1. Introduction
Mosquitoes are pivotal vectors of human diseases, transmitting a range of life-threatening illnesses, including malaria (Anopheles), dengue, chikungunya, and Zika virus (Aedes aegypti), as well as lymphatic filariasis (Culex) (Tolle, 2009; Socha et al., 2022; Singh et al., 2024). These diseases place a severe global burden, particularly in tropical and subtropical areas where conditions favor mosquito proliferation (Mackey et al., 2014; Naik et al., 2023).
The mosquito lifecycle presents a strategic target for control, with larvae developing in stagnant water before maturing into adults (Day, 2016; Liu, 2024). Intervening at the larval stage offers a practical, effective approach to reduce disease transmission. Targeting breeding sites and using microbial larvicides can significantly curb disease spread, bolstering global public health efforts. However, mosquito control faces challenges, particularly the increasing resistance of mosquito populations to chemical insecticides (Karunamoorthi and Sabesan, 2013; Dusfour et al., 2019; Ranson and Lissenden, 2016). Over-reliance on chemicals like pyrethroids, organophosphates, and carbamates has fostered resistant strains in species like Aedes aegypti, Anopheles gambiae, and Culex quinquefasciatus (Hua, 2018; Al Nazawi, 2019; Shroff et al., 2020), undermining control efforts in high-burden areas.
Moreover, the environmental impact of chemical insecticides is significant, as non-target organisms, including beneficial species, are harmed, disrupting ecosystems (Sánchez-Bayo, 2012; Punniyakotti et al., 2024). Residual chemical contamination in soil and water poses long-term ecological risks (Ali et al., 2021). This highlights the urgent need for sustainable, targeted, eco-friendly alternatives, such as microbial larvicides and integrated vector management.
Bacillus species, particularly Bacillus sonorensis, Bacillus paramycoides, Bacillus tequilensis, and Bacillus rugosus, have emerged as effective, environmentally safe biocontrol agents (Benelli et al., 2016; Thakur et al., 2020). These species produce secondary metabolites, including antimicrobial peptides and enzymes, which can target mosquito larvae (Dadasoglu et al., 2013; Dahmana et al., 2020; Rajagopal et al., 2020; Colvin et al., 2020; Alharbi et al., 2024). Unlike chemical insecticides, Bacillus species degrade naturally in aquatic environments, preventing harmful residue buildup and offering an eco-friendly solution (Brar, 2006). They also reduce the risk of resistance through strategic use, either alone or in combination, to enhance effectiveness and minimize ecological impact (Obeagu and Obeagu, 2024).
Extremophilic Bacillus species, thriving in harsh conditions, are particularly promising for vector control. Known for their production of bioactive compounds and resilience in extreme environments, these species, such as Bacillus thuringiensis and Bacillus sphaericus, have been extensively studied for their ability to produce insecticidal toxins targeting mosquito larvae (Bravo et al., 2007). Thermophilic bacilli, such as Bacillus and Geobacillus, are notable for their heat-stable enzymes and potential in biodegradation and fermentation applications (Harirchi et al., 2022).
The ability of Bacillus species to form biofilms enhances their efficacy by providing sustained bioactive compound release (Malakar et al., 2023). Synergistic combinations with other biological agents or environmentally friendly pesticides may delay resistance development and improve vector control outcomes (Narkhede et al., 2017). Genetic modifications of Bacillus species, such as enhanced toxin production in Bacillus thuringiensis, show promise in overcoming resistance (Federici et al., 2010). Bacillus-based biocontrol agents, including Bti and Bs, offer eco-friendly, targeted, biodegradable alternatives to chemical pesticides, ensuring safety for humans, non-target organisms, and ecosystems (Lacey, 2007; Brühl, 2020). Despite their promise, further research is required to optimize these agents’ production, deployment, and integration into comprehensive vector management programs.
The present study was designed to assess the effectiveness, ecological viability, and potential obstacles associated with the use of microbial control agents. Specifically, the research focused on evaluating six Bacillus species (B. licheniformis, B. stercoris, B. sonorensis, B. paramycoides, B. tequilensis, and B. rugosus) isolated from mosquito environments for their genetic compatibility and potential as larvicides in mosquito vector management. The study also aims to explore the role of these larvicides in reducing the transmission of mosquito-borne diseases, mitigating insecticide resistance, and providing an eco-friendly alternative to chemical pesticides. Furthermore, the study seeks to highlight the integration of these microbial agents with complementary vector management strategies, emphasizing their contributions to sustainable public health and ecological preservation.
2. Materials and Methods
Sample Collection
Water samples were collected from a hot spring at Ain Al-Hara (Geographic location: 20°27’43.9” N, 40°28’16.1” E), located in Gomaigh within the province of Al-Lith, is positioned in the western part of the Saudi Arabia Kingdom. The temperature and pH of the sample collection sites were examined by the portable thermometer and pH meter (
Table 1). Environmental samples were collected in sterilized 500 mL plastic containers and then transported to the laboratory in an icebox at 4°C to preserve microbial viability until processed for
Bt. isolation within 24 hours.
Isolation of Bacillus Species
Sample Pre-treatment: To enrich for spore-forming bacteria, water samples were heated at 80°C for 10 minutes to eliminate non-spore-forming microorganisms.
Plating and Culture: Serial dilutions of the treated samples (10-1 to 10-6) were prepared using sterile saline (0.5%) and 100 µL of each dilution was spread-plated onto nutrient agar (NA) and Luria-Bertani (LB) agar plates. Plates were incubated at 30°C for 24–48 hours.
Colony Selection and Purification: Morphologically distinct colonies with rough, raised textures typical of Bacillus species were selected and subcultured on fresh agar plates until pure cultures were obtained.
Morphological and Molecular Characterization of Bacillus Species
Gram Staining: Isolates were examined under a microscope to confirm their Gram-positive nature.
Spore Staining: Malachite green staining was performed to detect spore-forming ability.
Molecular Identification: DNA was extracted from the isolates by Qiagen QIAamp@ DNA Mini Kit (at. No. 51304). Bacterial 16S rRNA gene was amplified using universal primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-TACGGYTACCTTGTTACGACTT-3’) in a polymerase chain reaction (PCR). PCR products were visualized on a 1% agarose gel and sequenced. Sequences were compared against the NCBI BLAST database to confirm species identity (
Table 2).
Isolates were cultured in nutrient broth (NB) at 30°C with shaking at 150 rpm for 24 hours. Cultures were centrifuged at 4,000 × g for 10 minutes, and the bacterial pellet was resuspended in sterile saline to prepare standardized suspensions of 106–108 CFU/mL, as determined by optical density (OD600). Genomic sequences of Bacillus species were retrieved from available databases such as NCBI. Complete genome sequences were aligned using the Multiple Sequence Alignment tool in MEGA software (Tamura et al., 2021). Alignment was performed to identify conserved regions across the genomes, which serve as the basis for the phylogenetic analysis. The aligned sequences were used to construct a phylogenetic tree using the Neighbor-Joining (NJ) method (Saitou and Nei 1987). The tree was built based on evolutionary distances calculated using the Kimura 2-parameter model, which considers both transitions and transversions (Kimura 1980).
Rearing and Larvicidal Bioassay of Aedes aegypti
Laboratory-reared Aedes aegypti mosquitoes were maintained at Dengue fever and vector control unit, King Abdulaziz University, Jeddah, Saudi Arabia Kingdom under controlled environmental conditions (27 ± 2°C temperature, 75 ± 5% relative humidity, and a 12:12 hour light-dark cycle) (Mahyoub et al., 2023). Newly hatched larvae were transferred to plastic trays (30 × 20 × 5 cm) containing 500 mL of dechlorinated water. The larvae were fed daily with powdered fish food (e.g., TetraMin) or finely ground dog biscuits. Water was replaced every two days to prevent microbial contamination and oxygen depletion. Third- or fourth-instar larvae were used for larvicidal bioassays to ensure uniformity in developmental stage and susceptibility.
Stock solutions of microbial larvicides or test compounds were prepared by suspending appropriate concentrations of Bacillus species isolates (e.g., B. licheniformis, B. stercoris, B. sonorensis, B. paramycoides, B. tequilensis, and B. rugosus) in sterile dechlorinated water. Serial dilutions were prepared to achieve the desired test concentrations (10, 40, 80, 120 and 150 ppm). Groups of 25 larvae (third or fourth instar) were placed in disposable 500 mL plastic cups or small trays containing 200 mL of test solution. Control groups were exposed to dechlorinated water without larvicides. Each concentration was tested in triplicate, and negative controls (dechlorinated water) were included in each experiment. Larvae were observed for mortality at 48 hours post-treatment. Larvae were considered dead if they did not exhibit any movement upon gentle prodding with a needle.
Data Analysis
Dose-response curves were generated, and LC50 and LC90 values were calculated using probit analysis (Hayat et al., 2022). Comparisons of efficacy among Bacillus species were made using one-way ANOVA followed by Tukey’s post hoc test (p < 0.05).
3. Results
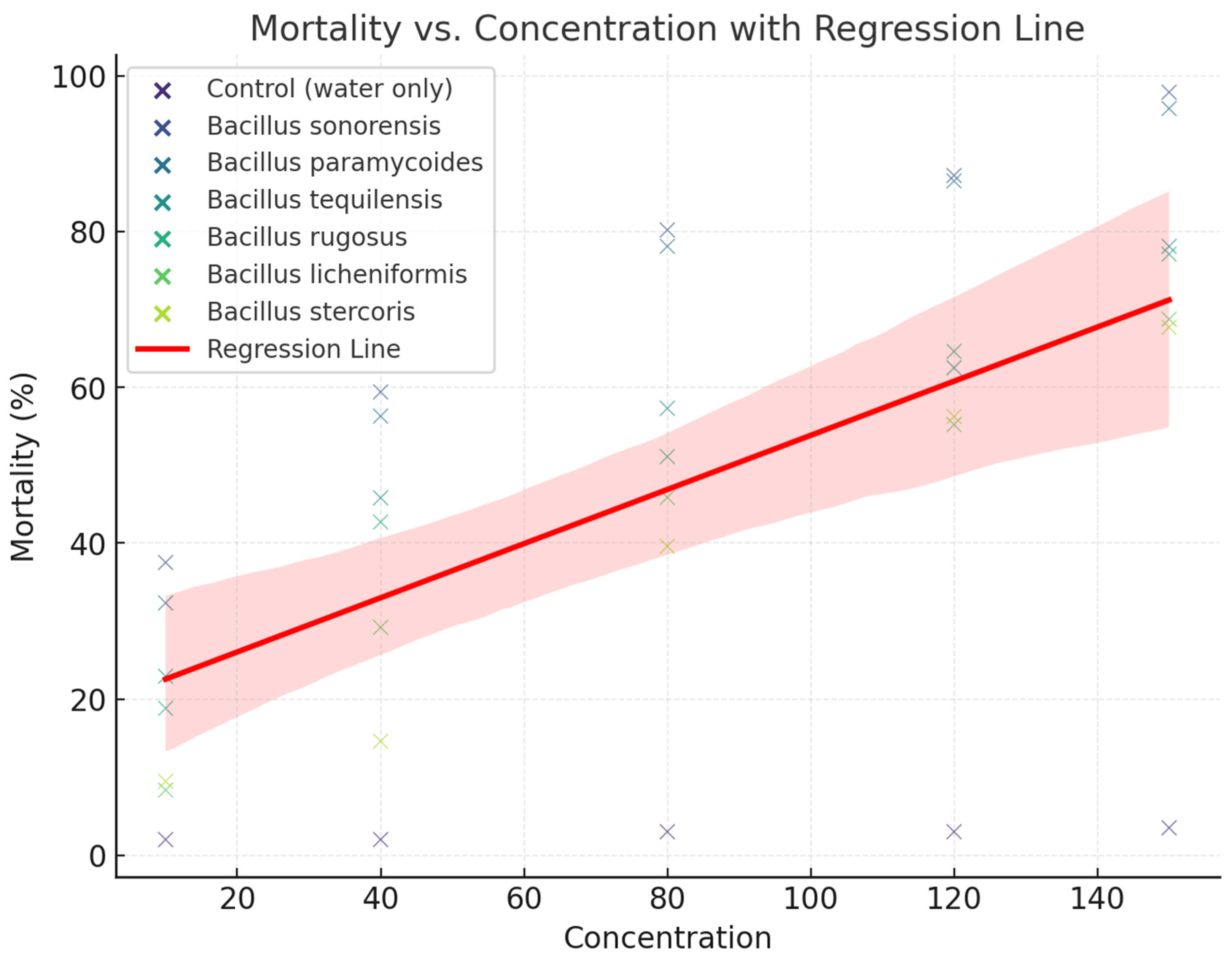
The relationship between concentration (ppm) and mortality (%) for various
Bacillus species tested against mosquito larvae was evaluated (
Figure 1). The scatterplot includes data points representing observed mortality for individual concentrations, color-coded by species. A regression line (in red) with a shaded confidence interval is overlaid to indicate the overall trend. The regression line suggests a significant positive correlation between concentration and mortality across the tested
Bacillus species. The dose-response relationship confirms the efficacy of the tested
Bacillus species as larvicides.
B. sonorensis and
B. paramycoides may be more potent as they achieve higher mortality at lower concentrations, suggesting greater larvicidal potential (
Figure 2). These findings support the use of microbial larvicides in mosquito control programs, with species-specific effectiveness allowing tailored application strategies. The variability in mortality across species highlights the need for optimizing concentration levels for each species to achieve maximum efficacy.
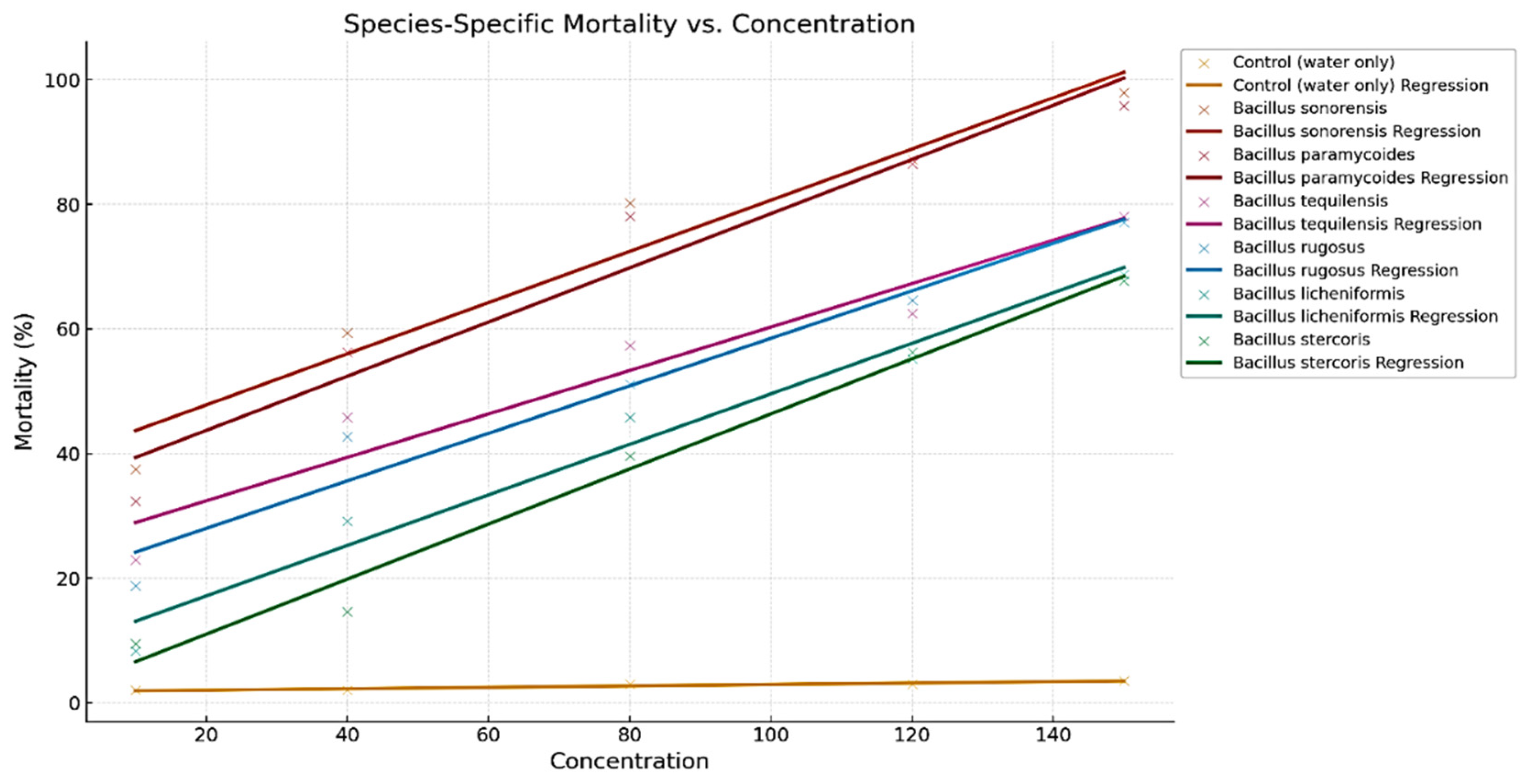
Different
Bacillus species affect the mortality of
A. aegypti larvae at increasing concentrations. Each species is represented by its data points (scatter) and a corresponding regression line, capturing the overall trend in larvicidal activity (
Figure 2). As the concentration increases, the percentage of larval mortality rises for all species, which demonstrates a concentration -dependent effect. Both,
B. sonorensis and
B. paramycoides show a steeper increase in mortality with rising concentrations compared to other species. This suggests these species are more potent at lower concentrations.
B. tequilensis and
B. rugosus show moderate slopes, indicating they are effective but require slightly higher concentrations to achieve similar levels of mortality. In contrast,
B. licheniformis and
B. stercoris show the least steep regression lines, meaning they are less effective at lower concentrations and may need significantly higher concentrations to achieve comparable mortality rates. The flat regression line for the control confirms no larvicidal activity in the absence of
Bacillus species, validating the experimental setup.
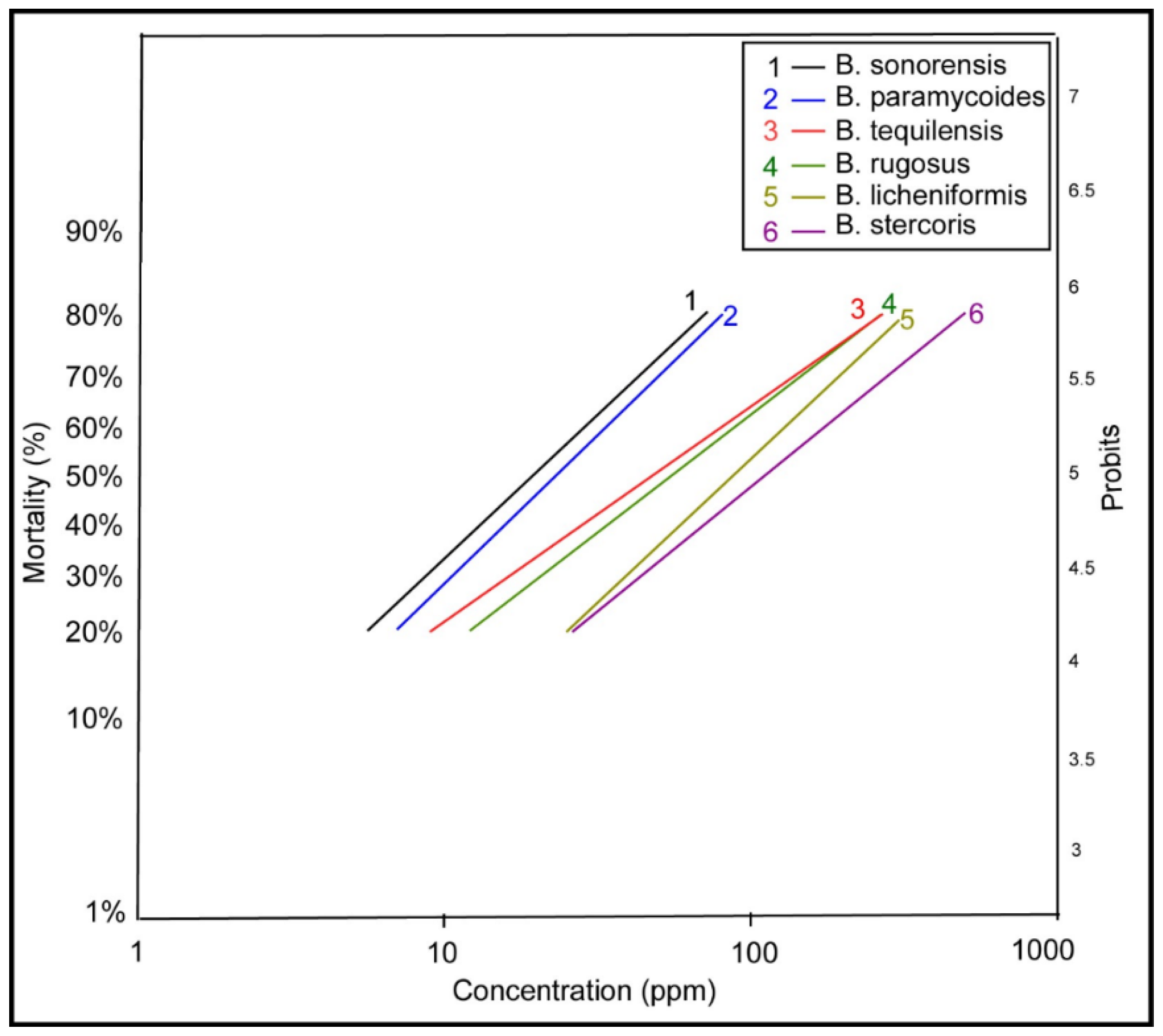
Table 3 presents the probit analysis results for different
Bacillus species used in larvicidal tests against
A. aegypti larvae. Key parameters like LC
50 and LC
90 values, slope (±SE), and chi-square values provide a clear picture of the larvicidal potential of each species.
B. sonorensis shows strong effectiveness, with LC
50 and LC
90 values only slightly smaller than
B. paramycoides. Thus,
B. sonorensis has the lowest LC
50 (19.72 ppm) and LC
90 (132.96 ppm), indicating it is the most effective species at killing larvae at lower concentrations. Moreover,
B. tequilensis and
B. rugosus are moderately effective, requiring higher concentrations to achieve similar mortality, as reflected by their LC
50 (48.17 ppm and 55.93 ppm) and LC
90 (636.17 ppm and 579.04 ppm) values.
Bacillus licheniformis and
Bacillus stercoris are the least effective, with LC
50 values of 87.44 ppm and 98.29 ppm, respectively, indicating the need for higher doses to achieve significant larval mortality. Additionally,
Bacillus stercoris has the steepest slope (2.87), meaning its response to increasing concentrations is more consistent and predictable compared to other species. The slopes for other species range between 1.14 and 1.59, reflecting variability in their concentration-mortality relationships. All chi-square values fall within acceptable ranges, confirming the reliability of the probit model for analyzing the data (
Table 3 and
Figure 3).
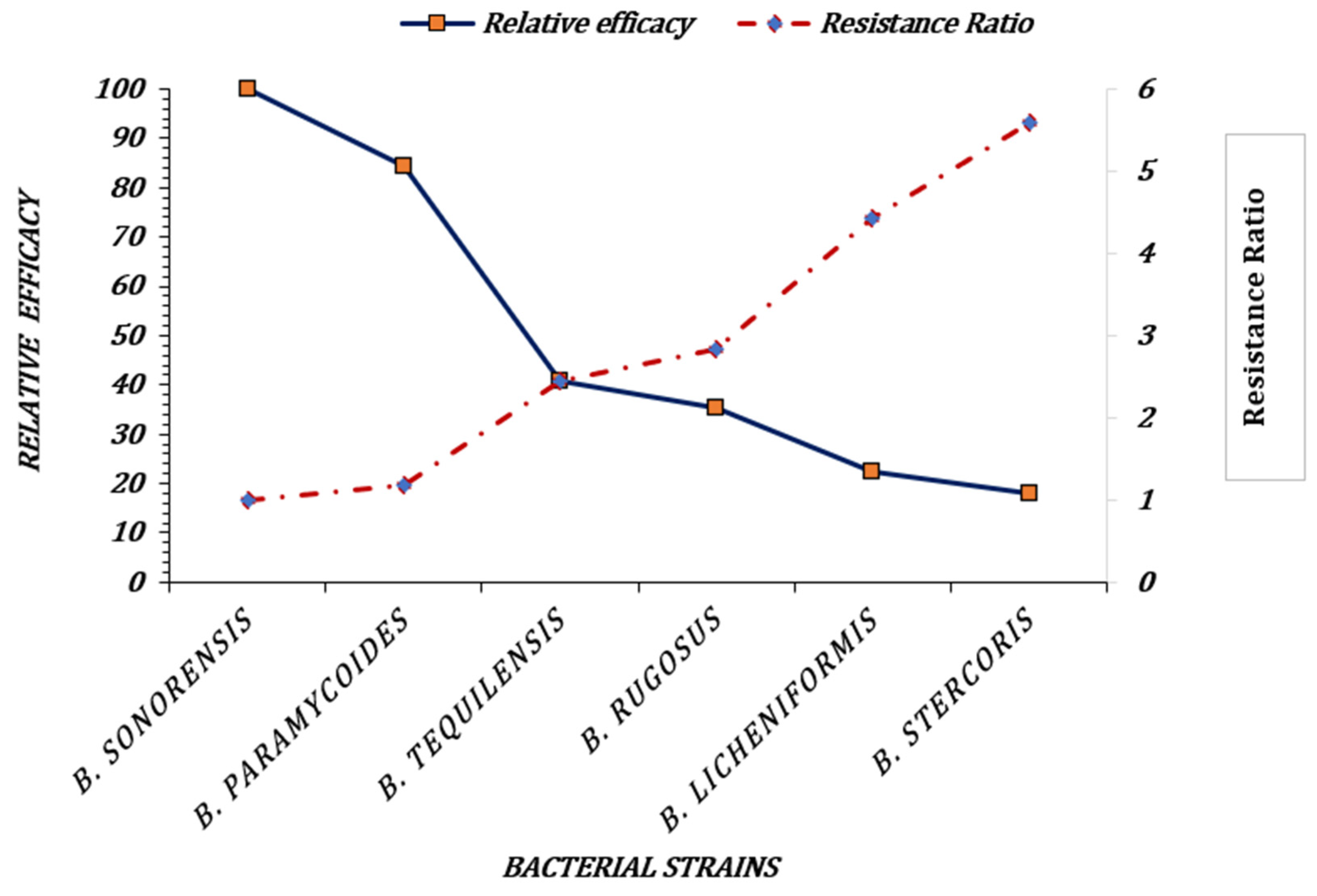
Our results indicate that the larvicidal potency (Toxicity Index) varies significantly between species (
Figure 4). For example,
B. sonorensis is much more toxic than
B. licheniformis and
B. stercoris, which have the lowest indices. The variation in resistance ratios shows that some species are more effective against resistant mosquito populations. For instance,
B.s stercoris has a higher resistance ratio (4.96), indicating lower efficacy in the presence of resistance, compared to
B. sonorensis (Resistance Ratio: 1).
In the same context, the strong negative correlation between the toxicity index and resistance ratio implies that as the toxicity index increases, the resistance ratio decreases. In simpler terms, more toxic species (like B. sonorensis) tend to maintain higher efficacy even against resistant mosquito populations, making them better candidates for larvicidal applications. Consequently, the regression model confirms that the toxicity index is a significant predictor of the resistance ratio. For every unit increase in the toxicity index, the resistance ratio decreases by approximately 0.039. This inverse relationship further supports the observation that highly toxic species like B. sonorensis are less affected by resistance, whereas species with lower toxicity (e.g., B. stercoris) are more prone to reduced efficacy. Thus, B. sonorensis emerges as the most effective species, with the highest toxicity index (100) and the lowest resistance ratio (1). This species is ideal for use in environments with resistant mosquito populations. Conversely, B. stercoris and B. licheniformis exhibit limited effectiveness due to their low toxicity and higher susceptibility to resistance. Overall, the strong correlation and regression results suggest prioritizing species with higher toxicity index values in mosquito control programs. These species not only achieve higher mortality rates but also maintain their effectiveness in the face of resistance development.
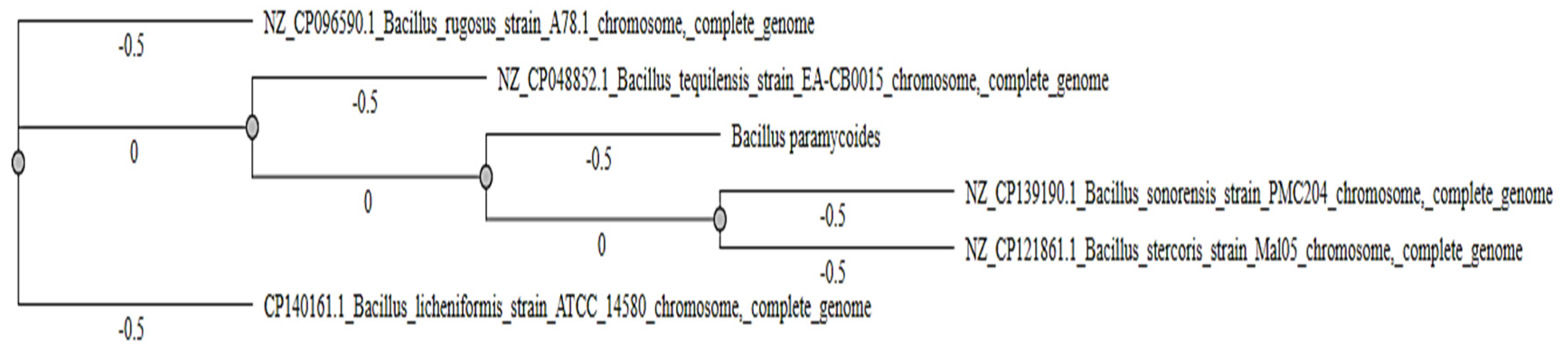
The phylogenetic tree demonstrates the evolutionary relationships among various
Bacillus species based on their genomic sequences (
Figure 5). The tree was constructed using complete genome sequences, showcasing distinct clusters that represent varying levels of genetic similarity among the analyzed species. Each branch point in the tree represents a shared ancestor, while the branch lengths reflect genetic distances between the species.
The results indicate that
B. rugosus (NZ_CP096590.1) and
B. tequilensis (NZ_CP048852.1) are closely related evolutionarily, forming a tight cluster with little genetic divergence. These two species also share a more distant ancestor with
B. paramycoides, which appears on a separate branch but remains relatively close in evolution. The distinct grouping of
B.sonorensis and
B. stercoris indicates these species share a common ancestor but are genetically distinct from the other clusters.
B. licheniformis (CP140161.1) occupies the most basal position in the tree, with longer branch lengths, indicating significant genomic divergence from the other species (
Figure 5).
Furthermore, the unique positioning of B. licheniformis and the B. sonorensis, B. stercoris cluster underscores the genetic diversity within the genus, which may translate into varied biological functions. The clustering of B. rugosus and B. tequilensis suggests potential similarities in functional properties, including their efficacy as larvicidal agents against mosquito species.
4. Discussion
Mosquitoes represent one of the most critical threats to global public health because they transmit a broad spectrum of life-threatening diseases (Bhatt et al., 2013; Brühl, 2020). Their ability to spread pathogens is directly linked to their life cycle, adaptability, and ecological preferences. The economic and social impact of these diseases is substantial. Beyond the direct health burden, they hinder economic development in endemic regions by increasing healthcare costs, reducing productivity, and creating cycles of poverty (Alonso and Tanner 2013; Shepard et al., 2013). The present study focused on microbial control agents derived from Bacillus species as highly effective and environmentally sustainable tools for mosquito control. These bioalternatives offer specificity, biodegradability, and minimal impact on non-target species, addressing many challenges associated with chemical insecticides (Brar et al., 2006). As a preliminary measure, environmental parameters like temperature and pH were monitored, as these are key factors influencing microbial and mosquito larval presence. The geographic focus of this study, Ain Al-Hara in Al-Lith province, adds significant value to understanding microbial diversity in extreme environments such as hot springs. Hot springs have been identified as unique ecosystems capable of supporting thermophilic microorganisms, including Bacillus species (Vishnivetskaya et al., 2000). Hot spring temperatures often create a unique selection pressure, favoring thermophilic and spore-forming bacteria like Bacillus, as described by Ward et al. (1998). In contrast, lower temperatures and neutral pH conditions in urban and agricultural water bodies tend to support a broader microbial diversity (Mehetre et al., 2018; Katak et al., 2021).
The results proved that the regression analysis demonstrates a strong dose-response relationship across all Bacillus species tested. The species B. sonorensis and B. paramycoides exhibit higher larvicidal activity at lower concentrations compared to others, indicating superior potency. In this case, B. sonorensis achieved high mortality rates with minimal dosage, aligning with findings in previous studies (Lacey et al., 2007) where specific Bacillus species were reported to exhibit significant efficacy against mosquito larvae. Similar to the findings here, Mittal et al. (2003) demonstrated that Bacillus species, including Bti and B. sphaericus (Bs), exhibit a strong dose-response relationship, with mortality increasing significantly with concentration. The strong dose-response trend with reduced required concentrations minimizes the environmental footprint of microbial larvicides, ensuring that non-target organisms remain unaffected (Thakur et al., 2020). This is in line with the findings by Sabbahi et al. (2022), which demonstrated the eco-friendliness of microbial agents like Bti. Thus, combining multiple Bacillus species with varying toxin profiles, as observed here, could reduce the risk of mosquito resistance, as noted in previous studies (Silva-Filha et al., 2021).
Probit analysis offers a powerful statistical method for evaluating the dose-response relationship in larvicidal studies, providing critical parameters such as LC
50, LC
90, slope (±SE), and chi-square values to quantify larvicidal potential. The analysis of six
Bacillus species reveals significant variation in larvicidal efficacy, with
B. sonorensis emerging as the most potent species against
A. aegypti larvae, followed by
B. paramycoides, while
B. licheniformis and
B. stercoris are less effective
(Table 3 and Figure 3). B. sonorensis exhibits the lowest LC
50 (19.72 ppm) and LC
90 (132.96 ppm) values, indicating its high efficacy at lower concentrations. These results align with studies by
Falqueto et al., (2021), where
Bacillus species producing Cry toxins showed potent larvicidal activity due to efficient toxin binding in the mosquito midgut.
B. tequilensis and
B. rugosus demonstrate moderate efficacy, requiring higher concentrations to achieve similar mortality. Similar findings were reported by
Mittal et al. (2003), where dose variations among
Bacillus species were attributed to differences in toxin production. The slopes for other species, ranging from 1.14 (
B. tequilensis) to 1.59 (
B. paramycoides), reflect moderate variability in their dose-response relationships. These variations may arise from differences in toxin activity, receptor specificity, or environmental factors affecting larvicidal action (
Bravo et al., 2007).
The results of
Bacillus species highlight significant differences in their toxicity and resistance ratios when used as larvicidal agents against
A. aegypti (
Figure 4). These metrics are critical for assessing their efficacy, particularly in environments where mosquito populations exhibit varying levels of resistance to larvicides.
B. sonorensis stands out as the most toxic species, with the highest toxicity index (100) and a resistance ratio of 1, indicating consistent effectiveness against both susceptible and resistant mosquito populations. In contrast,
B. licheniformis and
B. stercoris exhibit much lower toxicity indices (22.55 and 20.06, respectively), suggesting significantly reduced efficacy. These findings align with studies by
Ferreira et al., (2013) and
Silva-Filha et al., (2021), which highlighted that toxin production and receptor-binding affinity are key determinants of larvicidal activity. The resistance ratio provides an important measure of how well a
Bacillus species performs in the presence of resistant mosquito populations. A resistance ratio of 4.96 for
B. stercoris indicates its reduced effectiveness in such contexts, possibly due to diminished toxin-receptor interactions in resistant larvae (
Canton et al., 2015; Guo et al., 2020). The wide variation in toxicity and resistance ratios among the species studied here mirrors patterns observed in earlier research. For example, two recent researches demonstrated that
Bti species with higher Cry toxin production consistently outperformed
Bs species in controlling resistant mosquito populations
(Deshayes et al., 2017; Dacey et al., 2020). The low resistance ratio of
B. sonorensis suggests its potential for use in areas where traditional larvicides have failed due to resistance.
Notably, the phylogenetic analysis of
Bacillus species provides critical insights into their evolutionary relationships, genetic diversity, and potential functional implications (
Figure 5). The tree structure, branch lengths, and clustering patterns suggest varying degrees of genetic divergence among the species, with distinct evolutionary relationships highlighted in the clustering of
B. rugosus,
B. tequilensis, and
B. paramycoides as well as the separate grouping of
B. sonorensis and
B. stercoris. The phylogenetic tree places
B. rugosus and
B. tequilensis in a tight cluster with minimal branch lengths, indicating high genetic similarity. Such close relationships suggest shared evolutionary pressures and potentially similar functional properties, such as their production of bioactive metabolites. Previous studies, such as
Pham et al. (2021), emphasize that closely related
Bacillus species often exhibit overlapping ecological niches and biocontrol potential. While
B. paramycoides is on a separate branch, it remains relatively close to the
B. rugosus and
B. tequilensis cluster, suggesting a more recent divergence from a shared ancestor. This finding aligns with the work of
Ngurube et al. (2020), where intermediate positions in phylogenetic trees were linked to adaptive radiation events among
Bacillus species. The separation of
B. sonorensis and
B. stercoris into a distinct cluster reflects their shared evolutionary history but genetic divergence from the other species. This grouping could indicate the development of unique traits, possibly associated with specific environmental adaptations, as suggested by
Bravo et al. (2007). Overall, the close evolutionary relationship between
B. rugosus,
B. tequilensis, and
B. paramycoides suggests a possible overlap in their larvicidal efficacy, making them prime candidates for further investigation in mosquito control. Correspondingly, the divergence of
B. sonorensis and
B. stercoris may indicate specialization to unique ecological niches or the evolution of novel traits that could expand their utility in vector control or other biotechnological applications (
Katak et al., 2021). Experimental studies should investigate whether the phylogenetic clustering corresponds to functional similarities, particularly in larvicidal efficacy and toxin production. Whole-genome comparisons could identify genetic markers associated with larvicidal activity, enabling the optimization of these species for mosquito control. Understanding the ecological roles of these species in their native habitats could inform sustainable deployment strategies.
Recent research has illuminated the diverse and promising applications of Bacillus species in viral control, highlighting their potential as safe and effective agents in both medical and environmental contexts. Notably, Bacillus species have exhibited antiviral properties through several novel mechanisms, expanding our understanding of their capabilities.
One such mechanism involves the synthesis of exopolysaccharides, which have demonstrated the capacity to inhibit viral adhesion to host cells. For instance, B. licheniformis KCTC 12816BP produces an exopolysaccharide that exhibits antiviral activity against influenza A virus (Zeriouh et al., 2014; Thérien et al., 2020; Ajuna et al., 2024). Additionally, certain Bacillus species generate innovative antiviral peptides, with a peptide isolated from B. amyloliquefaciens displaying broad-spectrum antiviral efficacy against both enveloped and non-enveloped viruses (Wang et al., 2023). Furthermore, biofilms produced by Bacillus can establish a physical barrier against viral particles, potentially mitigating viral transmission on surfaces. This characteristic, observed in B. suBtilis species, could be harnessed for the development of antiviral coatings (Ajuna et al., 2024).
Bacillus species also possess the capacity to modulate host immune responses, thereby enhancing antiviral defenses. They can stimulate the production of interferons in the human immune system (Prabhakaran et al., 2025) and activate natural killer (NK) cells (Karačić et al., 2024). Some Bacillus probiotics have been found to stimulate the production of type I interferons, crucial components of the innate antiviral response. This effect has been observed with B. suBtilis natto, which demonstrated potential in bolstering resistance to influenza virus infection (Prabhakaran et al., 2025). Moreover, these probiotics can activate NK cells, augmenting the body’s ability to combat viral infections. Studies involving B. coagulans GBI-30, 6086 have shown enhanced NK cell activity and potential protection against common viral infections (Jensen et al., 2017; Karačić et al., 2024). In the realm of biotechnology, Bacillus spores are being explored as safe and effective delivery systems for antiviral agents. Researchers have developed methods to display antiviral proteins on the surface of Bacillus spores, creating stable and safe antiviral formulations (Khodavirdipour et al., 2022). This approach has shown promise in delivering neutralizing antibodies against viruses such as influenza.
From an environmental perspective, Bacillus species are being investigated for their potential to reduce viral contamination in various settings. Certain strains have demonstrated the ability to diminish viral loads in water systems (Behardien, 2008). For example, B. pumilus S8-07 has shown efficacy in removing human adenovirus from water, suggesting potential applications in water treatment and sanitation (Behardien et al., 2011). These findings collectively underscore the multifaceted and promising roles of Bacillus species in viral control, emphasizing their potential as safe and effective agents in both medical and environmental applications and reinforce the potential of Bacillus species as eco-friendly and effective alternatives for mosquito and viral control. Their specificity, biodegradability, and high efficacy at low concentrations make them valuable tools in integrated vector management programs (Schorkopf et al., 2016; Almeida et al., 2020; Ibiene et al., 2021; Silva-Filha et al., 2021; Miranda et al., 2024).
Anticipating future developments, the promising performance of these extremophile Bacillus species in controlled laboratory environments sets the stage for expansive field trials and practical implementations. The shift from experimental settings to real-world applications necessitates meticulous attention to potential ecological repercussions, the strategic management of resistance, and the harmonization with established vector control initiatives. Moreover, an in-depth investigation into the molecular mechanisms underlying larvicidal efficacy could pave the way for the engineering of even more potent and highly targeted biocontrol agents.
5. Conclusions
This study highlights the significant larvicidal potential of extremophile Bacillus species, particularly B. sonorensis and B. paramycoides, which exhibited high efficacy at lower concentrations and consistent performance against resistant mosquito populations. By integrating phylogenetic insights with larvicidal efficacy data, this research provides a foundation for the strategic use of microbial agents in mosquito control. Experimental validation, functional genomic studies, and field trials will be crucial for optimizing the application of these species. The findings also emphasize the importance of sustainable deployment strategies that leverage the unique properties of each species, particularly in regions where resistance to traditional insecticides is prevalent. The results of this study reinforce the critical role of Bacillus species as eco-friendly and effective alternatives for mosquito control. Their specificity, biodegradability, and high efficacy at low concentrations make them indispensable tools in integrated vector management (IVM) programs. Future research should focus on understanding the ecological roles and molecular mechanisms underlying the larvicidal activity of these species, ensuring their continued success in combating mosquito-borne diseases.
Funding
This research was funded by Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, grant number MoE-IF-UJ-R2-22-04100088-1.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number MoE-IF-UJ-R2-22-04100088-1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ajuna, H. B., Lim, H. I., Moon, J. H., Won, S. J., Choub, V., Choi, S. I., … & Ahn, Y. S. (2024). The prospect of antimicrobial peptides from Bacillus species with biological control potential against insect pests and diseases of economic importance in agriculture, forestry and fruit tree production. Biotechnology & Biotechnological Equipment, 38(1), 2312115.
- Almeida, J. S., Mohanty, A. K., Kerkar, S., Hoti, S. L., & Kumar, A. (2020). Current status and future prospects of bacilli-based vector control. Asian pacific journal of tropical medicine, 13(12), 525-534.
- Behardien, L. (2008). Investigation into the bacterial contamination in a spring water distribution system and the application of bioremediation as treatment technology (Doctoral dissertation, Cape Peninsula University of Technology).
- Behardien, L., Paulse, A., Jackson, V., Khan, S., & Khan, W. Investigation into the microbial contamination in a spring water distribution system, Western Cape, South Africa. African Journal of Microbiology Research 2011, 5(20), 3200–3214.
- Bravo, A., Gill, S. S., & Soberón, M. (2007). Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon, 49(4), 423-435.
- Brühl, C. A., Després, L., Frör, O., Patil, C. D., Poulin, B., Tetreau, G., & Allgeier, S. (2020). Environmental and socioeconomic effects of mosquito control in Europe using the biocide Bacillus thuringiensis subsp. israelensis (Bti). Science of the total environment, 724, 137800.
- Federici, B. A., Park, H. W., & Bideshi, D. K. (2010). Overview of the basic biology of Bacillus thuringiensis with emphasis on genetic engineering of bacterial larvicides for mosquito control. Open Toxinology Journal, 3(2), 83-100.
- Harirchi, S., Sar, T., Ramezani, M., Aliyu, H., Etemadifar, Z., Nojoumi, S. A., … & Taherzadeh, M. J. (2022). Bacillales: from taxonomy to biotechnological and industrial perspectives. Microorganisms, 10(12), 2355.
- Ibiene, A. A., Lawson, S. D., Enyinnaya, S. O., Amos, F. E., Nnodim, L., & Uzah, G. A (2021). Isolation and Use of Bacillus thuringiensis for the Production of Bio-Insecticide in Control of Mosquito Larvae.
- Jensen, G. S., Cash, H. A., Farmer, S., & Keller, D. (2017). Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. Journal of inflammation research, 107-117.
- Karačić, V., Miljaković, D., Marinković, J., Ignjatov, M., Milošević, D., Tamindžić, G., & Ivanović, M. (2024). Bacillus species: Excellent biocontrol agents against tomato diseases. Microorganisms, 12(3), 457.
- Khodavirdipour, A., Chamanrokh, P., Alikhani, M. Y., & Alikhani, M. S. (2022). Potential of Bacillus suBtilis against SARS-CoV-2–a sustainable drug development perspective. Frontiers in Microbiology, 13, 718786.
- Lacey, L. A. (2007). Bacillus thuringiensis serovar israelensis and Bacillus sphaericus for mosquito control. Journal of the American Mosquito Control Association, 23(1), 133-163.
- Malakar, C., Deka, S., & Kalita, M. C. (2023). Role of biosurfactants in biofilm prevention and disruption. In Advancements in Biosurfactants Research (pp. 481-501). Cham: Springer International Publishing.
- Miranda, L. S., Rudd, S. R., Mena, O., Hudspeth, P. E., Barboza-Corona, J. E., Park, H. W., & Bideshi, D. K. (2024). The perpetual vector mosquito threat and its eco-friendly nemeses. Biology, 13(3), 182.
- Narkhede, C. P., Patil, C. D., Suryawanshi, R. K., Koli, S. H., Mohite, B. V., & Patil, S. V. (2017). Synergistic effect of certain insecticides combined with Bacillus thuringiensis on mosquito larvae. Journal of Entomological and Acarological Research, 49(1).
- Prabhakaran, V. S., Park, K. B., Kim, K. Y., Jung, W. J., & Han, Y. S (2025). The role of Bacillus species in the management of plant-parasitic nematodes. Frontiers in Microbiology, 15, 1510036.
- Schorkopf, D. L. P., Spanoudis, C. G., Mboera, L. E., Mafra-Neto, A., Ignell, R., & Dekker, T. (2016). Combining attractants and larvicides in biodegradable matrices for sustainable mosquito vector control. PLoS Neglected Tropical Diseases, 10(10), e0005043.
- Silva-Filha, M. H. N. L., Romão, T. P., Rezende, T. M. T., Carvalho, K. D. S., Gouveia de Menezes, H. S., Alexandre do Nascimento, N., … & Bravo, A. (2021). Bacterial toxins active against mosquitoes: Mode of action and resistance. Toxins, 13(8), 523.
- Thérien, M., Kiesewalter, H. T., Auria, E., Charron-Lamoureux, V., Wibowo, M., Maróti, G., … & Beauregard, P. B. (2020). Surfactin production is not essential for pellicle and root-associated biofilm development of Bacillus suBtilis. Biofilm, 2, 100021.
- Wang, X., Hao, G., Zhou, M., Chen, M., Ling, H., & Shang, Y. (2023). Secondary metabolites of Bacillus suBtilis L2 show antiviral activity against pseudorabies virus. Frontiers in Microbiology, 14, 1277782.
- Zeriouh, H., de Vicente, A., Pérez-García, A., & Romero, D. (2014). Surfactin triggers biofilm formation of B acillus suBtilis in melon phylloplane and contributes to the biocontrol activity. Environmental microbiology, 16(7), 2196-2211.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).