Submitted:
23 January 2025
Posted:
29 January 2025
You are already at the latest version
Abstract
Background and Objective: Lung cancer continues to be a major global health issue, with a pressing need for improved diagnostic and prognostic methods to enhance early detection and patient outcomes. The objective of this survey is to review and evaluate the current methods and models used in lung cancer diagnosis and prognosis, focusing on their strengths, limitations, and potential for future advancements. Methods: A systematic review of the literature was conducted across key databases, focusing on studies that utilize deep learning architectures, such as CNN, GoogleNet, VGG-16, U-Net, and machine learning algorithms, including XGBoost, SVM, KNN, ANN, and Random Forest. The review synthesized findings from these studies to assess the effectiveness and limitations of these computational models in the context of lung cancer detection. Results: The review identified several strengths in current models, including high accuracy in controlled environments and potential for early detection. However, significant limitations were also highlighted, such as issues with model interpretability, a lack of real-world validation, and challenges in integrating diverse diagnostic techniques. These gaps indicate the need for further research to enhance the applicability and reliability of AI-driven models in clinical settings. Conclusions: Advanced computational methods, particularly those utilizing deep learning and machine learning, hold transformative potential for lung cancer diagnosis and prognosis. However, to fully realize this potential, future research must address current challenges, such as improving model interpretability and ensuring robust validation in real-world scenarios. By overcoming these obstacles, AI-driven approaches can significantly improve patient care and outcomes in lung cancer treatment.
Keywords:
1. Introduction
2. Literature Survey
| Year | Title of Paper | Objective | Limitations | Insights/Results | Dependent Variable | Independent Variables | Future Research Directions | Other Variables | Related RQs |
|---|---|---|---|---|---|---|---|---|---|
| 21 | Explainable artificial intelligence (XAI) in deep learning-based medical image analysis[13] | Overview of XAI in deep learning for medical image analysis | Limited generalizability of findings | Framework for classifying XAI methods; future opportunities identified | XAI effectiveness | Deep learning methods | Further development of XAI techniques | Anatomical locations, interpretability factors | RQ1_XAI Importance of in imaging |
| 22 | Human treelike tubular structure segmentation: A comprehensive review and future perspectives[14] | Review of datasets and algorithms for tubular structure segmentation | Potential bias in selected studies | Comprehensive dataset and algorithm review; challenges and future directions discussed | Segmentation accuracy | Imaging modalities (MRI, CT, etc.) | Exploration of new segmentation algorithms | Types of tubular structures (airways, blood vessels) | RQ2_Segmentation_Techniques |
| 23 | Multi-task deep learning for medical image computing and analysis: A review[15] | Summarize multi-task deep learning applications in medical imaging | Performance gaps in some tasks | Identification of popular architectures; outstanding performance noted in several areas | Medical image processing outcomes | Multiple related tasks | Addressing performance gaps in current models | Specific application areas (brain, chest, etc.) | RQ3_Multi-task learning in imaging |
| 22 | The COVID-19 epidemic analysis and diagnosis using deep learning: A systematic literature review[16] | Assess DL applications for COVID-19 diagnosis | Underutilization of certain features | Categorization of DL techniques; highlighted state-of-the-art studies; numerous challenges noted | COVID-19 detection accuracy | Various DL techniques | Investigation of underutilized features | Imaging sources (MRI, CT, X-ray) | RQ4_Deep learning for COVID-19 |
| 23 | The enlightening role of explainable artificial intelligence in medical & healthcare domains[17] | Analyze XAI techniques in healthcare to enhance trust | Limited focus on non-XAI methods | Insights from 93 articles; importance of interpretability in medical applications emphasized | Trust in AI systems | Machine learning models | Exploration of more XAI algorithms in healthcare | Factors influencing trust in AI systems | RQ1_Trust in AI systems |
| 23 | Aggregation of aggregation methods in computational pathology[18] | Review aggregation methods for whole-slide image analysis | Variability in methods discussed | Proposed general workflow; categorization of aggregation methods | WSI-level predictions | Computational methods | Recommendations for aggregation methods | Contextual application in computational pathology | RQ2_Segmentation_Techniques |
| 22 | COVID-19 image classification using deep learning: Advances, challenges and opportunities[19] | Review DL techniques for COVID-19 image classification | Challenges in manual detection | Summarizes state-of-the-art advancements; discusses open challenges in image classification | COVID-19 classification accuracy | DL algorithms (CNNs, etc.) | Suggestions for improving classification techniques | Types of imaging modalities (CXR, CT) | RQ4_Classification techniques |
| 22 | Harmony search: Current studies and uses on healthcare systems[20] | Survey applications of harmony search in healthcare | Potential limitations of search algorithms | Identifies strengths and weaknesses; proposes a framework for HS in healthcare | Optimization outcomes | Harmony search variants | Future research in optimizing healthcare applications | Applications in various healthcare domains | RQ5_Optimization in healthcare systems |
| 21 | A survey on incorporating domain knowledge into deep learning for medical image analysis[21] | Summarize integration of medical domain knowledge into deep learning models for various tasks | Limited datasets in medical imaging | Effective integration of medical knowledge enhances model performance | Model accuracy | Domain knowledge, model architecture | Explore more robust integration methods and domain-specific adaptations | Specific tasks: diagnosis, segmentation | RQ1_Integration of domain knowledge |
| 23 | Machine learning for administrative health records: A systematic review of techniques and applications[22] | Analyze machine learning techniques applied to Administrative Health Records (AHRs) | Limited breadth of applications due to data modality | AHRs can be valuable for diverse healthcare applications despite existing limitations in techniques | Model performance | Machine learning techniques, applications | Investigate connections between AHR studies and develop unified frameworks for analysis | Specific AHR types and health informatics application | RQ5_Applications in Health Records |
| 23 | Machine intelligence and medical cyber-physical system architectures for smart healthcare[23] | Provide a comprehensive overview of MCPS in healthcare, focusing on design, enabling technologies, and applications | Challenges in security, privacy, and interoperability | MCPS enhances continuous care in hospitals, with applications in telehealth and smart cities | System reliability | Architecture layers, technologies | Research on improving interoperability and security protocols in MCPS | Specific healthcare applications | RQ5_Optimization in Healthcare Systems. |
| 22 | Neural Natural Language Processing for unstructured data in electronic health records: A review[24] | Summarize neural NLP methods for processing unstructured EHR data | Challenges in processing diverse and noisy unstructured data | Advances in neural NLP methods outperform traditional techniques in EHR applications like classification and extraction | NLP task performance | EHR structure, data quality | Further development of interpretability and multilingual capabilities in NLP models for EHR | Characteristics of unstructured data | RQ4_NLP techniques in EHRs. |
| 21 | Precision health data: Requirements, challenges and existing techniques for data security and privacy[25] | Explore requirements and challenges for securing precision health data | Regulatory compliance and privacy concerns | Importance of secure and ethical handling of sensitive health data to maintain public trust and effective precision health systems | Data security | Privacy techniques, regulations | Identify more efficient privacy-preserving machine learning techniques suitable for health data | Ethical guidelines | RQ5_Optimization in healthcare systems, |
| 23 | Transforming medical imaging with Transformers? A comparative review of key properties[26] | Review the application of Transformer models in medical imaging tasks | Comparatively new field with limited comprehensive studies | Transformer models show potential in medical image analysis, outperforming traditional CNNs in certain applications | Image analysis accuracy | Model architecture, task type | Investigate hybrid models combining Transformers and CNNs for enhanced performance | Specific applications in medical imaging | RQ1_Advanced imaging techniques |
| 22 | A review: The detection of cancer cells in histopathology based on machine vision[27] | Review machine vision techniques for detecting cancer cells in histopathology images | Manual detection methods are time-consuming and error-prone | Machine vision provides automated and consistent detection of cancer cells, improving speed and accuracy in histopathology | Detection accuracy | Image preprocessing, segmentation techniques | Explore advancements in deep learning for improved accuracy in histopathology analysis | Characteristics of cancer cells | RQ2_Segmentation_Techniques |
| 20 | Deep learning in generating radiology reports: A survey[28] | Investigate automated models for generating coherent radiology reports using deep learning | Challenges in integrating image analysis and natural language generation | Combining CNNs for image analysis with RNNs for text generation has advanced automated reporting in radiology | Report quality | Image features, textual datasets | Develop better evaluation metrics and integrate patient context into report generation | Contextual factors in radiology reporting | RQ4_Automation in radiology reporting, RQ2_Segmentation_Techniques |
| 21 | A Comprehensive Analysis of Identifying Lung Cancer via Different Machine Learning Approaches[29] | To survey different machine learning approaches for lung cancer detection using medical image processing | Limited dataset sizes and variability in imaging | Deep neural networks are effective for cancer detection | Detection accuracy | Machine learning algorithms, image processing techniques | Explore hybrid models for improved accuracy | Image quality, patient demographics | RQ2_Segmentation_Techniques, RQ4_Automation in Radiology Reporting |
| 22 | Automating Patient-Level Lung Cancer Diagnosis in Different Data Regimes[30] | To automate lung cancer classification and improve patient-level diagnosis accuracy | Subjectivity in radiologist assessments; limited generalizability of methods | Proposed end-to-end methods improved patient-level diagnosis | Malignancy score | CT scan input, classification techniques | Investigate different data regimes and their impact on performance | Patient history, demographic data | RQ2_Segmentation_Techniques, RQ3_Multi-task learning in imaging |
| 23 | Machine Learning Approaches in Early Lung Cancer Prediction: A Comprehensive Review[31] | To review various machine learning algorithms for early lung cancer detection | Variability in model performance across datasets | SVM and ensemble methods show high accuracy | Early detection accuracy | Machine learning techniques used, dataset characteristics | Development of real-time prediction models | Clinical integration factors | RQ2_Segmentation_Techniques, RQ4_Automation in Radiology Reporting |
| 22 | A Comprehensive Survey on Various Cancer Prediction Using Natural Language Processing Techniques[32] | To explore NLP techniques for early lung cancer prediction | Limited applicability of some techniques in real-world settings | Data mining techniques enhance prediction abilities | Prediction accuracy | NLP techniques, data sources | Focus on improving NLP techniques for better predictions | Environmental factors, genetic predisposition | RQ4 Automation in radiology reporting |
| 23 | A Review of Deep Learning-Based Multiple-Lesion Recognition from Medical Images[27] | To review deep learning methods for multiple-lesion recognition | Complexity in recognizing multiple lesions | Advances in deep learning significantly aid in lesion recognition | Recognition accuracy | Medical imaging methods, lesion characteristics | Develop methods for better multiple-lesion recognition | Patient age, lesion type | RQ4_Automation in Radiology Reporting |
| 22 | An aggregation of aggregation methods in computational pathology[18] | Review aggregation methods for WSIs | Limited context on novel methods | Comprehensive categorization of aggregation methods | WSI-level labels | Tile predictions | Explore hybrid aggregation techniques | CPath use cases | RQ2_Segmentation_Techniques, RQ5_Optimization in Healthcare Systems. |
| 33 | Data mining and machine learning in heart disease prediction[33] | Survey ML and data mining techniques for heart disease prediction | Potential overfitting in small datasets | Several ML techniques yield promising predictive performance | Prediction accuracy | Data sources, features | Investigate integration of diverse data types | Health metrics | RQ3_Multi-task Learning in Imaging |
| 21 | The role of AI in precision medicine: Applications and challenges[34] | Analyze applications of AI in precision medicine | Ethical concerns regarding bias | AI can optimize treatment strategies and improve patient outcomes | Treatment effectiveness | Patient data types, AI algorithms | Future studies to address bias and enhance model transparency | Clinical settings | RQ5_Optimization in Healthcare Systems |
| 23 | Advances in medical image analysis: A comprehensive survey[35] | Comprehensive review of recent advances in medical image analysis techniques | Limitations in the scope of reviewed studies | Highlights the importance of advanced techniques like DL in medical imaging | Image analysis outcomes | Imaging methods | Integration of Imaging and Genomic Data in Cancer Detection Using AI Models | RQ3_Multi-task learning in imaging2023 |
| Title of Paper | Objective | Limitations | Insights/Results | Dependent Variable | Independent Variables | Future Research Directions | Other Variables | Year |
|---|---|---|---|---|---|---|---|---|
| Data resources and computational methods for lncRNA-disease association prediction[36] | Review lncRNA-disease associations and computational methods for prediction | Limited focus on specific diseases; evolving methods may not cover all recent advancements | Overview of 64 methods categorized into five groups; highlights challenges and future trends | lncRNA-disease association prediction | lncRNA features, disease types | Improve prediction accuracy and expand to more diseases | Data sources | 2023 |
| Data synthesis and adversarial networks: A review and meta-analysis in cancer imaging[37] | Assess GANs and adversarial training in addressing cancer imaging challenges | Limited scope of analysis; may not cover all potential GAN applications | Identifies challenges in cancer imaging; proposes SynTRUST framework for validation rigour | Cancer imaging outcomes | GAN techniques, imaging data | Explore novel GAN applications in cancer imaging | Data quality | 2023 |
| Explainable, trustworthy, and ethical machine learning for healthcare: A survey[38] | Review explainable and interpretable ML techniques in healthcare | Lack of standardization in methodologies; ethical concerns may be context-dependent | Highlights importance of transparency and trust in ML; discusses security and ethical issues | Trust in ML systems | ML techniques, application areas | Develop standardized evaluation metrics for explainable ML | Ethical considerations | 2023 |
| Machine learning in medical applications: A review of state-of-the-art methods[39] | Comprehensive review of ML applications in medical diagnostics | Rapidly evolving field; may miss the latest technologies | Discusses five major medical applications; emphasizes improving reliability and accuracy in diagnostics | Diagnostic accuracy | ML models, disease types | Explore integration of ML with clinical workflows | Healthcare outcomes | 2023 |
| Survey of explainable artificial intelligence techniques for biomedical imaging with deep neural networks[40] | Review XAI techniques for improving trust in DNN-based medical imaging diagnostics | Complexity of DNNs may limit interpretability; potential biases in training data | Discusses challenges in adopting DNNs; categorizes XAI techniques; highlights future research needs in interpretability | Model predictions | DNN architectures, imaging features | Enhance interpretability and regulatory compliance | Trustworthiness | 202 |
| Deep learning-based lung image registration: A review[41] | Review DL methods for lung image registration | Few comprehensive frameworks for lung registration | Comprehensive survey of DL methods categorized by supervision type | Lung image registration accuracy | DL methods, supervision types | Development of versatile DL frameworks for lung images | Evaluation metrics, datasets | 2021 |
| Deep learning for chest X-ray analysis: A survey[42] | Review DL applications in chest X-ray analysis | Varied quality and methodologies in studies | Categorization of tasks and datasets used in chest X-ray analysis | X-ray analysis accuracy | DL methods, types of tasks | Address gaps in dataset utilization and model applicability | Clinical requirements | 2021 |
| Deep learning for computational cytology: A survey[43] | Survey DL applications in computational cytology | Limited integration of DL methods in clinical practice | Overview of over 120 publications in cytology using DL methods | Cytology image analysis accuracy | DL techniques, public datasets | Explore clinical implementation and real-world testing | Evaluation metrics | 2021 |
| Recent advancement in cancer diagnosis using ML and DL techniques[44] | Review ML/DL advancements in cancer diagnosis | Varied effectiveness across cancer types and modalities | Detailed review of cancer detection methods and benchmark datasets | Cancer diagnosis accuracy | ML/DL techniques, cancer types | Further research on underexplored cancer types | Performance indicators | 2021 |
| A narrative review on ARDS in COVID-19 using AI[45] | Analyze AI models for ARDS in COVID-19 lungs | Lack of clinical validation for some AI models | Discusses AI applications in diagnosing ARDS and their workflow considerations | ARDS diagnosis accuracy | AI models, imaging modalities | Improvement of AI models considering comorbidities | Comorbidities | 2021 |
| A survey of deep learning models in medical therapeutic areas[46] | Identify therapeutic areas for DL applications in medicine | Limited by the quality of included studies | Increasing trend in DL publications; focus on oncology and image analysis | Diagnostic and treatment outcomes | DL models, therapeutic areas | Expand to less researched medical fields | Publication trends | 2021 |
| Computational Traditional Chinese Medicine diagnosis: A literature survey[47] | Review computational approaches in TCM diagnosis | Need for standardized methodologies in TCM diagnosis | Systematic summary of computational TCM methods and future directions | TCM diagnosis accuracy | Diagnostic approaches, computational methods | Standardization and validation of TCM computational models | Smart healthcare trends | 202 |
| Role of machine learning in medical research: A survey[48] | Review machine learning techniques in medical applications | Focus on recent work may overlook older valuable techniques | Identifies a shift toward deep learning dominance in medical data analysis | Application effectiveness | Machine learning, deep learning models | Investigate more diverse applications of ML in medicine | Medical datasets | 2021 |
| Transformers in medical imaging: A survey[49] | Review applications of Transformers in medical imaging | Complexity of implementation and adaptation from NLP | Highlights advantages of Transformers over CNNs in capturing global context for medical imaging tasks | Imaging performance | Transformer architectures, medical tasks | Address challenges in adaptation and optimization of Transformers | Image modalities | 2023 |
| A comprehensive survey of intestine histopathological image analysis using machine vision approaches[50] | Review ML methods for intestinal histopathological image analysis | Need for standardization in datasets and methodologies | Discusses various ML methods and their applications in analyzing intestinal histopathology images | Diagnostic accuracy | ML methods, histopathological datasets | Improve dataset quality and explore advanced ML techniques | Colon cancer | 2023 |
| Artificial intelligence in cancer diagnosis and therapy: Current status and future perspective[51] | Explore AI's role in cancer diagnosis and treatment | Challenges in data mining and clinical integration | Highlights AI's potential in enhancing cancer diagnosis and therapy through personalized approaches | Treatment effectiveness | AI, ML applications, cancer types | Overcome challenges for AI integration in clinical practice | Cancer types | 2023 |
| Brain tumor segmentation of MRI images: A comprehensive review on the application of AI tools[52] | Review AI methods for brain tumor detection using MRI | Need for more trained professionals in the field | Summarizes performance of various AI techniques for tumor segmentation and classification in MRI images | Segmentation accuracy | AI methods, MRI imaging | Enhance training programs for professionals using AI techniques | Brain tumors | 2023 |
| Leveraging 6G, extended reality, and IoT big data analytics for healthcare: A review[53] | Analyze the impact of 6G, XR, and IoT on healthcare systems | Limited reviews on convergence of these technologies | Identifies novel healthcare services and future applications of 6G, XR, and IoT analytics | Healthcare service quality | 6G, XR, IoT technologies | Explore synergistic applications of these technologies in healthcare | Telehealth services | 2023 |
| Application of uncertainty quantification to AI in healthcare: A review of last decade (2013–2023)[54] | Review uncertainty techniques in AI models for healthcare | Scarcity of studies on physiological signals | Highlights the importance of uncertainty quantification for reliable medical predictions and decisions | Prediction accuracy | AI models (Bayesian, Fuzzy, etc.) | Investigate uncertainty quantification in physiological signals | Medical predictions | 2023 |
| Clinical applications of graph neural networks in computational histopathology: A review[55] | Examine the use of graph neural networks in histopathological analysis | Limited understanding of contextual feature extraction | Summarizes clinical applications and proposes improved graph construction methods | Diagnostic accuracy | Graph neural networks | Further research on model generalization in histopathology | Histopathological images | 2023 |
| Recent advances and clinical applications of deep learning in medical image analysis[56] | Summarize recent advances in deep learning for medical imaging tasks | Lack of large annotated datasets | Reviews the effectiveness of deep learning techniques in various medical imaging applications | Imaging performance | Deep learning models | Address dataset limitations and enhance model robustness | Medical imaging | 2022 |
| Recent progress in transformer-based medical image analysis[57] | Discuss transformer applications in medical image analysis | Still emerging technology with challenges | Highlights how transformers outperform traditional methods in medical image tasks | Classification accuracy | Transformer models | Explore new applications in diverse medical imaging tasks | Image modalities | 2022 |
| A comprehensive review on recent approaches for cancer drug discovery associated with AI[58] | Review AI methods in anticancer drug discovery | Complexities in modeling for various cancer types | Discusses the role of AI in enhancing drug discovery processes | Drug discovery effectiveness | AI techniques (ML, DL, molecular docking) | Investigate AI applications in diverse cancer types | Drug interactions | 2023 |
| A comprehensive review of deep learning in colon cancer[59] | Analyze deep learning techniques for colon cancer diagnosis | Limited studies on diverse data sources | Provides an overview of popular architectures and applications in colon cancer analysis | Diagnosis accuracy | Deep learning models | Address data diversity in colon cancer studies | Cancer types | 2023 |
| A state-of-the-art survey of neural networks for whole-slide image analysis[60] | Review neural network methods for whole-slide image analysis | Lack of focus on specific ANN architectures | Summarizes common ANN methods and datasets for WSI analysis | Image analysis accuracy | ANN architectures | Explore potential of visual transformers in WSI analysis | WSI datasets | 2023 |
| A survey and taxonomy of 2.5D approaches for lung segmentation and nodule detection[61] | Discuss 2.5D techniques for lung segmentation and nodule detection | Need for more comprehensive techniques | Provides a taxonomy of 2.5D methods for improved lung cancer diagnostics | Detection accuracy | 2D and 3D imaging techniques | Further development of 2.5D methods | CAD systems | 2023 |
| A survey, review, and future trends of skin lesion segmentation and classification[62] | Review CAD systems for skin lesion analysis | Challenges in evaluating minimal datasets | Analyzes trends in segmentation and classification methods for skin lesions | Classification accuracy | Deep learning and machine learning methods | Enhance dataset quality and evaluation metrics | Skin cancer | 2022 |
| Lung nodule diagnosis and cancer histology classification from CT data by CNNs: A survey[63] | Examine CNN contributions to lung nodule diagnosis and histology classification | Lack of publicly accessible data | Reviews the effectiveness of CNNs in lung cancer diagnostics and highlights key challenges | Diagnostic accuracy | CNN architectures and CT data | Improve data accessibility and reproducibility in studies | Cancer types | 2022 |
3. Systematic Literature Review Methodology
3.1. Overview
- i.
- What are the current methods and models utilized for lung cancer diagnosis and prognosis?
- ii.
- What are the strengths and limitations of these methods and models?
- iii.
- How can these methods and models be improved or developed in the future?
- iv.
- What are the specific applications of deep learning architectures and machine learning algorithms in lung cancer detection?
- v.
- What are the gaps and challenges in the current literature on lung cancer diagnosis and prognosis?
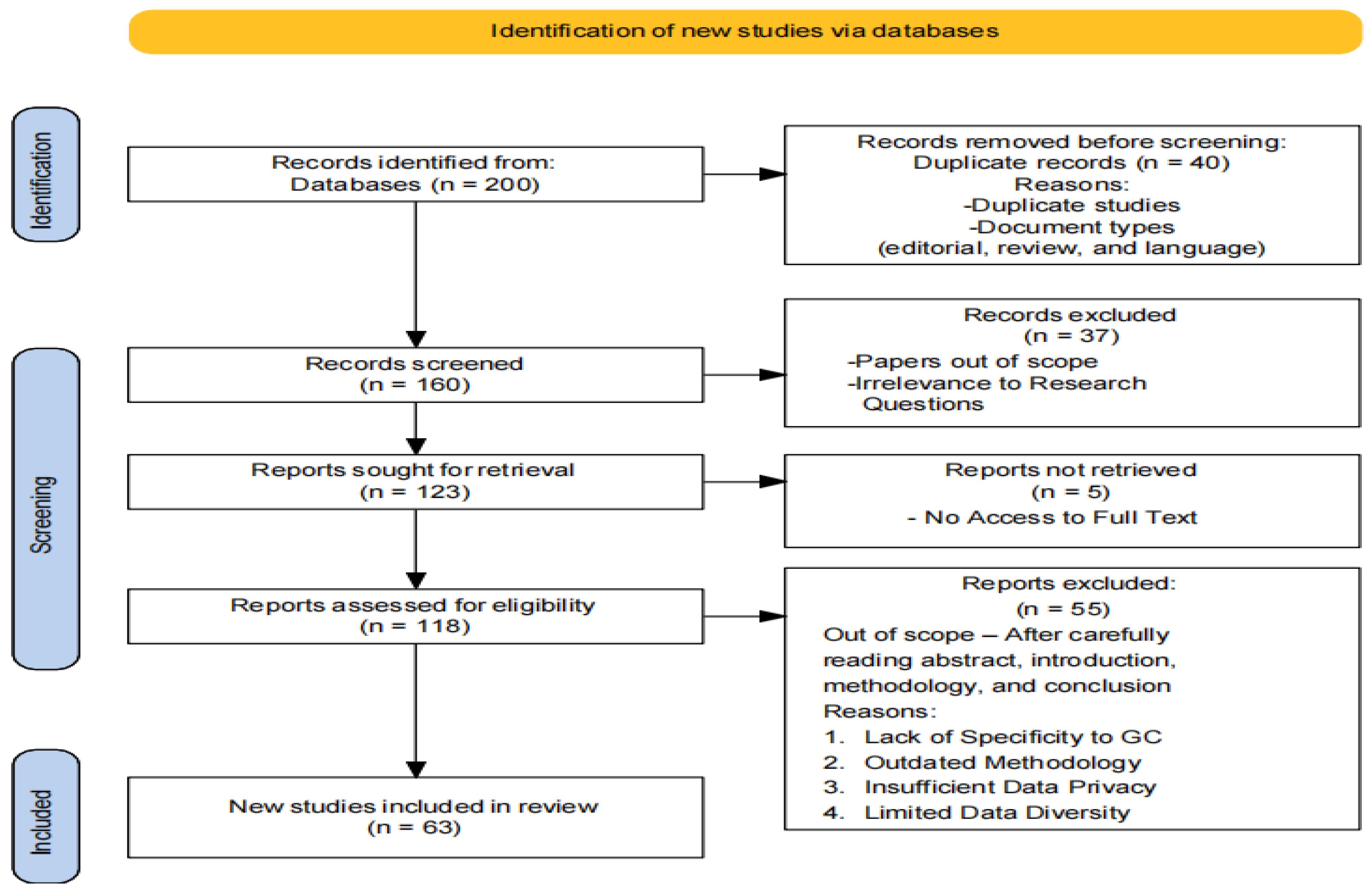
3.2. PRISMA Workflow
3.3. Inclusion and Exclusion Criteria
- Research employing deep learning architectures (e.g., CNN, GoogleNet, VGG-16, U-Net) and machine learning algorithms (e.g., XGBoost, SVM, KNN, ANN, Random Forest, hybrid models).
- Publications within a specified timeframe to capture recent advancements.
- Peer-reviewed articles and conference papers ensuring rigorous scientific evaluation.
- Studies not directly related to lung cancer diagnosis and prognosis.
- Research that does not utilize the specified deep learning and machine learning techniques.
- Non-peer-reviewed articles, opinion pieces, and editorials.
- Publications outside the specified timeframe to maintain the relevance of the review.
3.4. Descriptive Statistics of Selected Papers
4. Methodologies in Lung Cancer Detection and Classification
4.1. Machine Learning
4.2. Deep Learning
4.3. Strengths and Limitations of the Methodologies
| Methodology | Strengths | Limitations |
|---|---|---|
| Machine Learning | Ability to learn patterns and relationships in data. | Reliance on labeled datasets for training. |
| Generalization of knowledge for prediction. | Limited capability to handle complex and high-dimensional data. | |
| Well-established algorithms and techniques. | Lack of interpretability in complex models. | |
| Deep Learning | Ability to automatically extract useful features from raw data. | Requires large amounts of labeled training data. |
| Capable of handling complex and high-dimensional data. | Computationally intensive and requires significant computing resources. | |
| Achieves state-of-the-art performance in various domains. | Lack of interpretability in deep neural networks. | |
| Effective in image analysis and sequential data tasks. | Prone to overfitting with insufficient training data. |
5. Discussion and Survey Analysis
| Model | Accuracy | Results | |
|---|---|---|---|
| 2023 | LeNet | 97.88% | LeNet for classification |
| 2023 | VGG16 | 99.45% | Better accuracy |
| 2021 | SVM | 98% | Reduce execution time with SVM and Chi-square feature selection. |
| 2021 | GoogleNet | 94.38% | Higher accuracy with transfer learning |
| 2020 | KNN | 96.5% | Hybrid with GA for enhanced classification |
6. Research Challenges and Opportunities
7. Conclusion
Acknowledgements
References
- S. Nageswaran et al., “Lung cancer classification and prediction using machine learning and image processing,” Biomed Res. Int., vol. 2022, 2022. [CrossRef]
- D. Jamil, S. Palaniappan, S. S. Zia, A. Lokman, and M. Naseem, “Reducing the Risk of Gastric Cancer Through Proper Nutrition-A Meta-Analysis.,” Int. J. Online \& Biomed. Eng., vol. 18, no. 7, 2022. [CrossRef]
- V. K. Raghu et al., “Validation of a Deep Learning--Based Model to Predict Lung Cancer Risk Using Chest Radiographs and Electronic Medical Record Data,” JAMA Netw. Open, vol. 5, no. 12, pp. e2248793--e2248793, 2022. [CrossRef]
- B. S. Chhikara and K. Parang, “Global Cancer Statistics 2022: the trends projection analysis,” Chem. Biol. Lett., vol. 10, no. 1, p. 451, 2023.
- M. N. D. J. S. P. Sanjoy Kumar Debnath and S. B. and A. Lokman, “Prediction Model for Gastric Cancer via Class Balancing Techniques,” Int. J. Comput. Sci. Netw. Secur., vol. 23, no. 01, pp. p53-63, 2023. https://doi.org/07_book/202301/20230108.pdf.
- H. Sung et al., “Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA. Cancer J. Clin., vol. 71, no. 3, pp. 209–249, 2021. [CrossRef]
- M. S. AL-Huseiny and A. S. Sajit, “Transfer learning with GoogLeNet for detection of lung cancer,” Indones. J. Electr. Eng. Comput. Sci., vol. 22, no. 2, pp. 1078–1086, 2021. [CrossRef]
- D. Chauhan and V. Jaiswal, “An efficient data mining classification approach for detecting lung cancer disease,” in 2016 International Conference on Communication and Electronics Systems (ICCES), 2016, pp. 1–8.
- D. Jamil, “Diagnosis of Gastric Cancer Using Machine Learning Techniques in Healthcare Sector: A Survey,” Informatica, vol. 45, 2022. [CrossRef]
- W. Rahane, H. Dalvi, Y. Magar, A. Kalane, and S. Jondhale, “Lung cancer detection using image processing and machine learning healthcare,” in 2018 International Conference on Current Trends towards Converging Technologies (ICCTCT), 2018, pp. 1–5.
- S. Makaju, P. W. C. Prasad, A. Alsadoon, A. K. Singh, and A. Elchouemi, “Lung cancer detection using CT scan images,” Procedia Comput. Sci., vol. 125, pp. 107–114, 2018. [CrossRef]
- P. K. Vikas and P. Kaur, “Lung cancer detection using chi-square feature selection and support vector machine algorithm,” Int. J. Adv. Trends Comput. Sci. Eng., 2021.
- B. H. M. der Velden, H. J. Kuijf, K. G. A. Gilhuijs, and M. A. Viergever, “Explainable artificial intelligence (XAI) in deep learning-based medical image analysis,” Med. Image Anal., vol. 79, p. 102470, 2022. [CrossRef]
- H. Li, Z. Tang, Y. Nan, and G. Yang, “Human treelike tubular structure segmentation: A comprehensive review and future perspectives,” Comput. Biol. Med., vol. 151, p. 106241, 2022. [CrossRef]
- Y. Zhao, X. Wang, T. Che, G. Bao, and S. Li, “Multi-task deep learning for medical image computing and analysis: A review,” Comput. Biol. Med., vol. 153, p. 106496, 2023.
- Heidari, N. J. Navimipour, M. Unal, and S. Toumaj, “The COVID-19 epidemic analysis and diagnosis using deep learning: A systematic literature review and future directions,” Comput. Biol. Med., vol. 141, p. 105141, 2022. [CrossRef]
- S. Ali, F. Akhlaq, A. S. Imran, Z. Kastrati, S. M. Daudpota, and M. Moosa, “The enlightening role of explainable artificial intelligence in medical \& healthcare domains: A systematic literature review,” Comput. Biol. Med., p. 107555, 2023. [CrossRef]
- M. Bilal et al., “An aggregation of aggregation methods in computational pathology,” Med. Image Anal., p. 102885, 2023. [CrossRef]
- P. Aggarwal, N. K. Mishra, B. Fatimah, P. Singh, A. Gupta, and S. D. Joshi, “COVID-19 image classification using deep learning: Advances, challenges and opportunities,” Comput. Biol. Med., vol. 144, p. 105350, 2022.
- M. T. Abdulkhaleq et al., “Harmony search: Current studies and uses on healthcare systems,” Artif. Intell. Med., vol. 131, p. 102348, 2022. [CrossRef]
- X. Xie, J. Niu, X. Liu, Z. Chen, S. Tang, and S. Yu, “A survey on incorporating domain knowledge into deep learning for medical image analysis,” Med. Image Anal., vol. 69, p. 101985, 2021. [CrossRef]
- A. Caruana, M. Bandara, K. Musial, D. Catchpoole, and P. J. Kennedy, “Machine learning for administrative health records: A systematic review of techniques and applications,” Artif. Intell. Med., p. 102642, 2023. [CrossRef]
- T. A. Shaikh, T. Rasool, and P. Verma, “Machine intelligence and medical cyber-physical system architectures for smart healthcare: Taxonomy, challenges, opportunities, and possible solutions,” Artif. Intell. Med., p. 102692, 2023.
- I. Li et al., “Neural natural language processing for unstructured data in electronic health records: a review,” Comput. Sci. Rev., vol. 46, p. 100511, 2022. [CrossRef]
- Thapa and S. Camtepe, “Precision health data: Requirements, challenges and existing techniques for data security and privacy,” Comput. Biol. Med., vol. 129, p. 104130, 2021. [CrossRef]
- J. Li, J. Chen, Y. Tang, C. Wang, B. A. Landman, and S. K. Zhou, “Transforming medical imaging with Transformers? A comparative review of key properties, current progresses, and future perspectives,” Med. Image Anal., vol. 85, p. 102762, 2023.
- W. He et al., “A review: The detection of cancer cells in histopathology based on machine vision,” Comput. Biol. Med., vol. 146, p. 105636, 2022. [CrossRef]
- M. M. A. Monshi, J. Poon, and V. Chung, “Deep learning in generating radiology reports: A survey,” Artif. Intell. Med., vol. 106, p. 101878, 2020. [CrossRef]
- G. Paliwal and U. Kurmi, “A Comprehensive Analysis of Identifying Lung Cancer via Different Machine Learning Approach,” in 2021 10th International Conference on System Modeling \& Advancement in Research Trends (SMART), 2021, pp. 691–696.
- A. Pardyl, D. Rymarczyk, Z. Tabor, and B. Zieliński, “Automating patient-level lung cancer diagnosis in different data regimes,” in International Conference on Neural Information Processing, 2022, pp. 13–24.
- S. N. A. Shah and R. Parveen, “An extensive review on lung cancer diagnosis using machine learning techniques on radiological data: state-of-the-art and perspectives,” Arch. Comput. Methods Eng., vol. 30, no. 8, pp. 4917–4930, 2023.
- K. Jabir and A. T. Raja, “A Comprehensive Survey on Various Cancer Prediction Using Natural Language Processing Techniques,” in 2022 8th International Conference on Advanced Computing and Communication Systems (ICACCS), 2022, vol. 1, pp. 1880–1884.
- Zhao, W. Wu, L. Liang, X. Cai, Y. Chen, and W. Tang, “Prediction model of clinical prognosis and immunotherapy efficacy of gastric cancer based on level of expression of cuproptosis-related genes,” Heliyon, vol. 9, no. 8, 2023. [CrossRef]
- J. Lorkowski, O. Kolaszyńska, and M. Pokorski, “Artificial intelligence and precision medicine: A perspective,” in Integrative Clinical Research, Springer, 2021, pp. 1–11.
- A. Kazerouni et al., “Diffusion models in medical imaging: A comprehensive survey,” Med. Image Anal., vol. 88, p. 102846, 2023. [CrossRef]
- N. Sheng et al., “Data resources and computational methods for lncRNA-disease association prediction,” Comput. Biol. Med., vol. 153, p. 106527, 2023.
- R. Osuala et al., “Data synthesis and adversarial networks: A review and meta-analysis in cancer imaging,” Med. Image Anal., vol. 84, p. 102704, 2023. [CrossRef]
- K. Rasheed, A. Qayyum, M. Ghaly, A. Al-Fuqaha, A. Razi, and J. Qadir, “Explainable, trustworthy, and ethical machine learning for healthcare: A survey,” Comput. Biol. Med., vol. 149, p. 106043, 2022. [CrossRef]
- L. Benning, A. Peintner, and L. Peintner, “Advances in and the Applicability of Machine Learning-Based Screening and Early Detection Approaches for Cancer: A Primer,” Cancers (Basel)., vol. 14, no. 3, p. 623, 2022. [CrossRef]
- S. Nazir, D. M. Dickson, and M. U. Akram, “Survey of explainable artificial intelligence techniques for biomedical imaging with deep neural networks,” Comput. Biol. Med., vol. 156, p. 106668, 2023. [CrossRef]
- H. Xiao et al., “Deep learning-based lung image registration: A review,” Comput. Biol. Med., p. 107434, 2023. [CrossRef]
- Çall\i, E. Sogancioglu, B. van Ginneken, K. G. van Leeuwen, and K. Murphy, “Deep learning for chest X-ray analysis: A survey,” Med. Image Anal., vol. 72, p. 102125, 2021. [CrossRef]
- H. Jiang, Y. Zhou, Y. Lin, R. C. K. Chan, J. Liu, and H. Chen, “Deep learning for computational cytology: A survey,” Med. Image Anal., vol. 84, p. 102691, 2023. [CrossRef]
- Painuli, S. Bhardwaj, and others, “Recent advancement in cancer diagnosis using machine learning and deep learning techniques: A comprehensive review,” Comput. Biol. Med., vol. 146, p. 105580, 2022. [CrossRef]
- H. Ramdani, N. Allali, L. Chat, and S. El Haddad, “Covid-19 imaging: A narrative review,” Ann. Med. Surg., vol. 69, p. 102489, 2021. [CrossRef]
- Y. Kumar, S. Gupta, R. Singla, and Y. C. Hu, “A Systematic Review of Artificial Intelligence Techniques in Cancer Prediction and Diagnosis,” Arch. Comput. Methods Eng., vol. 29, no. 4, pp. 2043–2070, 2022. [CrossRef]
- Q. Zhang, J. Zhou, and B. Zhang, “Computational traditional Chinese medicine diagnosis: a literature survey,” Comput. Biol. Med., vol. 133, p. 104358, 2021. [CrossRef]
- A. Garg and V. Mago, “Role of machine learning in medical research: A survey,” Comput. Sci. Rev., vol. 40, p. 100370, 2021. [CrossRef]
- Shamshad et al., “Transformers in medical imaging: A survey,” Med. Image Anal., vol. 88, p. 102802, 2023. [CrossRef]
- Y. Jing et al., “A comprehensive survey of intestine histopathological image analysis using machine vision approaches,” Comput. Biol. Med., p. 107388, 2023. [CrossRef]
- M. Sufyan, Z. Shokat, and U. A. Ashfaq, “Artificial intelligence in cancer diagnosis and therapy: Current status and future perspective,” Comput. Biol. Med., p. 107356, 2023. [CrossRef]
- R. Ranjbarzadeh, A. Caputo, E. B. Tirkolaee, S. J. Ghoushchi, and M. Bendechache, “Brain tumor segmentation of MRI images: A comprehensive review on the application of artificial intelligence tools,” Comput. Biol. Med., vol. 152, p. 106405, 2023. [CrossRef]
- F. Ahmad, W. Rafique, R. U. Rasool, A. Alhumam, Z. Anwar, and J. Qadir, “Leveraging 6G, extended reality, and IoT big data analytics for healthcare: A review,” Comput. Sci. Rev., vol. 48, p. 100558, 2023. [CrossRef]
- S. Seoni, V. Jahmunah, M. Salvi, P. D. Barua, F. Molinari, and U. R. Acharya, “Application of uncertainty quantification to artificial intelligence in healthcare: A review of last decade (2013--2023),” Comput. Biol. Med., p. 107441, 2023. [CrossRef]
- X. Meng and T. Zou, “Clinical applications of graph neural networks in computational histopathology: A review,” Comput. Biol. Med., vol. 164, p. 107201, 2023. [CrossRef]
- X. Chen et al., “Recent advances and clinical applications of deep learning in medical image analysis,” Med. Image Anal., vol. 79, p. 102444, 2022. [CrossRef]
- Z. Liu, Q. Lv, Z. Yang, Y. Li, C. H. Lee, and L. Shen, “Recent progress in transformer-based medical image analysis,” Comput. Biol. Med., p. 107268, 2023. [CrossRef]
- S. Pandiyan and L. Wang, “A comprehensive review on recent approaches for cancer drug discovery associated with artificial intelligence,” Comput. Biol. Med., vol. 150, p. 106140, 2022. [CrossRef]
- Pacal, D. Karaboga, A. Basturk, B. Akay, and U. Nalbantoglu, “A comprehensive review of deep learning in colon cancer,” Comput. Biol. Med., vol. 126, p. 104003, 2020. [CrossRef]
- W. Hu et al., “A state-of-the-art survey of artificial neural networks for whole-slide image analysis: from popular convolutional neural networks to potential visual transformers,” Comput. Biol. Med., vol. 161, p. 107034, 2023. [CrossRef]
- R. J. Suji, S. S. Bhadauria, and W. W. Godfrey, “A survey and taxonomy of 2.5 D approaches for lung segmentation and nodule detection in CT images,” Comput. Biol. Med., p. 107437, 2023.
- M. K. Hasan, M. A. Ahamad, C. H. Yap, and G. Yang, “A survey, review, and future trends of skin lesion segmentation and classification,” Comput. Biol. Med., vol. 155, p. 106624, 2023.
- S. Tomassini, N. Falcionelli, P. Sernani, L. Burattini, and A. F. Dragoni, “Lung nodule diagnosis and cancer histology classification from computed tomography data by convolutional neural networks: A survey,” Comput. Biol. Med., vol. 146, p. 105691, 2022. [CrossRef]
- S. B. Lunge et al., “Therapeutic application of machine learning in psoriasis: A Prisma systematic review,” J. Cosmet. Dermatol., vol. 22, no. 2, pp. 378–382, 2023. [CrossRef]
- Li, S. : Supervisor, X. Wang, and M. Graeber, “Interpretable Radiomics Analysis of Imbalanced Multi-modality Medical Data for Disease Prediction,” no. March, 2022, [Online]. Available: https://ses.library.usyd.edu.au/handle/2123/28187.
- H. Witten, E. Frank, and M. A. Hall, Data Mining Practical Machine Learning Tools and Techniques Third Edition. Morgan Kaufmann, 2017.
- T. J. Saleem and M. A. Chishti, “Exploring the applications of Machine Learning in Healthcare,” Int. J. Sensors Wirel. Commun. Control, vol. 10, no. 4, pp. 458–472, 2020. [CrossRef]
- U. Kose and J. Alzubi, Deep Learning for Cancer Diagnosis. Springer Singapore, 2020.
- A. Ameri, “A deep learning approach to skin cancer detection in dermoscopy images,” J. Biomed. Phys. Eng., vol. 10, no. 6, pp. 801–806, 2020. [CrossRef]
- Y. Wu, B. Chen, A. Zeng, D. Pan, R. Wang, and S. Zhao, “Skin Cancer Classification With Deep Learning: A Systematic Review,” Front. Oncol., vol. 12, 2022. [CrossRef]
- A. Shimazaki et al., “Deep learning-based algorithm for lung cancer detection on chest radiographs using the segmentation method,” Sci. Rep., vol. 12, no. 1, p. 727, 2022. [CrossRef]
- M. Nishio et al., “Computer-aided diagnosis of lung nodule using gradient tree boosting and Bayesian optimization,” PLoS One, vol. 13, no. 4, p. e0195875, 2018. [CrossRef]
| Challenges | Opportunities |
|---|---|
| Limited dataset size and lack of annotated labels | Acquire larger datasets with annotated semantic labels for improved generalizability |
| Scalability and efficiency of algorithms | Explore hybrid models combining deep learning and conventional ML algorithms |
| Validation in clinical settings with medical experts | Integrate Decision Support Systems and CAD systems in operational clinical environments |
| Limited interpretability of models | Validate and assess models on diverse and high-volume datasets for extensive interpretability adoption |
| Resource utilization in healthcare organizations | Develop robust and efficient algorithms to optimize resource utilization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





