Submitted:
04 February 2025
Posted:
05 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Recombinant F. hepatica FABP (Fh15)
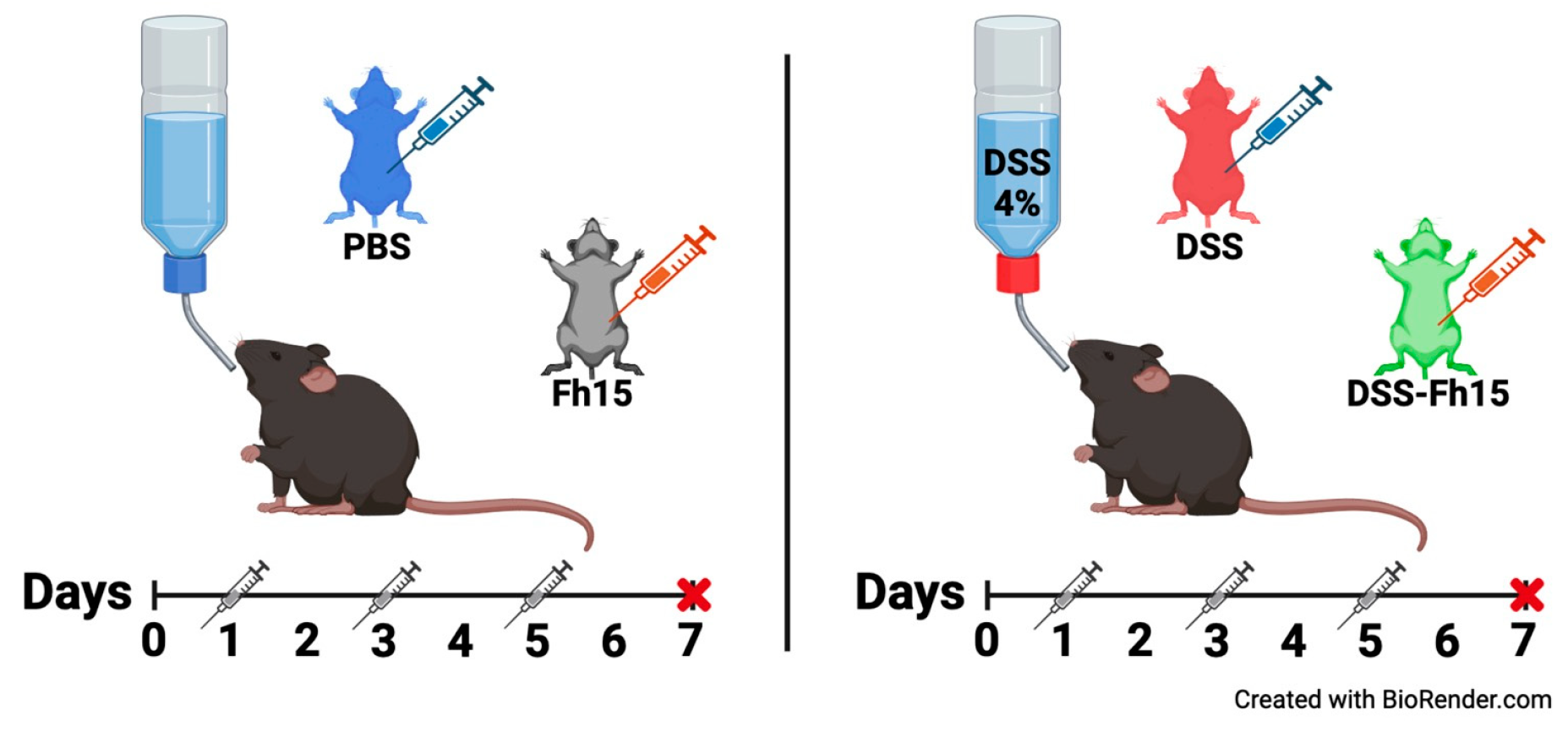
2.3. Dextran Sulfate Sodium (DSS) Colitis Induction and Fh15 Treatment
2.4. Disease Activity Index (DAI)
2.5. Macroscopic Score and Histopathological Scoring
2.6. Serum Myeloperoxidase and Chitinase-3 Like-Protein-1 Concentration
2.7. Colonic Cytokines Gene Expression
2.8. Colon Immune Cells Infiltration and Calcium Binding Protein Marker
2.9. Statistical Analysis
3. Results
3.1. Fh15 Treatment Reduces Disease Activity Index in DSS-Induced UC Mice
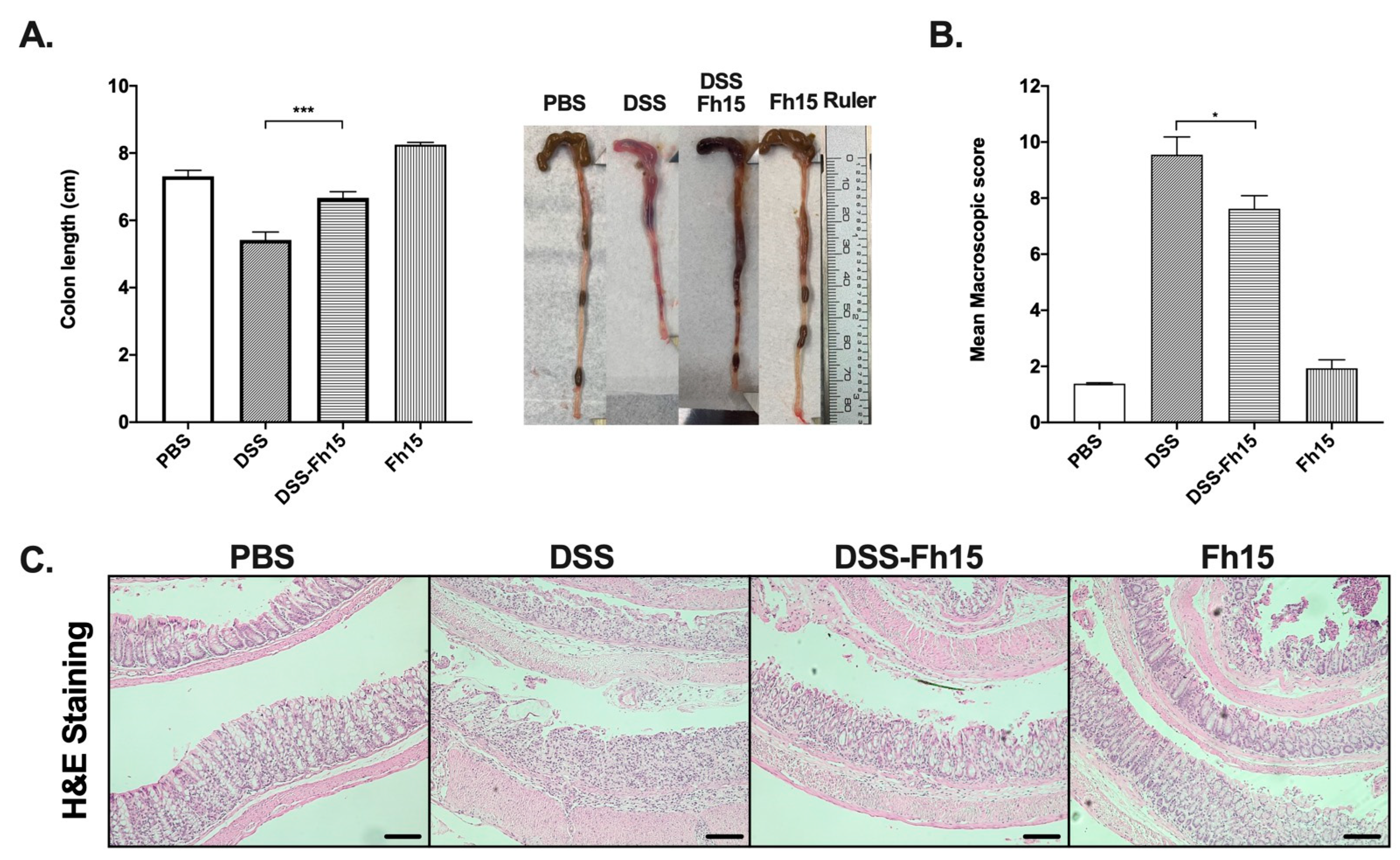
3.2. Fh15 Significantly Prevents Colon Shortening and Decreases Macroscopic Score in DSS-Induced UC Mice
3.3. Fh15 Ameliorates Histological Alterations in DSS-Induced UC Mice
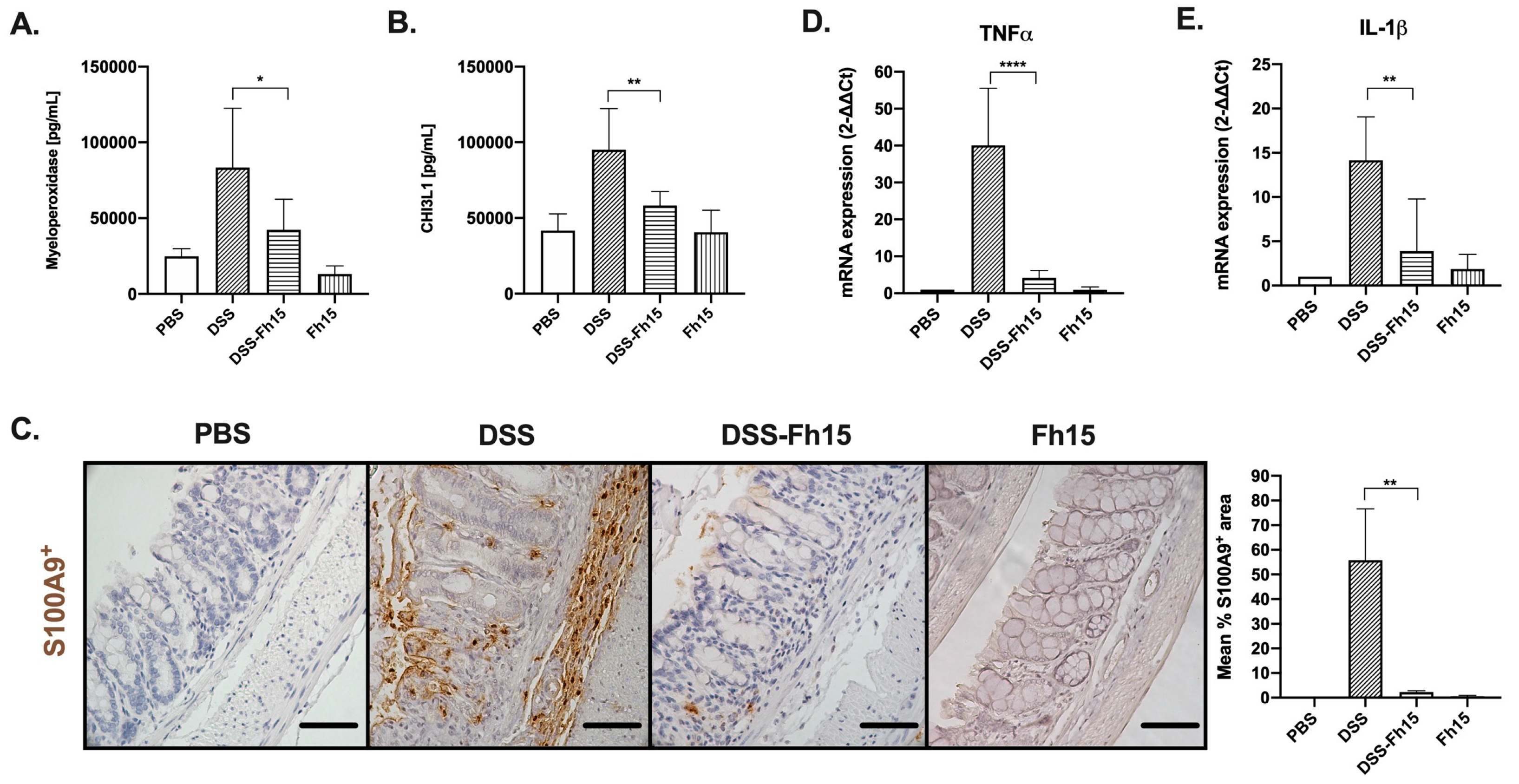
3.4. Fh15 Decreases Serum Levels of Myeloperoxidase and CHI3L1, While Suppressing S100A9 and Pro-Inflammatory Cytokines in Colonic Tissues of DSS-Induced UC Mice
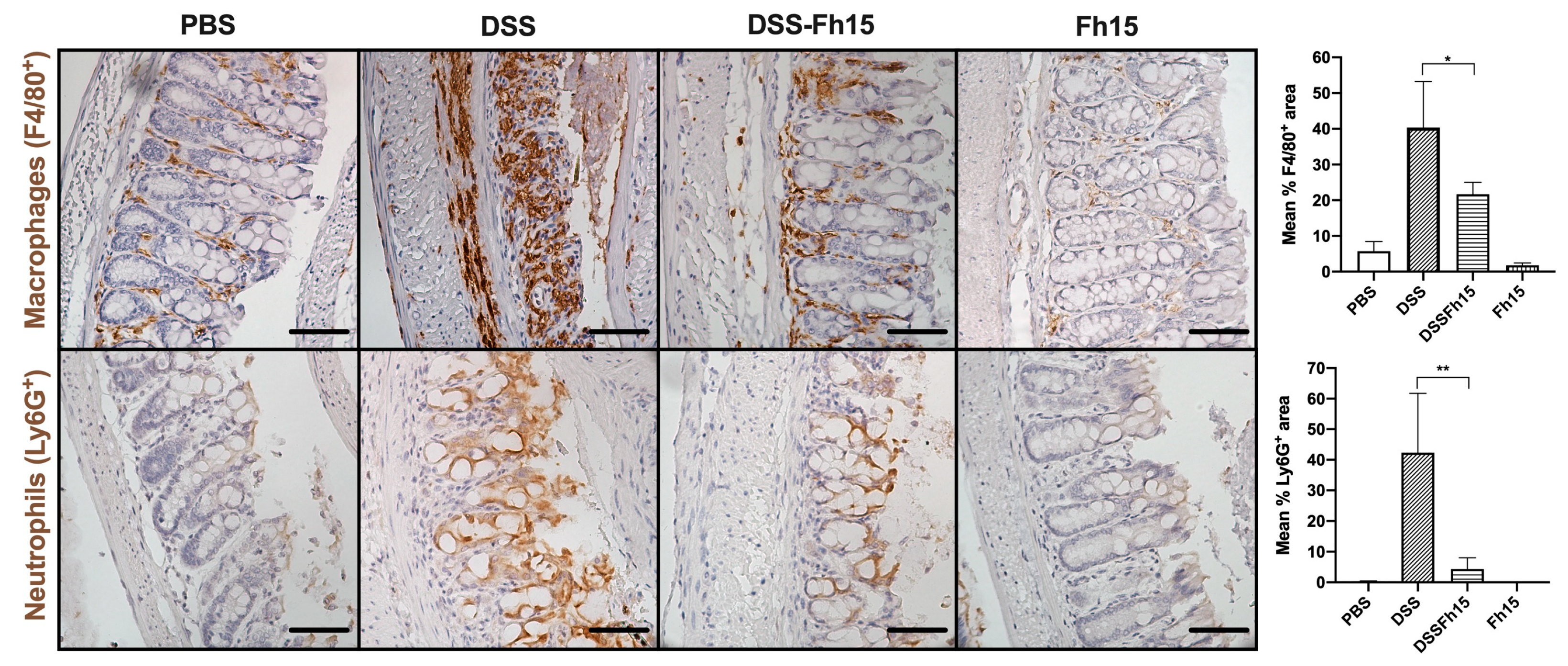
3.6. Fh15 Modulates Colonic Tissue Immune Cell Infiltration of DSS-Induced UC Mice
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Basso, P.J.; Fonseca, M.T.C.; Bonfá, G.; Alves, V.B.F.; Sales-Campos, H.; Nardini, V.; et al. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Brazilian J Med Biol Res 2014, 47, 727–737. [Google Scholar] [CrossRef]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Reinink, A.R.; Lee, T.C.; Higgins, P.D.R. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease. Inflamm Bowel Dis 2016, 22, 1859–1869. [Google Scholar] [CrossRef]
- Pugliese, N.; Roda, G.; Peyrin-Biroulet, L.; Danese, S. Emerging therapies for the treatment of ulcerative colitis. Expert Opin Emerg Drugs 2020, 25, 71–79. [Google Scholar] [CrossRef]
- Capron, M.; Béghin, L.; Leclercq, C.; Labreuche, J.; Dendooven, A.; Standaert, A.; et al. Safety of P28GST, a protein derived from a schistosome helminth parasite, in patients with Crohn’s disease: A pilot study (ACROHNEM). J Clin Med 2019, 9, 41. [Google Scholar] [CrossRef]
- Radtke, D.; Thuma, N.; Schülein, C.; Kirchner, P.; Ekici, A.B.; Schober, K.; et al. Th2 single-cell heterogeneity and clonal distribution at distant sites in helminth-infected mice. Elife 2022, 11. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016, 138, 666–675. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Summers, R.W.; Elliott, D.E.; Qadir, K.; Urban, J.F.; Thompson, R. The possible link between de-worming and the emergence of immunological disease. J Lab Clin Med 2002, 139, 334–338. [Google Scholar] [CrossRef]
- Khan, W.I.; Blennerhasset, P.A.; Varghese, A.K.; Chowdhury, S.K.; Omsted, P.; Deng, Y.; et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun 2002, 70, 5931–5937. [Google Scholar] [CrossRef]
- Walsh, K.P.; Brady, M.T.; Finlay, C.M.; Boon, L.; Mills, K.H.G. Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J Immunol 2009, 183, 1577–1586. [Google Scholar] [CrossRef]
- Lund, M.E.; O’Brien, B.A.; Hutchinson, A.T.; Robinson, M.W.; Simpson, A.M.; Dalton, J.P.; et al. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS ONE 2014, 9, e86289. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Tonks, P.; Jones, F.M.; O'Shea, H.; Hutchings, P.; Fulford, A.J.C.; et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol 1999, 21, 169–176. [Google Scholar] [CrossRef]
- Kuijk, L.M.; Klaver, E.J.; Kooij, G.; van der Pol, S.M.A.; Heijnen, P.; Bruijns, S.C.M.; et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 2012, 51, 210–218. [Google Scholar] [CrossRef]
- Moreels, T.G. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 2004, 53, 99–107. [Google Scholar] [CrossRef]
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.A.; Weinstock, J.V. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef]
- Flynn, R.J.; Mulcahy, G.; Welsh, M.; Cassidy, J.P.; Corbett, D.; Milligan, C.; et al. Co-infection of cattle with Fasciola hepatica and Mycobacterium bovis—Immunological consequences. Transbound Emerg Dis 2009, 56, 269–274. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Z.; Wan, S.; Wu, F.; Chen, W.; Zhang, B.; et al. Exosomes derived from dendritic cells treated with Schistosoma japonicum soluble egg antigen attenuate DSS-induced colitis. Front Pharmacol 2017, 8. [Google Scholar] [CrossRef]
- Roig, J.; Saiz, M.L.; Galiano, A.; Trelis, M.; Cantalapiedra, F.; Monteagudo, C.; et al. Extracellular vesicles from the helminth Fasciola hepatica prevent DSS-induced acute ulcerative colitis in a T-lymphocyte independent mode. Front Microbiol 2018, 9. [Google Scholar] [CrossRef]
- Ramos-Benitez, M.J.; Ruiz-Jimenez, C.; Rosado-Franco, J.J.; Ramos-Pérez, W.D.; Mendez, L.B.; Osuna, A.; et al. Fh15 blocks the lipopolysaccharide-induced cytokine storm while modulating peritoneal macrophage migration and CD38 expression within spleen macrophages in a mouse model of septic shock. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Armina-Rodriguez, A.; Ocasio-Malavé, C.; Méndez-Torres, L.B.; Valdés-Fernández, B.; Espino, A.M. Fasciola hepatica Fh15 promotes survival in a mouse septic shock model and downregulates inflammatory cytokines. J Immunol 2023, 210, 82.02–82.02. [Google Scholar] [CrossRef]
- Rosado-Franco, J.J.; Armina-Rodriguez, A.; Marzan-Rivera, N.; Burgos, A.G.; Spiliopoulos, N.; Dorta-Estremera, S.M.; et al. Recombinant Fasciola hepatica fatty acid binding protein as a novel anti-inflammatory biotherapeutic drug in an acute gram-negative nonhuman primate sepsis model. Microbiol Spectr 2021, 9, e0191021. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 2014, 104. [Google Scholar] [CrossRef]
- Storr, M.A.; Keenan, C.M.; Zhang, H.; Patel, K.D.; Makriyannis, A.; Sharkey, K.A. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 2009, 15, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Carson, F.L.; Hladik Cappellano, C. Histotechnology: A Self-Instructional Text, 3rd ed.; American Society for Clinical Pathology Press: Hong Kong, 2009. [Google Scholar]
- Sann, H.; von Erichsen, J.; Hessmann, M.; Pahl, A.; Hoffmeyer, A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Sci 2013, 92, 708–718. [Google Scholar] [CrossRef]
- Schaid, T.R.; LaCroix, I.; Hansen, K.C.; D’Alessandro, A.; Moore, E.E.; Sauaia, A.; et al. A proteomic analysis of NETosis in trauma: Emergence of SerpinB1 as a key player. J Trauma Acute Care Surg 2022. [Google Scholar] [CrossRef]
- Mizoguchi, E. Chitinase 3–like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 2006, 130, 398–411. [Google Scholar] [CrossRef]
- Viennois, E.; Chen, F.; Laroui, H.; Baker, M.T.; Merlin, D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: Lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes 2013, 6, 360. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.; Wang, J.; Qin, Z.; Wang, J.; Lu, Y.; et al. Suppression colitis and colitis-associated colon cancer by anti-S100A9 antibody in mice. Front Immunol 2017, 8. [Google Scholar] [CrossRef]
- Choi, S.G.; Tittle, T.; Garcia-Prada, D.; Kordower, J.H.; Melki, R.; Killinger, B.A. Alpha-synuclein aggregates are phosphatase resistant. bioRxiv Prepr Serv Biol 2024. [CrossRef]
- Heinzel, F.P.; Sadick, M.D.; Holaday, B.J.; Coffman, R.L.; Locksley, R.M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med 1989, 169, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Jovicic, N.; Jeftic, I.; Jovanovic, I.; Radosavljevic, G.; Arsenijevic, N.; Lukic, M.L.; et al. Differential immunometabolic phenotype in Th1 and Th2 dominant mouse strains in response to high-fat feeding. PLoS ONE 2015, 10, e0134089. [Google Scholar] [CrossRef] [PubMed]
- Corral-Ruiz, G.M.; Sánchez-Torres, L.E. Fasciola hepatica-derived molecules as potential immunomodulators. Acta Trop 2020, 210, 105548. [Google Scholar] [CrossRef]
- Donnelly, S.; Stack, C.M.; O’Neill, S.M.; Sayed, A.A.; Williams, D.L.; Dalton, J.P. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J 2008, 22, 4022–4032. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Driss, V.; El Nady, M.; Delbeke, M.; Rousseaux, C.; Dubuquoy, C.; Sarazin, A.; et al. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal Immunol 2016, 9, 322–335. [Google Scholar] [CrossRef]
- Long, S.R.; Liu, R.D.; Kumar, D.V.; Wang, Z.Q.; Su, C.-W. Immune protection of a helminth protein in the DSS-induced colitis model in mice. Front Immunol 2021, 12. [Google Scholar] [CrossRef]
- Coronado, S.; Barrios, L.; Zakzuk, J.; Regino, R.; Ahumada, V.; Franco, L.; et al. A recombinant cystatin from Ascaris lumbricoides attenuates inflammation of DSS-induced colitis. Parasite Immunol 2017, 39. [Google Scholar] [CrossRef]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 2018, 175, 400–415.e13. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur J Pharmacol 2020, 877, 173090. [Google Scholar] [CrossRef]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.-I.; Kühl, A.A.; Erben, U.; et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis 2015, 21, 1297–1305. [Google Scholar] [CrossRef]
- Webb, L.V.; Ley, S.C.; Seddon, B. TNF activation of NF-κB is essential for development of single-positive thymocytes. J Exp Med 2016, 213, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol 2013, 183, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J Immunol 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chu, C.; Teng, F.; Bessman, N.J.; Goc, J.; Santosa, E.K.; et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 2019, 568, 405–409. [Google Scholar] [CrossRef]
- Rosales, C.; Demaurex, N.; Lowell, C.A.; Uribe-Querol, E. Neutrophils: Their role in innate and adaptive immunity. J Immunol Res 2016, 2016, 1469780. [Google Scholar] [CrossRef]
- Ren, X.; Manzanares, L.D.; Piccolo, E.B.; Urbanczyk, J.M.; Sullivan, D.P.; Yalom, L.K.; et al. Macrophage–endothelial cell crosstalk orchestrates neutrophil recruitment in inflamed mucosa. J Clin Invest 2023, 133. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular mechanisms, role in physiology and pathology. Biochem 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franzè, E.; Di Grazia, A.; et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Mizutani, T.; Mizuta, S.; Miyamoto, A.; Murata, S.; Ano, T.; et al. Neutrophils and the S100A9 protein critically regulate granuloma formation. Blood Adv 2016, 1, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.-C.; Simon, M.-M.; Tessier, P.A.; Girard, D. Damage-associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. J Immunol 2011, 186, 3622–3631. [Google Scholar] [CrossRef] [PubMed]

| Weight loss | Stool Consistency | Bleeding | Score |
|---|---|---|---|
| < 2% | Normal | Negative hemoccult | 0 |
| > 2% - <5% | Softer stool | Positive hemoccult/ no visible blood | 1 |
| > 5% - <10% | Moderate Diarrhea | Visual blood in stool | 2 |
| > 10% - <15% | Diarrhea | Fresh rectal bleeding | 3 |
| > 15% | - | - | 4 |
| Macroscopic damage | Score |
|---|---|
| Colon length | >=6cm = 0pt, <6cm = 1pt, or <5cm = 2pt |
| Inflamed length | Centimeters measurement |
| Bowel thickness | Millimeters measurement |
| Adhesion | 0= No Adhesion, 1= Mild, 2= Moderate, or 3= Severe |
| Hemorrhage | Present= 1 or Absent = 0 |
| Fecal blood | Present= 1 or Absent = 0 |
| Diarrhea | Present= 1 or Absent = 0 |
| Score | Extent of inflammation |
Infiltration neutrophils + lympho-histiocytes | Extent of crypt damage | Crypt abscesses | Sub-mucosal edema | Loss of goblet cells |
Reactive epithelial hyperplasia |
|---|---|---|---|---|---|---|---|
| 0 | None | None | None | None | None | None | None |
| 1 | Mucosa | Focal | Basal one third | Focal | Focal | Focal | Focal |
| 2 | Mucosa+submucosa | Multifocal | Basal two thirds | Multifocal | Multifocal | Multifocal | Multifocal |
| 3 | Mucosa+submucosa+ muscle layer |
Diffuse | Entire crypt damage | - | Diffuse | Diffuse | Diffuse |
| 4 | Transmura | - | Crypt damage +ulceration |
- | - | - | - |
| Gene | Primers | Sequence 5’- 3’ |
|---|---|---|
| GAPDH | Forward | CATGGCCTTCCGTGTTCCTA |
| Reverse | CCTGCTTCACCACCTTCTTGAT | |
| TNF-α | Forward | AAGCCTGTAGCCCACGTCGTA |
| Reverse | AGGTACAACCCATCGGCTGG | |
| IL-1β | Forward | GAAATGCCACCTTTTGACAGTG |
| Reverse | TGGATGCTCTCATCAGGACAG |
| Marker | Target | Host species | IHC Dilution | Cat # | Vendor |
|---|---|---|---|---|---|
| F4/80 | Macrophages | Rabbit | 1:100 | 70076S | CST |
| LY6G | Neutrophils | Rabbit | 1:75 | 87048S | CST |
| S100A9 | Calcium binding protein A9 | Rabbit | 1:800 | 73425S | CST |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





