Submitted:
31 January 2025
Posted:
03 February 2025
You are already at the latest version
Abstract
Ambrosia beetles bore into trees, excavating galleries where they farm fungi as their sole source of nutrition. These mutualistic fungi typically do not cause significant damage to host trees; however, since their invasion into the U.S., the beetle Xyleborus glabratus has vectored its fungal partner, Harringtonia lauricola, which has acted as a devastating plant pathogen resulting in the deaths of over 500 million trees. Here, we show differences in mycangial colonization of the indigenous X. affinis ambrosia beetle by H. lauricola, and the native fungal species, H. aguacate and Raffaelea arxii. While X. affinis was a good host for H. lauricola, the related ambrosia beetle, X. ferrugineus, was only marginally colonized by H. lauricola. X. affinis beetles neither fed on, nor were colonized by, the distantly related fungus, Magnaporthe oryzae. Mycangial colonization was affected by the nutritional state of the fungus. A novel method for direct quantification of mycangial contents based on cell cytometry was developed and validated. The method was used to confirm mycangial colonization and to demonstrate alternating fungal partner switching, which showed significant variation and dynamic turnover. X. affinis pre-oral mycangial pouches were visualized using fluorescent and light microscopy, revealing that newly emerged pupae displayed uncolonized mycangia prior to feeding, whereas beetles fed H. lauricola contained single-celled fungi within 6 h post-feeding. Mixed populations of fungal cells were seen in the mycangia of beetles following alternating colonization. Nuclear counter-staining revealed insect cells surrounding the mycangia. These data highlight variation and specificity in ambrosia beetle-fungal pairings and provide a facile method for direct quantification of mycangial contents.
Keywords:
1. Introduction
2. Materials and Methods
Insect Rearing and Fungal Strains and Culture Conditions
Experimental Mycangia Colonization, Determination of Colony Forming Units (CFUs), and Cell Cytometry Assays
Microscopy
Data Analysis
3. Results
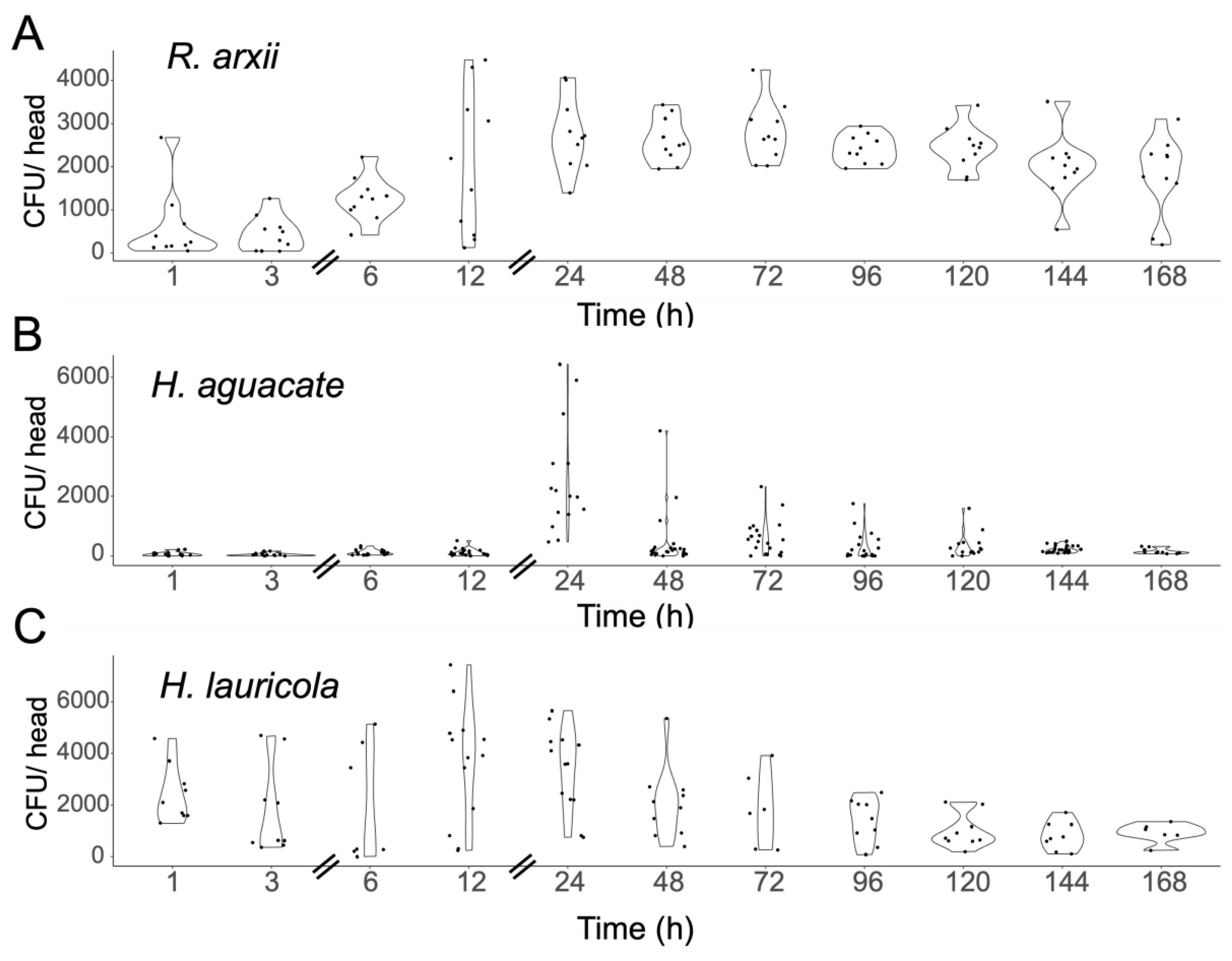
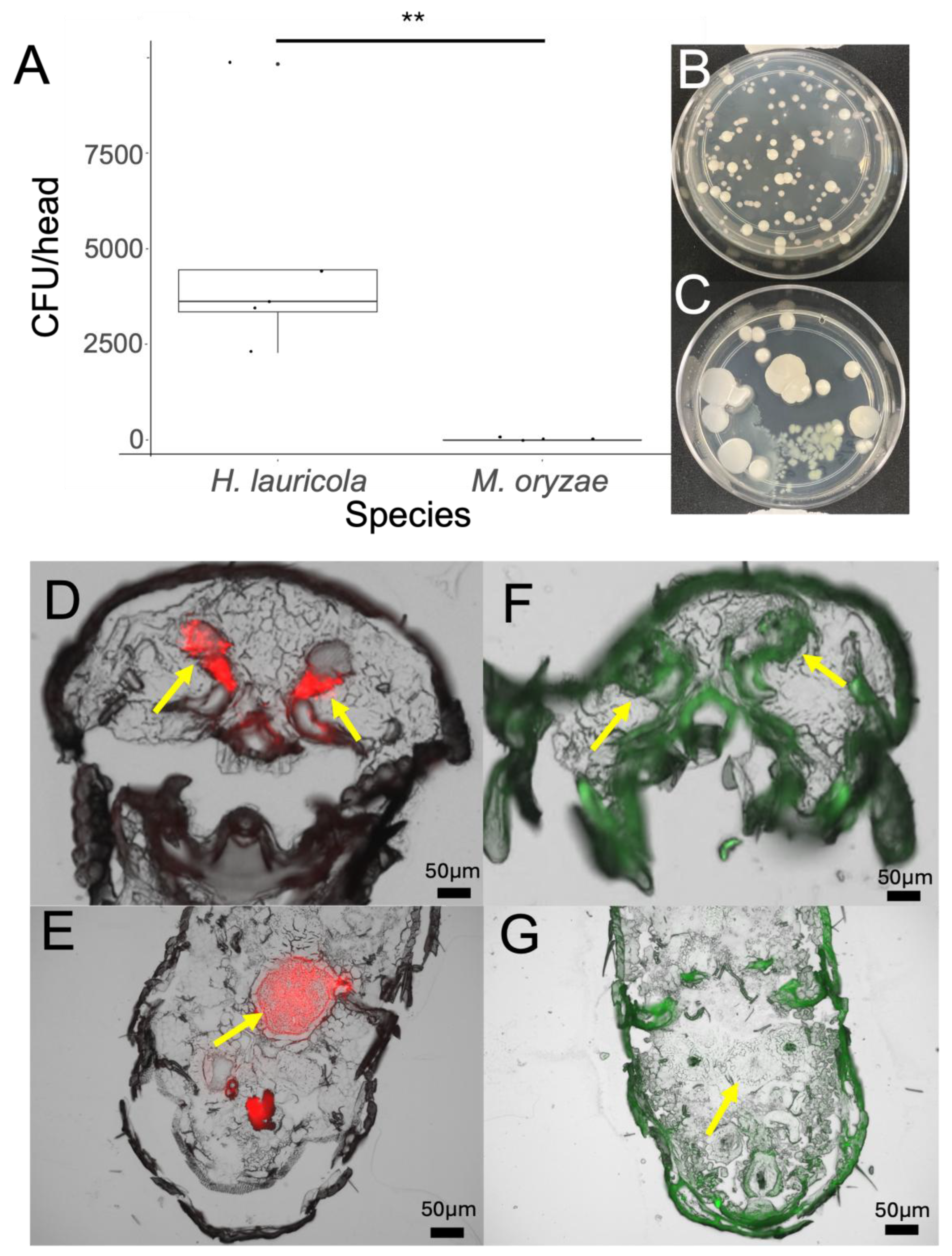
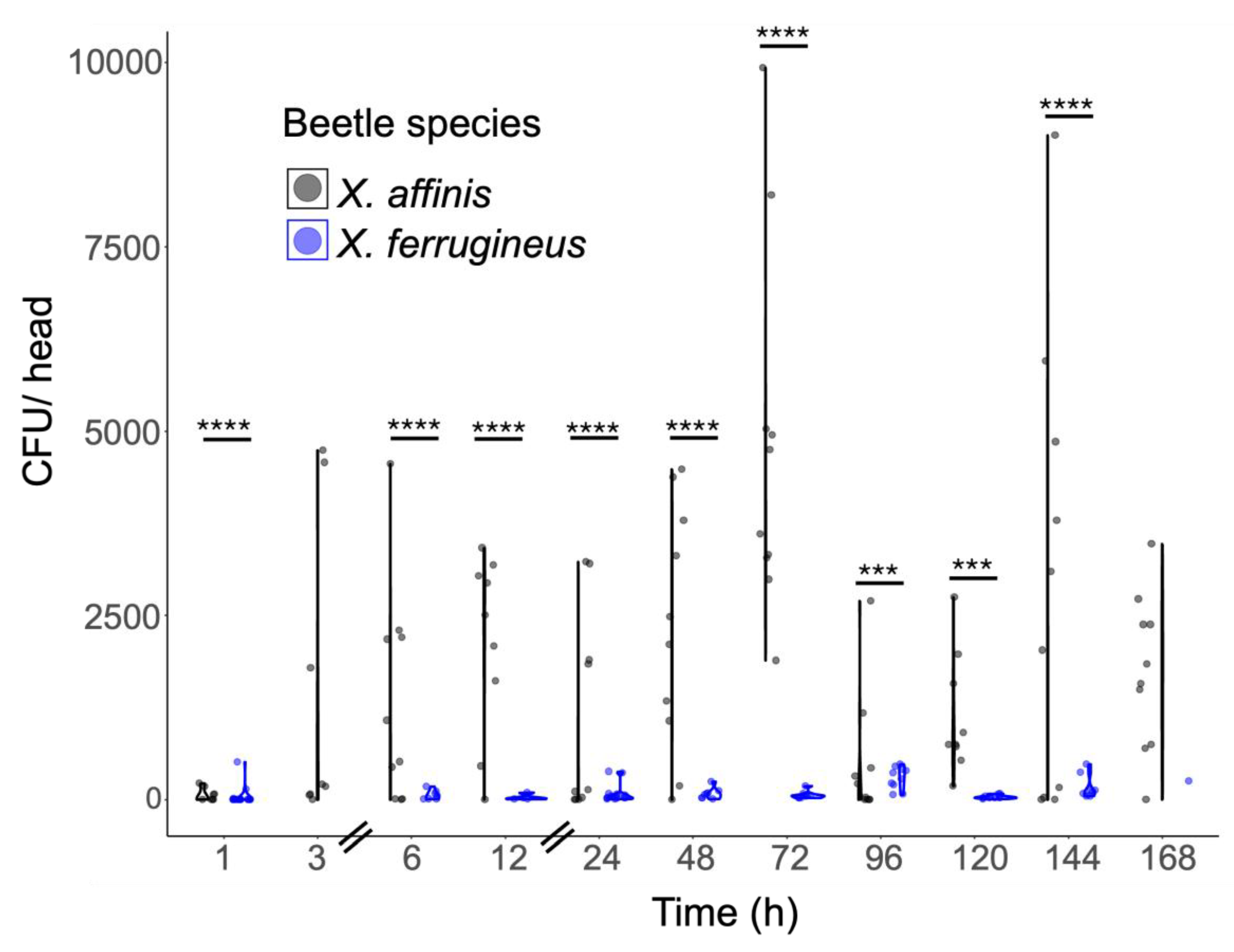
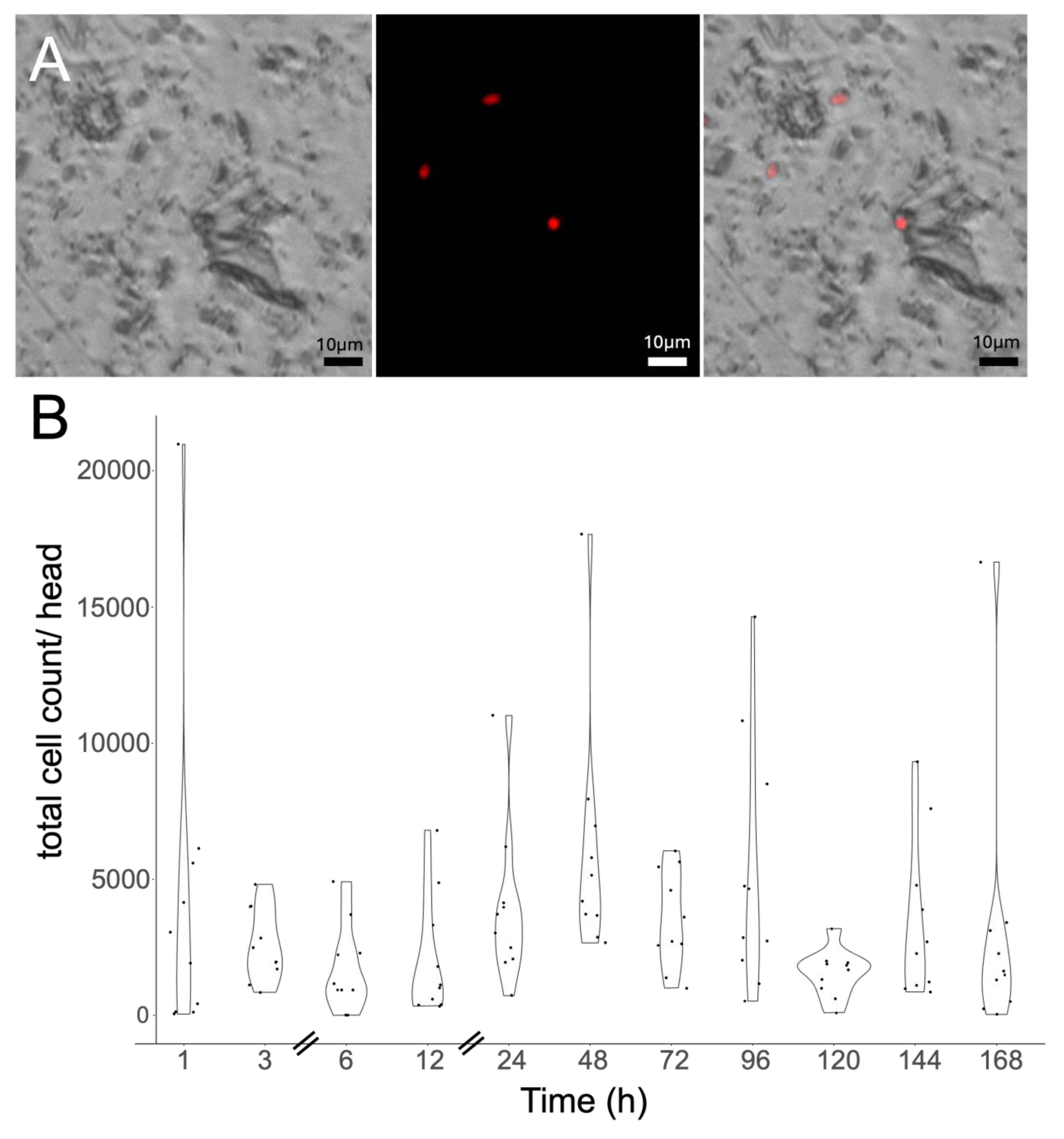
Mycangial Colonization Is Dependent upon Beetle and Fungal Partner Species
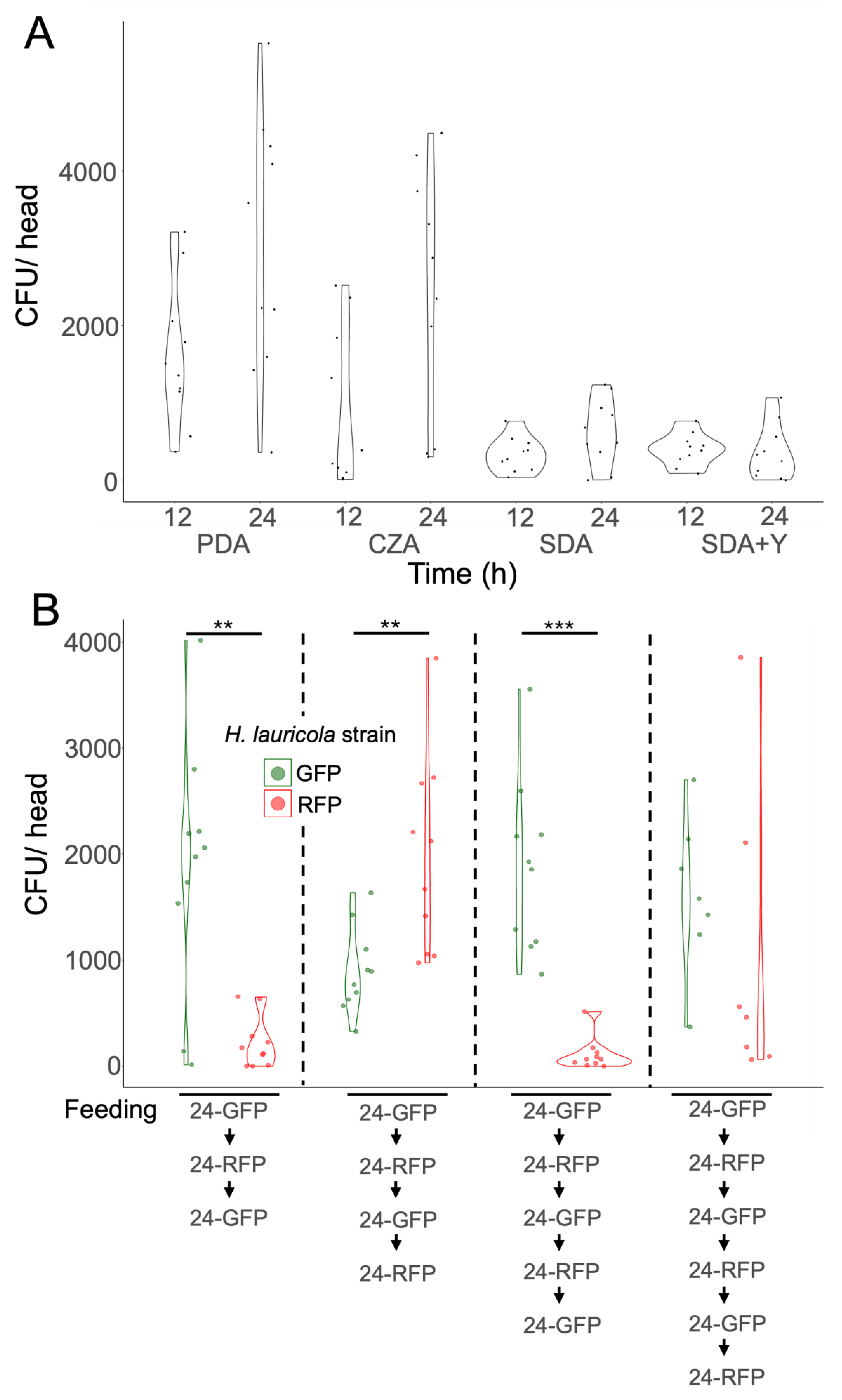
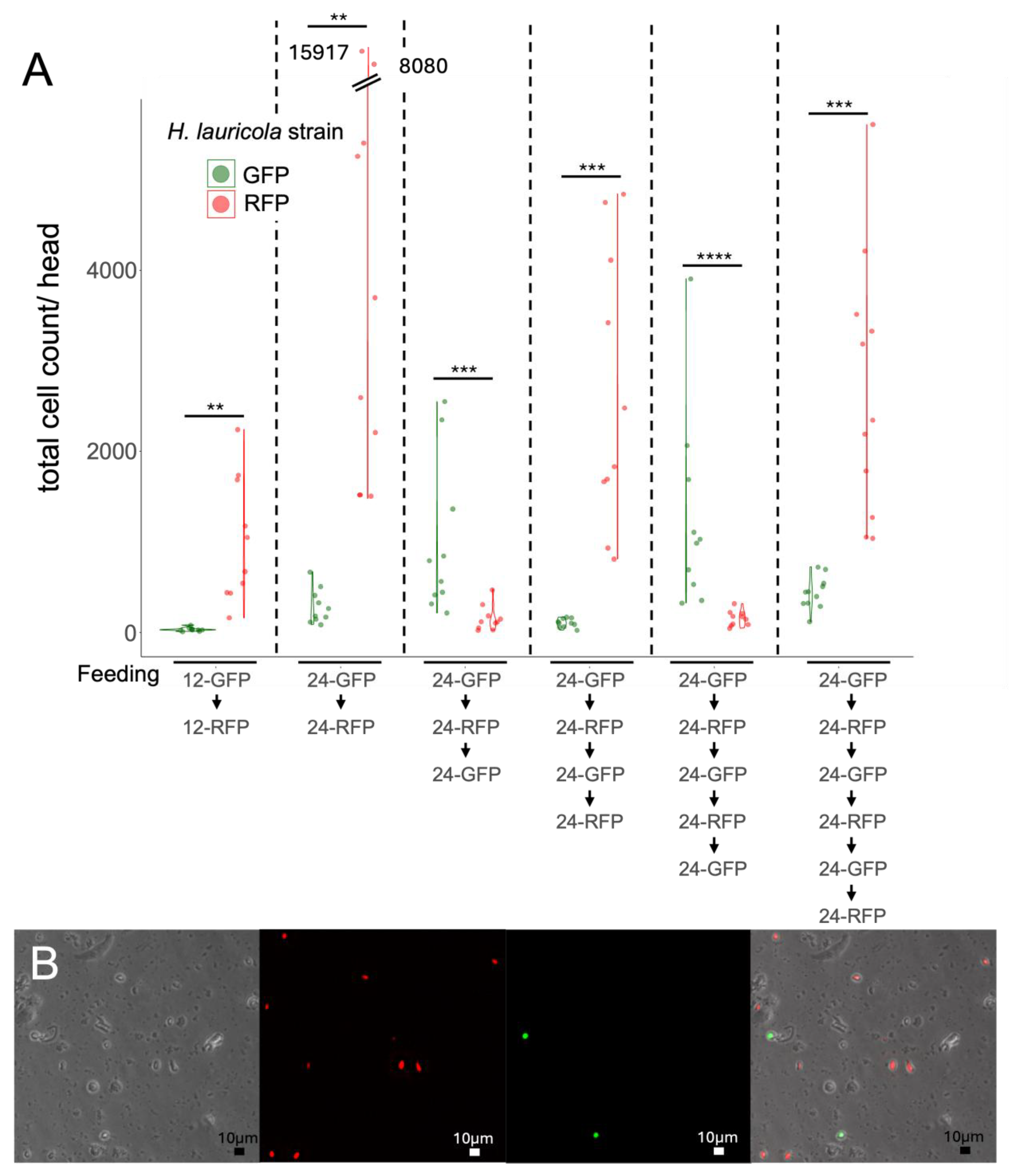
Mycangial Colonization Is Affected by the Nutritional State of the Partner Fungus, and Long-Term Switching of Partner Fungi Can Occur
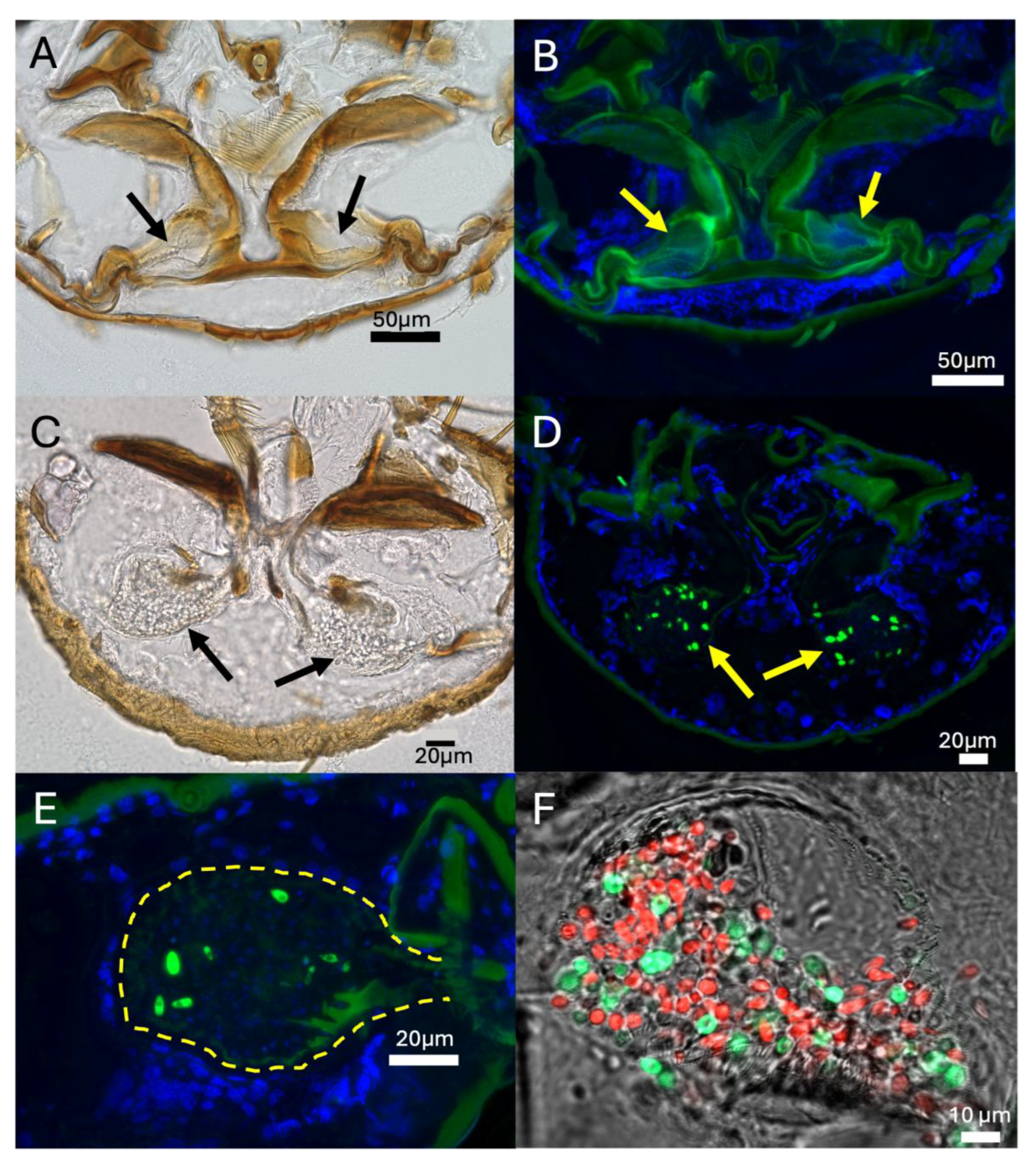
Application of Cell Cytometry to Quantify Mycangial Content and Imaging of the Mycangia
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzurenko, M.; Hulcr, J. Ambrosia beetles. Current Biology 2022, 32, R61-R62.
- Joseph, R.; Keyhani, N. Fungal mutualisms and pathosystems: life and death in the ambrosia beetle mycangia. Applied Microbiology and Biotechnology 2021, 105, 3393-3410, doi:10.1007/s00253-021-11268-0. [CrossRef]
- Vanderpool, D.; Bracewell, R.R.; McCutcheon, J.P. Know your farmer: Ancient origins and multiple independent domestications of ambrosia beetle fungal cultivars. Molecular Ecology 2018, 27, 2077-2094, doi:10.1111/mec.14394. [CrossRef]
- Biedermann, P.; Vega, F.; Douglas, A. Ecology and Evolution of Insect-Fungus Mutualisms. Annual Review of Entomology, Vol 65 2020, 65, 431-455, doi:10.1146/annurev-ento-011019-024910. [CrossRef]
- Seibold, S.; Muller, J.; Baldrian, P.; Cadotte, M.; Stursova, M.; Biedermann, P.; Krah, F.; Bassler, C. Fungi associated with beetles dispersing from dead wood - Let’s take the beetle bus! Fungal Ecology 2019, 39, 100-108, doi:10.1016/j.funeco.2018.11.016. [CrossRef]
- Skelton, J.; Johnson, A.; Jusino, M.; Bateman, C.; Li, Y.; Hulcr, J. A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts. Proceedings of the Royal Society B-Biological Sciences 2019, 286, doi:10.1098/rspb.2018.2127. [CrossRef]
- Carrillo, J.; Rugman-Jones, P.; Husein, D.; Stajich, J.; Kasson, M.; Carrillo, D.; Stouthamer, R.; Eskalen, A. Members of the Euwallacea fornicatus species complex exhibit promiscuous mutualism with ambrosia fungi in Taiwan. Fungal Genetics and Biology 2019, 133, doi:10.1016/j.fgb.2019.103269. [CrossRef]
- Saucedo-Carabez, J.R.; Ploetz, R.C.; Konkol, J.L.; Carrillo, D.; Gazis, R. Partnerships between ambrosia beetles and fungi: lineage-specific promiscuity among vectors of the laurel wilt pathogen, Raffaelea lauricola. Microb Ecol 2018, doi:10.1007/s00248-018-1188-y. [CrossRef]
- Kostovcik, M.; Bateman, C.; Kolarik, M.; Stelinski, L.; Jordal, B.; Hulcr, J. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. Isme Journal 2015, 9, 126-138, doi:10.1038/ismej.2014.115. [CrossRef]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive tree diseases associated with ambrosia and bark beetles: black swan events in tree pathology? Plant Disease 2013, 97, 856-872, doi:10.1094/Pdis-01-13-0056-Fe. [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Narvaez, T.; Duncan, R.E.; Saucedo, R.J.; Campbell, A.; Mantilla, J.; Carrillo, D.; Kendra, P.E. Presence and prevalence of Raffaelea lauricola, cause of laurel wilt, in different species of ambrosia beetle in Florida, USA. J Econ Entomol 2017, doi:10.1093/jee/tow292. [CrossRef]
- Pena, J.E.; Carrillo, D.; Duncan, R.E.; Capinera, J.L.; Brar, G.; Mclean, S.; Arpaia, M.L.; Focht, E.; Smith, J.A.; Hughes, M.; et al. Susceptibility of Persea spp. and other Lauraceae to attack by redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Florida Entomologist 2012, 95, 783-787, doi:Doi 10.1653/024.095.0334. [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the Southeastern United States. Plant Disease 2008, 92, 215-224, doi:10.1094/Pdis-92-2-0215. [CrossRef]
- Saucedo, J.R.; Ploetz, R.C.; Konkol, J.L.; Angel, M.; Mantilla, J.; Menocal, O.; Carrillo, D. Nutritional symbionts of a putative vector, Xyloborus bispinatus, of the laurel wilt pathogen of avocado, Raffaelea lauricola. Symbiosis 2017, 1-10, doi:10.1007/s13199-017-0514-3. [CrossRef]
- Joseph, R.; Bansal, K.; Keyhani, N.O. Host switching by an ambrosia beetle fungal mutualist: Mycangial colonization of indigenous beetles by the invasive laurel wilt fungal pathogen. Environ Microbiol 2023, 25, 1894-1908, doi:10.1111/1462-2920.16401. [CrossRef]
- Huang, Y.; Skelton, J.; Hulcr, J. Lipids and small metabolites provisioned by ambrosia fungi to symbiotic beetles are phylogeny-dependent, not convergent. Isme Journal 2020, 14, 1089-1099, doi:10.1038/s41396-020-0593-7. [CrossRef]
- Huang, Y.; Skelton, J.; Hulcr, J. Multiple evolutionary origins lead to diversity in the metabolic profiles of ambrosia fungi. Fungal Ecology 2019, 38, 80-88, doi:10.1016/j.funeco.2018.03.006. [CrossRef]
- Mayers, C.; Harrington, T.; Mcnew, D.; Roeper, R.; Biedermann, P.; Masuya, H.; Bateman, C. Four mycangium types and four genera of ambrosia fungi suggest a complex history of fungus farming in the ambrosia beetle tribe Xyloterini. Mycologia 2020, 112, 1104-1137, doi:10.1080/00275514.2020.1755209. [CrossRef]
- Li, Y.; Ruan, Y.; Kasson, M.; Stanley, E.; Gillett, C.; Johnson, A.; Zhang, M.; Hulcr, J. Structure of the Ambrosia Beetle (Coleoptera: Curculionidae) Mycangia Revealed Through Micro-Computed Tomography. Journal of Insect Science 2018, 18, doi:10.1093/jisesa/iey096. [CrossRef]
- Spahr, E.; Kasson, M.; Kijimoto, T. Micro-computed tomography permits enhanced visualization of mycangia across development and between sexes inEuwallaceaambrosia beetles. Plos One 2020, 15, doi:10.1371/journal.pone.0236653. [CrossRef]
- Hulcr, J.; Stelinski, L.L. The Ambrosia symbiosis: from evolutionary ecology to practical management. Annu Rev Entomol 2017, 62, 285-303, doi:10.1146/annurev-ento-031616-035105. [CrossRef]
- Rodrigues, A.; Johnson, A.J.; Joseph, R.A.; Li, Y.; Keyhani, N.O.; Stanley, E.L.; Weiss, B.; Kaltenpoth, M.; Smith, M.E.; Hulcr, J. Fungal symbiont community and absence of detectable mycangia in invasive Euplatypus ambrosia beetles. Symbiosis 2023, 90, 305-319.
- Kasson, M.T.; Wickert, K.L.; Stauder, C.M.; Macias, A.M.; Berger, M.C.; Simmons, D.R.; Short, D.P.G.; DeVallance, D.B.; Hulcr, J. Mutualism with aggressive wood-degrading Flavodon ambrosius (Polyporales) facilitates niche expansion and communal social structure in Ambrosiophilus ambrosia beetles. Fungal Ecology 2016, 23, 86-96, doi:10.1016/j.funeco.2016.07.002. [CrossRef]
- Bateman, C.; Huang, Y.T.; Simmons, D.R.; Kasson, M.T.; Stanley, E.L.; Hulcr, J. Ambrosia beetle Premnobius cavipennis (Scolytinae: Ipini) carries highly divergent ascomycotan ambrosia fungus, Afroraffaelea ambrosiae gen. nov et sp nov (Ophiostomatales). Fungal Ecology 2017, 25, 41-49, doi:10.1016/j.funeco.2016.10.008. [CrossRef]
- Li, Y.; Bateman, C.; Skelton, J.; Jusino, M.; Nolen, Z.; Simmons, D.; Hulcr, J. Wood decay fungus Flavodon ambrosius (Basidiomycota: Polyporales) is widely farmed by two genera of ambrosia beetles. Fungal Biology 2017, 121, 984-989, doi:10.1016/j.funbio.2017.08.004. [CrossRef]
- Menocal, O.; Cruz, L.F.; Kendra, P.E.; Berto, M.; Carrillo, D. Flexibility in the ambrosia symbiosis of Xyleborus bispinatus. Front Microbiol 2023, 14, 1110474, doi:10.3389/fmicb.2023.1110474. [CrossRef]
- Cruz, L.F.; Menocal, O.; Mantilla, J.; Ibarra-Juarez, L.A.; Carrillo, D. Xyleborus volvulus (Coleoptera: Curculionidae): Biology and Fungal Associates. Appl Environ Microbiol 2019, 85, doi:10.1128/AEM.01190-19. [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, 2016.
- Hughes, M.A.; Riggins, J.J.; Koch, F.H.; Cognato, A.I.; Anderson, C.; Formby, J.P.; Dreaden, T.J.; Ploetz, R.C.; Smith, J.A. No rest for the laurels: symbiotic invaders cause unprecedented damage to southern USA forests. Biological Invasions 2017, 19, 2143-2157, doi:10.1007/s10530-017-1427-z. [CrossRef]
- Jiang, Z.-R.; Kinoshita, S.; Sasaki, O.; Cognato, A.I.; Kajimura, H. Non-destructive observation of the mycangia of Euwallacea interjectus (Blandford) (Coleoptera: Curculionidae: Scolytinae) using X-ray computed tomography. Entomological Science 2019, 22, 173-181.
- Spahr, E.; McLaughlin, S.; Tichinel, A.; Kasson, M.; Kijimoto, T. Staining and Scanning Protocol for Micro-Computed Tomography to Observe the Morphology of Soft Tissues in Ambrosia Beetles. Bio-Protocol 2023, 13, doi:10.21769/BioProtoc.4584. [CrossRef]
- Bateman, C.; Sigut, M.; Skelton, J.; Smith, K.; Hulcr, J. Fungal Associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) Are Spatially Segregated on the Insect Body. Environmental Entomology 2016, 45, 883-890, doi:10.1093/ee/nvw070. [CrossRef]
- Carrillo, D.; Duncan, R.E.; Ploetz, J.N.; Campbell, A.F.; Ploetz, R.C.; Pena, J.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathology 2014, 63, 54-62, doi:10.1111/ppa.12073. [CrossRef]
- Skelton, J.; Jusino, M.; Li, Y.; Bateman, C.; Thai, P.; Wu, C.; Lindner, D.; Hulcr, J. Detecting Symbioses in Complex Communities: the Fungal Symbionts of Bark and Ambrosia Beetles Within Asian Pines. Microbial Ecology 2018, 76, 839-850, doi:10.1007/s00248-018-1154-8. [CrossRef]
- Harrington, T.C.; Yun, H.Y.; Lu, S.S.; Goto, H.; Aghayeva, D.N.; Fraedrich, S.W. Isolations from the redbay ambrosia beetle, Xyleborus glabratus, confirm that the laurel wilt pathogen, Raffaelea lauricola, originated in Asia. Mycologia 2011, 103, 1028-1036, doi:10.3852/10-417. [CrossRef]
- Harrington, T.C.; Fraedrich, S.W. Quantification of propagules of the laurel wilt fungus and other mycangial fungi from the Redbay ambrosia beetle, Xyleborus glabratus. Phytopathology 2010, 100, 1118-1123, doi:10.1094/Phyto-01-10-0032. [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Perez-Martinez, J.M.; Fernandez, R. Management of laurel wilt of avocado, caused by Raffaelea lauricola. European Journal of Plant Pathology 2017, 149, 133-143, doi:10.1007/s10658-017-1173-1. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




