Submitted:
04 February 2025
Posted:
05 February 2025
You are already at the latest version
Abstract
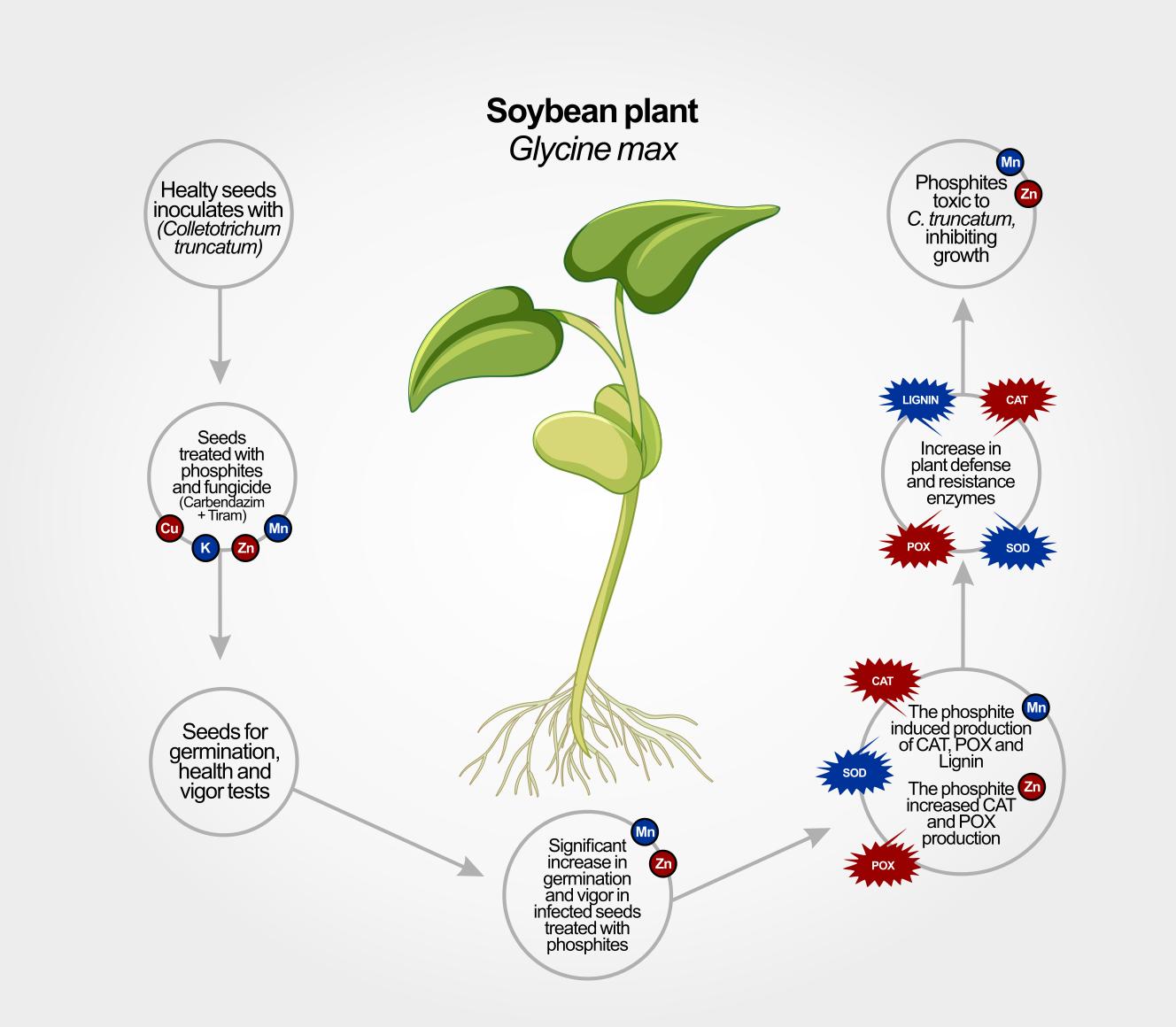
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Source and Doses of Phosphites and Fungicide
2.3. Inoculation of Soybean Seeds with C. truncatum
2.4. Seed Treatments
2.5. Effects of Phosphites on Seed Health and Germination
2.6. Emergence and Development of Soybean Seedlings Under Greenhouse Conditions
2.7. Fungitoxic Activity of Phosphites
2.8. Activation of Biochemical Defense Mechanisms
2.9. Statistical Analysis
3. Results
3.1. Effects of Phosphites on Seed Health and Germination
3.2. Emergence and Development of Soybean Seedlings Under Greenhouse Conditions
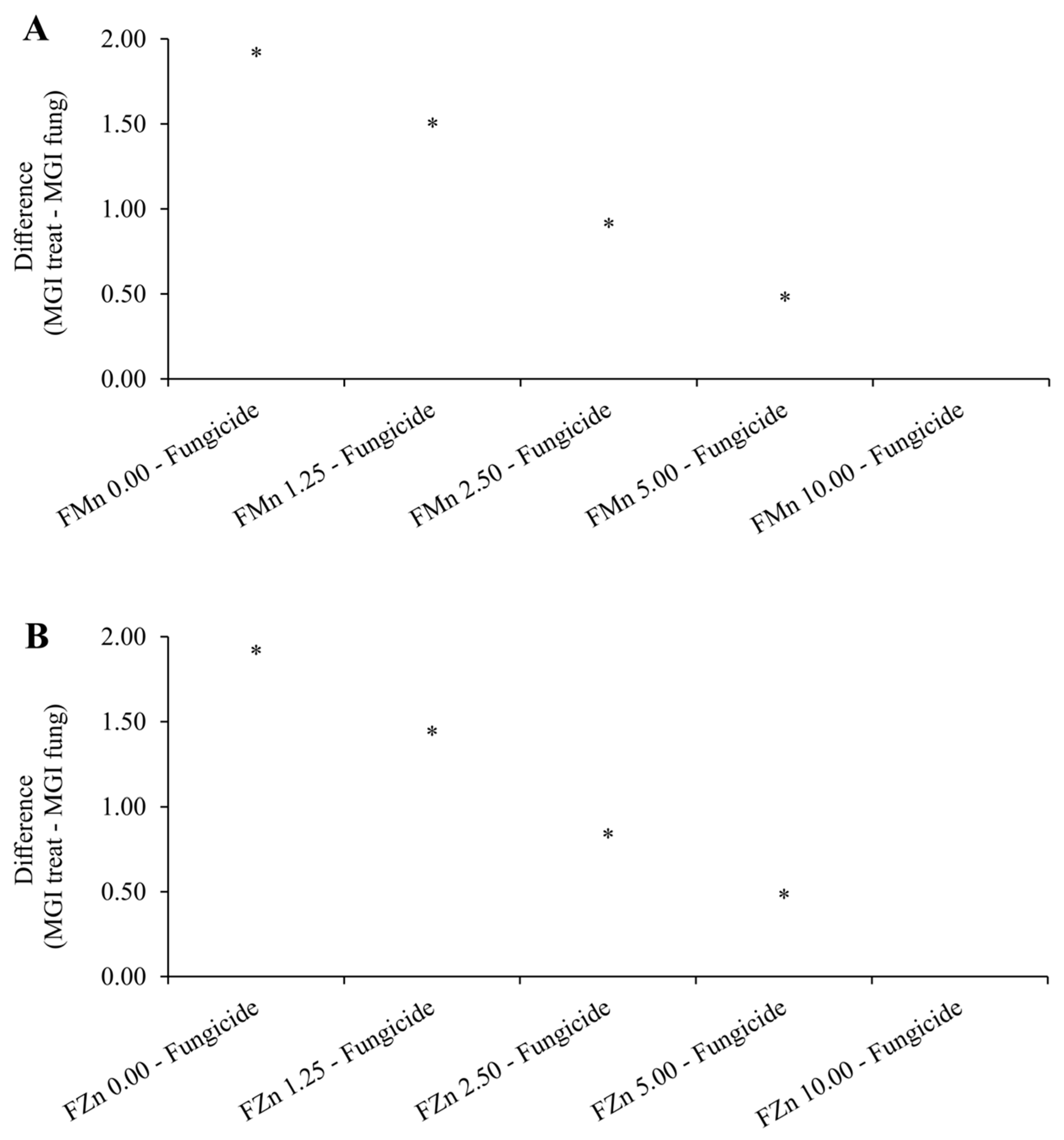
3.3. Fungitoxic Activity of Phosphites
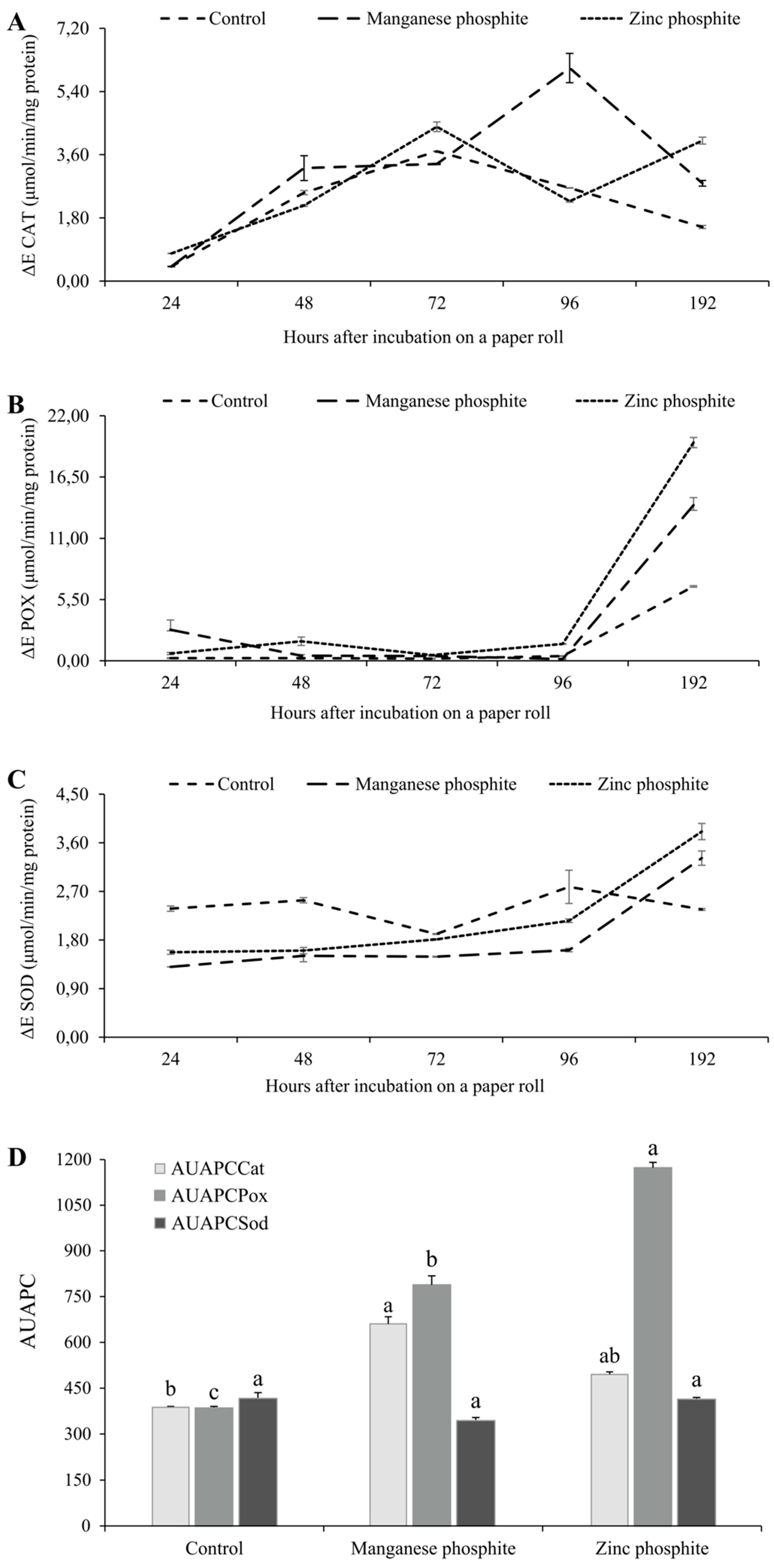
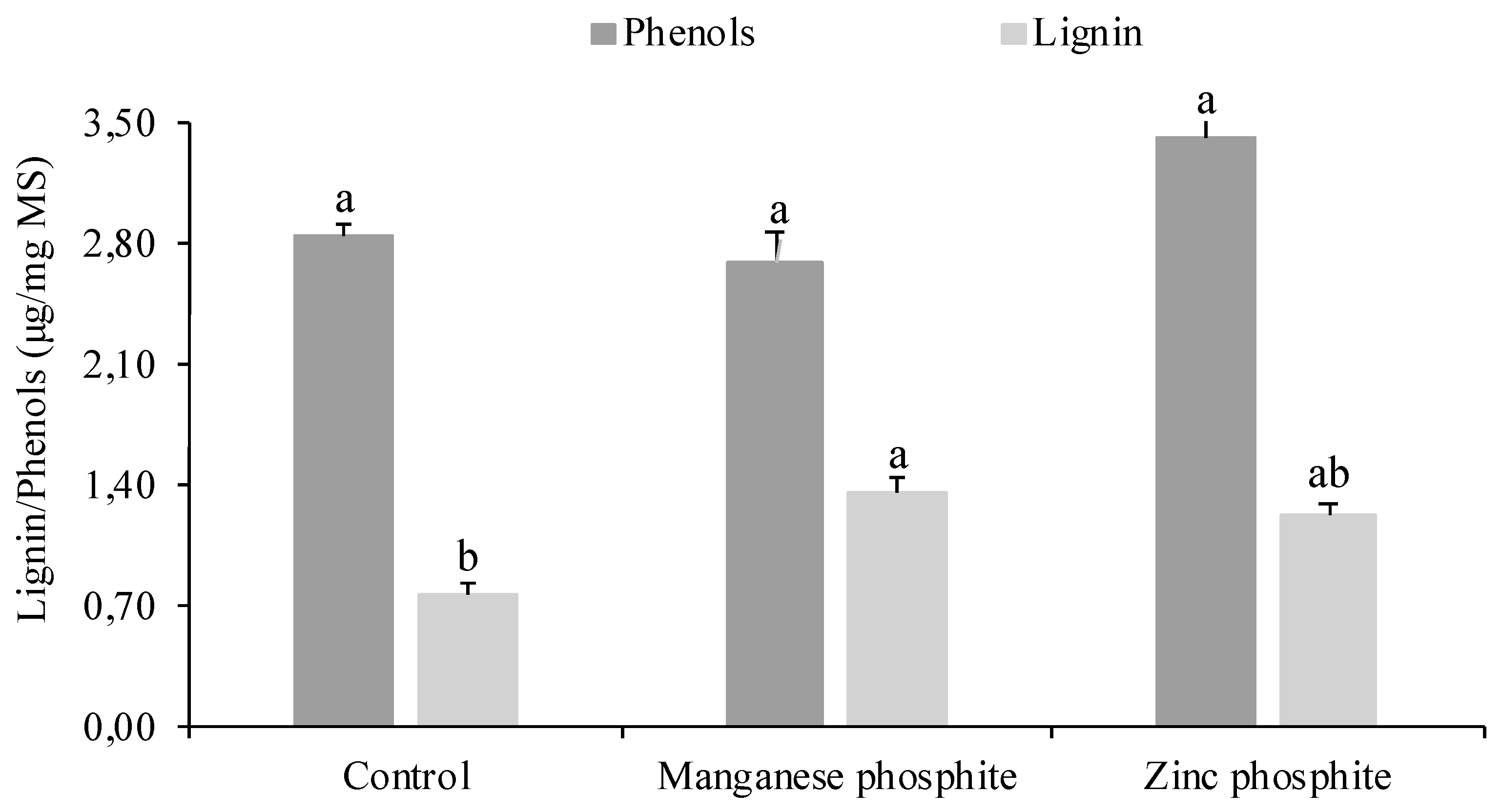
3.4. Activation of Biochemical Defense Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H., Li, H., Liu, Z., Zhang, C., Zhang, S., Atkinson, PM, 2023. A novel Greenness and Water Content Composite Index (GWCCI) for soybean mapping from single remotely sensed multispectral images. Remote Sensing of Environment, 295. [CrossRef]
- Belewu, MA., Belewu, KY, 2007. Comparative physico-chemical evaluation of tiger-nut, soybean and coconut milk sources. International Journal of Agriculture and Biology. 9, 785-7.
- Pagano, MC., Miransari, M., 2016. The importance of soybean production worldwide. In: Miransari, M. (Ed.) Abiotic and Biotic Stresses in Soybean Production. Cambridge, MA, USA: Academic Press, 1-26. [CrossRef]
- Inglada, J., Arias, M., Tardy, B., Hagolle, O., Valero, S., Morin, D., Dedieu, G., Sepulcre, G., Bontemps, S., Defourny, P., Koetz, B., 2015. Assessment of an operational system for crop type map production using high temporal and spatial resolution satellite optical imagery. Remote Sens. 7, 12356-12379. [CrossRef]
- Matton, N., Canto, G., Waldner, F., Valero, S., Morin, D., Inglada, J., Arias, M., Bontemps, S., Koetz, B., Defourny, P., 2015. An automated method for annual cropland mapping along the season for various globally-distributed agrosystems using high spatial and temporal resolution time series. Remote Sens. 7, 13208-13232. [CrossRef]
- Rogério, F., de Castro, RRL., Massola Júnior, NS., Boufleur, TR., dos Santos, RF., 2024. Multiple resistance of Colletotrichum truncatum from soybean to QoI and MBC fungicides in Brazil. Journal of Phytopathology, 172, e13341. [CrossRef]
- Hartman, GL., Sinclair, JB., Rupe, JC., 1999. Compendium of Soybean Diseases, 4th ed.; APS Press: St. Paul, MN, USA. [CrossRef]
- Billores, SDO., Joshi, P., Bhatia, VS., Ramesh, A., 2011. Soybean Based Intercropping Systems in India- A Review. Soybean Research, 9, 1-30.
- Dias, MD., Pinheiro, VF., Cafe-Filho, AC., 2016. Impact of anthracnose on the yield of soybean subjected to chemical control in the north region of Brazil. Summa Phytopathologica. 42, 1, 18-23. [CrossRef]
- Dias, MD., Dias-Neto, JJ., Santos, MDM., Formento, AN., Bizerra, LVAS., Fonseca, MEN., Boiteux, LS., Café-Filho, AC., 2019. Current Status of Soybean Anthracnose Associated with Colletotrichum truncatum in Brazil and Argentina. Plants (Basel). 8, 11, 459. [CrossRef]
- Silva, AC., Souza, PE., Machado, JC., Silva, BM., Pinto, JEBP., 2012. Effectiveness of essential oils in the treatment of Colletotrichum truncatum infected soybean seeds. Topical Plant Pathology. 37, 305-313. [CrossRef]
- Galli, JÁ., Panizzi, RC., Vieira, RD., 2007. Resistência de variedades de soja à morte de plântulas causada por Colletotrichum truncatum. Arquivos do Instituto Biológico, 74, 163-165. [CrossRef]
- Sharma, SK., Gupta, GK., Ramteke, R., 2011. Colletotrichum truncatum [(Schw.) Andrus and W.D. Moore], the causal agent of anthracnose of soybean [Glycine max (L.) Merrill.] –a review. Soybean Research, 9, 31-52. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1974Articles/Phyto64n01_154.pdf.
- Júnior, MBd., Resende, MLV., Pozza, EA., 2021. Effect of temperature on Colletotrichum truncatum growth, and evaluation of its inoculum potential in soybean seed germination. Eur J Plant Pathol, 160, 999–1004. [CrossRef]
- Boufleur, TR., Ciampi-Guillardi, M., Tikami, Í., Rogério, F., Thon, MR., Sukno, SA., Massola Júnior, NS., Baroncelli, R., 2021. Soybean anthracnose caused by Colletotrichum species: Current status and future prospects. Mol Plant Pathol. 22, 4, 393-409. [CrossRef]
- Pereira, CE., Oliveira, JÁ., Rosa, MCM., Oliveira, GE., Neto, JC., 2009. Tratamento fungicida de sementes de soja inoculadas com Colletotrichum truncatum. Ciência Rural, 39, 2390-2395. [CrossRef]
- Yang, HC., Hartman, GL., 2015. Methods and evaluation of soybean genotypes for resistance to Colletotrichum truncatum. Plant Disease, 99, 143-148. [CrossRef]
- Poti, T., Mahawan, K., Cheewangkoon, R., Arunothayanan, H., Akimitsu, K., Nalumpang, S., 2020. Detection and molecular characterization of carbendazim-resistant Colletotrichum truncatum isolates causing anthracnose of soybean in Thailand. Journal of Phytopathology, 168, 267–278.
- Mello, FE., Mathioni, SM., Matos, VORL., 2024. Sensitivity of Colletotrichum plurivorum and C. truncatum isolated from soybean in Brazil to SDHIs and DMIs fungicides. Trop. plant pathol. 49, 83–92. [CrossRef]
- Begum, MM., Sariah, M., Puteh, AB., Zainal Aabidin, MA., Rahman, MA., Siddiqui, Y., 2010. Field performance of bio-primed seeds to supress Colletotrichum truncatum causing dampping-off and seedling stand of soybean. Biological Control. 53, 18-23. [CrossRef]
- Dalio, RJD., Ribeiro Júnior, PM., Resende, MLV., Silva, AC., Blumer, S., Pereira, VF., Osswald, W., Pascholati, SF., 2012. O triplo modo de ação dos fosfitos em plantas. In: Wilmar C. Luz. (Org.). Revisão Anual de Patologia de Plantas, 20, 206-242.
- Buffara, CRS., Angelotti, F., Tessmann, DJ., Souza, CD., Vida, JB., 2013. Atividade de fosfito de potássio na pré e pós-infecção de Phakospsora euvitis em folhas de videira. Semina: Ciências Agrárias, 34, 6, 3333-3340. [CrossRef]
- Magalhães, LPP., de Lima Pereira Sales, N., Barroso, PD., da Silva, RAF., Pinho, DB., Zanuncio, JC., Silva, AC., 2024. Pseudoplagiostoma humilis sp. nov., a New Fungal Species Causing Shoot Blight and Dieback in Anacardium humile in Brazil. Curr Microbiol 81, 378. [CrossRef]
- Brasil. 2009. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Brasília: Secretaria de Defesa Agropecuária. Mapa/ACS. 399 p.
- Machado, JC., Oliveira, JÁ., Vieira, MGCG., Alves, MC., 2001. Inoculação artificial de sementes de soja por fungos, utilizando solução de manitol. Revista Brasileira de Sementes, Londrina, 23, 2, 95-101. [CrossRef]
- Michel, BE., Radcliffe, DA., 1995. Computer program relating solute potential to solution composition for five solutes. Agronomy Journal, 87, 1, 131-136. [CrossRef]
- Maguire, JD., 1962. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Science, 2, 2, 176-177. [CrossRef]
- Araújo, L., Valdebenito-Sanhueza, RM., Stadnik, MJ., 2010. Avaliação de formulações de fosfito de potássio sobre Colletotrichum gloeosporioides in vitro e no controle pós-infeccional da mancha foliar de Glomerella em macieira. Tropical Plant Pathology. v. 35, n. 1, p. 54-59. [CrossRef]
- Araújo, L., Stadnik, MJ., Borsato, LC., Valdebenito-Sanhueza, RM., 2008. Fosfito de potássio e ulvana no controle da mancha foliar da gala em macieira. Tropical Plant Pathology. 33, 2, 148-152. [CrossRef]
- Torres-Calzada, C., Tapia-Tussel, R., Higuera-Ciapara, I., Nexticapan-Garcez, A., Perez- Brito, D., 2015. Sensitivity of Colletotrichum truncatum to four fungicides and characterization of thiabendazole-resistant isolates. Plant Disease. 99, 1590-1595. [CrossRef]
- Bradford, MM., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, Washington, 72, 1/2, 248-254. [CrossRef]
- Urbanek, H., Kuzniak-Gebarowska, E., Herka, H., 1991. Elicitation of defence responses in common bean leaves by Botrytis cinerea polygalacturonase. Acta Phisiologiae Plantarum. 13, 1, 43-50.
- Chance, B., Maehley, AC., 1955. Assay of catalases and peroxidases. Methods in Enzymology, 2, 764-775.
- Havir, EA., McHale, NA., 1987. Biochemical and developmental characterization of multiple forms of catalase in toacco leaves. Plant Physiology, 84, 2, 450-455. [CrossRef]
- Gianopolitis, CN., Ries, SK., 1977. Superoxide dismutases: I. Occourrence in higher plants. Plant Phisiology, 59, 2, 309-314. [CrossRef]
- Shaner, G., Finney, RF., 1977. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathology, 67, 1051-1056.
- Doster, MA., Bostock, RM., 1988. Quantification of lignin formation in almond bark in response to wounding and infection by Phytophthora species. Phytopathology, 78, 473-477. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1988Articles/Phyto78n04_473.PDF.
- Spanos, GA., Wrolstad, RE., 1990. Influence of processing and storage on the phenolic composition of Thompson sedless grape juice. Journal of Agricultural & Food Chemistry, 38, 7, 1565-1571. https://pubs.acs.org/doi/10.1021/jf00097a030.
- R Development Core Team, 2009. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- Yáñez-Juárez, MG., López-Orona, CA., Ayala-Tafoya, F., Partida-Ruvalcaba, L., Velázquez-Alcaraz, TJ., Medina-López, R., 2018. Phosphites as alternative for the management of phytopathological problems. Revista mexicana de fitopatología. 36, 1, 79-94. [CrossRef]
- Carmona, MA., Sautua, FJ., Grijalba, PE., Cassina, M., Pérez-Hernández, O., 2017. Effect of potassium and manganese phosphites in the control of Pythium damping-off: a feasible alternative to fungicide seed treatments. Pest Management Science. 74, 366-374. 10.1002/ps.4714.
- da Silva, AC., Resende, MLV., de Souza, PE., Silva, NCN., Silva, MB., Vitorino, LRR., 2013. Coffee-leaf extract and phosphites on the curative control of powdery mildew in eucalyptus mini-stumps. For. Path., 43, 297-305. [CrossRef]
- Yogev, E., Sadowsky, A., Solel, Z., Oren, Y., Orbach, Y., 2006. The performance of potassium phosphite for controlling Alternaria brown spot of citrus fruit. J. Plant Dis. Prot. 113, 207-213. [CrossRef]
- Monteiro, ACA., Resende, MLV., Valente, TCT., Ribeiro Júnior, PM., Pereira, VF., Costa, JR., Silva, JAG., 2016. Manganese phosphite in coffee defence against Hemileia vastatrix, the coffee rust fungus: Biochemical and molecular analyses. Journal of Phytopathology. 164, 1043-1053. [CrossRef]
- Silva, OC., Santos, HAA., Dalla Pria, M., May-De Mio, LL., 2011. Potassium phosphite for control of downy mildew of soybean. Crop Protection. 30, 598-604. [CrossRef]
- Abbasi, PA., Lazarovits, G., 2006. Seed treatment with phosphonate (AG3) suppresses Pythium damping-off of cucumber seedlings. Plant Disease. 90, 459-464. [CrossRef]
- Mayton, H., Amirkhani, M., Loos, M., Johnson, B., Fike, J., Johnson, C., Myers, K., Starr, J., Bergstrom, GC., Taylor, A., 2022. Evaluation of Industrial Hemp Seed Treatments for Management of Damping-Off for Enhanced Stand Establishment. Agriculture. 12, 591. [CrossRef]
- Lobato, MC., Olivieri, FP., González Altamiranda, EA., Wolski, EA., Daleo, GR., Caldiz, DO., Andreu, AB., 2008. Phosphite compounds reduce disease severity in potato seed tubers and foliage. European Journal of Plant Pathology. 122, 349-358. [CrossRef]
- Simonetti, E., Pin Viso, N., Montecchia, M., Zilli, C., Balestrasse, K., Carmona, M., 2015. Evaluation of native bacteria and manganese phosphite for alternative control of charcoal root rot of soybean. Microbiological Research. 180, 40-48. [CrossRef]
- McDonald, AE., Grant, BR., Plaxton, WC., 2001. Phosphite (phosphorous acid): its relevance in the environment and agriculture and influence on plant phosphate starvation response. Journal Plant Nutrition, 24, 1505-1519. [CrossRef]
- Tkaczyk, M., Kubiak, KA., Sawicki, J., Nowakowska, JA., Oszako, T., 2016. The use of phosphates in forestry. Forest Research Papers. 77, 76-81. [CrossRef]
- Whiley, AW., Hargreaves, PA., Pegg, KG., Doogan, VJ., Ruddle, LJ., Saranah, JB., Langdon, PW., 1995. Changing sink strengths influence translocation of phosphonate in avocado (Persea Americana Mill.) trees. Aust. J. Agric. Res. 46, 1079-1090. [CrossRef]
- Förster, H., Adaskaveg, J., Kim, D., Stanghellini, M., 1998. Effect of Phosphite on Tomato and Pepper Plants and on Susceptibility of Pepper to Phytophthora Root and Crown Rot in Hydroponic Culture. Plant. Dis. 82, 1165–1170. [CrossRef]
- Guest, DI., Grant, BR., 1991. The complex action of phosphonates as antifungal agents. Biol. Rev. 66, 159-187.
- Malusa, E., Tosi, L., 2005. Phosphorous acid residues in apples after foliar fertilization: results of field trials. Food Addit. Contam. 22, 541-548. https://pubmed.ncbi.nlm.nih.gov/16019827/.
- Marks, GC., Smith, W., 1992. Metalaxyl and phosphonate as prophylactic and curative agents against stem infection of Leucadendron caused by Phytophthora cinnamomi. Aust. J. Exp. Agric. 32, 255-259. [CrossRef]
- Howard, K., Dell, B., Hardy, GE., 2000. Phosphite and mycorrhizal formation in seedlings of three Australian Myrtaceae. Aust J Bot. 48, 6, 725-729. [CrossRef]
- Tambascio, C., Covacevich, F., Lobato, MC., De Lasa, C., Caldiz, D., Dosio, G., Andreu, A., 2014. The application of K phosphites to seed tubers enhanced emergence, early growth and mycorrhizal colonization in potato (Solanum tuberosum). Am J Plant Sci. 5, 132-137. [CrossRef]
- Martinez, S., 2016. Effects of combined application of potassium phosphite and fungicide on stem and sheath disease control, yield, and quality of rice. Crop Prot. 89, 259-264. [CrossRef]
- Liljeroth, E., Lankinen, Å., Wiik, L., Burra, DD., Alexandersson, E., Andreasson, E., 2016. Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop Prot. 86, 42-55. [CrossRef]
- Puerari, HH., Dias-Arieira, C., Tavares Silva, CA., Arieira, JO., Biela, F., Poletine, JP., 2013. Ecolife nd manganese phosphite in the control of Meloidogyne javanica and in the development of soybean cultivars susceptible and resistant to the nematode. Nematropica. 43, 105-112. https://www.cabidigitallibrary.org/doi/pdf/10.5555/20133372345.
- Silva, AC., Resende, MLV., Souza, PE., Possa, KF., Júnior, MBS., 2016. Plant extract, zinc phosphite and zinc sulphate in the control of powdery mildew in the eucalyptus. Revista Ciência Agronômica, 47, 1, 93-100. [CrossRef]
- King, M., Reeve, W., Van der Hoek, MB., Williams, N., McComb, J., O’Brien, PA., Hardy, GE., 2010. Defining the phos phite-regulated transcriptome of the plant pathogen Phyto phthora cinnamomi. Mol Genet Genomics 284:425-35. [CrossRef]
- Lobato, MC., Olivieri, FP., Daleo, GR., Andreu, AB., 2010. Antimicrobial activity of phosphites against different potato pathogens. Journal of Plant Diseases and Protection. 117, 102-109. [CrossRef]
- Dobrowolski, MP., Shearer, BL., Colquhoun, IJ., O’Brienand, PA., Hardy, GESTJ., 2008. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant. Pathol. 58, 928-936. [CrossRef]
- Brown, S., Koike, ST., Ochoa, OE., Laemmlen, F., Michelmore, RW., 2004. Insensitivity to the fungicide fosetyl-aluminum in California isolates of the lettuce downy mildew pathogen Bremia lactucae. Plant Dis. 88, 502-508. [CrossRef]
- Duvenhage, JA., 1994. Monitoring the Resistance of Phytophthora cinnamomito Fosetyl-Al and H3PO3. Duivelskloof: Yearbook, South African Avocado Growers’ Association. 17, 35-37.
- Perez, V., Mamdouh, AM., Huet, JC., Pernollet, JC., Bompeix, G., 1995. Enhanced secretion of elicitins by Phytophthora fungi exposed to phosphonate. Cryptogamie. Mycologia. 16, 191-994. ⟨hal-02707440⟩.
- Pilbeam, RA., Howard, K., Shearer, BL., Hardy, GEJ., 2011. Phosphite stimulated histological responses of Eucalyptus marginata to infection by Phytophthora cinnamomi. Tree. 25, 1121-1131. [CrossRef]
- Machinandiarena, MF., Lobato, MC., Feldman, ML., Daleo, GR., Andreu, AB., 2012. Potassium phosphite primes defense responses in potato against Phytophthora infestans. Journal of Plant Physiology, 169, 14, 1417-1424. [CrossRef]
- Han, X., Xi, Y., Zhang, Z., Mohammadi, M., Joshi, J., Borza, T., Wang-Pruski, G., 2021. Effects of phosphite as a plant biostimulant on metabolism and stress response for better plant performance in Solanum tuberosum. Ecotox. Environ. Safety 210, 111873. [CrossRef]
- Daniel, R., Guest. D., 2006. Defence responses induced by potassium phosphonate in Phytophthora palmivora-cha llenged Arabidopsis thaliana. Physiologycal and Molecular Plant Pathology, 67, 194-201. [CrossRef]
- Zhao, H., Wu, L., Chai. T., Zhang. Y., Tan, J., Ma, S., 2012. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese- hyperacumulatior Phytolacca americana. Journal of Plant Physiology. 169, 1243-1252. [CrossRef]
- Demirevska-Kepova. K., Simova-Stoilova, L., Stoyanova, Z., Holzer, R., Feller, U., 2004. Biochemical changes in barley plants after excessive supply of copper and manganese. Enviromental and Experimental Botany, 52, 3, 253-266. [CrossRef]
- Rengel, Z., Graham, RD., Pedler, JF., 1993. Manganese nutrition and accumulation of phenolics and lignina as related to differential resistance of wheat genotypes to the take-all fungus. Plant and Soil, 151, 255-263. [CrossRef]
- Dordas, C., 2008. Role of nutrients in controlling plant diseases in sustainable agriculture: A review. Agronomy for Sustainable Development, 28, 1, 33-46. [CrossRef]
- Stangarlin, JR., Kuhn, OJ., Toledo, MV., Portz, RL., Schan-Estrada, KRF., Pascholati, SF., 2011. A defesa vegetal contra fitopatógenos. Scientia Agraria Paranaensis, 10, 18-46.
- Griffith, J., Coffey, M., Grant, B., 1993. Phosphonate inhibition as a function of phosphate concentration in isolates of Phytophthora palmivova. J. Gen. Microbiol. 139, 2109-2116. [CrossRef]
- Varadarajan, DK., Karthikeyan, AS., Matilda, PD., Raghothama, KG., 2002. Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 129, 3, 1232-1240. [CrossRef]
- Thao, HTB., Yamakawa, T., 2009. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 55, 2, 228-234. [CrossRef]
- Rossall, S., Qing, C., Paneri, M., Bennett, M., Swarup, R., 2016. A ‘growing’ role for phosphites in promoting plant growth and development. Acta Hortic. 1148. [CrossRef]
- Mohammadi, M., Zhang, Z., Xi, Y., Han, H., Lan, F., Zhang, B., Wang-Pruski, G., 2019. Effects of potassium phosphite on biochemical contents and enzymatic activities of Chinese potatoes inoculated by Phytophthora infestans. Appl. Ecol. Environ. Res, 17, 4499-4514. [CrossRef]
- Mohammadi, M., Han, X., Zhang, Z., Xi, Y., Boorboori, M., Wang-Pruski G., 2020. Phosphite.
- Application Alleviates Pythophthora infestans by Modulation of Photosynthetic and Physio-Biochemical Metabolites in Potato Leaves. Pathogens. 9, 170.
| Treatments | Germination (%) | Incidence (%) | Incidence Reduction (%) |
|---|---|---|---|
| Inoculated Control | 25 ± 1.0d* | 96 ± 3.7e* | 0 |
| Uninoculated control | 85 ± 3.4a | 4 ± 4.4a | 95 |
| Fungicide | 83 ± 10.8a | 27 ± 6.9b | 72 |
| Copper phosphite | 69 ± 3.4b | 70 ± 7.2d | 27 |
| Manganese phosphite | 81 ± 3.4a | 29 ± 5.7b | 69 |
| Potassium phosphite | 59 ± 10.8c | 49 ± 4.8c | 49 |
| Zinc phosphite | 85 ± 5.2a | 33 ± 4.1b | 65 |
| Treatments | ESI* | IS* (%) | FS* (%) | H*(cm) | R*(cm) | BA*(g) | BR*(g) |
|---|---|---|---|---|---|---|---|
| Control inoculated | 21.5 ± 1.2d* | 32.2 ± 1.5e* | 34.0 ± 4.1e* | 24.3 ± 0.7c* | 20.4 ± 2.5c* | 2.9 ± 0.4c* | 1.6 ± 0.1d* |
| Uninoculated control | 65.7 ± 2.8a | 93.0 ± 2.9a | 95.0 ± 2.1a | 28.9 ± 1.7b | 32.8 ± 1.1a | 7.5 ± 0.7a | 5.1 ± 0.6a |
| Fungicide | 45.9 ± 3.2b | 71.0 ± 3.5b | 76.7 ± 5.6b | 30.3 ± 0.7a | 30.2 ± 3.0a | 6.8 ± 0.7a | 3.8 ± 0.7b |
| Copper phosphite | 33.3 ± 5.4c | 51.5 ± 7.0d | 55.0 ± 9.6d | 28.6 ± 1.0b | 25.0 ± 2.1b | 4.6 ± 0.6b | 2.6 ± 0.8c |
| Manganese phosphite | 44.1 ± 2.7b | 62.5 ± 7.5c | 80.2 ± 1.2b | 30.5 ± 1.6a | 30.2 ± 0.9a | 7.0 ± 0.3a | 4.2 ± 0.2b |
| Potassium phosphite | 39.0 ± 5.8c | 60.0 ± 8.2c | 64.0 ± 6.9c | 30.2 ± 2.5a | 24.4 ± 4.0b | 5.8 ± 0.97a | 2.4 ± 0.3c |
| Zinc phosphite | 47.7 ± 2.9b | 75.7 ± 5.3b | 80.0 ± 3.3b | 31.9 ± 1.6a | 30.5 ± 0.1a | 6.5 ± 0.2a | 3.7 ± 0.4b |
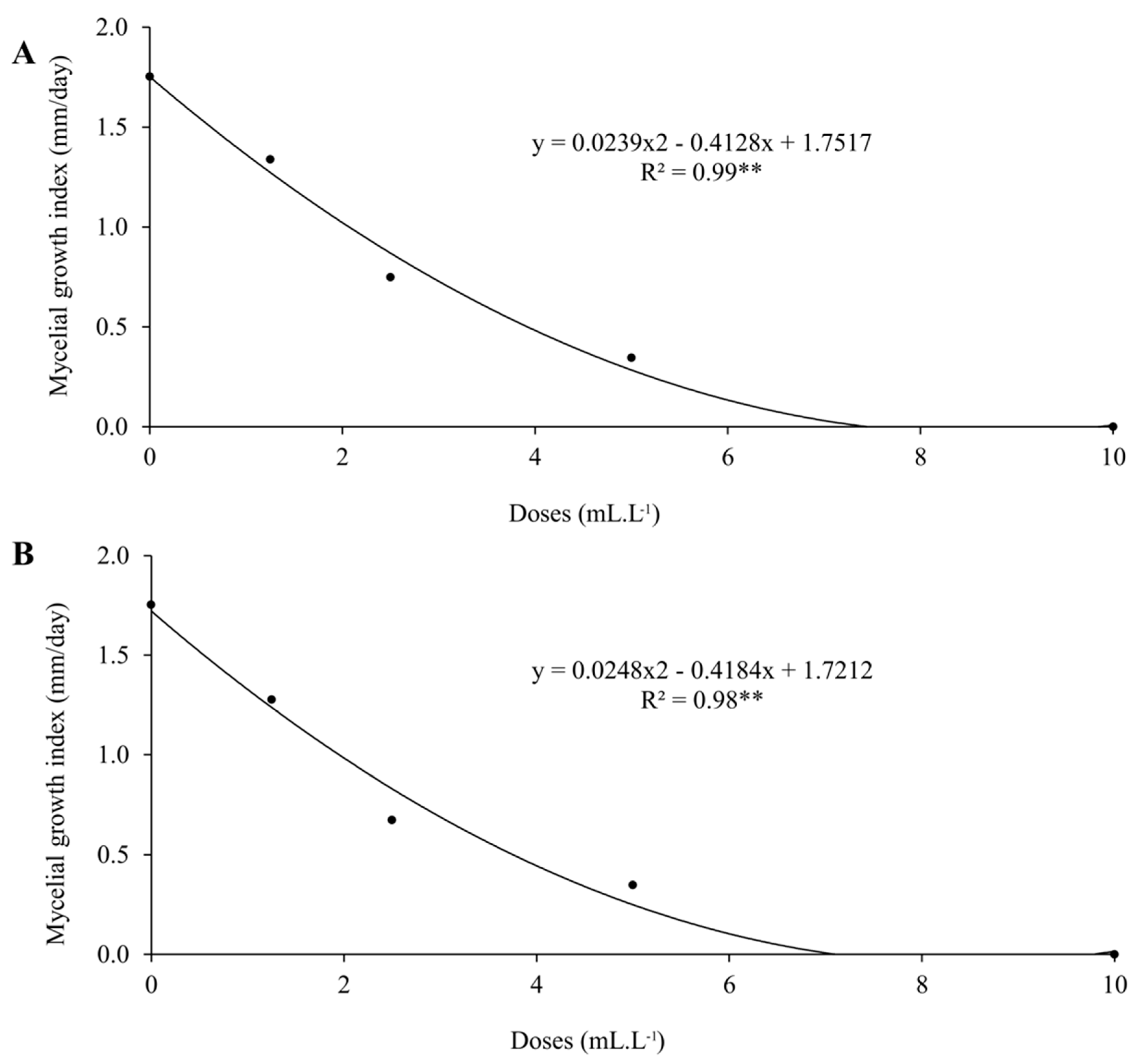
| Treatments | LD50 (mL.L-1) | MCI (mL.L-1) |
|---|---|---|
| Manganese phosphite | 2.48 | 8.64 |
| Zinc phosphite | 2.35 | 8.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





