1. Introduction
Minoxidil was initially used as an antihypertensive medication in the 1970s due to its potent oral vasodilatory action. After its development and commercialization, adverse effects such as the growth of hair in various parts of the body began to be reported. Scientific evidence demonstrated its powerful effect in inducing hypertrichosis and hirsutism when the drug was ingested, leading to excessive growth of fine, dark, or coarse hair in localized areas or across the entire body, in both men and women. As a result, topical formulations of the medication began to be developed and marketed [
1,
2,
3,
4].
In Colombia, minoxidil is approved by the National Institute for Food and Drug Surveillance (INVIMA) as a medication for the treatment of alopecia, and there is sufficient evidence proven by clinical trials to corroborate its effectiveness [
5]. However, this ingredient cannot be used in cosmetic products, as Colombian regulations adhere to international lists of ingredients that can or cannot be incorporated into cosmetics [
6]. An example of this is the Cosmetic Ingredient Database (CosIng) of the European Commission, which states that minoxidil and its salts should not be used in cosmetic products due to their antihypertensive activity, as the absorption of minoxidil after application to the scalp could lead to systemic effects [
2,
7,
8]. Furthermore, the Scientific Committee on Cosmetic and Non-Food Products Intended for Consumers (SCCNFP), which advises the European Commission, stated on January 21, 1998, that “minoxidil and its salts should not be used in cosmetic products due to their antihypertensive activity and because the absorption of minoxidil after application to the scalp could lead to systemic effects”. The SCCNFP also provides relevant toxicity data, including significant cutaneous absorption in humans, fetotoxicity and maternal toxicity from subcutaneous injections in rats, and cardiotoxicity in long-term oral studies in rats and dogs [
9].
Additionally, every cosmetic product must declare on the label or packaging its qualitative composition, which should correspond to the content and nature of the product. In the event that the content does not match what is authorized, it will be considered an adulterated product and will be considered adulterated and subject to sanctions by the competent authorities in accordance with the relevant regulations [
6,
10].
However, there are different molecules derived from minoxidil that are approved by CosIng, including Minoxidil Hyaluronamide, Minoxidil Succinoyl Decapeptide-18, Minoxidil Succinoyl Oligopeptide-143, and Minoxidil Oxothiazolidinecarboxylate [
7]. Despite several approved derivatives listed above, the most used in cosmetic products is usually Oxothiazolidinecarboxylate due to its ease of incorporation into cosmetic products. Additionally, it is the most effective among all the derivatives in terms of hair growth [
4].
Despite these regulatory restrictions currently in place in Colombia and other countries, it has been found that minoxidil is still illegally used in cosmetic products for hair growth to enhance effectiveness and achieve better short-term results. Therefore, it has become of great importance to assess the use of unauthorized ingredients in cosmetics, and various research efforts have been undertaken to develop and validate methods for evaluating the presence of prohibited compounds such as minoxidil in these types of products [
11,
12,
13,
14,
15,
16].
Various studies have reported the presence of minoxidil and other unauthorized compounds in cosmetic products [
11,
13,
14,
16]. Wu CS et al. (2013) [
16] determined the presence of seven prohibited substances in cosmetic products, including minoxidil, hydrocortisone, spironolactone, estrone, canrenone, triamcinolone acetonide, and progesterone. Of the 37 products evaluated, minoxidil was found in eight of them, and concomitantly with spironolactone in one product. In a study by De Orsi D. et al. (2008) [
17], six cosmetic products (two creams and four lotions) sold on internet websites were analyzed to determine the presence of minoxidil, progesterone, estrone, spironolactone, canrenone, hydrocortisone, and triamcinolone acetonide. Not only was the presence of various prohibited substances demonstrated, but the percentages of these substances were extremely high. Furthermore, in a study by Park HN et al. (2018) [
11], 76 samples of products claimed to be hair growth treatments were analyzed, and the results once again showed the presence of unapproved compounds in cosmetics. It was found that around 10% of the samples were adulterated with five compounds, including minoxidil. In these types of research, the use of different analytical techniques has been reported, including liquid chromatography coupled with tandem mass spectrometry (LC-MS) [
11,
16,
17], UV spectrophotometry (12), capillary electrophoresis [
13,
15], and high-performance liquid chromatography-DAD (HPLC-DAD) [
14,
17].
In this study, the goal was to develop and validate a rapid, cost-effective, and reliable method with suitable selectivity for the determination of minoxidil in multi-matrix cosmetic products for hair growth. To achieve this, high-performance liquid chromatography with reverse-phase coupled to UV detection (HPLC-UV) was employed.
2. Materials and Methods
2.1. Reagents:
Secondary standard grade certified reference material (CRM) minoxidil was obtained from Sigma-Aldrich. Raw minoxidil material was donated by Licol Laboratories (Medellín, Colombia). HPLC grade and analytical grade methanol were obtained from Merck Chemical Supplies (Darmstadt, Germany). Niacinamide, biotin, onion extract, rosemary extract, and Minoxidil Oxothiazolidinecarboxylate were donated by Samara Cosmetic (Medellín, Colombia). The other ingredients for placebo preparation were obtained from LyM and LyF chemicals (Medellín, Colombia). The hair growth cosmetic products were purchased from beauty stores, pharmacies, and online retailers.
2.2. Method Validation
2.2.1. Placebo Preparation
The ingredients were weighed in the quantities described in
Table 1. First, water was weighed, and then each of the other ingredients was individually weighed and dissolved in the water. Between the addition of each ingredient, the mixture was stirred for approximately 2 minutes. Finally, it was packaged and labeled for subsequent use in method validation.
2.2.2. Chromatographic System
Analysis was performed using a Waters HPLC Refurbished, equipped with a UV detector to 280 nm (2487), an isocratic pump (515), an autosampler and a column thermostat. The analytical column (Waters Symmetry 4.6 mm x 75 mm x 3.5 µm) was thermostated at 25° C. The mobile phase consisted of methanol: water with 2 % acetic acid (35:65). After preparing the mobile phase, it was filtered through 0.45 µm nylon filters and ultrasonic for 5 minutes to degas [
18].
2.2.3. Minoxidil Stock Solution
12.5 mg of minoxidil CMR were weighed and placed in a 50 mL flask, 40 ml of methanol was added and shaken in ultrasound for 5 min, then made up to volume with methanol to obtain a stock solution with a concentration of 0.25 mg/mL.
2.2.4. Minoxidil Calibration Curve
Aliquots of 100 µL, 200 µL, 400 µL, 600 µL and 800 µL were taken from the stock solution. Each aliquot was adjusted with a remaining volume of methanol to obtain a final volume of 1 mL, leaving final concentrations of 0.025, 0.05, 0.1, 0.15, and 0.2 mg/mL equivalent to active percentages of 10 %, 20 %, 40 %, 60 %, 80 % and 100 %.
2.2.5. Selectivity
Preparation of placebo: 250 mg of placebo were weighed into a 50 mL flask, and approximately 40 mL of methanol was added. The mixture was subjected to ultrasound for 5 minutes and then made up to volume with methanol. It was filtered through 0.45 µm nylon filters and 20 µL of this solution were injected into the HPLC.
Preparation of the spiked sample: 12.5 mg were weighed into a 50 mL flask and dissolved in a small volume of methanol. Then, in the same flask, 250 mg of placebo were weighed, approximately 40 mL of methanol was added, and the mixture was subjected to ultrasound for 5 minutes. It was then made up to volume with methanol, filtered through 0.45 µm nylon filters, and 20 µL of this solution were injected into the HPLC [
19,
20].
2.2.6. Linearity and Accuracy
To evaluate the linearity parameter, minoxidil solutions were prepared at five concentration levels: 100%, 75%, 50%, 25%, and 10%. To prepare the 100% concentration, 125 mg of minoxidil raw material was weighed into a 50 mL flask. Enough methanol was added to dissolve it, and 2500 mg of placebo was added to the same flask. Then, it was sonicated for 5 minutes and topped up with methanol. Subsequently, a 1 mL aliquot was taken, transferred to a 10 mL flask, and topped up with methanol. Finally, it was filtered using 0.45 µm nylon filters, and 20 µL of this solution was injected into the HPLC. This procedure was performed in triplicate for each of the concentration levels to be prepared [
19,
20].
2.2.7. Limits of Detection and Quantification
For the determination of LOD (Limit of Detection) and LOQ (Limit of Quantification), the standard deviation of the placebo (obtained from the selectivity test data) and the slope of the calibration curve were used. The detection limit was determined using the equation [
19,
20]:
L: Limit of detection (LOD) or limit of quantification (LOQ)
K= constant, 10 for LOQ and 3 for LOD
Sb= Placebo standard deviation (selectivity)
b= Calibration curve slope (linearity)
2.2.8. Precision
Samples were prepared at concentrations equivalent to 50 %, 75 %, and 100 %, as described in section 2.2.6, Linearity, and accuracy [
19,
20].
Samples were prepared at concentrations equivalent to 100 %, as described in section
2.2.6. Linearity and Accuracy. The variation of two factors, day (two days), and researchers (three researchers), was evaluated [
19,
20].
2.2.9. Robustness
To evaluate the method's robustness, a Youden-Steiner factorial design was conducted, as represented in
Table 2. The treatment of the samples was carried out following the description in each of the assays (columns 1-8). The percentage recovery was calculated for each assay [
19,
20].
Where:
|
A: Sample preparation 16 mg/25*4/10 mL |
a: Sample preparation 32 mg/25*2/10 mL |
|
B: Ultrasound 5 min |
b: Ultrasound 2 min |
|
C: Room temperature bath |
c: Hot water bath (50°C c.a.) |
|
D: Pre-dissolution of minoxidil in methanol |
d: No pre-dissolution of minoxidil in methanol |
|
E: Injection volume 20 uL |
e: Injection volume 10 uL |
|
F: Flow rate of MP 1.0 mL/min |
f: Flow rate of MP 1.1 mL/min |
|
G: New sample filters |
g: Reused sample filters |
2.3. Determination of Minoxidil in Hair Growth Products
For each product, 50 mg were weighed and transferred to a 10 mL flask. 8 mL of methanol was added, and it was sonicated for 5 minutes. Then, it was topped up, filtered using 0.45 µm nylon filters, and 20 µL of this solution was injected into the HPLC. Each product was evaluated in triplicate by three different analysts.
2.4. Statistical Analysis
Results were expressed as the mean of repetitions, and standard deviation and coefficients of variation were calculated. For linearity, R and R2 were calculated, and additionally, an analysis of variance (ANOVA) and a student’s t-test were performed to evaluate the slope and intercept. For accuracy, the percentage recovery was evaluated, and a Cochran's G test was conducted to assess the influence of concentration levels on variances. A reduced factorial design of Youden-Steiner was used for robustness. All calculations were performed at Microsoft Excel.
3. Results and Discussion
3.1. Method Validation
As part of the compliance verification for legal requirements, the assessment of efficacy and safety of cosmetic products, as well as contributing to active cosmetovigilance through the determination of the use of prohibited substances in cosmetics, it becomes necessary to develop analytical methods that can be easily applied in post-marketing surveillance and control stages, in addition to being reliable and economical. For this reason, we proceeded to carry out assays for the determination of minoxidil using UV spectrophotometry (data not shown); however, good selectivity was not achieved to differentiate minoxidil base from its derivatives. In this regard, we proceeded to develop and validate a method that would meet the minimum quality parameters according to the ICH for the determination of the analyte of interest by HPLC-UV [
18,
19,
20].
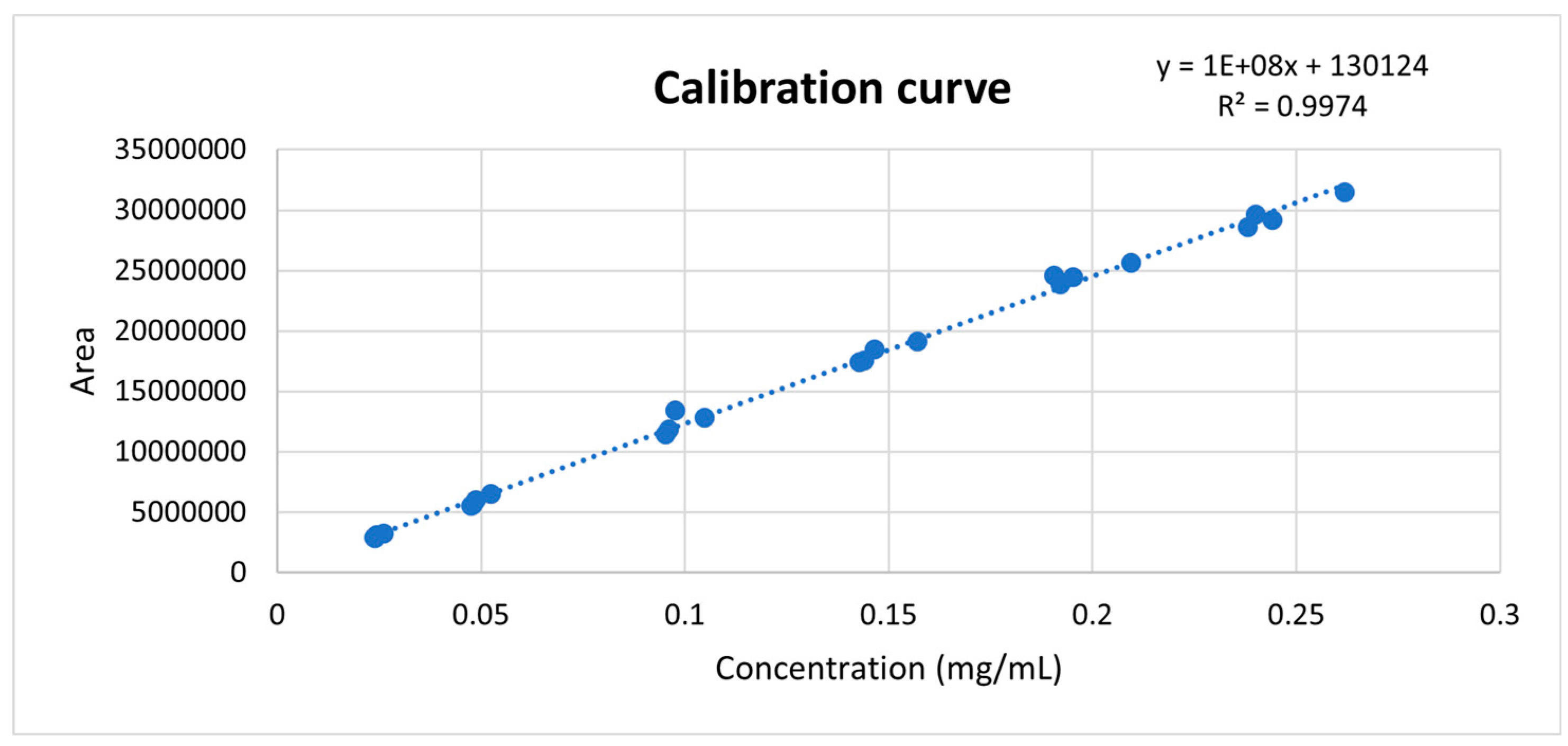
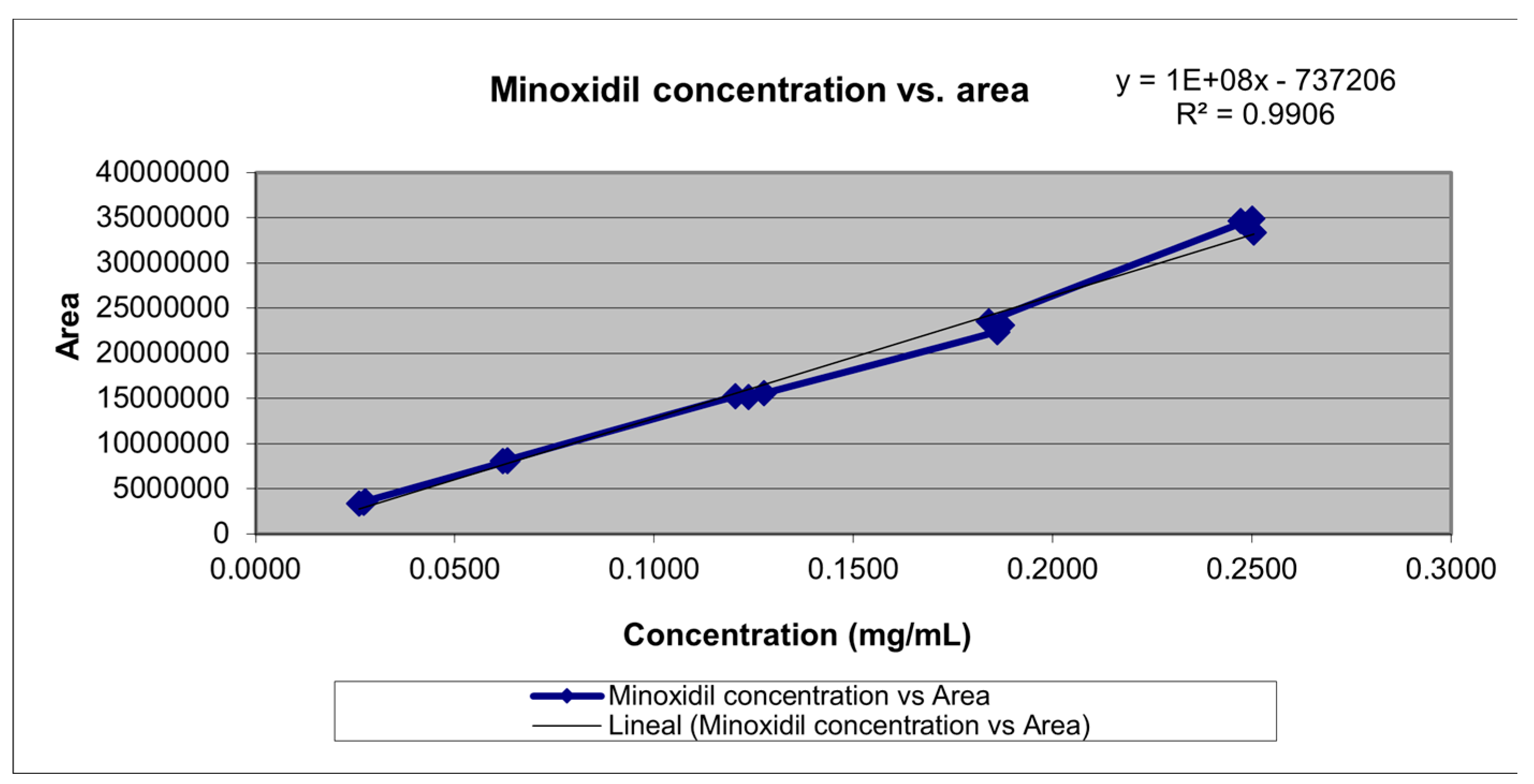
With the calibration curve (
Figure 1), the range of concentrations with linear behavior was determined. In addition to this, the slope and intercept of the straight-line equation were used to calculate the concentrations or recovery percentages of minoxidil from the obtained areas. This allowed the identification and quantification of minoxidil in the evaluated products.
3.1.1. Selectivity
The placebo formulation was prepared considering the different ingredients used in the evaluated products, aiming to create a matrix similar to hair growth cosmetic products. This was done to determine potential interferences in the quantification of the analyte.
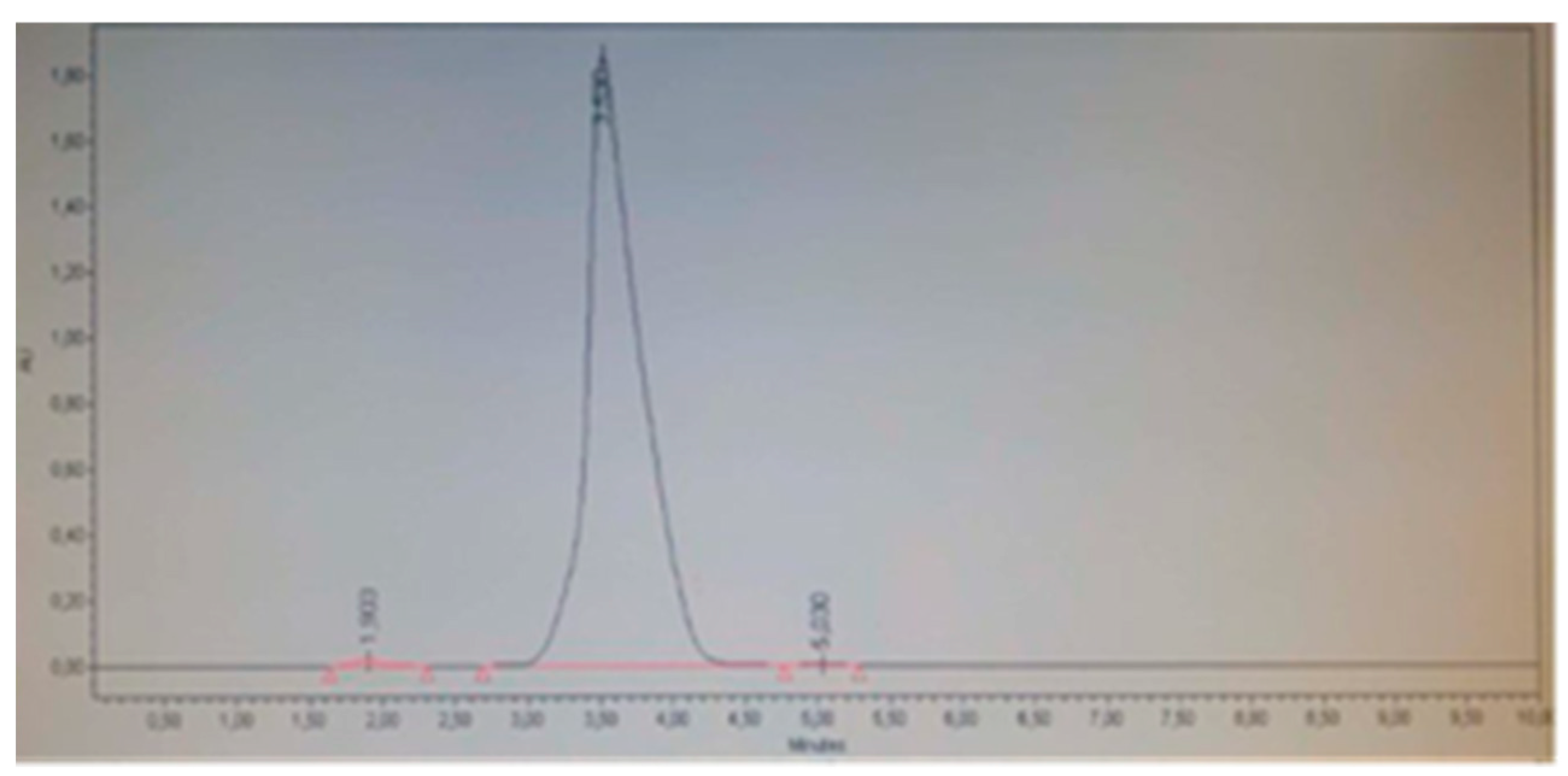
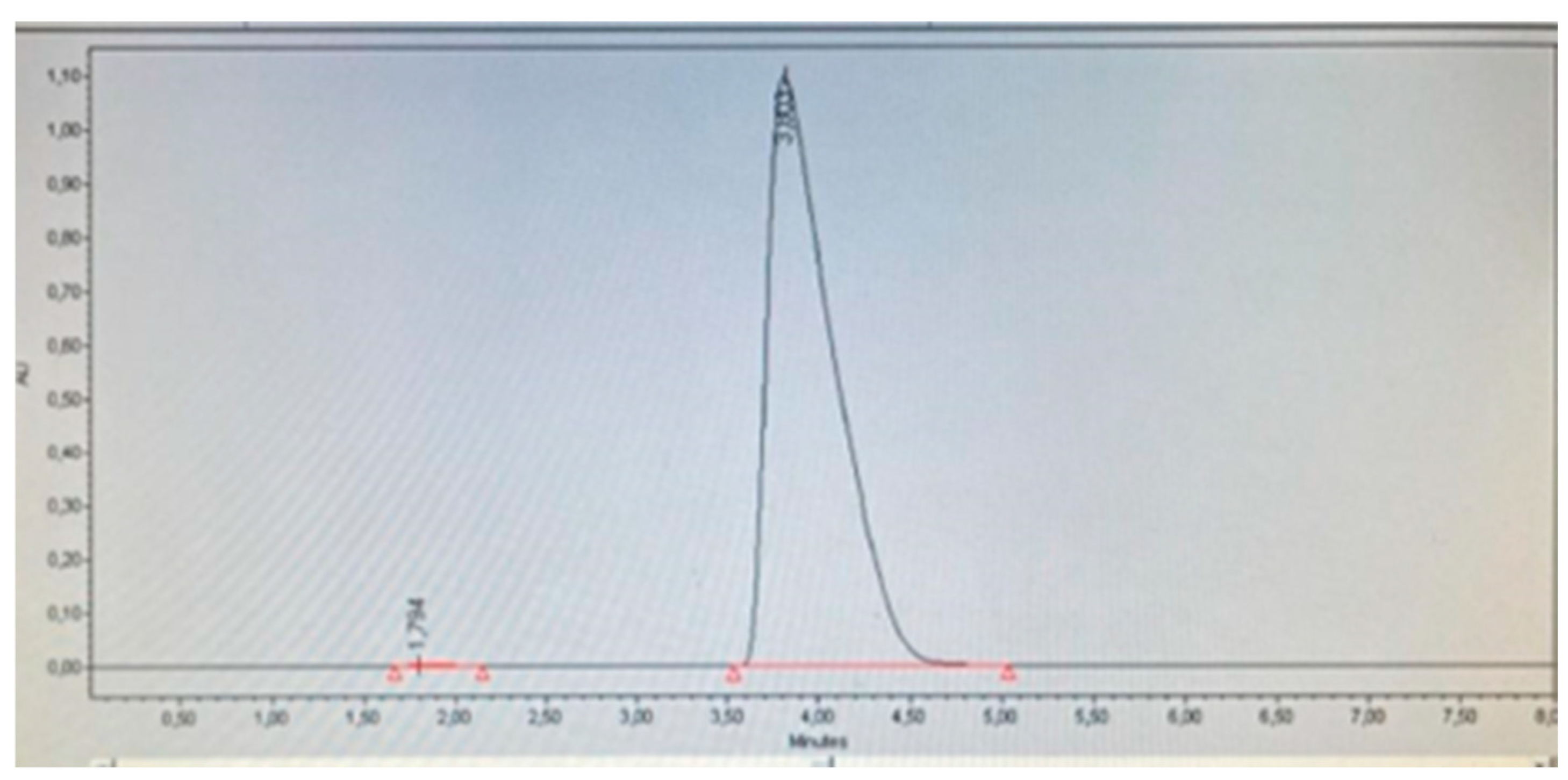
Figure 2 shows that the placebo does not interfere with the detection of the analyte of interest, as no significant peaks are observed in the retention time (tR) between 3.8-4.0 that could interfere with the detection and quantification of minoxidil, in comparison with the height and area ratio observed in the spiked placebo (
Figure 3). Therefore, it can be concluded that the placebo does not interfere with the measurements, indicating the selectivity of the method.
It was determined that the placebo spiked with the analyte at a concentration of 0.25 mg/mL did not present interferences in the detection and quantification of minoxidil, allowing the real recovery of the analyte (98.56 %), with a retention time (RT) for minoxidil of 3.803 according to the standard, showing that despite being within the matrix, there was no matrix effect that significantly increased or decreased the recovery of the analyte (
Figure 3). In this sense, Park HN et al. (2018) [
11] demonstrated the selectivity of their analytical method in a similar manner by comparing the matrix before and after adding the analyte of interest to identify potential interferences present in the sample. The results showed that there were no interference peaks at the target retention time (RT).
3.1.2. Linearity
Linear regression allows evaluating the fit to the proposed linear model through the correlation coefficient (R) indicated at the top of
Table 3. In this case, considering products with a matrix composed of a large number of different ingredients, the obtained R value is adequate, indicating the data's non-variability and their accommodation to the defined acceptance criteria. Using least squares, the equation of the straight-line y = 135270081.5x – 737205.57 was obtained, which proportionally relates the instrumental response 'y' (area) to the analyte concentration 'x', as observed in
Figure 4.
Another key statistical measure derived from linear regression is the coefficient of determination (R^2), which reflects the goodness of fit of a model to the variable being explained. The obtained value of R^2 demonstrates that there is a direct relationship between the analyte concentration and the instrumental response obtained. Additionally, an analysis of variance (ANOVA) was conducted to statistically demonstrate the existence of a non-zero slope and the linear relationship of the results obtained. This was confirmed, as observed in
Table 3, where the calculated F1 is greater than the tabulated F, thus rejecting the null hypothesis representing a slope equal to zero. On the other hand, the calculated F2 is less than the tabulated F, confirming the null hypothesis, which, in this case, represents the existence of a linear relationship in the data. The t-test was also employed to demonstrate that the method responds to changes in concentration, meaning that the slope is different from zero (calculated t > tabulated t for the slope) and that the confidence interval of the slope does not include zero. Additionally, it was observed that the method is proportional, as the confidence interval of the intercept includes zero and the calculated t is less than the tabulated t for the intercept. It is concluded that the method is linear within the evaluated concentration range.
3.1.3. Accuracy
To evaluate accuracy, a criterion of acceptance was established for the recovery percentage of 95-105 %.
Table 4 shows mean of the results obtained for the 5 levels evaluated, standard deviations, coefficients of variation, and variances for each of the measurements, meeting the established parameters and showing better recoveries (between 100.3 and 104.3%) than those reported in the literature. In this regard, Park HN et al. (2018) [
11] developed and validated a method for the determination of 12 hair growth compounds in adulterated products by UHPLC-MS/MS, and in the assessment of the accuracy parameter, they observed recoveries between 81-117 % for minoxidil, values that were defined as acceptable for method validation. Comparing our method with the one mentioned in the present study, it can be said that ours has greater reliability. Additionally, in the present study, the Cochran's G test was applied to evaluate the variances at the five concentration levels, concluding that the concentration factor does not influence the variability of the results; therefore, the method is accurate.
3.1.4. Precision—Repeatability
The repeatability parameter was evaluated at 50, 75, and 100 %, and a global coefficient of variation equal to or less than 3% was established as the acceptance criterion. The obtained global coefficient of variation (2.6%) for this parameter falls within the established range, as observed in
Table 5. Therefore, it is concluded that the percentages found in the repetitions of the samples analyzed did not exhibit significant variability.
3.1.5. Intermediate Precision
In the current study, intermediate precision was assessed considering two variables: the analyst and the day. The acceptance criterion for the evaluation of intermediate precision was defined as a global coefficient of variation less than or equal to twice the coefficient of variation for repeatability, that is, 5.2 %, and the coefficient of variation for each subgroup less than or equal to 3%. In
Table 6, it is observed that analysts 1 and 3 obtained a similarly low coefficient of variation between them; however, analyst 2 had higher inter-day variations. Despite this, the average global coefficient of variation is below the established acceptance criterion. Therefore, the variability that occurs when conducting the assay on different days and with different analysts is low, which does not significantly impact the results. Based on the results obtained in this parameter, it is concluded that the method is precise.
In the previously mentioned study for the validation of a method for the determination of non-permitted compounds using UHPLC-MS/MS, precision was measured considering inter-day parameters, evaluating on three different days, and intra-day parameters that were determined from repeated measurements on a single day. The results obtained show that the intra-day precision was below 4%, and for the inter-day case, it was at 11%. Both values were considered suitable to determine that the method is precise 11, however, both results are higher than those obtained in our study. Moreover, Patterson SC et al. (2005)15 found in a method they developed for determining Minoxidil, CV% values similar to those we found (Intra-day: 0.0114-0.0133 and inter-day: 0.0243-0.0329).
3.1.6. Detection and Quantification Limits
According to equation 1, a detection limit of 8.03x10-6 mg/mL (0.00803 ppm) and a quantification limit of 0.000027 mg/mL (0.027 ppm) were obtained. Therefore, the method allows for the detection and quantification of small amounts of the analyte under study.
3.1.7. Robustness
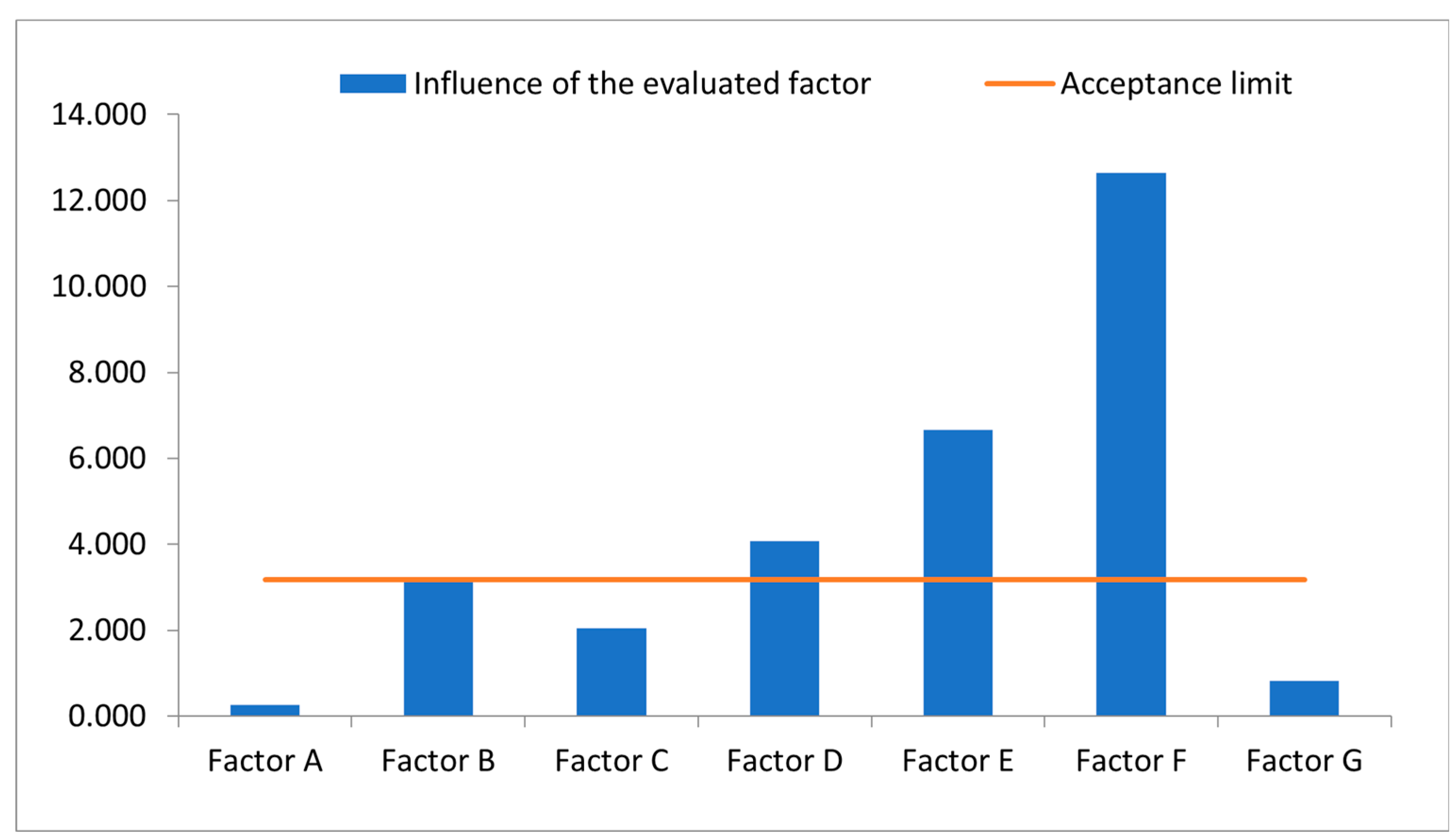
The objective of the robustness test was to determine which processes or steps of the method are critical and may affect the recovery of the analyte, and thus the accuracy of the measurement. For this parameter, a reduced Youden-Steiner factorial design was used, evaluating 7 variables in 8 assays, and the % recovery was determined as the response variable (
Table 7). Furthermore, as an acceptance criterion, it was defined that the influence of a factor is relevant and considered significant if, when comparing the value of the effect with the expression s√2, where s is the standard deviation of repeatability, the differences exceed in absolute value the result of this expression. With this evaluation, it was observed that the relevant factors in the method application were reducing the time in ultrasound (Factor B), pre-dissolving minoxidil in methanol before weighing or adding the placebo (Factor D), injection volume (Factor E), and flow change (Factor F) (
Figure 5). For this reason, especially these parameters must be kept unchanged as they significantly influence the results obtained, while other factors such as: modification of the aliquot (Factor A), control of temperature in ultrasound (Factor C), and the use of new or used filters (Factor G), do not significantly influence. In this sense, for the method to be reliable, the significant factors must be rigorously followed when performing quantification (
Table 7 and
Figure 5).
3.2. Determination of Minoxidil in Hair Growth Products
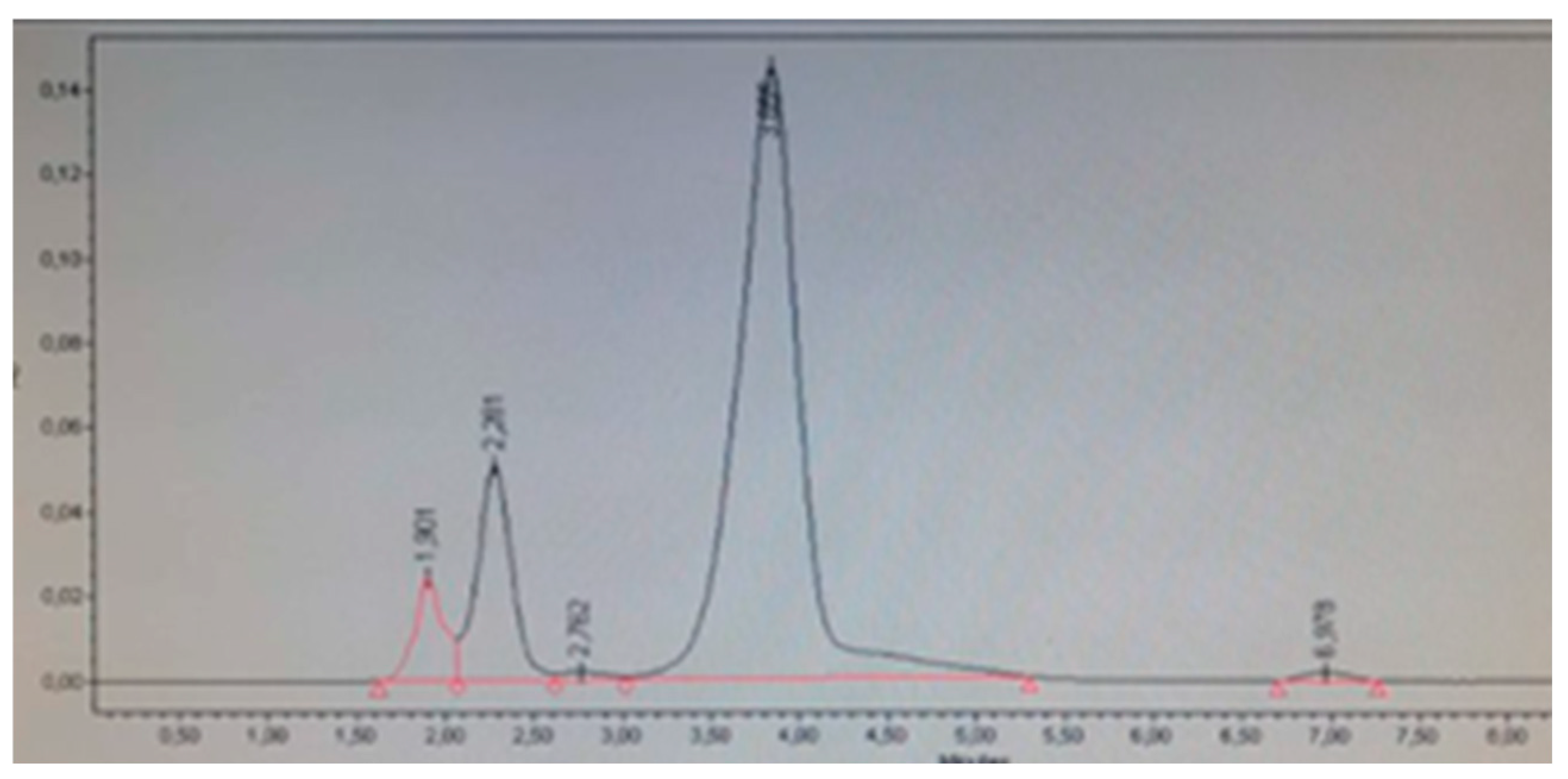
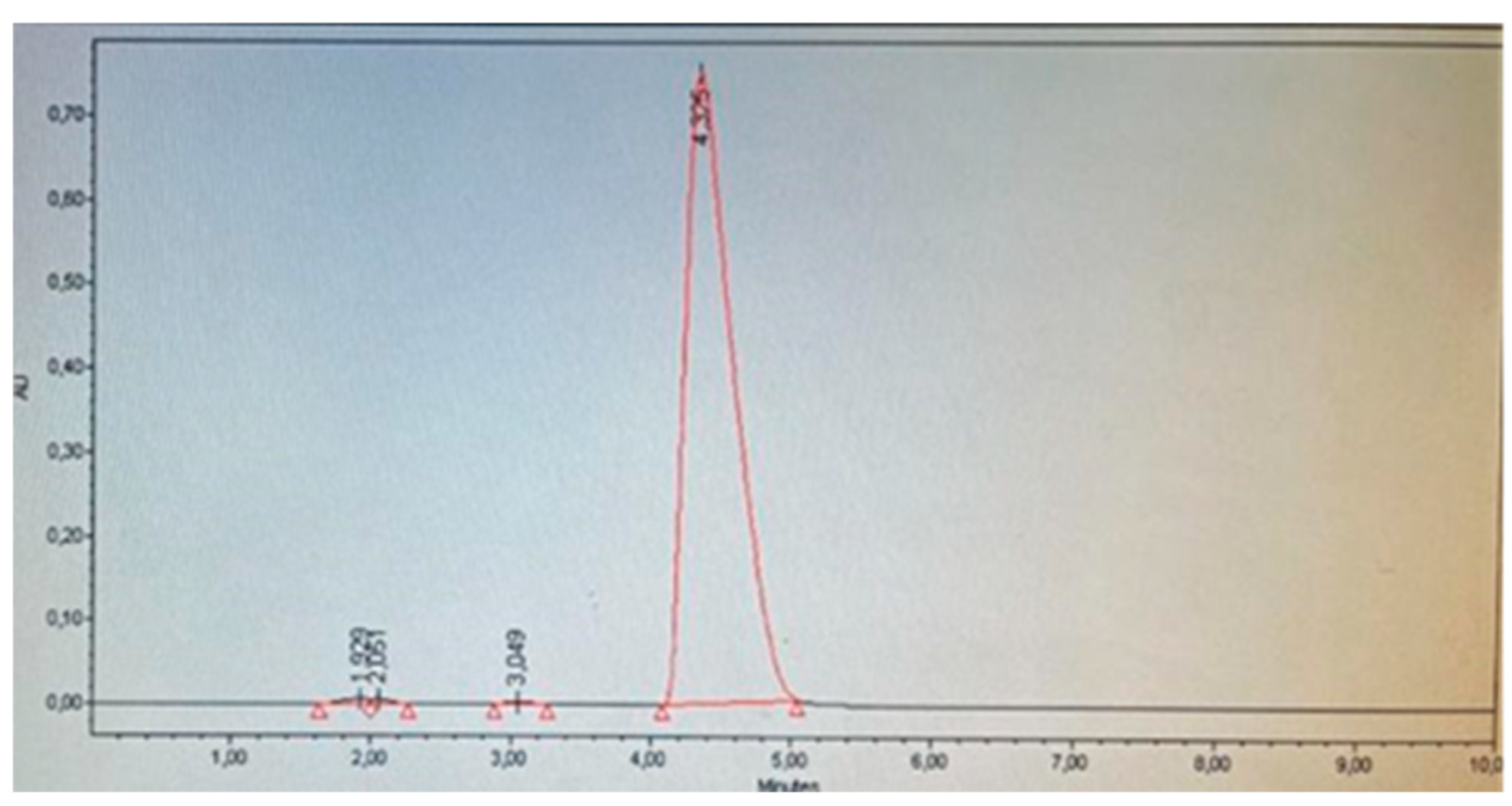
Eleven products were selected from market: two medications declaring to contain 5 % Minoxidil and nine cosmetic hair growth products with different matrices. All products were purchased from websites, beauty stores, hair salons, and barber shops. As selection criteria, products suspected to contain Minoxidil and cosmetic hair growth products declaring Minoxidil were considered, even if it was not specified in the ingredient list whether it is a derivative of Minoxidil or corresponds to the base Minoxidil. According to the method validation, it was found that the base minoxidil had an approximate retention time (tR) of 3.8, while minoxidil oxothiazolidinecarboxylate, in addition to this peak, presented another peak of lower intensity at a tR of 2.5-3.0. This latter peak helped differentiate whether the products contained base minoxidil or minoxidil oxothiazolidinecarboxylate (
Figure 6 and
Figure 7).
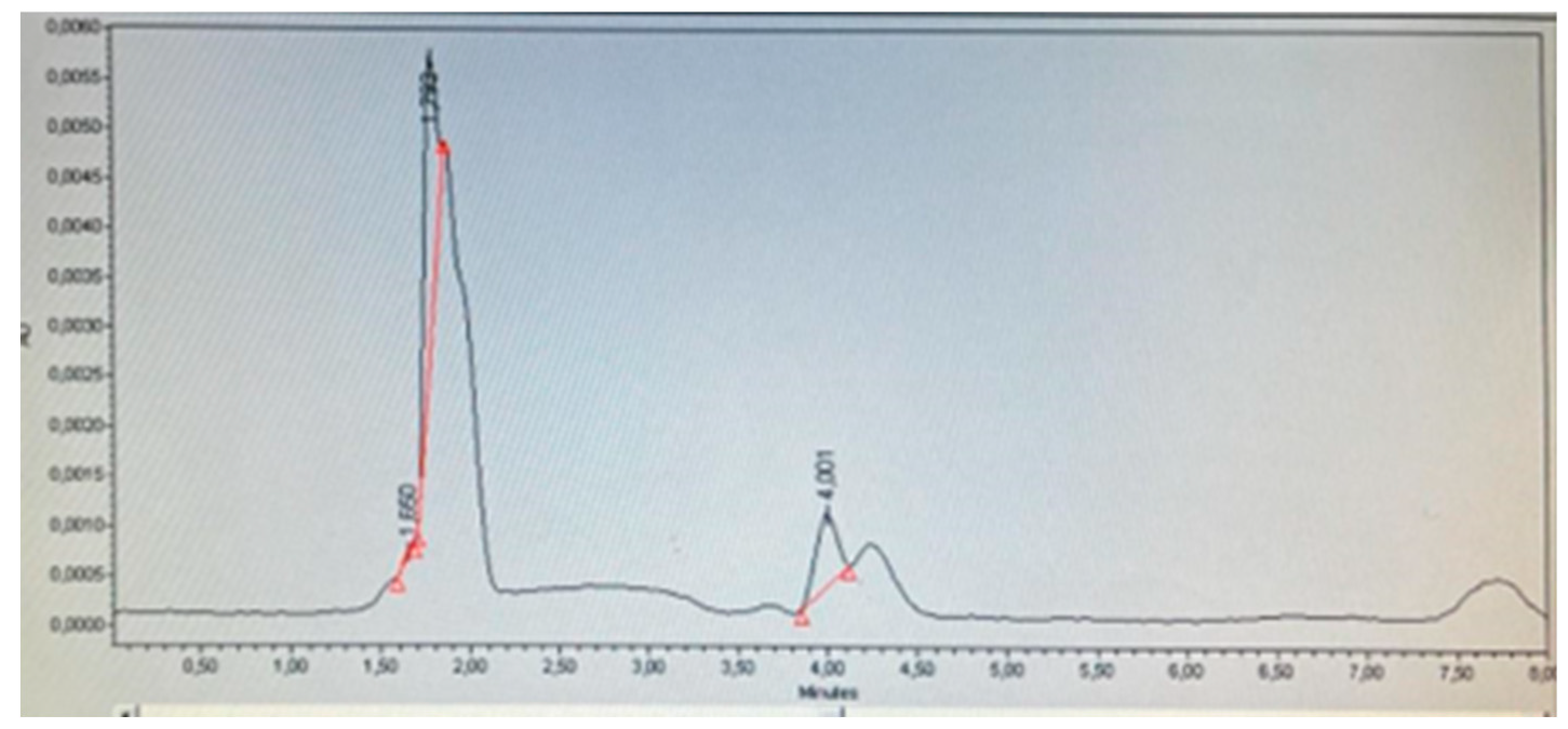
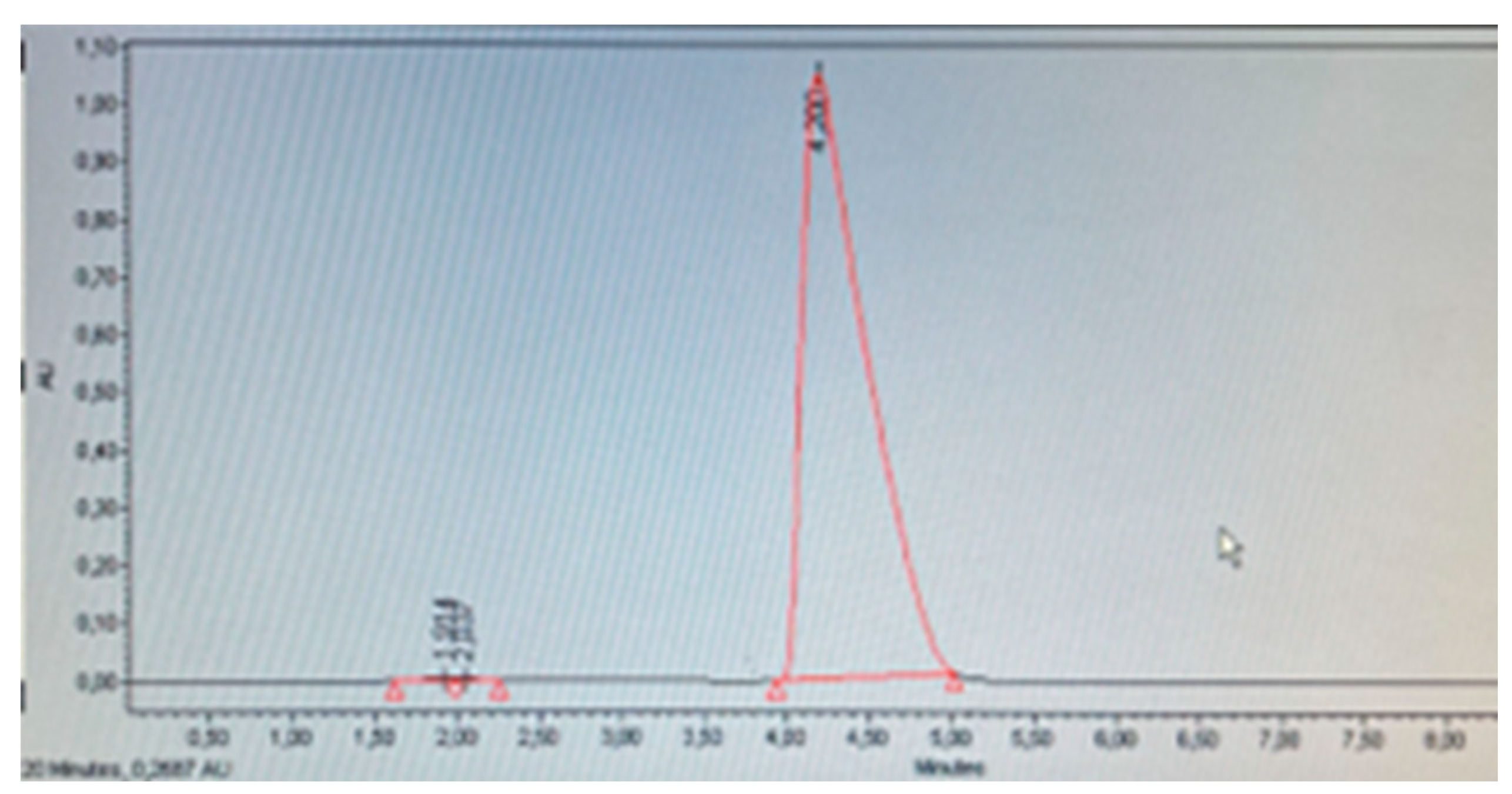
Table 8 shows the results of the determination of Minoxidil and its derivative Minoxidil oxothiazolidinecarboxylate. It was identified that products A, E, F, G, and K do not contain Minoxidil, which corresponds to what is declared on their respective labels. Products C and H (
Figure 8) declare on their label to contain Minoxidil oxothiazolidinecarboxylate, and through identification and quantification, it was corroborated that this compound was present and not the base Minoxidil (
Table 8). However, product D did not present the characteristic peak at tR 2.5-3.0 indicative of the derivative Minoxidil oxothiazolidinecarboxylate, therefore, it is presumed that this cosmetic product contains base Minoxidil (
Figure 7). This adverse analytical result is compounded by the fact that this product showed appearance problems, presenting a large amount of precipitated white crystals, which were separated by filtration, washed, and dried in an oven at 60°C. Subsequently, a solution was prepared with these crystals and injected using the same chromatographic system, presenting a retention time corresponding to base Minoxidil. These findings suggest that this product does not comply with international standards for cosmetic ingredients, as discussed, this substance is prohibited for use in cosmetics [
6,
7].
Figure 8.
Product D chromatogram.
Figure 8.
Product D chromatogram.
Figure 9.
Product H chromatogram.
Figure 9.
Product H chromatogram.
Product B declares to contain minoxidil; however, its ingredient list does not specify whether it is minoxidil oxothiazolidinecarboxylate or another approved derivative. Upon verification on the website of INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos de Colombia), its Mandatory Health Notification (NSO), the search yielded no results, which raises suspicions of possible regulatory non-compliance. Additionally, the analysis of this product showed the specific peak for base Minoxidil, indicating that this product likely contains the prohibited form of minoxidil in cosmetics.
Two medications, topical solutions of minoxidil, products I and J, were also analyzed. These were evaluated as reference products for the identification of minoxidil. Indeed, it was confirmed that these products, being medications rather than cosmetics, contain declared base minoxidil at a concentration of 5 %. Drug product I, which is illegally imported into the country as it lacks a sanitary registration, had a found percentage of Minoxidil of 1.10±0.02%, while medication J, a domestically manufactured product with sanitary registration, had a found percentage of 5.29±0.10 %. According to the individual USP monograph, these medications should have between 90 – 110% of the labeled quantity, meaning between 4.5 – 5.5% of Minoxidil per formulation. Therefore, product I, illegally imported into the country, does not meet the quality characteristics for the assay according to the USP 18.
In the results obtained in this research, it was found that presumably 2 out of the 9 cosmetic products analyzed contained the prohibited analyte base minoxidil, i.e., 22.22 % of the analyzed products, which is consistent with what has been reported in other studies [
11,
13,
16,
17].
In this regard, De Orsi et al. (2008) determined the presence of seven prohibited substances in cosmetic products, including the substances evaluated: minoxidil, hydrocortisone, spironolactone, estrone, canrenone, triamcinolone acetonide, and progesterone, found that of the six products evaluated, minoxidil was present in four of them, and concomitantly with spironolactone in two product [
17]. Not only did these products demonstrate the presence of different prohibited substances, but also the percentages of these substances were extremely high. Finally, another study conducted in 2018 analyzed 76 samples of products claimed to be hair growth treatments. Once again, the results demonstrated the presence of unapproved compounds in cosmetics. It was found that around 10 % of the samples were adulterated with five prohibited ingredients, including minoxidil [
11]. Finally, with this work we hope to contribute to the inspection, surveillance, and post-market control activities of cosmetic products, with the aim of reducing regulatory non-compliance due to the use of prohibited ingredients, leading to reducing the trend of the number of fraudulent products in the international markets.
4. Conclusions
This work allowed for the development, standardization, and validation of an HPLC-UV analysis methodology that is much simpler, faster, and more economical compared to other methods reported for the identification and quantification of minoxidil in cosmetic products and medications. The results obtained in this research coincide with what has been reported in the literature regarding the use of prohibited substances in cosmetic products. Additionally, it is worth noting that it was rigorously determined whether the presence of minoxidil corresponded to the base molecule or to a derivative such as minoxidil oxothiazolidinecarboxylate. This distinction is not present in other related articles that have evaluated the presence of prohibited substances like minoxidil; which could lead to erroneous results. However, it is suggested to verify these results with other more selective techniques such as HPLC-DAD or LC-MS, which can support these claims. It is hoped that the results obtained will contribute to the generation of cosmetovigilance activities, cosmetic control, and inspection, as an important strategy to prevent illegality or regulatory non-compliance by manufacturers.
Author Contributions
Conceptualization, J.C.M.-G.; methodology, J.C.M.-G, M.L-L, D.M.T-L and T. R-A; validation, M.L-L, D.M.T-L and T. R-A.; formal analysis, J.C.M.-G; investigation, J.C.M.-G, M.L-L, D.M.T-L and T. R-A; resources J.C.M.-G; data curation, J.C.M.-G and M.L-L; writing—original draft preparation, M.L-L, D.M.T-L and T. R-A; writing—review and editing, J.C.M.-G; supervision, J.C.M.-G; project administration, J.C.M.-G; funding acquisition, J.C.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted with financial support from the Research Vice-Rectorate of the University of Antioquia - Medellín, Colombia. Call for research projects submitted by undergraduate students of the Faculty of Pharmaceutical and Food Sciences of the University of Antioquia, year 2022: CIFAL 334.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors acknowledge to Licol Laboratories (Medellín, Colombia and Samara Cosmetic (Medellín, Colombia) for all the materials donated for the development of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-Label Use of Topical Minoxidil in Alopecia: A Review. Am J Clin Dermatol. 2019, 20, 237–250. [CrossRef]
- Sanabria BD, Palmegiani E, Seron AF, et al. Prospective cardiovascular evaluation with 24-hour Holter and 24-hour ambulatory blood pressure monitoring in men using 5-mg oral minoxidil for androgenetic alopecia. J Am Acad Dermatol. 2023, 88, 436–437. [CrossRef]
- Sattur S, Talathi A, Shetty G, et al. Comparative Clinical Study Evaluating the Efficacy and Safety of Topical 5% Cetosomal Minoxidil and Topical 5% Alcohol-Based Minoxidil Solutions for the Treatment of Androgenetic Alopecia in Indian Men. Cureus. 2023, 15, 1–8. [CrossRef]
- Abdullah, A. Comparative Study of the Online Over-The-Counter Hair Loss Products. J Dermatology Res Ther. 2020;6(1). [CrossRef]
- Ministerio de la Protección Social - Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA). Normas Farmacológicas. Colombia: https://app.invima.gov.co/ovirtual/public/NormasfarmacologicasMARZO2023.pdf; 2023:1-445.
- Comision de la Comunidad Andina. Decisión 833. Armonización de Legislaciones En Materia de Productos Cosméticos.; 2018:1-18.
- European Commission. CosIng - Cosmetics Ingredients [database online]. https://ec.europa.eu/growth/tools-databases/cosing/advanced. Accessed February 6, 2024.
- da Silva Prado L, Grivicich I, Miri JM, et al. Toxicological assessment of minoxidil: A drug with therapeutic potential besides alopecia. Food Chem Toxicol. 2023;182(November). [CrossRef]
- SCCNFP. Opinion of the Scientific Committee on Cosmetic and Non-Food Products Intended for Consumers Concerning Minoxidil and Salts Adopted by the Plenary Session of the SCCNFP.; 1998.
- Ministerio de salud - Republica de Colombia. Decreto 677. Colombia; 1995.
- Park HN, Lee JH, Park SK, et al. Development and validation of rapid and simultaneous method for determination of 12 hair-growth compounds in adulterated products by UHPLC–MS/MS. Forensic Sci Int. 2018;284:129-135. [CrossRef]
- Zaheer ZA, Mirza S, Moazzam I, et al. UV-Spectrophotometric determination of minoxidil and its application to the assay in pharmaceutical dosage forms. Der Pharma Chem. 2012, 4, 568–573.
- Gibson G, Ramstad T, Mills KA, et al. A method for the determination of minoxidil in hair-regrowth formulations by micellar electrokinetic capillary chromatography. Farmaco. 2005, 60, 847–853. [CrossRef]
- Gagliardi L, Amato A, Turchetto L. Simultaneous determination of minoxidil and tretinoin in pharmaceutical and cosmetic formulations by reversed-phase HPLC. Anal Lett. 1991, 24, 1825–1835. [CrossRef]
- Patterson SC, Ramstad T, Mills KA. Development and validation of a procedure for the determination of minoxidil in hair-regrowth formulations using two variants of capillary zone electrophoresis. Farmaco. 2005;60(6-7):547-554. [CrossRef]
- Wu CS, Jin Y, Zhang JL, et al. Simultaneous determination of seven prohibited substances in cosmetic products by liquid chromatography-tandem mass spectrometry. Chinese Chem Lett. 2013, 24, 509–511. [CrossRef]
- De Orsi D, Pellegrini M, Pichini S, et al. High-performance liquid chromatography-diode array and electrospray-mass spectrometry analysis of non-allowed substances in cosmetic products for preventing hair loss and other hormone-dependent skin diseases. J Pharm Biomed Anal. 2008, 48, 641-648. [CrossRef]
- United States Pharmacopeial Convention. Monografia Individual Minoxidil. In: Farmacopea de Los Estados Unidos de América: USP 42, NF 37. Rockville, Md: United States Pharmacopeial Convention; 2022.
- International Conference on Harmonization - ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1).; 1996:17. https://doi.org/2_R1/Step4/Q2_R1__Guideline.pdf.
- United States Pharmacopeial Convention. VALIDACIÓN DE PROCEDIMIENTOS FARMACOPEICOS. In: Farmacopea de Los Estados Unidos de América: USP 42, NF 37. ; 2022.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).