Submitted:
04 February 2025
Posted:
05 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Toxicity Data Screening
2.2. Derivation of Tissue-Based Criteria
2.3. Derivation of WQC for Dieldrin
2.4. Ecological Risk Assessment
2.5. Data Analysis
3. Results and Discussion
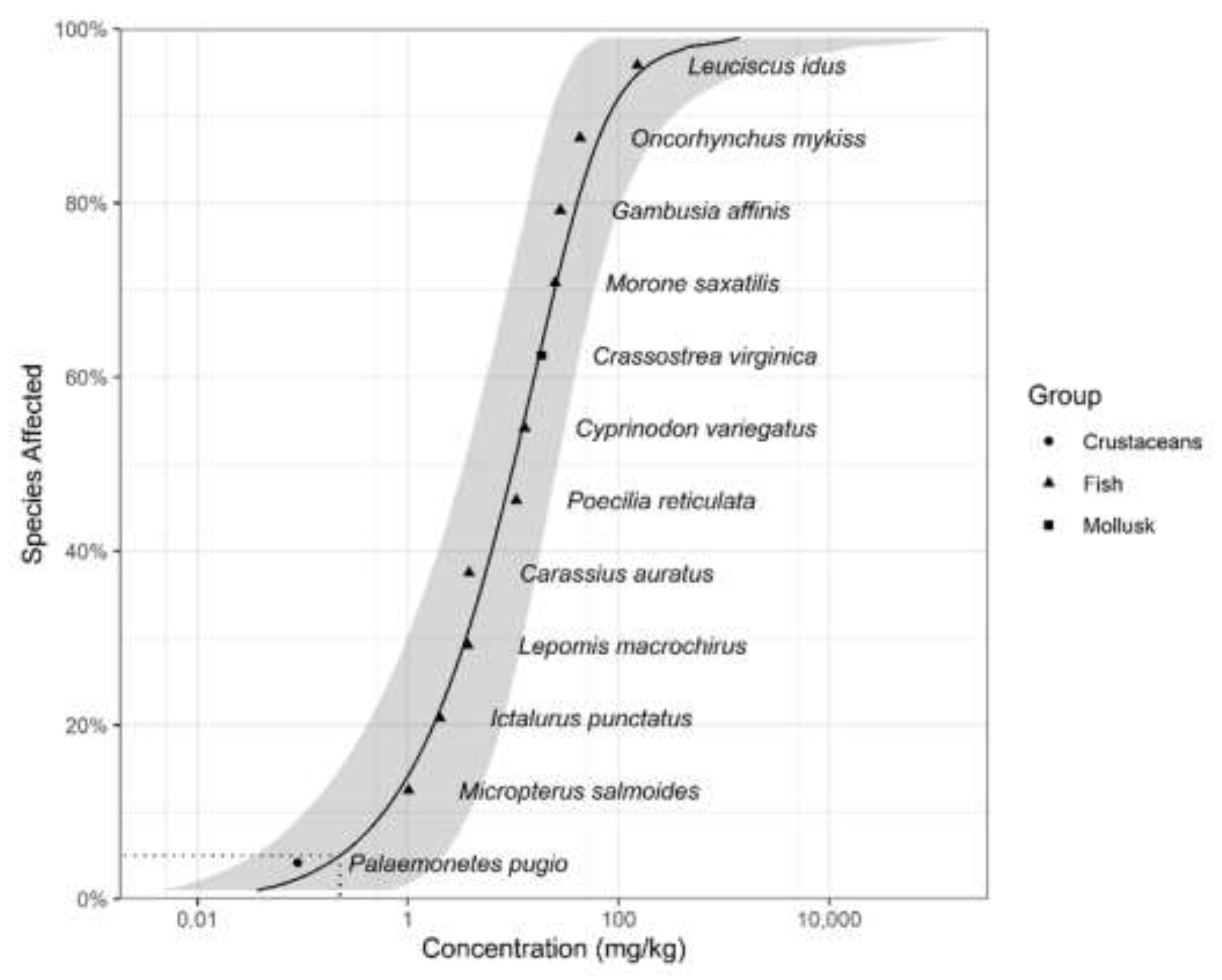
3.1. WQC for Protection of Aquatic Life
| Species | Common name | NOEC (mg/kg) | Taxa | Reference |
|---|---|---|---|---|
| Palaemonetes pugio | Grass shrimp | 0.09 | Crustaceans | [41] |
| Micropterus salmoides | Largemouth bass | 1.01 | Fishes | [42] |
| Ictalurus punctatus | Channel catfish | 2 | Fishes | [43] |
| Lepomis macrochirus | Bluegill | 3.7 | Fishes | [44] |
| Carassius auratus | Godfish | 3.8 | Fishes | [44] |
| Poecilia reticulata | Guppy | 10.7 | Fishes | [45] |
| Cyprinodon variegatus | Sheepshead minnow | 12.8 | Fishes | [41] |
| Crassostrea virginica | Eastern oyster | 18.6 | Mollusks | [46] |
| Morone saxatilis | Striped bass | 25 | Fishes | [47] |
| Gambusia affinis | Mosquito fish | 28 | Fishes | [48] |
| Oncorhynchus mykiss | Rainbow trout | 43 | Fishes | [49] |
| Leuciscus idus | Golden ide | 151 | Fishes | [50] |
3.2. WQC for Protection Of Aquatic-Dependent Wildlife
3.3. Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Honeycutt, M.; Shirley, S. Dieldrin. In Encyclopedia of Toxicology; 2014; pp. 107-110.
- ASTDR. Toxicological Profile for Aldrin and Dieldrin; 2022.
- Petrocelli, S.R.; Anderson, J.W.; Hanks, A.R. Controlled food-chain transfer of dieldrin residues from phytoplankters to clams. Marine Biology 1975, 31, 215–218. [Google Scholar] [CrossRef]
- Petrocelli, S.R.; Anderson, J.W.; Hanks, A.R. Biomagnification of dieldrin residues by food-chain transfer from clams to blue crabs under controlled conditions. Bulletin of Environmental Contamination and Toxicology 1975, 13, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Jandric, Z.; Chhem-Kieth, S.; Vreysen, M.J.; Rathor, M.N.; Gilles, J.R.; Cannavan, A. Anopheles arabiensis egg treatment with dieldrin for sex separation leaves residues in male adult mosquitoes that can bioaccumulate in goldfish (Carassius auratus auratus). Environ Toxicol Chem 2013, 32, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.H. Hazard identification of the potential for dieldrin carcinogenicity to humans. Environ Res 2014, 131, 188–214. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Mehinto, A.C.; Denslow, N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Molecular and Cellular Endocrinology 2020, 507. [Google Scholar] [CrossRef] [PubMed]
- Evangelista de Duffard, A.M.; Duffard, R. Behavioral toxicology, risk assessment, and chlorinated hydrocarbons. Environ Health Perspect 1996, 104 Suppl 2, 353–360. [Google Scholar] [CrossRef]
- Deck, A.T.; Reinke, E.N.; McCain, W.C. Wildlife Toxicity Assessment for Aldrin and Dieldrin. In Wildlife Toxicity Assessments for Chemicals of Military Concern; 2015; pp. 367-384.
- Ulugov, U.A.; Bobritskaya, L.S.; Sinitsky, J. Inventory of Obsolete Pesticide Warehouses in Tajikistan and Implications for Removal of Contaminated Soil. J Health Pollut 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Navarrete, I.A.; Tee, K.A.M.; Unson, J.R.S.; Hallare, A.V. Organochlorine pesticide residues in surface water and groundwater along Pampanga River, Philippines. Environ Monit Assess 2018, 190, 289. [Google Scholar] [CrossRef] [PubMed]
- Ogbeide, O.; Uhunamure, G.; Okundaye, F.; Ejeomo, C. First report on probabilistic risk assessment of pesticide residues in a riverine ecosystem in South-South Nigeria. Chemosphere 2019, 231, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.D.; Chakraborty, P.; Uralbekov, B.; Satybaldiev, B.; Sallach, J.B.; Thornton Hampton, L.M.; Jeffries, M.; Kolok, A.S.; Bartelt-Hunt, S.B. Legacy and current pesticide residues in Syr Darya, Kazakhstan: Contamination status, seasonal variation and preliminary ecological risk assessment. Water Res 2020, 184, 116141. [Google Scholar] [CrossRef]
- Oginawati, K.; Susetyo, S.H.; Rahmawati, S.I.; Kurniawan, S.B.; Abdullah, S.R.S. Distribution of organochlorine pesticide pollution in water, sediment, mollusk, and fish at Saguling Dam, West Java, Indonesia. Toxicol Res 2022, 38, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Portella, R.B.; Almeida, P.; Pinto, C.O.; Gubert, P.; Santos da Silva, J.D.; Nakamura, T.C.; do Rego, E.L. Human milk contamination by nine organochlorine pesticide residues (OCPs). J Environ Sci Health B 2020, 55, 530–538. [Google Scholar] [CrossRef] [PubMed]
- El-Saeid, M.H.; Hassanin, A.S.; Bazeyad, A.Y. Levels of pesticide residues in breast milk and the associated risk assessment. Saudi J Biol Sci 2021, 28, 3741–3744. [Google Scholar] [CrossRef]
- Al Antary, T.M.; Alawi, M.A.; Kiwan, R.; Haddad, N.A. Monitoring of Organochlorine Pesticide Residues in Human Breast Milk in the Northern Governorates of Jordan in 2019/2020 Compared with the Results of 2015 Study. Bull Environ Contam Toxicol 2021, 106, 1071–1076. [Google Scholar] [CrossRef]
- Padayachee, K.; Reynolds, C.; Mateo, R.; Amar, A. A global review of the temporal and spatial patterns of DDT and dieldrin monitoring in raptors. Sci Total Environ 2023, 858, 159734. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.F.; Dietz, R.; Sonne, C.; Letcher, R.J.; Roos, A.M.; Simon, M.; Rosing-Asvid, A.; Ferguson, S.H.; McKinney, M.A. Feeding and biological differences induce wide variation in legacy persistent organic pollutant concentrations among toothed whales and polar bear in the Arctic. Science of The Total Environment 2024, 908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Fang, L.; Wang, J.; Liu, W.; Yuan, H.; Jantunen, L.; Li, Y.-F. Residues of Currently and Never Used Organochlorine Pesticides in Agricultural Soils from Zhejiang Province, China. Journal of Agricultural and Food Chemistry 2012, 60, 2982–2988. [Google Scholar] [CrossRef]
- Niu, L.; Xu, C.; Zhu, S.; Liu, W. Residue patterns of currently, historically and never-used organochlorine pesticides in agricultural soils across China and associated health risks. Environ Pollut 2016, 219, 315–322. [Google Scholar] [CrossRef]
- Wang, L.; Cao, G.; Liu, L.-Y.; Zhang, Z.-F.; Jia, S.-M.; Fu, M.-Q.; Ma, W.-L. Cross-regional scale studies of organochlorine pesticides in air in China: Pollution characteristic, seasonal variation, and gas/particle partitioning. Science of The Total Environment 2023, 904, 166709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, R.; Guan, Y.; Zhuang, T.; Wang, Y.; Tan, R.; Wang, J.; Zhou, R.; Wang, B.; Xu, J.; et al. The profiling of elements and pesticides in surface water in Nanjing, China with global comparisons. Science of The Total Environment 2021, 774, 145749. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, F.; Yu, R.-Q.; Sun, X.; Wu, Y. Bioaccumulation of legacy organic contaminants in pregnant Indo-Pacific humpback dolphins (Sousa chinensis): Unique features on the transplacental transfer. Science of The Total Environment 2021, 785, 147287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, L.; Wu, J.; Fan, C. Residual levels, tissue distribution and risk assessment of organochlorine pesticides (OCPs) in edible fishes from Taihu Lake, China. Environ Monit Assess 2013, 185, 9265–9277. [Google Scholar] [CrossRef]
- Han, X.; Xu, L.; Deng, A.; Xing, P.; Xu, Y. Centurial deposition records of polychlorinated biphenyls and organochlorine pesticides in sediment cores from a plateau deep-water lake of China: Significance of anthropogenic impacts, transformation signals and ecological risks revealed by full congener analysis. Science of The Total Environment 2024, 926. [Google Scholar] [CrossRef]
- CCME. Canadian Sediment Quality Guidelines for the Protection of Aquatic Life-dieldrin. 1999.
- USEPA. Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: Dieldrin. 2003.
- USEPA. Wildlife Fact Sheet for Dieldrin: (Wildlife). 1995.
- Yang, M.; Mu, Y.; Wu, F. Derivation on Criteria Maximum Concentration of Four Typical Organochlorine Pesticides for Protecting Aquatic Organisms. Journal of Kunming University of Science and Technology (Natural Science) 2019, 44, 111–118. [Google Scholar] [CrossRef]
- Barnhart, B.; Flinders, C.; Ragsdale, R.; Johnson, G.; Wiegand, P. Deriving Human Health and Aquatic Life Water Quality Criteria in the United States for Bioaccumulative Substances: A Historical Review and Future Perspective. Environ Toxicol Chem 2021, 40, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Warne, M.S.J.; Chapman, P.M.; Chan, K.M.; Yu, S.; Leung, K.M.Y. Tissue-based environmental quality benchmarks and standards. Environmental Science and Pollution Research 2013, 21, 28–32. [Google Scholar] [CrossRef]
- USEPA. Tissue-Based Criteria for “Bioaccumulative” Chemicals. 2005.
- CCME. Protocol for the Derivation of Canadian Tissue Residue Guidelines for the Protection of Wildlife that Consume Aquatic Biota. 1998.
- Mackay, D.; Fraser, A. Kenneth Mellanby Review Award. Bioaccumulation of persistent organic chemicals: mechanisms and models. Environ Pollut 2000, 110, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Arnot, J.A.; Gobas, F.A.P.C. A Generic QSAR for Assessing the Bioaccumulation Potential of Organic Chemicals in Aquatic Food Webs. QSAR & Combinatorial Science 2003, 22, 337–345. [Google Scholar] [CrossRef]
- Thorley, J.; Schwarz, C. ssdtools: An R package to fit Species Sensitivity Distributions. Journal of Open Source Software 2018, 3. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing, 4.1.0; R Foundation for Statistical Computing: 2021.
- De Bruijn, J.; Busser, F.; Seinen, W.; Hermens, J. Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method. Environmental Toxicology and Chemistry 1989, 8, 499–512. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environmental Reviews 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Parrish, P.R.; Couch, J.A.; Forester, J.; Jr., J.M.P.; Cook, G.H. Dieldrin: Effects on Several Estuarine Organisms. Journal of the Southeastern Association of Fish and Wildlife Agencies 1977.
- Johnson, K.G.; Muller, J.K.; Price, B.; Ware, A.; Sepúlveda, M.S.; Borgert, C.J.; Gross, T.S. Influence of seasonality and exposure on the accumulation and reproductive effects of p,p′-dichlorodiphenyldichloroethane and dieldrin in largemouth bass. Environmental Toxicology and Chemistry 2007, 26, 927–934. [Google Scholar] [CrossRef]
- Shannon, L.R. Accumulation and elimination of dieldrin in muscle tissue of channel catfish. Bulletin of Environmental Contamination and Toxicology 1977, 17, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Gakstatter, J.H.; Weiss, C.M. The Elimination of DDT-C14, Dieldrin-C14, and Lindane-C14 from Fish following a Single Sublethal Exposure in Aquaria. Transactions of The American Fisheries Society 1967, 96, 301–307. [Google Scholar] [CrossRef]
- Burnett, K.M.; Liss, W.J. Multi-steady-state toxicant fate and effect in laboratory aquatic ecosystems. Environmental Toxicology and Chemistry 1990, 9, 637–647. [Google Scholar] [CrossRef]
- Mason, J.W.; Rowe, D.R. The accumulation and loss of dieldrin and endrin in the Eastern oyster. Archives of Environmental Contamination and Toxicology 1976, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Santerre, C.R.; Blazer, V.S.; Khanna, N.; Reinert, R.E.; Barrows, F.T. Absorption of Dietary Dieldrin by Striped Bass. Bulletin of Environmental Contamination and Toxicology 1997, 58, 334–340. [Google Scholar] [CrossRef]
- Metcalf, R.L. Chapter 2 - A Laboratory Model Ecosystem to Evaluate Compounds Producing Biological Magnification. In Essays in Toxicology, Hayes, W.J., Ed.; Elsevier: 1974; Volume 5, pp. 17-38.
- Mehrle, P.; Mayer, F.; Johnson, W.; Mayer, F.; Hamelink, J. Diet Quality in Fish Toxicology: Effects on Acute and Chronic Toxicity. In Aquatic Toxicology and Hazard Evaluation; ASTM International: 1977; Volume STP634-EB, p. 0.
- Freitag, D.; Ballhorn, L.; Geyer, H.; Korte, F. Environmental hazard profile of organic chemicals: An experimental method for the assessment of the behaviour of organic chemicals in the ecosphere by means of simple laboratory tests with 14C labelled chemicals. Chemosphere 1985, 14, 1589–1616. [Google Scholar] [CrossRef]
- Su, H.; Mu, Y.; Feng, C.; Zhu, Y.; Wang, H.; Wu, F.; Giesy, J.P. Tissue Residue Guideline for ∑DDT for Protection of Aquatic Birds in China. Human and Ecological Risk Assessment: An International Journal 2014, 20, 1629–1642. [Google Scholar] [CrossRef]
- Su, H.; Feng, C.; Chang, H.; Mu, Y.; Wu, F. Study on Tissue Residue Guidelines of DDTs for Protection of Aquatic Mammalian Species in China. Asian Journal of Ecotoxicology 2015, 10, 110–118. [Google Scholar] [CrossRef]
- Hung, C.L.H.; Lau, R.K.F.; Lam, J.C.W.; Jefferson, T.A.; Hung, S.K.; Lam, M.H.W.; Lam, P.K.S. Risk assessment of trace elements in the stomach contents of Indo-Pacific Humpback Dolphins and Finless Porpoises in Hong Kong waters. Chemosphere 2007, 66, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.A. Population biology of the Indo-Pacific Humpacked dolphin in Hong Kong waters. Journal of Wildlife Management 2000, 64, 1–65. [Google Scholar]
- Mendenhall, V.M.; Klaas, E.E.; McLane, M.A.R. Breeding success of barn owls (Tyto alba) fed low levels of DDE and dieldrin. Archives of Environmental Contamination and Toxicology 1983, 12, 235–240. [Google Scholar] [CrossRef]
- Wiese, I.H.; Basson, N.C.J.; Van Der Vyver, J.H.; Van Der Merwe, J.H. Toxicology and Dynamics of Dieldrin in the Crowned Guinea-fowl, Numida Meleagris (L). Phytophylactica 1969, 1, 161–175. [Google Scholar]
- Atkins, T.D. Effects of Dieldrin on Reproduction of Penned Hen Pheasants. Journal of Wildlife Management 1967, 31, 746. [Google Scholar] [CrossRef]
- Fergin, T.J.; Schafer, E.C. Toxicity of dieldrin to bobwhite quail in relation to sex and reproductive status. Archives of Environmental Contamination and Toxicology 1977, 6, 213–219. [Google Scholar] [CrossRef]
- Heinz, G.H.; Hill, E.F.; Contrera, J.F. Dopamine and norepinephrine depletion in ring doves fed DDE, dieldrin, and Aroclor 1254. Toxicology and Applied Pharmacology 1980, 53, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.M.; Arscott, G.H.; Tinsley, I.J. Effect of Dieldrin and Calcium on the Performance of Adult Japanese Quail (Coturnix coturnix japonica)1. Poultry Science 1976, 55, 212–219. [Google Scholar] [CrossRef]
- Dahlgren, R.B.; Linder, R.L. Effects of Dieldrin in Penned Pheasants through the Third Generation. Journal of Wildlife Management 1974, 38, 320. [Google Scholar] [CrossRef]
- Kreitzer, J.F.; Heinz, G.H. The effect of sublethal dosages of five pesticides and a polychlorinated biphenyl on the avoidance response of coturnix quail chicks. Environmental Pollution (1970) 1974, 6, 21–29. [Google Scholar] [CrossRef]
- Jefferies, D.J.; French, M.C. Changes Induced in the Pigeon Thyroid by p,p prime -DDE and Dieldrin. Journal of Wildlife Management 1972, 36, 24. [Google Scholar] [CrossRef]
- Davison, K.L.; Sell, J.L. DDT thins shells of eggs from mallard ducks maintained onad libitum or controlled-feeding regimens. Archives of Environmental Contamination and Toxicology 1974, 2, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Eden, W.G. Toxicity of Dieldrin to Chickens. Journal of Economic Entomology 1951, 44, 1013–1013. [Google Scholar] [CrossRef]
- Walker, A.I.T.; Stevenson, D.E.; Robinson, J.; Thorpe, E.; Roberts, M. The toxicology and pharmacodynamics of dieldrin (HEOD): Two-year oral exposures of rats and dogs. Toxicology and Applied Pharmacology 1969, 15, 345–373. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.I.T.; Thorpe, E.; Stevenson, D.E. The toxicology of dieldrin (HEOD). I. Long-term oral toxicity studies in mice. Food and Cosmetics Toxicology 1973, 11, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Wiese, I.; Basson, N.C.J.; Basson, P.; Naudé, T.W.; Maartens, B.P. The toxicology and pathology of dieldrin and photodieldrin poisoning in two antelope species. The Onderstepoort journal of veterinary research 1973, 40 1, 31–39. [Google Scholar]
- Murphy, D.A.; Korschgen, L.J. Reproduction, growth, and tissue residues of deer fed dieldrin. Journal of Wildlife Management 1970, 34, 887–903. [Google Scholar] [CrossRef]
- Davison, K.L. Growth, Hemoglobin, Body Composition and Vitamin A of Sheep Fed Dieldrin. Journal of Animal Science 1970, 31, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Bildstein, K.L.; Forsyth, D.J. Effects of dietary dieldrin on behavior of white-footed mice (Peromyscus leucopus) towards an avian predator. Bulletin of Environmental Contamination and Toxicology 1979, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kotonya, R.; Jensen, N.-E. No effect of dieldrin on progesterone production in gilts. Toxicology 1993, 81, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hurkat, P.C.; Joshi, G.P. Some Physiological and Behavior Studies on Rabbit (Oryctolagus cuniculus) after Dieldrin Administration. Indian Veterinary Journal 1977, 54(9), 709–714. [Google Scholar]
- Uzoukwu, M.; Sd, S. Dieldrin toxicosis: fetotoxicosis, tissue concentrations, and microscopic and ultrastructural changes in guinea pigs. American journal of veterinary research 1972, 33 3, 579–583. [Google Scholar]
- Klump, D.W.; Huasheng, H.; Humphrey, C.; Xinhong, W.; Codi, S. Toxic contaminants and their biological effects in coastal waters of Xiamen, China. I. Organic pollutants in mussel and fish tissues. Mar Pollut Bull 2002, 44, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Lamai, S.L.; Warner, G.F.S.; Walker, C.H. Effects of dieldrin on life stages of the African catfish, Clarias gariepinus (Burchell). Ecotoxicology and environmental safety 1999, 42 1, 22–29. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; van der Hoek, J.P. Residues of organochlorine pesticides (OCPs) in aquatic environment and risk assessment along Shaying River, China. Environ Geochem Health 2018, 40, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Weisner, O.; Arle, J.; Liebmann, L.; Link, M.; Schafer, R.B.; Schneeweiss, A.; Schreiner, V.C.; Vormeier, P.; Liess, M. Three reasons why the Water Framework Directive (WFD) fails to identify pesticide risks. Water Res 2022, 208, 117848. [Google Scholar] [CrossRef]
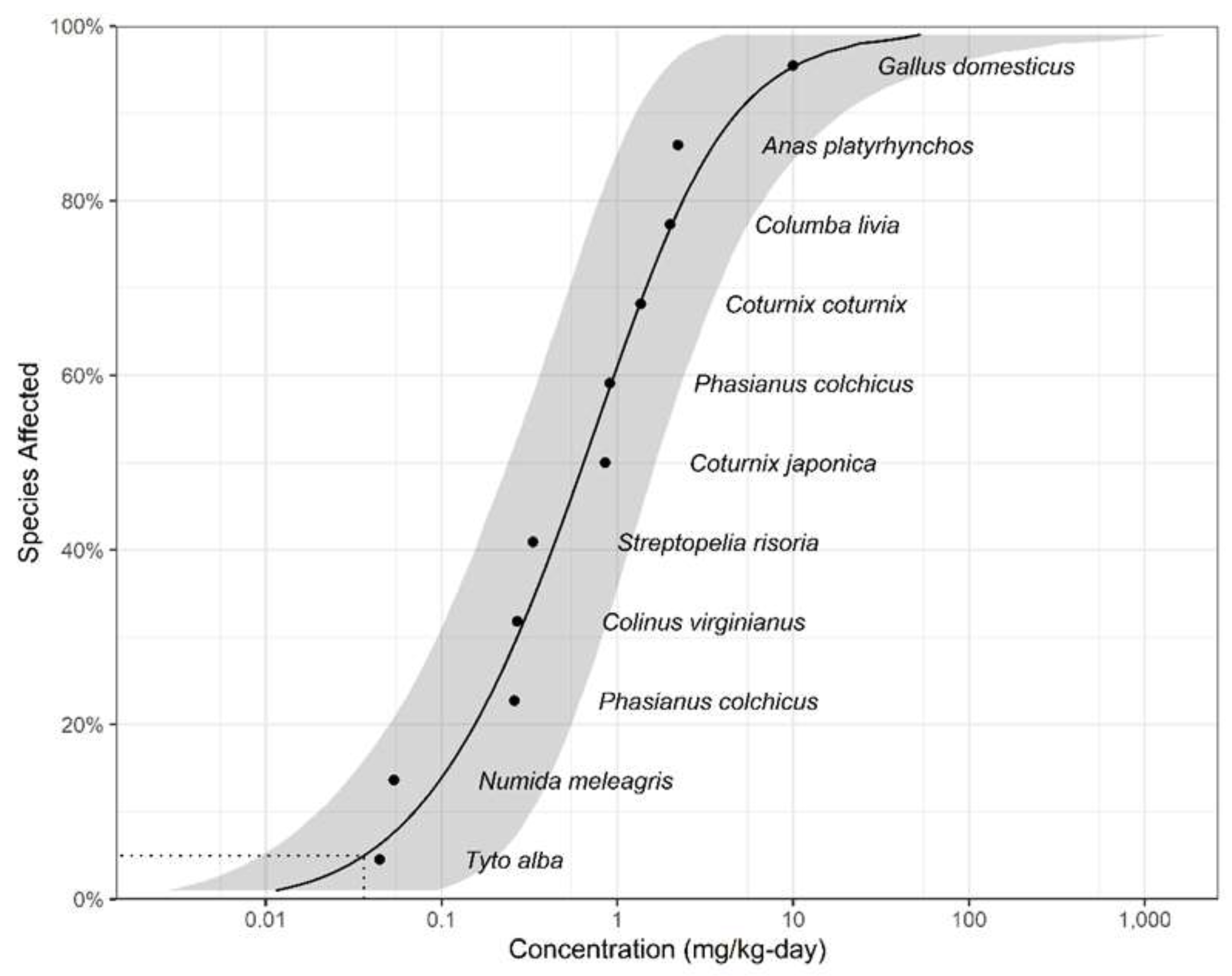
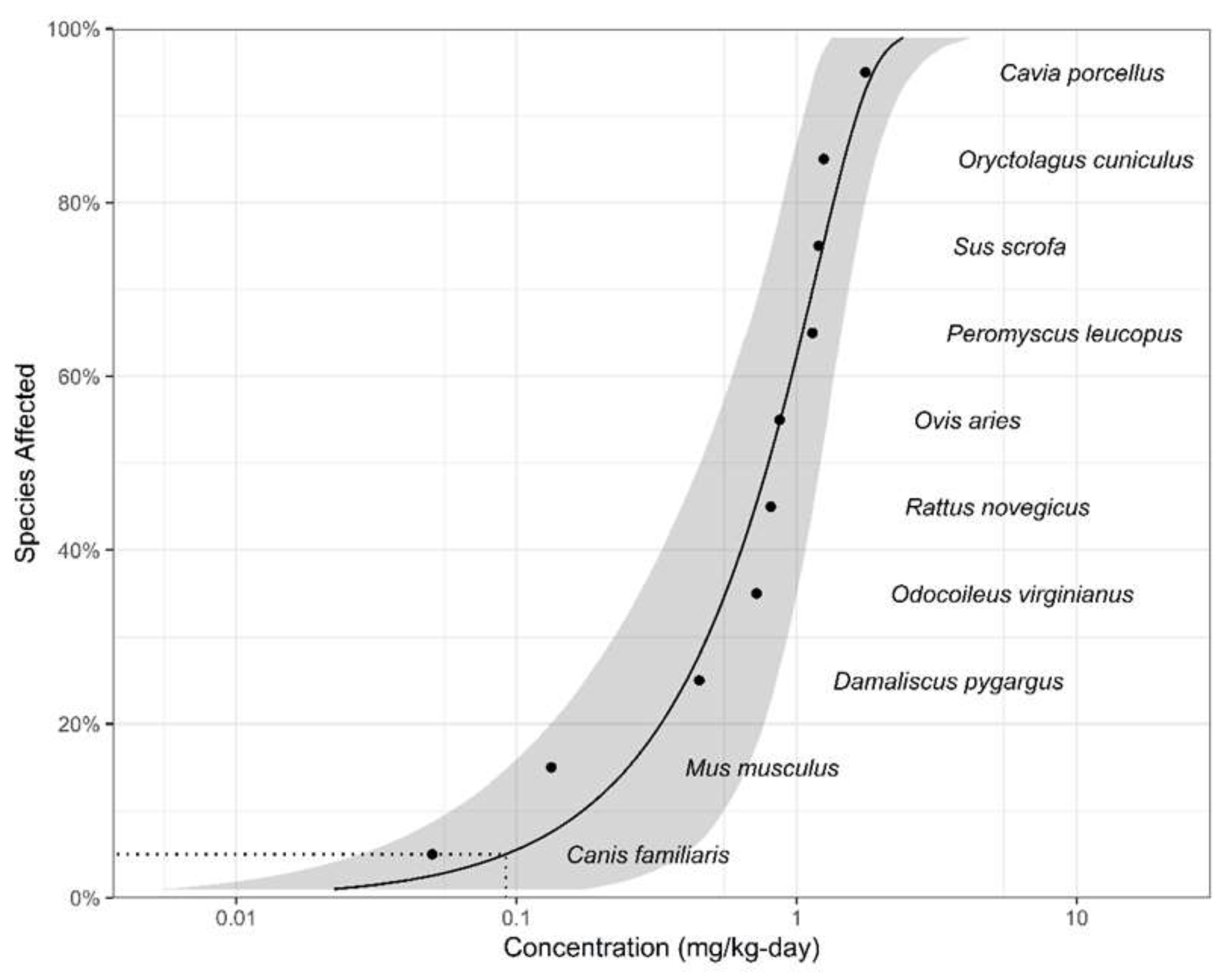
| Species | Common name | NOEC (mg/kg) | Taxa | Reference |
|---|---|---|---|---|
| Tyto alba | Barn owl | 0.0445 | Avian | [55] |
| Numida meleagris | Crowned guinea fowl | 0.0537 | Avian | [56] |
| Phasianus colchicus | Ring-necked pheasant | 0.26 | Avian | [57] |
| Colinus virginianus | Bobwhite quail | 0.27 | Avian | [58] |
| Streptopelia risoria | Ring dove | 0.331 | Avian | [59] |
| Coturnix japonica | Japanese quail | 0.852 | Avian | [60] |
| Phasianus colchicus | Pheasant | 0.905 | Avian | [61] |
| Coturnix coturnix | Quail | 1.36 | Avian | [62] |
| Columba livia | Homing pigeon | 2 | Avian | [63] |
| Anas platyrhynchos | Mallard | 2.21 | Avian | [64] |
| Gallus domesticus | Chicken | 10 | Avian | [65] |
| Canis familiaris | Dog | 0.05 | Mammalian | [66] |
| Mus musculus | Mouse | 0.133 | Mammalian | [67] |
| Damaliscus pygargus | Blesbuk | 0.449 | Mammalian | [68] |
| Odocoileus virginianus | White tailed deer | 0.72 | Mammalian | [69] |
| Rattus novegicus | Rat | 0.81 | Mammalian | [66] |
| Ovis aries | Sheep | 0.87 | Mammalian | [70] |
| Peromyscus leucopus | White footed mouse | 1.14 | Mammalian | [71] |
| Sus scrofa | Pig | 1.20 | Mammalian | [72] |
| Oryctolagus cuniculus | Rabbit | 1.25 | Mammalian | [73] |
| Cavia porcellus | Guinea pig | 1.76 | Mammalian | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





