in a number of species to become increasingly complete. The mouse retina comprises ~130 neuron types: the rod, 2 cones, 1 horizontal cell, 15 bipolar cells [10,37,38,42], ~63 amacrine cells [39] and ~45 ganglion cells [30–32]. Of these, all except the rods and cones are primarily known by a numbered identity (e.g. H1 horizontal cell, Type 1 bipolar cell, etc.). While aspects of these catalogues still require consensus and alignment across species, in principle a numerical system gives each type of neuron a unique and unmistakable identity. For example, a horizontal cell that is orthologous to the H1 of mice [43] also exists in lampreys44, zebrafish [40,45], chicken [14,25,46] and humans [40,47]. This understanding allows us to make powerful inferences across the vertebrate tree of life.
Recent transcriptomic studies (e.g. Refs [21,33,48,49]) investigated molecular relationships of vertebrate photoreceptors across a broad range of species. Among other findings, this work provided a molecular confirmation of what had long been suspected [4]: Many vertebrate photoreceptor types appear to be orthologous across multiple evolutionarily distant species. We believe that the time has come to inscribe this knowledge into the names of photoreceptor types.
Our Proposal
As a clear and systematic nomenclature for vertebrate photoreceptors, we suggest a numbering scheme that simultaneously mirrors the cell’s ancestry, their typical relative abundance in the eye, their development, and their systematically distinct postsynaptic wiring patterns (Box 3).
Ancestral red cones [4], renamed PR1, are probably the least derived type of cone. In most non-avian vertebrate eyes, barring rods, PR1 are the most abundant, and probably also most important type of photoreceptor [4,24,50]. PR1 typically, but not always (e.g. Ref [51]), expresses LWS-opsin, and the only sighted vertebrates thought to lack PR1 are rod-only species that typically live in extreme low-light conditions. Human daylight vision is overwhelmingly driven by PR1 cones [52], which include both their ‘L’ and ‘M’ variants [15]. In mouse, PR1 includes both the ‘green’ sensitive dorsal and predominantly ‘UV-sensitive’ ventral LWS cones [53–55].
Beyond PR1, the other three ‘original’ single cones [4,6] (ancestral green/RH2, blue/SWS2, UV/SWS1) are designated PR2-4, respectively. This order mirrors their typical spectral order2 (cf. Box 1), and their transcriptomic relatedness to PR1 [21], their anatomical wiring order in the retina (‘spectral block wiring’[1,13]), as well as the order of their typical numerical abundance and relative sizes (PR1≥PR2≥PR3≥PR4, e.g. in chicken [8] and adult zebrafish [56], see also Box 2). Moreover, this sequence mirrors the numerical order of cones’ postsynaptic targets, namely horizontal cells and bipolar cells. For example, the zebrafish H1 horizontal cell is the only one that connects with PR1 cones, alongside PR2,3 [45]. H2 then links with PR2-4 cones, while H3 links to PR3,4. Likewise, the Type 1 bipolar cell of mice is the only cone-bipolar cell that preferentially targets PR1 cones [37]. The numerical system also elucidates evolutionary loss of cone types, e.g. for eutherian mammals who retain only PR1 and PR4 [4].
After these original five photoreceptor types, the next most widespread photoreceptor type in vertebrates is the tetrapod double cone (except in birds where double cones are dominant)[24,27,28], and we suggest the designations PR5 and PR6 for the principal and accessory members, respectively. This system also mirrors their likely evolutionary order of appearance [24]. We posit that the double cone should receive two numbers (5 and 6) rather than one because (i) it consists of two constituent members [21,25,27], (ii) each member makes independent connections to postsynaptic targets [26], and (iii), the two members almost certainly differ in their physiological properties and roles in vision [17,28,57]; and (iv) the two cell types are morphologically[26] and molecularly [21,33] distinct.
Beyond these established photoreceptor types (see below), many amphibians have a ‘second-rod’ type known traditionally as the ‘green rod’ based on its microscopic appearance (and in contrast with the canonical or ‘red rod’)[58–60]. Tentatively designated PR7, the ancestry of this eighth type of photoreceptor remains unresolved. However, its exclusive presence in amphibians strongly suggests that unlike PR5/6, PR7 may have emerged after present-day amphibians diverged from all other tetrapods. Beyond PR7, further hints of yet to be defined photoreceptor types include a possible ‘extra’ type in some marsupials such as dunnarts [61] and subsets of photoreceptors found in some snakes [62,63] and geckos [64–66]. We posit that these, or others, should be added onto the end of the proposed scheme if and when appropriate.
Finally, we propose that rods be designated PR0. Despite rods’ substantial molecular differentiation from cones [21], both rods and cones share a common ancestry, and the presence of rods (PR0) and ancestral single cones (PR1-4) in cyclostomes [6,44,67] strongly suggests that both were present in the common ancestor of extant vertebrates. While some species feature additional rod-like photoreceptors, it remains unclear which, if any, represent genuine new types (see Box 3, Definitions).
A Final Word
We acknowledge that some common names for photoreceptor types are deeply engrained, both in the scientific literature but also in popular culture. For example, the terms of ‘L’, ‘M’ and ‘S’ cones of the human eye, although potentially misleading (discussed in Ref4), are unlikely to go away anytime soon, or perhaps ever. However, as a community we can endeavour to define the terms that we use in our communications by the simple and hopefully uncontroversial numbering scheme suggested here. Perhaps, in this way, it will eventually become more widely adopted.
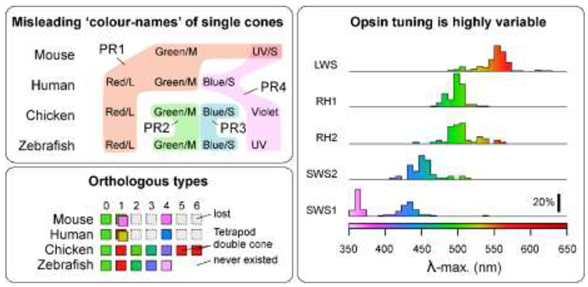
Box 1. Opsins and their spectral properties are poor indicators of cone identity
Comparison of humans, zebrafish, and mice illustrates the central problem. Humans and zebrafish have ‘red/L’, ‘green/M’ and ‘blue/S’ cones, while zebrafish additionally have ultraviolet (UV) cones4, but human ‘green/M’ and ‘blue/S’ cones are evolutionarily unrelated to zebrafish ‘green/M’ and ‘blue/S’ cones [21]. The opsins of human ‘green/M’ and ‘red/L’ cones are orthologous to zebrafish ‘red/L’ (all express LWS), and human ‘blue/S’ to zebrafish UV (both express SWS1). However, this match by opsins is fortuitous in the sense that both human and zebrafish cones, where present, consistently express variants of their ancestrally linked opsins: LWS, RH2, SWS2 and SWS1 for PR1-4, respectively [2]. By contrast, the opsin scheme falls apart in mice because mouse PR1 cones (which are often referred to as ‘green/M’ in reference to their ‘green-shifted’ LWS opsin) co-express the LWS and SWS1 opsins in the ventral retina[53–55]. The same ancestral neuron type PR1 therefore transitions from ‘functionally green’ to ‘functionally UV’ along the dorsal-ventral axis of the mouse retina. Moreover mice retain the ancestral UV-cone PR4, which like the SWS1-coexpressing PR1 cones are more concentrated in the ventral retina [68], but have distinct connectivity with second-order neurons[37,68,69]. Mice therefore have two types of UV-sensitive cones in direct proximity. Similarly, some fish species including cichlids and salmonids are known to switch opsin expression in individual cones, such that PR1 cones may express LWS or RH2 opsins, and PR4 cones may express SWS1 or SWS2 opsins depending on developmental stage or environmental cues [70–72]. While these examples illustrate the problem, they are not outliers in the vertebrate tree of life. The identity and wavelength specificity of expressed cone opsins is subject to routine variation [1,2], both across species (e.g. Ref [73] ) as well as within species (including by retinal region [53,54], life stage [74], season [75–77], and environment [78]). It further depends on an opsin’s associated chromophore (A1 or A2) [79,80], which can also vary seasonably and according to life stage. In fact, opsins and their properties are an evolutionary hotspot, varying as species enter new visual niches [2,81]. The identity or functional properties of opsins therefore do not reliably specify the identity of the neuron that expresses them.
A second issue is that a definition by ‘colour’ implies that wavelength selectivity is the only important characteristic of a photoreceptor. This is misleading4, because beyond wavelength selectivity, cone types systematically differ in their basic cellular physiology including their spatio-temporal properties [82–84], as well as in their developmental postsynaptic wiring [26,37,85] – all of which directly feed into their distinct roles in vision [50].
Figure Box 1: Left, Cone ‘colour names’ (top) do not necessarily align with orthologous neuron types across vertebrates (bottom, based on Ref4). Right, Sequence changes within vertebrate opsins can give rise to diverse spectral properties (based on Ref2). Note e.g. that a λ-max of ~500 nm is readily achieved by four out of five opsin families. Colour shadings as defined on the bottom right, by most common expressed opsin variant, independent of oil droplets, where present.
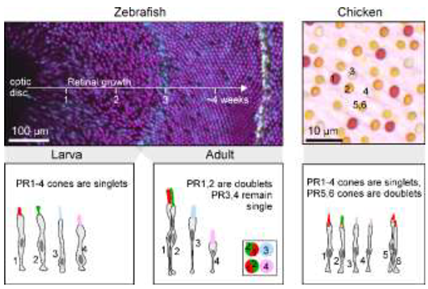
Box 2. Cellular morphology is an imperfect indicator of photoreceptor identity
Morphological definitions of photoreceptor types based on the shape of the outer segment (i.e., ‘rod’ vs. ‘cone’), association with other photoreceptors (e.g., ‘single’ vs. ‘double’ cones) or other cellular features are as problematic as opsin-based definitions. Photoreceptors can be grouped into ‘morphological types’, namely ‘single cones’ which tend to occur in isolation, ‘twin’ cones3 which comprise pairs made up of morphologically identical partners, and ‘double/triple cones’ consisting of asymmetric groups, often with ‘principal’ and ‘accessory’ members [3,8,28,86]. Single cones are occasionally further identified by other descriptors such as ‘long’, ‘short’ [87], and miniature [88] in reference to their size and/or vertical location in the outer retina. However, there are many factors that influence the anatomical arrangement of photoreceptors, and like opsin or spectral identity, none are reliably stable across or within species.
Zebrafish and chicken serve as two well studied examples. Adult zebrafish have a quasi-crystalline photoreceptor mosaic with PR1-4 cones arranged at a fixed 2:2:1:1 stoichiometry in the adult [13,89]. These six neurons are arranged in a tight lattice whereby PR1,2 pairs alternate rows with PR3 and PR4. Fishes have different patterns of PR1-4 cones that vary in spectral content and regularity depending on developmental stage and retinal location. PR1,2 pairs have been generally referred to as ‘paired cones’. If the two members have different morphology (usually with PR1 being larger than PR2) or visual pigment content, they have been called ‘double cones’. If they appear morphologically equal and contain the same visual pigment, they have been termed ‘twin cones’ [90].
In non-tetrapod vertebrates, all the above terms describe subsets of the same four ancestral cone types [21], PR1-4. One way to ascertain this is during development: In zebrafish, the retina does not start out in a crystalline arrangement that forces some cone types into pairs. Instead, larval cones are arranged independently [45,89,91]. This developmental history is inscribed into the adult retina as the ‘larval patch’ near the optic disc, which never rearranges [89,92]. Beyond this developmental evidence, the conclusion that all (zebra)fish cones are subsets of the same four ancestral ‘single’ cones is overwhelmingly supported by single cell transcriptomics [21,93]. By contrast, many tetrapods, with the notable exception of eutherian mammals, do have an ‘extra’ pair of cone photoreceptors, called ‘the tetrapod double cone’[24] (PR5,6). Unlike fish ‘double/twin/paired’ cones, this cone pair exists in parallel to PR1,2. Birds and most reptiles therefore have six ancestrally distinct types of cones. The four ‘original’ cones PR1-4 plus the two members of the tetrapod double cone PR5 and PR6. This view is supported by extensive morphological [8,17,26,27], behavioural [28,94] and molecular evidence [21,25,95].
Figure Box 2: Left, Developmental transition of zebrafish cone patterning from a mosaic with low regularity to a highly regular row lattice (top, mod from Ref [89]) and schematic summary of cone type arrangements at each stage (bottom, mod. from refs [13,45,96]). Right, oil droplets in chicken retina show five independent cone-type mosaics (top) and schematic summary of cone types (bottom, both mod. from Ref8).
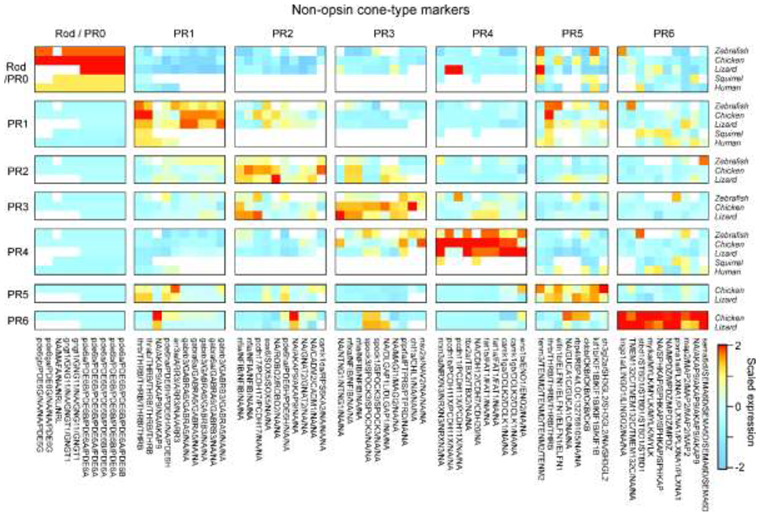
Box 3. Naming yet-to-be-identified photoreceptor types.
We recognize that the list of photoreceptor types presented in
Figure 1 is not exhaustive. For example, evidence suggests that the little skate, deep-sea fishes, marsupials, geckos, and snakes may possess unusual photoreceptor types that do not fall within any of the categories we propose. Furthermore, the photoreceptor types of only a minute fraction of the nearly 67,000 extant vertebrate species have been studied in any detail. Thus, the present classification system is intended to be open-ended, permitting the addition of more photoreceptor types as they are characterized.
The goal of our definition is to include ‘extra’ types that exist in parallel to the ancestral ones (such as tetrapod double cones PR5,6 or the amphibian ‘second rod’ PR7), but to exclude those that represent within-retina variation (e.g. human PR1-L versus PR1-M).
More generally, the proposed definition of photoreceptors into ancestral types is centrally anchored in their distinct transcriptomic signatures [21] across vertebrate ‘model’ species that are thought to be broadly representative for their clade: Humans (primates), squirrel (rodents), chicken (birds), brown anole lizard (non-avian reptiles), and zebrafish (teleosts). In these species, single cell transcriptomic data conforms with long standing insights into a photoreceptor’s morphological and functional properties (reviewed in Refs [4,13,14,16,24]). Together, this wealth of data renders it unlikely that their cell-type definitions will need to be revised in the light of possible future evidence. We therefore posit that these species, and others where corresponding insights exist (e.g. Ref [44]), can serve as a reference when defining cone types in other species.
Figure Box 3: Non-exhaustive list of possible marker genes for rods and cones (based on Ref21). Note that the second rod of amphibians PR7 is omitted because single-cell transcriptomic data remains outstanding.
In addition, transcriptomic datasets are in good agreement with functional genetic experiments on the development of cone identity. These experiments have shown that specific transcription factors control fate decisions in photoreceptor progenitors and are thus required for the generation of each cone type or for the expression of their unique set of genes; transcriptomic datasets confirm that each cone type retains specific expression of these transcription factors throughout their lifespan, likely actively controlling cone identity. Furthermore, evidence suggests that transcription factors and their cognate binding sites throughout the genome are interlocked and therefore highly resistant to evolutionary change. Thus, transcription factors and cis-regulatory ‘grammars’ are often relatively fixed, ancestral features of a cell type which are more persistent in evolution than the modules of ‘effector genes’ which they regulate, as the latter are more directly subject to present-day adaptive pressures [97,98]. Some examples of deeply conserved photoreceptor transcription factors follow:
Generation of rods/PR0 is dependent on Maf family transcription factors (e.g., NRL, MAFA, MAFB) and NR2E3 [99,100].
Generation of PR1 depends on THRB, a subunit of the thyroid-hormone receptor [101–103] as well as the transcriptional co-factor SAMD7 [48].
Generation of PR2 depends on SIX6/SIX7 [104,105].
Generation of PR3 depends on FOXQ2; both PR3 and FOXQ2 were lost in eutherian mammals but retained in monotremes [106–109].
Generation of PR4 has been shown to depend on TBX2 in zebrafish [49,110] and TBX2 expression is specific to PR4 in transcriptomic datasets across vertebrates (zebrafish, chicken, lizards, squirrels and primates).
Generation of PR5 depends on THRB; and similar to PR1, PR5 also expresses SAMD7.
PR6 expresses FOXQ2 and SKOR1, similar to PR3 and PR3,4, respectively.
Aside from the above, inferences about photoreceptor identity can nevertheless emerge from limited data such as those used in classical definitions (
Figure 1) and reference to typical patterns of photoreceptor properties across the vertebrate tree of life (reviewed e.g. in Refs [4,17,24,28]). For example:
Numerical abundance is usually PR1≥PR2>PR3≥PR4. If present, PR5,6 is usually PR1>PR5/6>PR4, except in birds, where the more typical pattern is PR5/6>PR1,2.
If cone types are missing, the likely order of evolutionary loss is PR2=PR3>PR4>PR1. In non-eutherian tetrapods, PR5,6 is usually present. PR5,6 are not known to occur individually.
Postsynaptic wiring appears to conform to ‘spectral blocks’ in the sense that ‘intermediate’ cones, if present, do not tend to be skipped. For example, a bipolar cell is unlikely to connect with PR1 and PR3 without also contacting PR2. In this order, rods and PR5,6 appear to group with PR1 (i.e. PR0/PR5,6-PR1-PR2-PR3-PR4). In birds, PR6 appears to additionally group with PR3 [46].
The spectral appearance of pigmented oil droplets, if present, generally correlates with cone-type identity, with PR1-PR4 exhibiting long to short-wavelength filtering properties, respectively, matching the spectral sensitivity of the corresponding opsins. PR4 usually has a clear oil droplet, devoid of light-absorbing carotenoid pigments. PR5 tend to have spectrally intermediate oil droplets and PR6 tend to have either absent or minute droplets, while frequently retaining carotenoid pigmentation in the mitochondrial aggregates of the ellipsoid [17].
References
- Baden, T. & Osorio, D. The Retinal Basis of Vertebrate Color Vision. Annu. Rev. Vis. Sci. 5, 177–200 (2019). [CrossRef]
- Hagen, J. F. D., Roberts, N. S. & Johnston, R. J. The evolutionary history and spectral tuning of vertebrate visual opsins. Dev. Biol. 493, 40–66 (2023). [CrossRef]
- Fang, M. et al. Retinal twin cones or retinal double cones in fish: misnomer or different morphological forms? Int. J. Neurosci. 115, 981–987 (2005).
- Baden, T. Ancestral photoreceptor diversity as the basis of visual behaviour. Nat. Ecol. Evol. 1–13 (2024). [CrossRef]
- Collin, S. P. et al. Ancient colour vision: multiple opsin genes in the ancestral vertebrates. Curr. Biol. 13, R864–R865 (2003).
- Warrington, R. E. et al. Visual opsin expression and morphological characterization of retinal photoreceptors in the pouched lamprey (Geotria australis, Gray). J. Comp. Neurol. 529, 2265–2282 (2021). [CrossRef]
- Reese, B. E. & Keeley, P. W. Design principles and developmental mechanisms underlying retinal mosaics. Biol. Rev. Camb. Philos. Soc. 90, 854–876 (2015). [CrossRef]
- Kram, Y. A. et al. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS ONE 5, e8992 (2010).
- Wässle, H., Puller, C., Müller, F. & Haverkamp, S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J. Neurosci. Off. J. Soc. Neurosci. 29, 106–17 (2009).
- Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–74 (2013).
- Bae, J. A. et al. Digital Museum of Retinal Ganglion Cells with Dense Anatomy and Physiology. Cell 173, 1293-1306.e19 (2018). [CrossRef]
- Takahashi, Y., Hisatomi, O., Sakakibara, S., Tokunaga, F. & Tsukahara, Y. Distribution of blue-sensitive photoreceptors in amphibian retinas. FEBS Lett. 501, 151–155 (2001).
- Baden, T. Circuit mechanisms for colour vision in zebrafish. Curr. Biol. 31, R807–R820 (2021).
- Seifert, M., Baden, T. & Osorio, D. The retinal basis of vision in chicken. Semin. Cell Dev. Biol. 106, 106–115 (2020). [CrossRef]
- Peng, Y.-R. et al. Molecular Classification and Comparative Taxonomics of Foveal and Peripheral Cells in Primate Retina. Cell 176, 1222-1237.e22 (2019).
- Brainard, D. H. Color and the Cone Mosaic. Annu. Rev. Vis. Sci. 1, 519–546 (2015).
- Toomey, M. B. & Corbo, J. C. Evolution, development and function of vertebrate cone oil droplets. Front. Neural Circuits 11, 97 (2017). [CrossRef]
- Okano, T., Kojima, D., Fukada, Y., Shichida, Y. & Yoshizawa, T. Primary structures of chicken cone visual pigments: Vertebrate rhodopsins have evolved out of cone visual pigments. Proc. Natl. Acad. Sci. U. S. A. 89, 5932–5936 (1992).
- Yokoyama, S. & Yokoyama, R. Adaptive Evolution of Photoreceptors and Visual Pigments in Vertebrates. Annu. Rev. Ecol. Syst. 27, 543–567 (1996). [CrossRef]
- Bowmaker, J. K. Evolution of vertebrate visual pigments. Vision Res. (2008) doi:10.1016/j.visres.2008.03.025.
- Tommasini, D., Yoshimatsu, T., Baden, T. & Shekhar, K. Comparative transcriptomic insights into the evolutionary origin of the tetrapod double cone. 2024.11.04.621990 Preprint at https://doi.org/10.1101/2024.11.04.621990 (2024). [CrossRef]
- Engström, K. Cone types and cone arrangements in teleost retinae. Acta Zool. (1963). [CrossRef]
- Lyall, A. H. Cone Arrangements in Teleost Retinae. J. Cell Sci. s3-98, 189–201 (1957).
- Baden, T. From water to land: Evolution of photoreceptor circuits for vision in air. PLOS Biol. 22, e3002422 (2024). [CrossRef]
- Yamagata, M., Yan, W. & Sanes, J. R. A cell atlas of the chick retina based on single-cell transcriptomics. eLife 10, 1–39 (2021).
- Günther, A. et al. Species–specific circuitry of double cone photoreceptors in two avian retinas. Commun. Biol. 7, 1–11 (2024). [CrossRef]
- Günther, A. et al. Double cones and the diverse connectivity of photoreceptors and bipolar cells in an avian retina. J. Neurosci. 41, 5015–5028 (2021). [CrossRef]
- Kelber, A. Bird colour vision – from cones to perception. Curr. Opin. Behav. Sci. 30, 34–40 (2019).
- Zeng, H. What is a cell type and how to define it? Cell 185, 2739–2755 (2022).
- Goetz, J. et al. Unified classification of mouse retinal ganglion cells using function, morphology, and gene expression. Cell Rep. 40, 111040 (2022). [CrossRef]
- Huang, W. et al. Linking transcriptomes with morphological and functional phenotypes in mammalian retinal ganglion cells. Cell Rep. 40, (2022).
- Baden, T. et al. The functional diversity of mouse retinal ganglion cells. Nature 1–21 (2016). [CrossRef]
- Liu, Y. et al. Avian photoreceptor homologies and the origin of double cones. Curr. Biol. (in press), (2025).
- Rheaume, B. A. et al. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat. Commun. 9, 2759 (2018). [CrossRef]
- Keeley, P. W., Eglen, S. J. & Reese, B. E. From Random to Regular: Variation in the Patterning of Retinal Mosaics. J. Comp. Neurol. 528, 2135–2160 (2020).
- Field, G. D. et al. Functional connectivity in the retina at the resolution of photoreceptors. Nature 467, 673–7 (2010).
- Behrens, C. et al. Connectivity map of bipolar cells and photoreceptors in the mouse retina. eLife 5, 1206–1217 (2016).
- Shekhar, K. et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 166, 1308-1323.e30 (2016).
- Yan, W. et al. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 40, 5177–5195 (2020). [CrossRef]
- Hahn, J. et al. Evolution of neuronal cell classes and types in the vertebrate retina. Nature 624, 415–424 (2023). [CrossRef]
- Franke, K. et al. Inhibition decorrelates visual feature representations in the inner retina. Nature 542, 439–444 (2017).
- Della Santina, L. et al. Glutamatergic Monopolar Interneurons Provide a Novel Pathway of Excitation in the Mouse Retina. Curr. Biol. 26, 2070–2077 (2016). [CrossRef]
- Chapot, C. A., Euler, T. & Schubert, T. How do horizontal cells ‘talk’ to cone photoreceptors? Different levels of complexity at the cone-horizontal cell synapse. J. Physiol. 595, 5495–5506 (2017).
- Wang, J. et al. Molecular characterization of the sea lamprey retina illuminates the evolutionary origin of retinal cell types. Nat. Commun. 15, 10761 (2024).
- Yoshimatsu, T. et al. Ancestral circuits for vertebrate color vision emerge at the first retinal synapse. Sci. Adv. 7, 6815–6828 (2021).
- Guenther, A. et al. Morphology and connectivity of retinal horizontal cells in two avian species. 2025.01.27.634460 Preprint at https://doi.org/10.1101/2025.01.27.634460 (2025). [CrossRef]
- Cowan, C. S. et al. Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cell 182, 1623-1640.e34 (2020). [CrossRef]
- Volkov, L. I. et al. Samd7 represses short-wavelength cone genes to preserve long-wavelength cone and rod photoreceptor identity. Proc. Natl. Acad. Sci. 121, e2402121121 (2024).
- Angueyra, J. M. et al. Transcription factors underlying photoreceptor diversity. eLife 12, e81579 (2023).
- Fornetto, C., Euler, T. & Baden, T. Vertebrate vision is ancestrally based on competing cone circuits. 2024.11.19.624320 Preprint at https://doi.org/10.1101/2024.11.19.624320 (2024). [CrossRef]
- Cheney, K. L. et al. Seeing Picasso: an investigation into the visual system of the triggerfish Rhinecanthus aculeatus. J. Exp. Biol. 225, jeb243907 (2022). [CrossRef]
- Hofer, H., Carroll, J., Neitz, J., Neitz, M. & Williams, D. R. Organization of the Human Trichromatic Cone Mosaic. J. Neurosci. 25, 9669–9679 (2005).
- Applebury, M. L. et al. The Murine Cone Photoreceptor: A Single Cone Type Expresses Both S and M Opsins with Retinal Spatial Patterning. Neuron 27, 513–523 (2000).
- Baden, T. et al. A tale of two retinal domains: near-optimal sampling of achromatic contrasts in natural scenes through asymmetric photoreceptor distribution. Neuron 80, 1206–1217 (2013). [CrossRef]
- Szél, A. et al. Unique topographic separation of two spectral classes of cones in the mouse retina. J. Comp. Neurol. 325, 327–42 (1992).
- Lagman, D., Callado-Pérez, A., Franzén, I. E., Larhammar, D. & Abalo, X. M. Transducin duplicates in the zebrafish retina and pineal complex: differential specialisation after the teleost tetraploidisation. PloS One 10, e0121330 (2015). [CrossRef]
- Seifert, M., Roberts, P. A., Kafetzis, G., Osorio, D. & Baden, T. Birds multiplex spectral and temporal visual information via retinal On- and Off-channels. Nat. Commun. 14, 5308 (2023).
- Yang, C. Y., Hassin, G. & Witkovsky, P. Blue-sensitive rod input to bipolar and ganglion cells of the Xenopus retina. Vision Res. 23, 933–941 (1983).
- Rozenblit, F. & Gollisch, T. What the salamander eye has been telling the vision scientist’s brain. Semin. Cell Dev. Biol. 106, 61–71 (2020).
- Donner, K. & Yovanovich, C. A. M. A frog’s eye view: Foundational revelations and future promises. Semin. Cell Dev. Biol. (2020). [CrossRef]
- Cowing, J. A., Arrese, C. A., Davies, W. L., Beazley, L. D. & Hunt, D. M. Cone visual pigments in two marsupial species: the fat-tailed dunnart (Sminthopsis crassicaudata) and the honey possum (Tarsipes rostratus). Proc. R. Soc. B Biol. Sci. 275, 1491–1499 (2008).
- Hauzman, E. Adaptations and evolutionary trajectories of the snake rod and cone photoreceptors. Semin. Cell Dev. Biol. 106, 86–93 (2020). [CrossRef]
- Walls, G. L. The Vertebrate Eye and Its Adaptive Radiation. xiv, 785 (Cranbrook Institute of Science, Oxford, England, 1942). [CrossRef]
- Dunn, R. F. Studies on the retina of the gecko Coleonyx variegatus. J. Ultrastruct. Res. 16, 685–692 (1966). [CrossRef]
- Kojima, K. et al. Evolutionary adaptation of visual pigments in geckos for their photic environment. Sci. Adv. 7, eabj1316 (2021).
- Röll, B. Gecko vision—retinal organization, foveae and implications for binocular vision. Vision Res. 41, 2043–2056 (2001).
- Fain, G. L. Lamprey vision: Photoreceptors and organization of the retina. Semin. Cell Dev. Biol. (2020). [CrossRef]
- Nadal-Nicolás, F. M. et al. True S-cones are concentrated in the ventral mouse retina and wired for color detection in the upper visual field. eLife 9, 1–30 (2020).
- Haverkamp, S. et al. The primordial, blue-cone color system of the mouse retina. J Neurosci 25, 5438–5445 (2005).
- Carleton, K. L. & Yourick, M. R. Axes of visual adaptation in the ecologically diverse family Cichlidae. Semin. Cell Dev. Biol. (2020). [CrossRef]
- Cheng, C. L., Gan, K. J. & Novales Flamarique, I. Thyroid Hormone Induces a Time-Dependent Opsin Switch in the Retina of Salmonid Fishes. Invest. Ophthalmol. Vis. Sci. 50, 3024–3032 (2009).
- Nandamuri, S. P., Yourick, M. R. & Carleton, K. L. Adult plasticity in African cichlids: Rapid changes in opsin expression in response to environmental light differences. Mol. Ecol. 26, 6036–6052 (2017). [CrossRef]
- Cortesi, F. et al. Visual system diversity in coral reef fishes. Semin. Cell Dev. Biol. (2020). [CrossRef]
- Frau, S., Novales Flamarique, I., Keeley, P., Muñoz-Cueto, J. & Reese, B. Straying from the flatfish retinal plan: Cone photoreceptor patterning in the common sole (Solea solea) and the Senegalese sole (Solea senegalensis). J. Comp. Neurol. 528, (2020).
- Arbogast, P., Flamant, F., Godement, P., Glösmann, M. & Peichl, L. Thyroid Hormone Signaling in the Mouse Retina. PLoS ONE 11, e0168003 (2016).
- Roberts, M. R., Srinivas, M., Forrest, D., Morreale de Escobar, G. & Reh, T. A. Making the gradient: Thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc. Natl. Acad. Sci. 103, 6218–6223 (2006).
- Yoshimura, T. Thyroid hormone and seasonal regulation of reproduction. Front. Neuroendocrinol. 34, 157–166 (2013).
- Dalton, B. E., Lu, J., Leips, J., Cronin, T. W. & Carleton, K. L. Variable light environments induce plastic spectral tuning by regional opsin coexpression in the African cichlid fish, Metriaclima zebra. Mol. Ecol. 24, 4193–204 (2015). [CrossRef]
- Morshedian, A. et al. Cambrian origin of the CYP27C1-mediated vitamin A1-to-A2 switch, a key mechanism of vertebrate sensory plasticity. R. Soc. Open Sci. 4, (2017).
- Enright, J. M. et al. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting Vitamin A1 into A2. Curr. Biol. 25, 3048–3057 (2015).
- Carleton, K. L., Escobar-Camacho, D., Stieb, S. M., Cortesi, F. & Justin Marshall, N. Seeing the rainbow: Mechanisms underlying spectral sensitivity in teleost fishes. J. Exp. Biol. 223, jeb193334 (2020).
- Herzog, T. et al. A heterogeneous population code at the first synapse of vision. 2024.05.03.592379 Preprint at https://doi.org/10.1101/2024.05.03.592379 (2024). [CrossRef]
- Yoshimatsu, T., Schröder, C., Nevala, N. E., Berens, P. & Baden, T. Fovea-like Photoreceptor Specializations Underlie Single UV Cone Driven Prey-Capture Behavior in Zebrafish. Neuron 107, 320-337.e6 (2020). [CrossRef]
- Baudin, J., Angueyra, J. M., Sinha, R. & Rieke, F. S-cone photoreceptors in the primate retina are functionally distinct from L and M cones. eLife 8, (2019).
- Li, Y. N., Tsujimura, T., Kawamura, S. & Dowling, J. E. Bipolar cell-photoreceptor connectivity in the zebrafish (Danio rerio) retina. J. Comp. Neurol. 520, 3786–3802 (2012). [CrossRef]
- Ali, M.-Ather. & Anctil, Michel. Retinas of Fishes : An Atlas. (Springer Berlin Heidelberg, 1976).
- Meier, A., Nelson, R. & Connaughton, V. P. Color Processing in Zebrafish Retina. Front. Cell. Neurosci. 12, 1–19 (2018).
- Stell, W. K. & Hárosi, F. I. Cone structure and visual pigment content in the retina of the goldfish. Vision Res. 16, 647-IN4 (1976). [CrossRef]
- Allison, W. T. et al. Ontogeny of cone photoreceptor mosaics in zebrafish. J. Comp. Neurol. 518, 4182–4195 (2010). [CrossRef]
- Flamarique, I. N. & Hawryshyn, C. W. No Evidence of Polarization Sensitivity in Freshwater Sunfish from Multi-unit Optic Nerve Recordings. Vision Res. 37, 967–973 (1997).
- Zimmermann, M. J. Y. et al. Zebrafish Differentially Process Color across Visual Space to Match Natural Scenes. Curr. Biol. 28, 2018-2032.e5 (2018). [CrossRef]
- Shand, J., Archer, M. A. & Collin, S. P. Ontogenetic changes in the retinal photoreceptor mosaic in a fish, the black bream, Acanthopagrus butcheri. J. Comp. Neurol. 412, 203–217 (1999).
- Ogawa, Y. & Corbo, J. C. Partitioning of gene expression among zebrafish photoreceptor subtypes. Sci. Rep. 11, 17340 (2021). [CrossRef]
- Lind, O., Chavez, J. & Kelber, A. The contribution of single and double cones to spectral sensitivity in budgerigars during changing light conditions. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 200, 197–207 (2014).
- Enright, J. M., Lawrence, K. A., Hadzic, T. & Corbo, J. C. Transcriptome profiling of developing photoreceptor subtypes reveals candidate genes involved in avian photoreceptor diversification. J. Comp. Neurol. 523, 649–668 (2015).
- Raymond, P. A. & Barthel, L. K. A moving wave patterns the cone photoreceptor mosaic array in the zebrafish retina. Int. J. Dev. Biol. 48, 935–945 (2004).
- Ogawa, Y. et al. Conservation of cis-regulatory codes over half a billion years of evolution. 2024.11.13.623372 Preprint at https://doi.org/10.1101/2024.11.13.623372 (2024). [CrossRef]
- Roberts, R. J. V., Pop, S. & Prieto-Godino, L. L. Evolution of central neural circuits: state of the art and perspectives. Nat. Rev. Neurosci. 23, 725–743 (2022). [CrossRef]
- Swaroop, A., Kim, D. & Forrest, D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci. 11, 563–576 (2010).
- Xie, S. et al. Knockout of Nr2e3 prevents rod photoreceptor differentiation and leads to selective L-/M-cone photoreceptor degeneration in zebrafish. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 1865, 1273–1283 (2019).
- Hudson, L. G., Santon, J. B., Glass, C. K. & Gill, G. N. Ligand-activated thyroid hormone and retinoic acid receptors inhibit growth factor receptor promoter expression. Cell 62, 1165–1175 (1990). [CrossRef]
- Ng, L. et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 27, 94–98 (2001).
- Suzuki, S. C. et al. Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc. Natl. Acad. Sci. 110, 15109–15114 (2013).
- Ogawa, Y. et al. Six6 and Six7 coordinately regulate expression of middle-wavelength opsins in zebrafish. Proc. Natl. Acad. Sci. 116, 4651–4660 (2019).
- Ogawa, Y., Shiraki, T., Kojima, D. & Fukada, Y. Homeobox transcription factor Six7 governs expression of green opsin genes in zebrafish. Proc. Biol. Sci. 282, 20150659 (2015). [CrossRef]
- Ogawa, Y., Shiraki, T., Fukada, Y. & Kojima, D. Foxq2 determines blue cone identity in zebrafish. Sci. Adv. 7, eabi9784 (2021).
- Yamazaki, A., Yamamoto, A., Yaguchi, J. & Yaguchi, S. cis-Regulatory analysis for later phase of anterior neuroectoderm-specific foxQ2 expression in sea urchin embryos. genesis 57, e23302 (2019).
- Wakefield, M. J. et al. Cone visual pigments of monotremes: filling the phylogenetic gap. Vis. Neurosci. 25, 257–264 (2008). [CrossRef]
- Davies, W. L. et al. Visual pigments of the platypus: A novel route to mammalian colour vision. Curr. Biol. 17, R161–R163 (2007). [CrossRef]
- Alvarez-Delfin, K. et al. Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc. Natl. Acad. Sci. U. S. A. 106, 2023–2028 (2009). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).