Submitted:
30 January 2025
Posted:
31 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Inhibin Immunogen Preparation
2.2. Experimental Design
 , Group B
, Group B  and control Group C
and control Group C  of Dezhou donkeys at 21, 28,34 and 40 days of experiment. Vertical bars represent standard error of mean (SEM). The values with ** indicate the difference (P < 0.001) whereas the values with * indicate difference (P < 0.05) between groups A, B and C. Arrows indicate primary and booster Inhibin (INH) immunziation at Ist and 23rd day of experiment.
of Dezhou donkeys at 21, 28,34 and 40 days of experiment. Vertical bars represent standard error of mean (SEM). The values with ** indicate the difference (P < 0.001) whereas the values with * indicate difference (P < 0.05) between groups A, B and C. Arrows indicate primary and booster Inhibin (INH) immunziation at Ist and 23rd day of experiment.
 , Group B
, Group B  and control Group C
and control Group C  of Dezhou donkeys at 21, 28,34 and 40 days of experiment. Vertical bars represent standard error of mean (SEM). The values with ** indicate the difference (P < 0.001) whereas the values with * indicate difference (P < 0.05) between groups A, B and C. Arrows indicate primary and booster Inhibin (INH) immunziation at Ist and 23rd day of experiment.
of Dezhou donkeys at 21, 28,34 and 40 days of experiment. Vertical bars represent standard error of mean (SEM). The values with ** indicate the difference (P < 0.001) whereas the values with * indicate difference (P < 0.05) between groups A, B and C. Arrows indicate primary and booster Inhibin (INH) immunziation at Ist and 23rd day of experiment.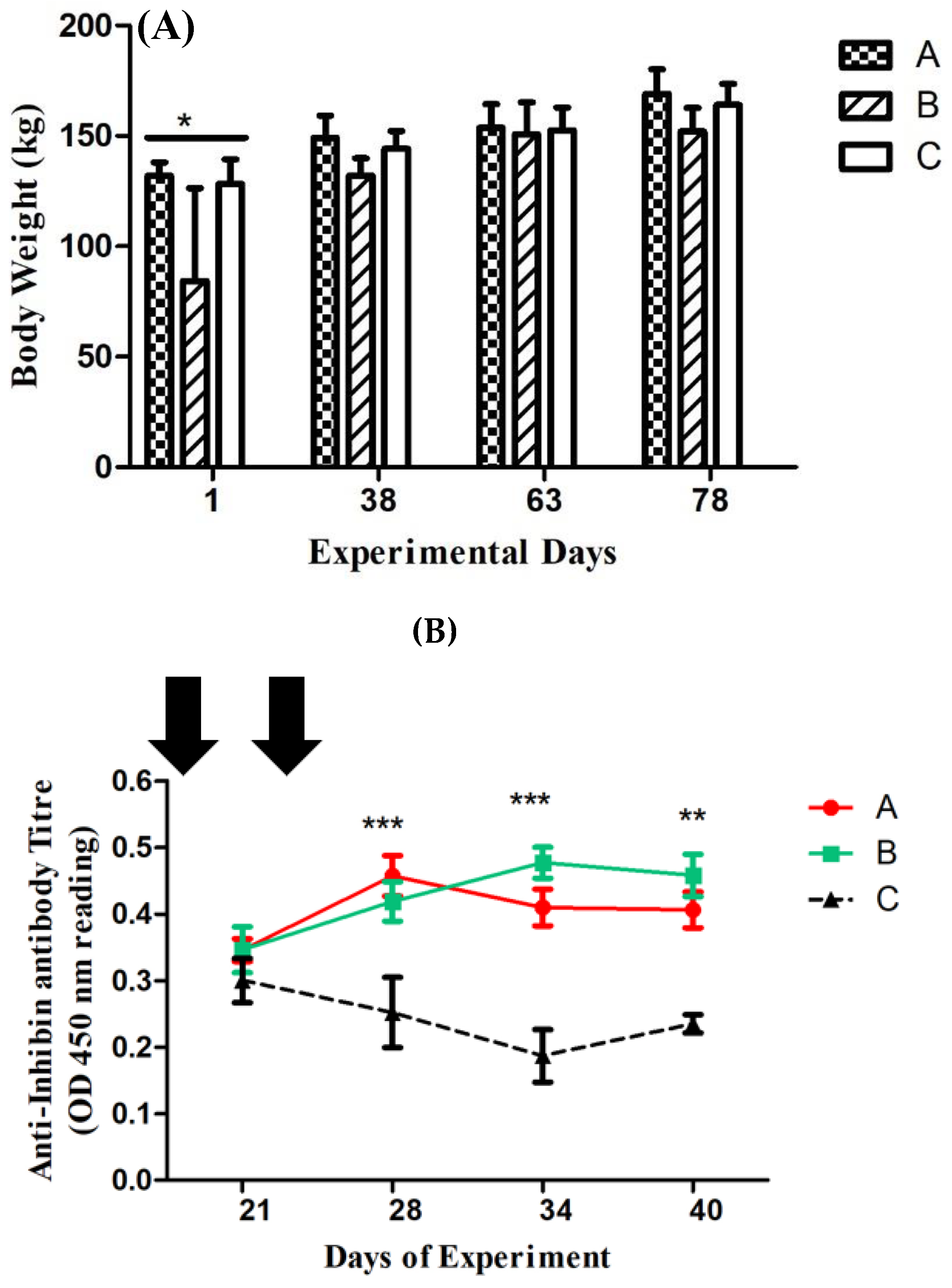
2.3. Measurement of Body Weight, Blood, and Testes Tissue Collection
2.4. Antibody Titer
2.5. Plasma Hormone Concentrations
2.6. Microscopy Performance
2.7. Statistical Analysis
3. Results
3.1. Body Weights
3.2. Anti-Inhibin Antibody Titer
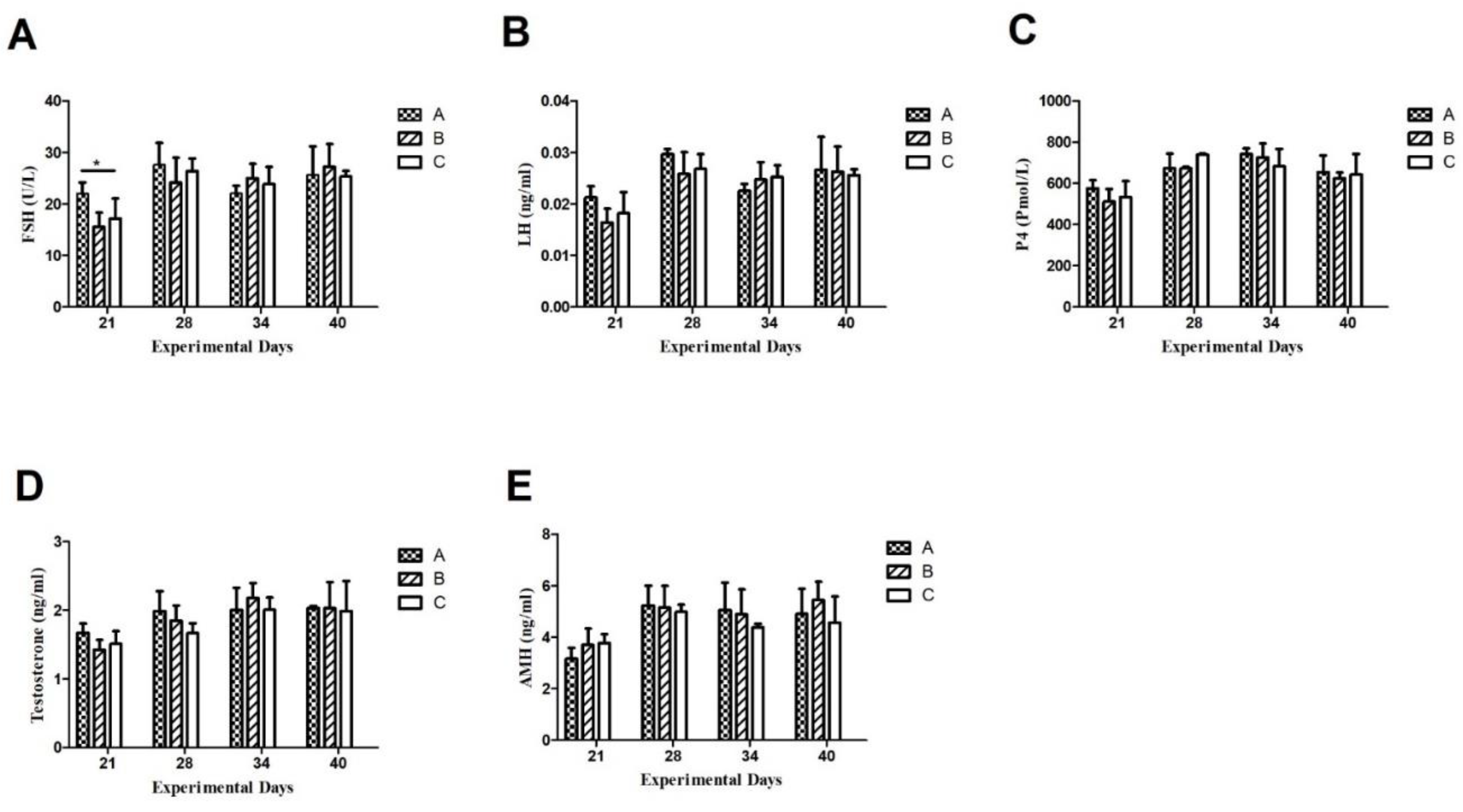
3.3. Plasma Hormone Concentrations
3.3.1. Follicle Stimulating Hormone (FSH)
3.3.2. Luteinizing Hormone (LH)
3.3.3. Progesterone (P4)
3.3.4. Testosterone (T)
3.3.5. AntiMullerian Hormone
3.3.6. Activin A
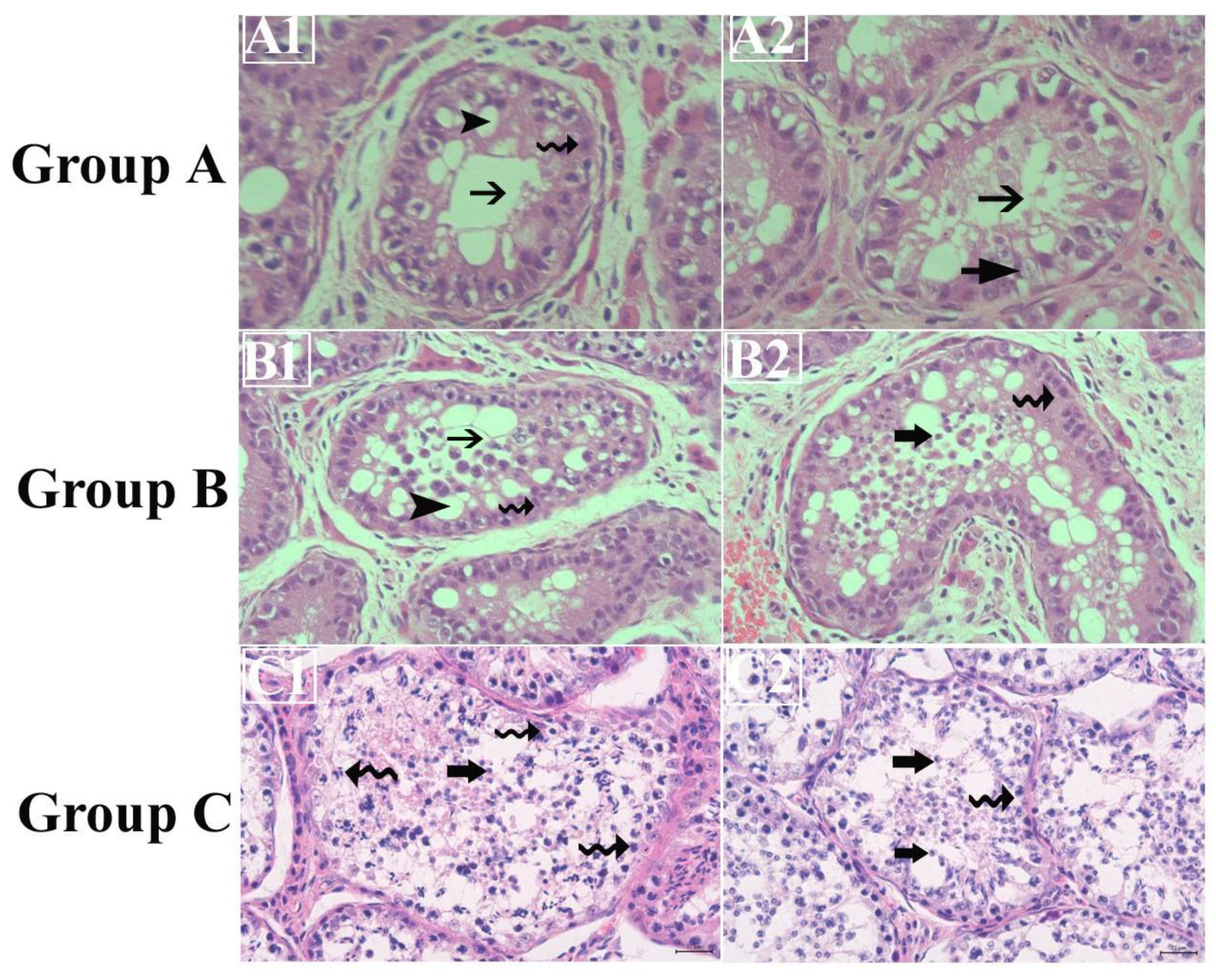
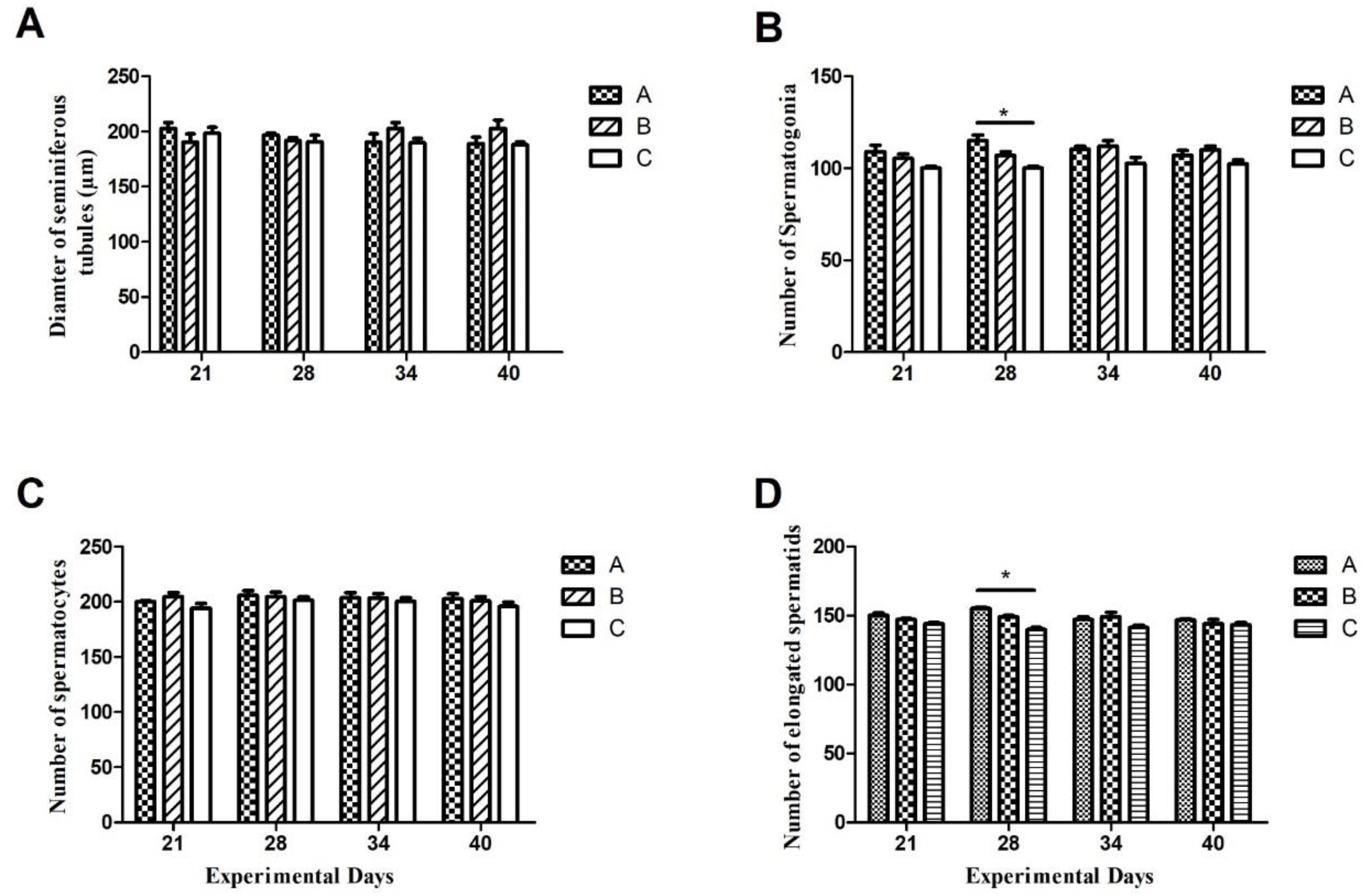
3.4. Germ cells Count and Variations in Seminiferous Epithelium
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, T.; Hu, W.; Hou, H.; Zhao, Z.; Shang, M.; Zhang, L. Identification and comparative analysis of long non-coding RNA in the skeletal muscle of two dezhou donkey strains. Genes 2020, 11, 508. [Google Scholar] [CrossRef]
- Tian, F.; Wang, J.; Li, Y.; Yang, C.; Zhang, R.; Wang, X.; Ju, Z.; Jiang, Q.; Huang, J.; Wang, C. Integrated analysis of mRNA and miRNA in testis and cauda epididymidis reveals candidate molecular markers associated with reproduction in Dezhou donkey. Livestock Science 2020, 234, 103885. [Google Scholar] [CrossRef]
- Zeng, L.; Dang, R.; Dong, H.; Li, F.; Chen, H.; Lei, C. Genetic diversity and relationships of Chinese donkeys using microsatellite markers. Archives Animal Breeding 2019, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Wu, F.; Zhou, Z.; Li, M.; Gao, Y.; Yin, G.; Yu, J.; Lei, C.; Dang, R. Expression profiles and polymorphic identification of the ACSL1 gene and their association with body size traits in Dezhou donkeys. Archives Animal Breeding 2020, 63, 377–386. [Google Scholar] [CrossRef]
- Clauss, M.; Zerbe, P.; Bingaman Lackey, L.; Codron, D.; Müller, D.W. Basic considerations on seasonal breeding in mammals including their testing by comparing natural habitats and zoos. Mammalian Biology 2021, 101, 373–386. [Google Scholar] [CrossRef]
- Tibary, A.; Sghiri, A.; Bakkoury, M.; Fite, C. Reproductive patterns in donkeys. In Proceedings of the Proceedings of the 9th International Congress of the World Equine Veterinary Association, 2006; pp. 311-319.
- Aissanou, S.; Besseboua, O.; Abdelhanine, A. Some reproductive characteristics in common donkey male (Equus asinus)-A mini review. Turkish Journal of Veterinary Research 2022, 6, 77–84. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Wei, Q.; Zhu, H.; Chen, Z.; Ahmad, E.; Zhendan, S.; Shi, F. The role of active immunization against inhibin α-subunit on testicular development, testosterone concentration and relevant genes expressions in testis, hypothalamus and pituitary glands in Yangzhou goose ganders. Theriogenology 2019, 128, 122–132. [Google Scholar] [CrossRef]
- Woodruff, T.K.; Mather, J.P. Inhibin, activin and the female reproductive axis. Annual review of physiology 1995, 57, 219–244. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, X.; Wei, Y.; Yu, J.; Li, H.; Chen, R.; Shi, Z. Studies on enhancing embryo quantity and quality by immunization against inhibin in repeatedly superovulated Holstein heifers and the associated endocrine mechanisms. Animal reproduction science 2013, 142, 10–18. [Google Scholar] [CrossRef]
- Chen, F.; Lu, J.; Guo, R.; Mei, C.; Guo, B.; Li, W.; Tsigkou, A.; Shi, Z. Rectifying cow infertility under heat stress by immunization against inhibin and supplementation of progesterone. Domestic Animal Endocrinology 2022, 80, 106726. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Ahmad, E.; Sattar, A.; Riaz, A.; Khan, J.A.; Naseer, Z.; Akhtar, M.F.; Abbas, M.; Shi, Z. Long term effects of immunization against inhibin on fresh and post-thawed semen quality and sperm kinematics during low and peak breeding seasons in Beetal bucks. Small Ruminant Research 2021, 201, 106442. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, E.; Arshad, U.; Riaz, A.; Akhtar, M.S.; Ahmad, T.; Khan, J.A.; Mohsin, I.; Shi, Z.; Sattar, A. Effects of immunization against inhibin α-subunit on ovarian structures, pregnancy rate, embryonic and fetal losses, and prolificacy rate in goats where estrus was induced during the non-breeding season. Animal Reproduction Science 2021, 224, 106654. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Z.; Ma, Z.; Ma, J.; Zhao, F. Immunization against inhibin promotes fertility in cattle: A meta-analysis and quality assessment. Frontiers in Veterinary Science 2021, 8, 687923. [Google Scholar] [CrossRef]
- Meng, J.; Feng, J.H.; Xiao, L.; Zhou, W.; Zhang, H.; Lan, X.; Wang, S. Active immunization with inhibin DNA vaccine promotes spermatogenesis and testicular development in rats. Journal of Applied Animal Research 2024, 52, 2360408. [Google Scholar] [CrossRef]
- Lovell, T.M.; Knight, P.G.; Groome, N.P.; Gladwell, R.T. Measurement of dimeric inhibins and effects of active immunization against inhibin α-subunit on plasma hormones and testis morphology in the developing cockerel. Biology of reproduction 2000, 63, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Baqerkhani, M.; Soleimanzadeh, A.; Mohammadi, R. Effects of intratesticular injection of hypertonic mannitol and saline on the quality of donkey sperm, indicators of oxidative stress and testicular tissue pathology. BMC Veterinary Research 2024, 20, 99. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Ahmad, E.; Ali, I.; Shafiq, M.; Chen, Z. The effect of inhibin immunization in seminiferous epithelium of Yangzhou goose ganders: a histological study. Animals 2021, 11, 2801. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, T.; Liu, A.; Liu, L. Role and regulatory mechanism of inhibin in animal reproductive system. Theriogenology 2023, 202, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.J.; Li, Y.; Toufaily, C.; Schang, G. Regulation of gonadotropins. In Oxford Research Encyclopedia of Neuroscience; 2019.
- Guo, R.; Chen, F.; Mei, C.; Dai, Z.; Yan, L.; Shi, Z. Conception rate and reproductive hormone secretion in Holstein cows immunized against inhibin and subjected to the ovsynch protocol. Animals 2020, 10, 313. [Google Scholar] [CrossRef]
- Li, D.; Qin, G.; Wei, Y.; Lu, F.; Huang, Q.; Jiang, H.; Shi, D.; Shi, Z. Immunisation against inhibin enhances follicular development, oocyte maturation and superovulatory response in water buffaloes. Reproduction, Fertility and Development 2011, 23, 788–797. [Google Scholar] [CrossRef]
- Anderson, R.; Groome, N.; Baird, D. Inhibin A and inhibin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono-ovulation. Clinical endocrinology 1998, 48, 577–584. [Google Scholar] [CrossRef]
- Medan, M.; Akagi, S.; Kaneko, H.; Watanabe, G.; Tsonis, C.; Taya, K. Effects of re-immunization of heifers against inhibin on hormonal profiles and ovulation rate. Reproduction 2004, 128, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Medan, M.S.; Watanabe, G.; Sharawy, S.; Taya, K. Immunization of goats against inhibin increased follicular development and ovulation rate. Journal of Reproduction and Development 2006, 52, 543–550. [Google Scholar] [CrossRef]
- Lazebny, O.; Kulikov, A.; Butovskaya, P.; Proshakov, P.; Fokin, A.; Butovskaya, M. Analysis of aggressive behavior in young Russian males using 250 SNP markers. Russian Journal of Genetics 2020, 56, 1118–1128. [Google Scholar] [CrossRef]
- O’Donnell, L.; Whiley, P.A.; Loveland, K.L. Activin A and sertoli cells: key to fetal testis steroidogenesis. Frontiers in Endocrinology 2022, 13, 898876. [Google Scholar] [CrossRef]
- Rodriguez, K.F.; Brown, P.R.; Amato, C.M.; Nicol, B.; Liu, C.-F.; Xu, X.; Yao, H.H.-C. Somatic cell fate maintenance in mouse fetal testes via autocrine/paracrine action of AMH and activin B. Nature Communications 2022, 13, 4130. [Google Scholar] [CrossRef]
- Shah, W.; Khan, R.; Shah, B.; Khan, A.; Dil, S.; Liu, W.; Wen, J.; Jiang, X. The molecular mechanism of sex hormones on Sertoli cell development and proliferation. Frontiers in endocrinology 2021, 12, 648141. [Google Scholar] [CrossRef]
- Arato, I.; Grande, G.; Barrachina, F.; Bellucci, C.; Lilli, C.; Jodar, M.; Aglietti, M.C.; Mancini, F.; Vincenzoni, F.; Pontecorvi, A. “In vitro” Effect of Different Follicle—Stimulating Hormone Preparations on Sertoli Cells: Toward a Personalized Treatment for Male Infertility. Frontiers in Endocrinology 2020, 11, 401. [Google Scholar] [CrossRef]
- Santi, D.; Crépieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-stimulating hormone (FSH) action on spermatogenesis: a focus on physiological and therapeutic roles. Journal of Clinical Medicine 2020, 9, 1014. [Google Scholar] [CrossRef]
- Wang, J.-M.; Li, Z.-F.; Yang, W.-X.; Tan, F.-Q. Follicle-stimulating hormone signaling in Sertoli cells: a licence to the early stages of spermatogenesis. Reproductive Biology and Endocrinology 2022, 20, 97. [Google Scholar] [CrossRef]
- Ni, F.-D.; Hao, S.-L.; Yang, W.-X. Molecular insights into hormone regulation via signaling pathways in Sertoli cells: With discussion on infertility and testicular tumor. Gene 2020, 753, 144812. [Google Scholar] [CrossRef]
- Olsen, O.E.; Hella, H.; Elsaadi, S.; Jacobi, C.; Martinez-Hackert, E.; Holien, T. Activins as dual specificity TGF-β family molecules: SMAD-activation via activin-and BMP-type 1 receptors. Biomolecules 2020, 10, 519. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, B.; Qi, Y.; Zhu, L.; Cui, X.; Liu, Z. Antagonistic effects of activin A and TNF-α on the activation of L929 fibroblast cells via Smad3-independent signaling. Scientific Reports 2020, 10, 20623. [Google Scholar] [CrossRef]
- Zeng, X.; Turkstra, J.; Tsigos, A.; Meloen, R.; Liu, X.; Chen, F.; Schaaper, W.; Guo, D.; van de Wiel, D. Effects of active immunization against GnRH on serum LH, inhibin A, sexual development and growth rate in Chinese female pigs. Theriogenology 2002, 58, 1315–1326. [Google Scholar] [CrossRef]
- Avital-Cohen, N.; Heiblum, R.; Argov, N.; Rosenstrauch, A.; Chaiseha, Y.; Mobarkey, N.; Rozenboim, I. The effect of active immunization against vasoactive intestinal peptide and inhibin on reproductive performance of young White Leghorn roosters. Poultry science 2011, 90, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Samir, H.; El Sayed, M.A.; Nagaoka, K.; Sasaki, K.; El-Maaty, A.M.A.; Karen, A.; Abou-Ahmed, M.M.; Watanabe, G. Passive immunization against inhibin increases testicular blood flow in male goats. Theriogenology 2020, 147, 85–91. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. International journal of molecular sciences 2021, 22, 12735. [Google Scholar] [CrossRef]
- Ali, A.; Derar, D.R.; Zeitoun, M.M.; Al-Sobayil, F. Impotentia generandi in male dromedary camels: FSH, LH and testosterone profiles and their association with clinical findings and semen analysis data. Theriogenology 2018, 120, 98–104. [Google Scholar] [CrossRef]
- Swelum, A.A.-A.; Saadeldin, I.M.; Zaher, H.A.; Alsharifi, S.A.; Alowaimer, A.N. Effect of sexual excitation on testosterone and nitric oxide levels of water buffalo bulls (Bubalus bubalis) with different categories of sexual behavior and their correlation with each other. Animal reproduction science 2017, 181, 151–158. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, Z.; Worku, T.; Davis, J.S.; Talpur, H.S.; Bhattarai, D.; Kadariya, I.; Hua, G.; Cao, J.; Dad, R.; Hussain, T. Role and mechanism of AMH in the regulation of Sertoli cells in mice. The Journal of Steroid Biochemistry and Molecular Biology 2017, 174, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, E.; Hermanowicz, A.; Komarowska, M.; Debek, W. Serum AMH in physiology and pathology of male gonads. International journal of endocrinology 2013, 2013, 128907. [Google Scholar] [CrossRef] [PubMed]
- Rey, R.A.; Grinspon, R.P. Normal male sexual differentiation and aetiology of disorders of sex development. Best Practice & Research Clinical Endocrinology & Metabolism 2011, 25, 221–238. [Google Scholar]
- Hui, H.-B.; Xiao, L.; Sun, W.; Zhou, Y.-J.; Zhang, H.-Y.; Ge, C.-T. Sox9 is indispensable for testis differentiation in the red-eared slider turtle, a reptile with temperature-dependent sex determination. Zoological Research 2021, 42, 721. [Google Scholar] [CrossRef]
- Shima, Y.; Miyabayashi, K.; Haraguchi, S.; Arakawa, T.; Otake, H.; Baba, T.; Matsuzaki, S.; Shishido, Y.; Akiyama, H.; Tachibana, T. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Molecular endocrinology 2013, 27, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cellular and molecular life sciences 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Banerjee, S.; Chaturvedi, C.M. Apoptotic mechanism behind the testicular atrophy in photorefractory and scotosensitive quail: Involvement of GnIH induced p-53 dependent Bax-Caspase-3 mediated pathway. Journal of Photochemistry and Photobiology B: Biology 2017, 176, 124–135. [Google Scholar] [CrossRef]
- Jiménez, R.; Burgos, M.; Barrionuevo, F.J. Circannual testis changes in seasonally breeding mammals. Sexual Development 2015, 9, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Frutos, E.; Seco-Rovira, V.; Martínez-Hernández, J.; Ferrer, C.; Serrano-Sánchez, M.I.; Pastor, L.M. Cellular modifications in spermatogenesis during seasonal testicular regression: an update review in mammals. Animals 2022, 12, 1605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




