Submitted:
30 January 2025
Posted:
30 January 2025
You are already at the latest version
Abstract
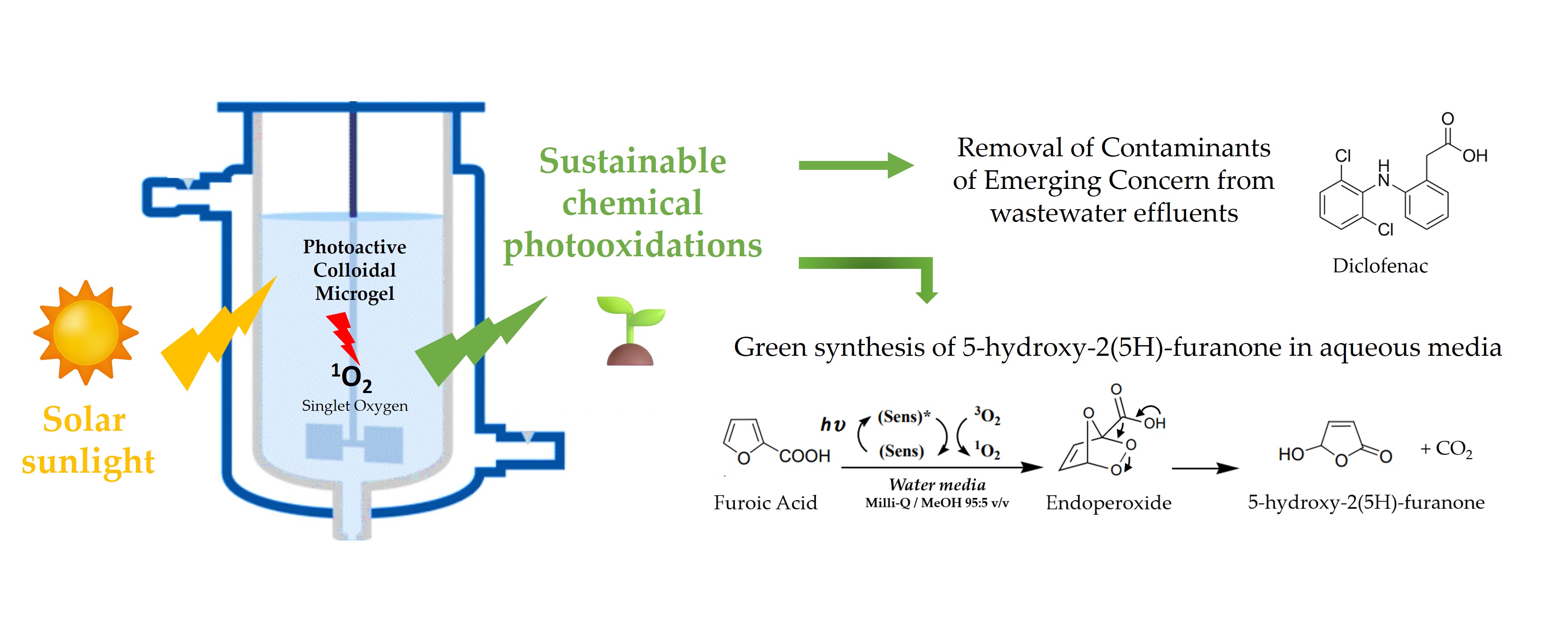
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents and Polymeric Precursors
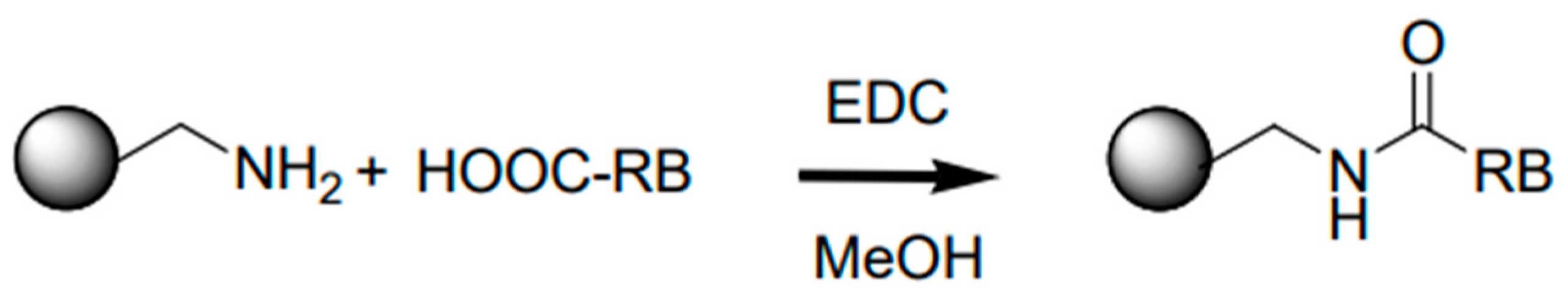
2.2. Synthesis of the Photoactive Colloidal Microgels
- Step 1. Synthesis of NIPAM-co-AEMA polymeric microgel
2.3. Characterization of the Colloidal Microgels
2.4. Evaluation the Photo-Oxidation Kinetics of the Photoactive Colloidal Microgels
2.5. Studies of Diclofenac Pollutant Photo-Degradation
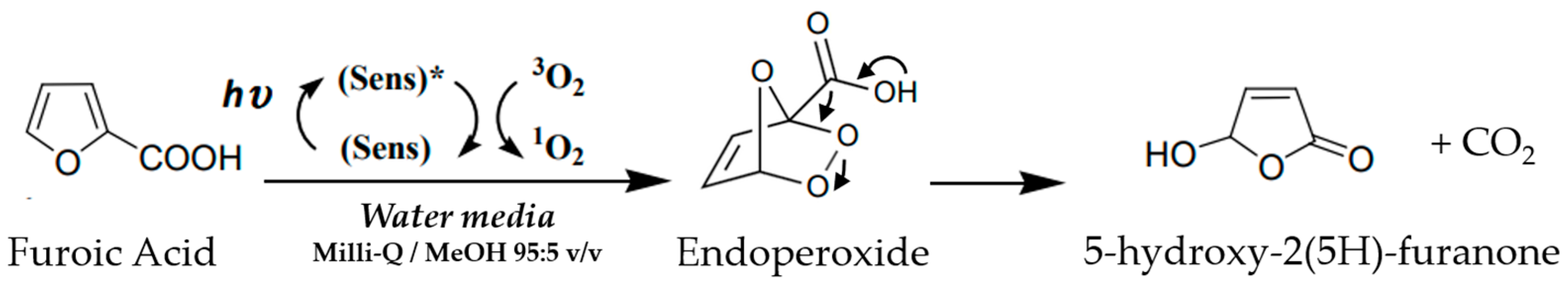
2.6. Photo-Oxidation of Furoic Acid and Green Synthesi 5-Hydroxy-2(5H)-Furanone
3. Results and Discussion
3.1. Synthesis and Characterization of the Photoactive Colloidal Microgels
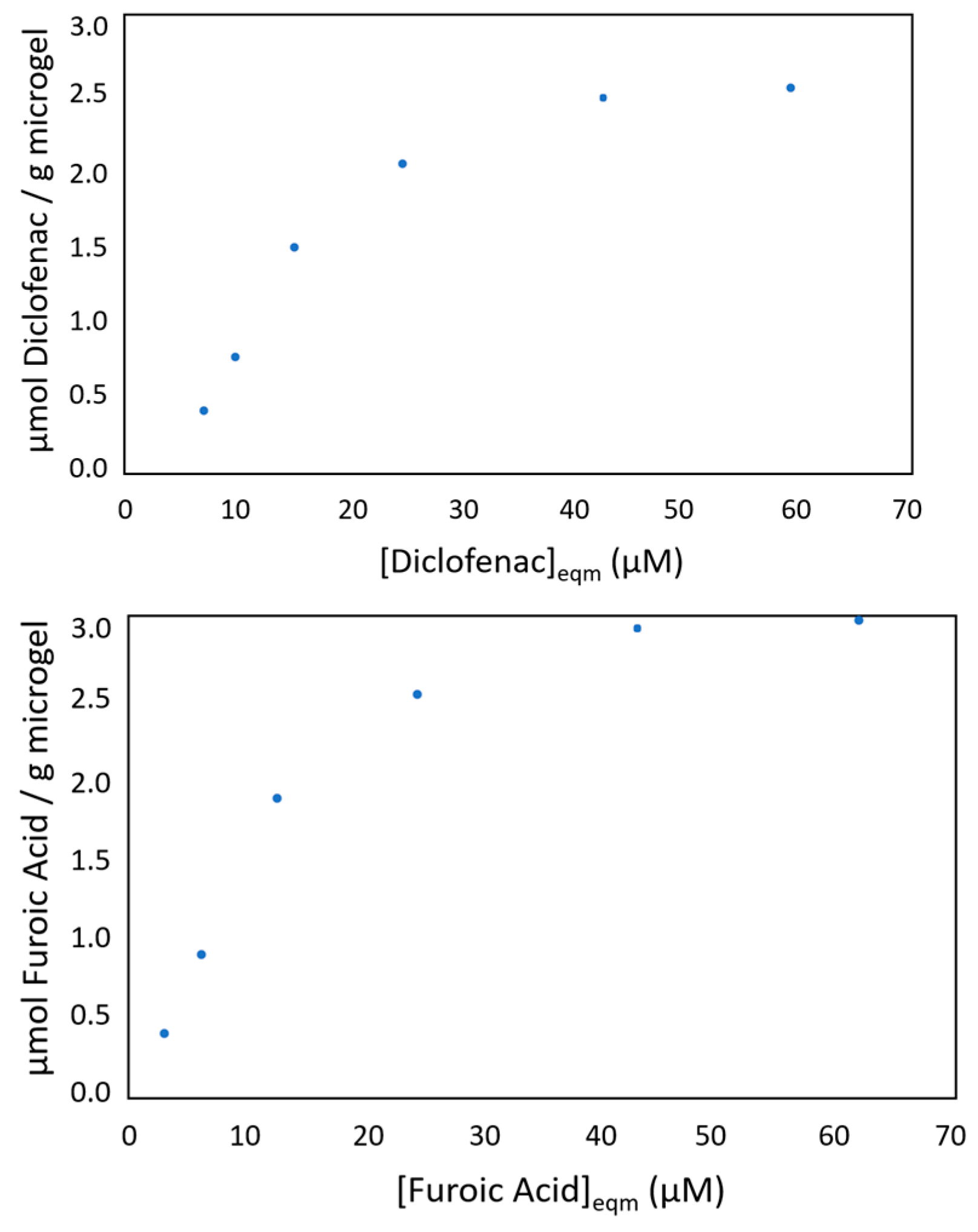
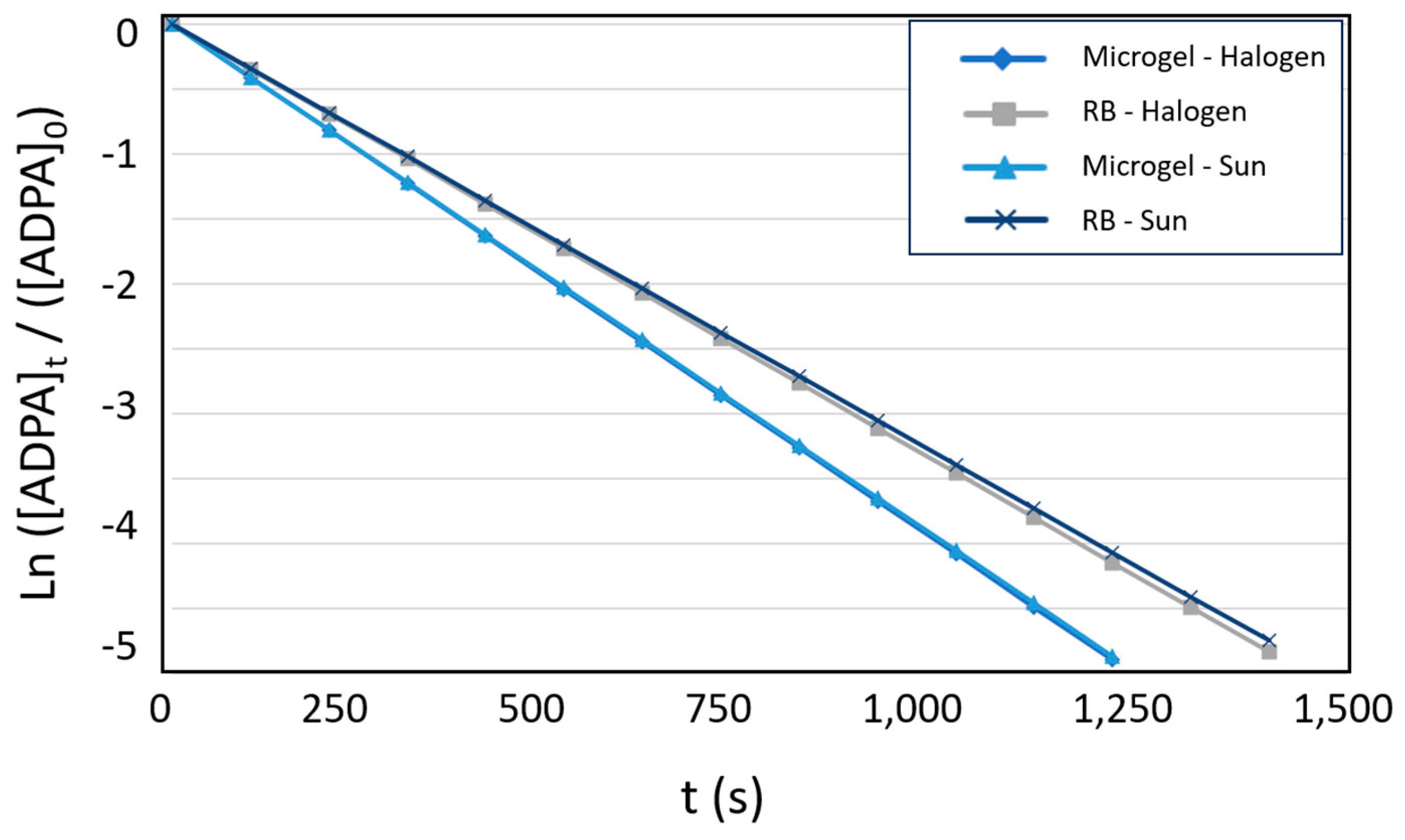
3.2. Evaluation the Photo-Oxidation Kinetics of the Photoactive Colloidal Microgels
3.3. Studies of Diclofenac Pollutant Photo-Degradation
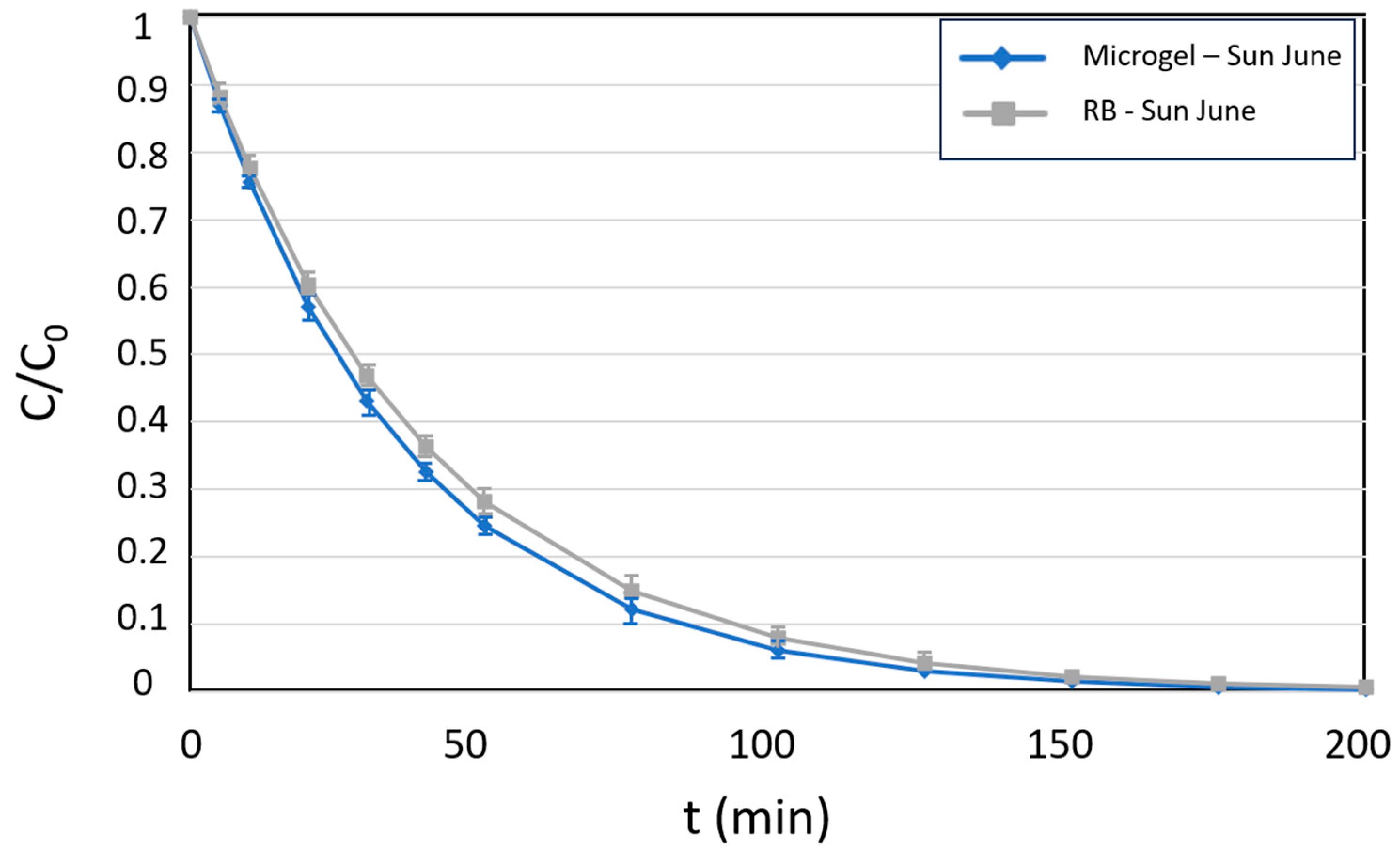
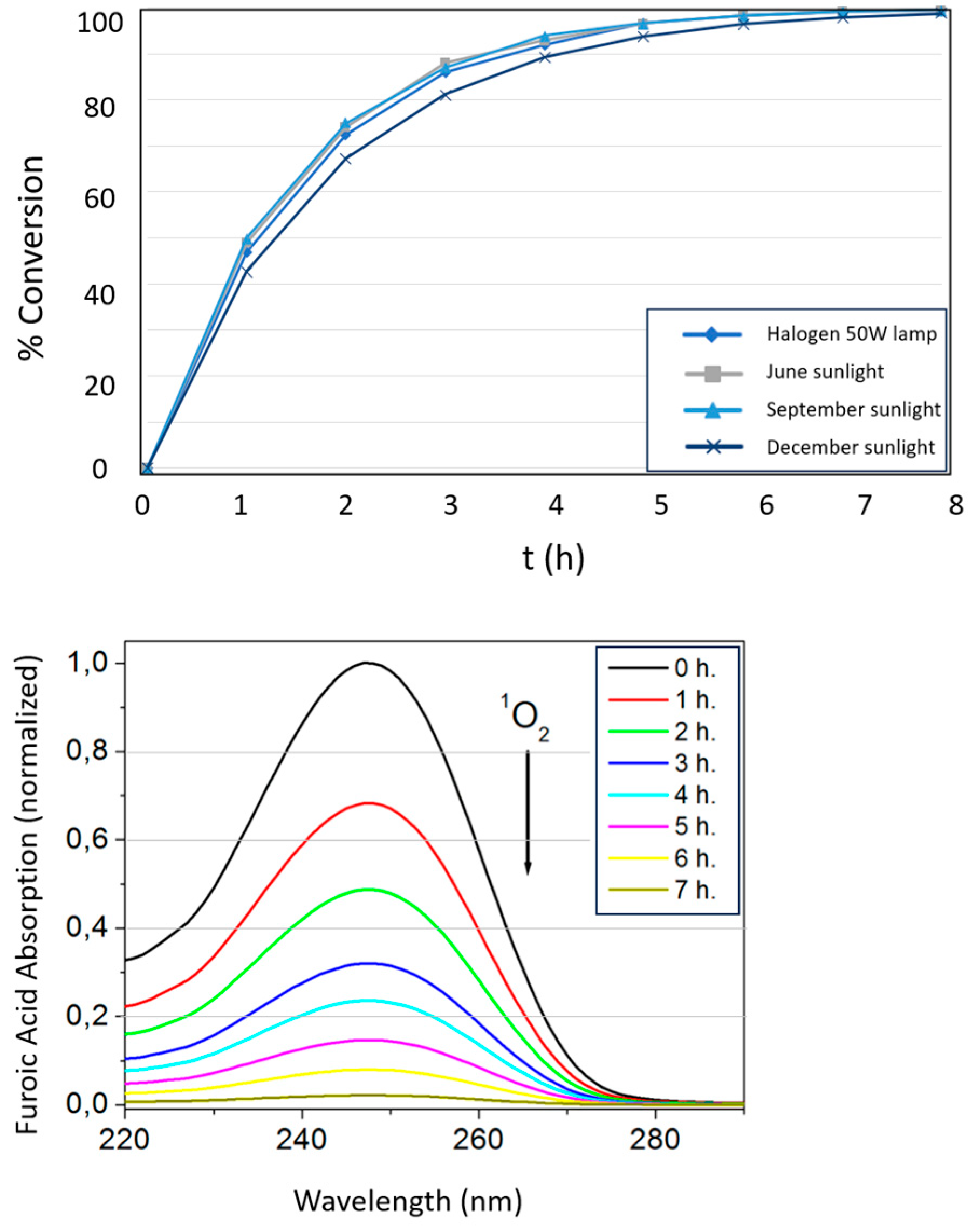
3.4. Studies of Photo-Oxidation of Furoic Acid and Green Synthesi 5-Hydroxy-2(5H)-Furanone
3.5. Energy Cost-Effective Calculations for Industrial Scale-Up
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CECs | Contaminants of Emerging Concern |
| WWTPs | Wastewater treatment plants |
| PNIPAM | Poly(N-isopropylacrylamide) |
| RB | Rose Bengal |
| EPs | Emerging pollutants |
| ADPA | 9,10-anthracenedipropionic acid |
| DLS | Dynamic light scattering |
| UV-Vis | Ultraviolet-visible spectroscopy |
| HPLC | High-Performance Liquid Chromatography |
| NIPAM | N-isopropylacrylamide |
| AEMA | Aminoethyl methacrylate |
| MBAM | N,N'-methylenebisacrylamide |
| EDC | 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide |
| MeOH | Methanol |
| t ½ | Half-reaction time |
| kobs | Observed rate constant or observed k |
| 1O2 or O₂(¹Δg) | Singlet oxygen |
| 3O2 | Molecular oxygen |
| hʋ | Light excitation or photon energy |
| TRL | Technology Readiness Level |
| CAPEX | Capital Expenditure |
| OPEX | Operational Expenditure |
References
- Anastas, P. T. , Warner, J. C., 2000, Green chemistry: theory and practice. Oxford university press.
- Andraos, J.; Matlack, A.S. Introduction to Green Chemistry; Taylor & Francis: London, United Kingdom, 2022. [Google Scholar]
- Ncube, A.; Mtetwa, S.; Bukhari, M.; Fiorentino, G.; Passaro, R. Circular Economy and Green Chemistry: The Need for Radical Innovative Approaches in the Design for New Products. Energies 2023, 16, 1752. [Google Scholar] [CrossRef]
- Zuin, V.G.; Eilks, I.; Elschami, M.; Kümmerer, K. Education in green chemistry and in sustainable chemistry: perspectives towards sustainability. Green Chem. 2021, 23, 1594–1608. [Google Scholar] [CrossRef]
- Lee, B.C.Y.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water 2021, 13, 2340. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: a review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Patel, N.A.V.E.E.N.; Khan, M.D.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging pollutants in aquatic environment: Source, effect, and challenges in biomonitoring and bioremediation-a review. Pollution 2020, 6, 99–113. [Google Scholar]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water – A critical review. Co-ord. Chem. Rev. 2021, 442. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, B.; Fang, W. Sunlight-driven photocatalytic conversion of furfural and its derivatives. Green Chem. 2024, 26, 6261–6288. [Google Scholar] [CrossRef]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F. Catalytic Scissoring of Lignin into Aryl Monomers. Adv. Mater. 2019, 31, e1901866. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- van Putten, R. J. , Van Der Waal, J. C., De Jong, E. D., Rasrendra, C. B., Heeres, H. J., de Vries, J. G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chemical reviews, 2013, 113(3), 1499-1597.
- Bozell, J. J. , Petersen, G. R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green chemistry, 2010, 12(4), 539-554.
- Fabregat, V.; Burguete, M.I.; Galindo, F.; Luis, S.V. Singlet oxygen generation by photoactive polymeric microparticles with enhanced aqueous compatibility. Environ. Sci. Pollut. Res. 2013, 21, 11884–11892. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Lu, G.-H.; Zong, M.-H.; Cui, W.-J.; Li, N. A plug-and-play chemobiocatalytic route for the one-pot controllable synthesis of biobased C4 chemicals from furfural. Green Chem. 2021, 23, 8604–8610. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Trombino, S.; Sole, R.; Di Gioia, M.L.; Procopio, D.; Curcio, F.; Cassano, R. Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis. Molecules 2023, 28, 2107. [Google Scholar] [CrossRef]
- Rozhkova, Y.A.; Burin, D.A.; Galkin, S.V.; Yang, H. Review of Microgels for Enhanced Oil Recovery: Properties and Cases of Application. Gels 2022, 8, 112. [Google Scholar] [CrossRef]
- Das, A.; Babu, A.; Chakraborty, S.; Van Guyse, J.F.R.; Hoogenboom, R.; Maji, S. Poly(N-isopropylacrylamide) and Its Copolymers: A Review on Recent Advances in the Areas of Sensing and Biosensing. Adv. Funct. Mater. 2024, 34, 2402432. [Google Scholar] [CrossRef]
- Guerron, A.; Giasson, S. Multiresponsive Microgels: Toward an Independent Tuning of Swelling and Surface Properties. Langmuir 2021, 37, 11212–11221. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Green Chemistry, Biocatalysis, and the Chemical Industry of the Future. ChemSusChem 2022, 15, e202102628. [Google Scholar] [CrossRef]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic Photocatalysts for the Oxidation of Pollutants and Model Compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Ghogare, A.A.; Greer, A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef]
- Al-Nu'Airat, J.; Oluwoye, I.; Zeinali, N.; Altarawneh, M.; Dlugogorski, B.Z. Review of Chemical Reactivity of Singlet Oxygen with Organic Fuels and Contaminants. Chem. Rec. 2020, 21, 315–342. [Google Scholar] [CrossRef]
- Fabregat, V. Enhancing Emerging Pollutant Removal in Industrial Wastewater: Validation of a Photocatalysis Technology in Agri-Food Industry Effluents. Appl. Sci. 2024, 14, 6308. [Google Scholar] [CrossRef]
- Fabregat, V. Exploring the Role of pH and Solar Light-Driven Decontamination with Singlet Oxygen in Removing Emerging Pollutants from Agri-Food Effluents: The Case of Acetamiprid. Physchem 2025, 5, 9. [Google Scholar] [CrossRef]
- Koizumi, H.; Shiraishi, Y.; Tojo, S.; Fujitsuka, M.; Majima, T.; Hirai, T. Temperature-Driven Oxygenation Rate Control by Polymeric Photosensitizer. J. Am. Chem. Soc. 2006, 128, 8751–8753. [Google Scholar] [CrossRef]
- Shiraishi, Y. , Suzuki, T., Hirai, T. Temperature-and pH-responsive photosensitization activity of polymeric sensitizers based on poly-N-isopropylacrylamide. Polymer, 2009, 50(24), 5758-5764.
- Shiraishi, Y.; Kimata, Y.; Koizumi, H.; Hirai, T. Temperature-Controlled Photooxygenation with Polymer Nanocapsules Encapsulating an Organic Photosensitizer. Langmuir 2008, 24, 9832–9836. [Google Scholar] [CrossRef]
- Koizumi, H.; Kimata, Y.; Shiraishi, Y.; Hirai, T. Temperature-controlled changeable oxygenation selectivity by singlet oxygen with a polymeric photosensitizer. Chem. Commun. 2007, 1846–1848. [Google Scholar] [CrossRef]
- Egil, A.C.; Carmignani, A.; Battaglini, M.; Sengul, B.S.; Acar, E.; Ciofani, G.; Ince, G.O. Dual stimuli-responsive nanocarriers via a facile batch emulsion method for controlled release of Rose Bengal. J. Drug Deliv. Sci. Technol. 2022, 74. [Google Scholar] [CrossRef]
- Don, T.-M.; Lu, K.-Y.; Lin, L.-J.; Hsu, C.-H.; Wu, J.-Y.; Mi, F.-L. Temperature/pH/Enzyme Triple-Responsive Cationic Protein/PAA-b-PNIPAAm Nanogels for Controlled Anticancer Drug and Photosensitizer Delivery against Multidrug Resistant Breast Cancer Cells. Mol. Pharm. 2017, 14, 4648–4660. [Google Scholar] [CrossRef]
- Maki, Y.; Dobashi, T. Poly (N-isopropylacrylamide)-Clay Nanocomposite Hydrogels with Patterned Thermo-Responsive Behavior. Trans. Mater. Res. Soc. Jpn. 2017, 42, 119–122. [Google Scholar] [CrossRef]
- Nowakowska, M. , Kȩpczyński, M., Da̧browska, M. Polymeric Photosensitizers, 5. Synthesis and Photochemical Properties of Poly [(N-isopropylacrylamide)-co-(vinylbenzyl chloride)] Containing Covalently Bound Rose Bengal Chromophores. Macromolecular Chemistry and Physics, 2001, 202(9), 1679-1688.
- Nowakowska, M.; Kepczynski, M.; Szczubialka, K. New polymeric photosensitizers. 73. [CrossRef]
- Nowakowska, M.; Szczubiałka, K. Photoactive polymeric and hybrid systems for photocatalytic degradation of water pollutants. Polym. Degrad. Stab. 2017, 145, 120–141. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Moya, P.; Marin, M.L.; Bosca, F. Synthesis of novel heterogeneous photocatalysts based on Rose Bengal for effective wastewater disinfection and decontamination. Catal. Today 2022, 413-415, 113948. [Google Scholar] [CrossRef]
- Flores, J. , Moya, P. , Bosca, F., Marin, M. L. Photoreactivity of new rose bengal-SiO2 heterogeneous photocatalysts with and without a magnetite core for drug degradation and disinfection. Catalysis Today, 2023, 413, 113994. [Google Scholar]
- Esser, P.; Pohlmann, B.; Scharf, H. The Photochemical Synthesis of Fine Chemicals with Sunlight. Angew. Chem. Int. Ed. Engl. 1994, 33, 2009–2023. [Google Scholar] [CrossRef]
- Roy, J.S.; Messaddeq, Y. The Role of Solar Concentrators in Photocatalytic Wastewater Treatment. Energies 2024, 17, 4001. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Gayathri, B.; Bharathi, V.D. Photocatalysis for removal of environmental pollutants and fuel production: a review. Environ. Chem. Lett. 2020, 19, 441–463. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Gayathri, B.; Bharathi, V.D. Photocatalysis for removal of environmental pollutants and fuel production: a review. Environ. Chem. Lett. 2020, 19, 441–463. [Google Scholar] [CrossRef]
- Lee, B.C.Y.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water 2021, 13, 2340. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Luis, S.V.; Galindo, F. Improving photocatalytic oxygenation mediated by polymer supported photosensitizers using semiconductor quantum dots as ‘light antennas’. RSC Adv. 2017, 7, 35154–35158. [Google Scholar] [CrossRef]
- Burguete, M.I.; Fabregat, V.; Galindo, F.; Izquierdo, M.A.; Luis, S.V. Improved polyHEMA–DAQ films for the optical analysis of nitrite. Eur. Polym. J. 2009, 45, 1516–1523. [Google Scholar] [CrossRef]
- Fabregat, V.; Izquierdo, M.A.; Burguete, M.I.; Galindo, F.; Luis, S.V. Quantum dot–polymethacrylate composites for the analysis of NOx by fluorescence spectroscopy. Inorganica Chim. Acta 2011, 381, 212–217. [Google Scholar] [CrossRef]
- Fabregat, V.; Izquierdo, M.Á.; Burguete, M.I.; Galindo, F.; Luis, S.V. Nitric oxide sensitive fluorescent polymeric hydrogels showing negligible interference by dehydroascorbic acid. Eur. Polym. J. 2014, 55, 108–113. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Galindo, F.; Luis, S.V. Influence of polymer composition on the sensitivity towards nitrite and nitric oxide of colorimetric disposable test strips. Environ. Sci. Pollut. Res. 2016, 24, 3448–3455. [Google Scholar] [CrossRef]
- Bradley, M.; Vincent, B. Poly(vinylpyridine) Core/Poly(N-isopropylacrylamide) Shell Microgel Particles: Their Characterization and the Uptake and Release of an Anionic Surfactant. Langmuir 2008, 24, 2421–2425. [Google Scholar] [CrossRef]
- Bradley, M.; Vincent, B.; Burnett, G. Uptake and Release of Anionic Surfactant into and from Cationic Core−Shell Microgel Particles. Langmuir 2007, 23, 9237–9241. [Google Scholar] [CrossRef]
- Burakowska, E.; Zimmerman, S.C.; Haag, R. Photoresponsive Crosslinked Hyperbranched Polyglycerols as Smart Nanocarriers for Guest Binding and Controlled Release. Small 2009, 5, 2199–2204. [Google Scholar] [CrossRef]
- Atkins, P. W. , De Paula, J., Keeler, J., 2023, Atkins' physical chemistry. Oxford university press.
- Available online:. Available online: https://www.aemet.es/documentos/es/serviciosclimaticos/datosclimatologicos/atlas_radiacion_solar/atlas_de_radiacion_24042012.pdf (accessed on 12 January 2025).
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA, 2003; Volume 66. [Google Scholar]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of techno-economic analysis and life cycle assessment for sustainable process design – A review. J. Clean. Prod. 2021, 317. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. Technoeconomic Analysis of the Recovery of Phenols from Olive Mill Wastewater through Membrane Filtration and Resin Adsorption/Desorption. Sustainability 2021, 13, 2376. [Google Scholar] [CrossRef]
- Fabregat, V.; Pagán, J.M. Technical–Economic Feasibility of a New Method of Adsorbent Materials and Advanced Oxidation Techniques to Remove Emerging Pollutants in Treated Wastewater. Water 2024, 16, 814. [Google Scholar] [CrossRef]
- Dirección General del Agua, Ministerio para la Transición Ecológica y el Reto Demográfico, 2020, Mejora de la eficiencia energética e integral de las plantas de tratamiento, regeneración y reutilización de aguas residuales – Informe complementario.

| Characterization Data | NIPAM-co-AEMA | NIPAM-co- AEMA-RB |
|
|---|---|---|---|
| Effective diameter (nm) | pH = 3 | 732 | 724 |
| Natural pH of microgel | 655 | 669 | |
| pH = 7 | 631 | 634 | |
| pH = 11 | 488 | 498 | |
|
Electrophoretic mobility (m2s-1V-1) |
pH = 3 | 0.15 | 0.18 |
| Natural pH of microgel | 0.02 | 0.07 | |
| pH = 7 | 0.01 | 0.03 | |
| pH = 11 | -0.09 | -0.32 | |
| % AEMA (titration) | 0.92 % | 0.92 % | |
| RB loading (titration) | 0 | 33 µmol RB /g polym. | |
| RB loading (UV-Vis) | 0 | 33 µmol RB /g polym. | |
| Photosensitizer | t ½ (s) | t (>99%) (s) | kobs (10-5 s-1) | |
|---|---|---|---|---|
|
Halogen lamp (sol simulated) |
Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | 170 | 1,128 | 408 | |
| Free RB | 200 | 1,332 | 346 | |
|
Sun natural irradiation (May 2024) |
Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | 171 | 1,135 | 406 | |
| Free RB | 204 | 1,357 | 339 | |
| Photosensitizer | Solar irradiation (kWh/m2) |
t (>99%) (min) | kobs (min-1) | |
|---|---|---|---|---|
|
Halogen lamp (sun simulated) |
Blank | 6.9 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 164 | 0.02808 | ||
| RB | 182 | 0.02530 | ||
|
Sun natural irradiation June |
Blank | 7.2 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 165 | 0.02791 | ||
| RB | 181 | 0.02544 | ||
|
Sun natural irradiation September |
Blank | 6.1 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 165 | 0.02788 | ||
| RB | 184 | 0.02532 | ||
|
Sun natural irradiation December |
Blank | 3.3 | - | - |
| NIPAM-co-AEMA | - | - | ||
| NIPAM-co-AEMA-RB | 169 | 0.02725 | ||
| RB | 188 | 0.02450 | ||
| Photosensitizer | Conversion of Furoic Acid at t=420 min | t ½ (min) | kobs (min-1) | |
|---|---|---|---|---|
|
Halogen lamp Irradiation = 6.9 kWh/m2 |
Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | >99% | 61 | 0.01143 | |
| RB | 12% | 2,277 | 0.00030 | |
|
June sunlight Irradiation = 7.5 kWh/m2 |
Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | >99% | 60 | 0.01140 | |
| RB | 13% | 2,090 | 0.00033 | |
|
September sunlight Irradiation = 6.1 kWh/m2 |
Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | >99% | 61 | 0.01134 | |
| RB | 2,498 | 0.00028 | ||
|
December sunlight Irradiation = 2.9 kWh/m2 |
Blank | - | - | - |
| NIPAM-co-AEMA | - | - | - | |
| NIPAM-co-AEMA-RB | 98% | 74 | 0.00931 | |
| RB | 9% | 3,087 | 0.00022 | |
| Process | Energy consumption (kWh/m3) Reactor 500 mL |
Reactor scale-up 1 m3. Energy consp. (kWh/m3) |
Reactor scale-up 100 m3. Energy consp. (kWh/m3) | Reactor scale-up 1 Hm3. Energy consp. (kWh/m3) |
|||
|---|---|---|---|---|---|---|---|
| Aspen | Six-Tenths | Aspen | Six-Tenths | Aspen | Six-Tenths | ||
| Diclofenac (halogen lamp) |
537 | 24.38 | 25.69 | 4.12 | 4.07 | 0.27 | 0.26 |
| Diclofenac (sunlight) |
253 | 12.48 | 12.09 | 2.01 | 1.92 | 0.12 | 0.12 |
| Furoic Acid (halogen lamp) |
1050 | 48.36 | 50.21 | 7.85 | 7.96 | 0.48 | 0.50 |
| Furoic Acid (sunlight) |
375 | 18.05 | 17.95 | 2.92 | 2.85 | 0.17 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




