1. Introduction
The genus of
Panax belongs to the Araliaceae family, which has 17 species of the
P. genus, including
P. ginseng,
P. quinquefolius, and
P. notoginseng, etc [
1]. Most members of this genus have medicinal properties and more than 150 ginsenosides have been identified and classified according to their structures, which can be divided into damarane, oktylon and oleanolic acid types [
2,
3].
P. notoginseng (Burk.) F. H. Chen is a traditional and valuable Chinese herbal medicine, mainly grown in Guangxi and Yunnan provinces in Southwest China. In
P. notoginseng, dalmarane-type tetracyclic triterpene saponins can be further divided into propanaxadiol-type saponins (PPD, such as Rb
1, Rb
2, Rb
3, Rd and Rc) and propanaxtriol-type saponins (PPT, such as Re and Rg
1) according to the presence of a hydroxyl group attached to C-6. The kinds and contents of saponins in different parts of
P. notoginseng are also different, and
P. notoginseng root (PNR) mainly contains PPD-type ginsenosides (Rb
1 and Rd) and PPT-type ginsenosides (Re, Rg
1 and R
1) [
4]. The major saponins in PNR mainly showed great polarity because of the different types and amounts of sugar moieties linked to the C-3, C-6 and C-20 positions of the dammarane triterpenoid backbone [
5]. After oral administration, the major saponins need to be hydrolyzed by digestive enzymes and intestinal microorganisms before they can become more active and easily absorbed minor ginsenosides, but the efficiency of these conversions is very low [
6].
Most studies have shown that the function of ginsenosides is closely related to the position, type and quantity of glycosidic bonds [
7]. The main ginsenosides contain more sugar groups, which leads to their low pharmacological activity and is not easy to be absorbed by human body. Compared with the main ginsenosides, the minor ginsenosides have better pharmacological activities such as anti-hypertension, anti-aging, etc [
8]. These minor saponins are produced mainly by deglycosylation, isomerization and dehydration [
9,
10]. The main ginsenosides can be hydrolyzed into minor ginsenosides by physical, chemical and biotransformation. Compared with other methods, biotransformation has the advantages of mild conversion conditions, product stability and pollution-free [
11].
In order to prepare minor ginsenosides, many microorganisms capable of transforming ginsenosides have been discovered and applied. In particular, the relatively safe strain of
Aspergillus niger is well known.
A. niger JGL8 isolated from
Gynostemma pentaphyllum can transform
Gynostemma pentaphyllum saponins to ginsenoside F
2 through GypV→ Rd→ F
2 [
12]. Ginsenoside Rb
1 can be transformed by extracellular enzymes produced by
A. niger WU-16. The transformation pathway of Rb
1 is Rb
1→ F
2, CK [
13].
A. Niger XD101 and its crude enzymes screened from the planting soil of
P. notoginseng also have the ability to hydrolyze ginsenoside Rb
1, and its transformation pathway is: Rb
1→ Rd→ F
2→ CK [
14]. The results of all these studies indicate the feasibility of using microorganisms to carry out the transformation of major saponins for the preparation of minor saponins. Moreover, previous studies has shown that there are four main enzymes used in the enzymatic production of minor ginsenosides, these four enzymes hydrolyze glycosidic bonds at different positions on major ginsenosides [
15,
16,
17,
18,
19,
20].
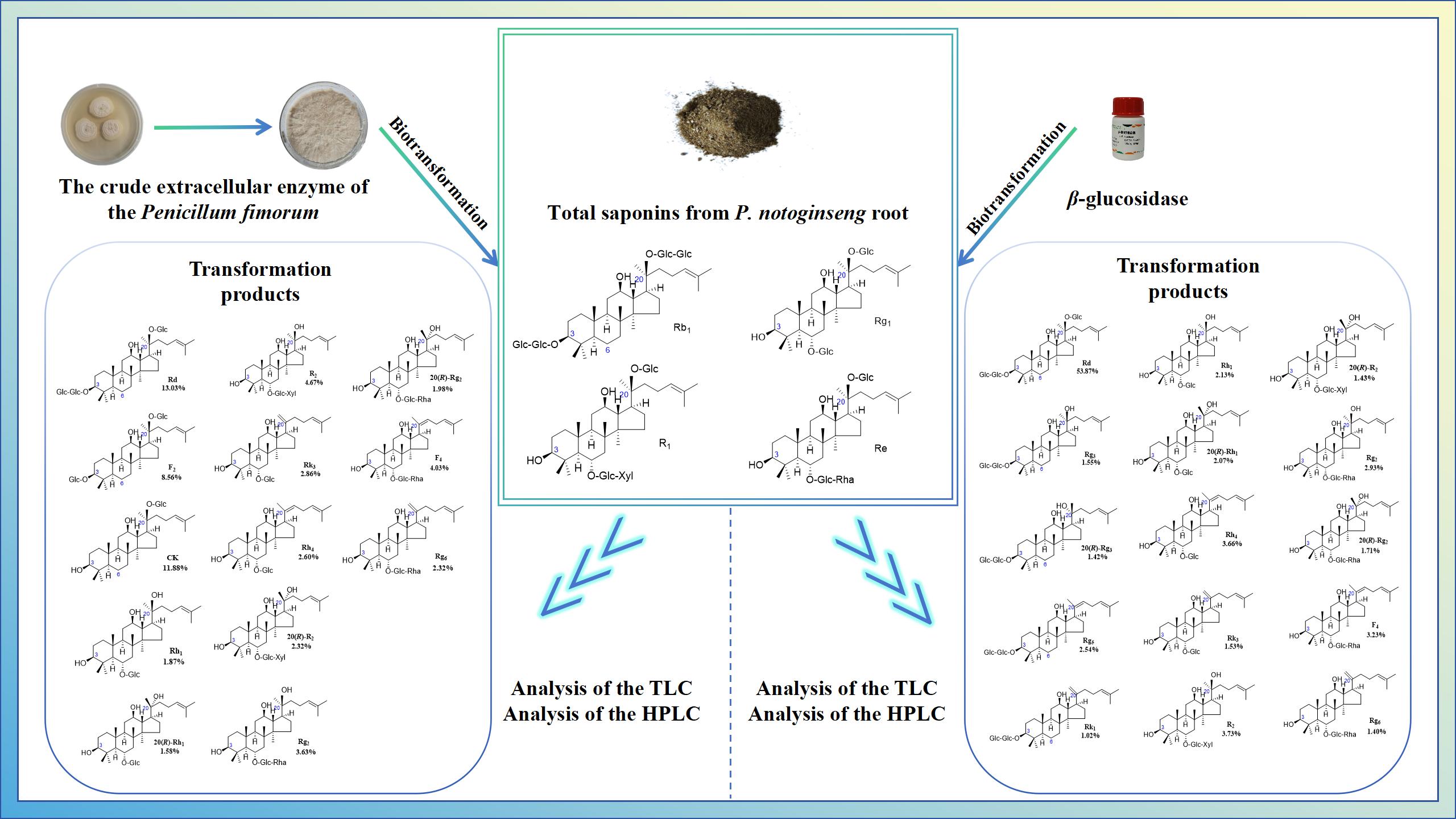
In this study, a strain of the plant endophytic fungus P. fimorum with excellent saponin transformation capacity was isolated from fresh Gastrodia elata, and its extracellular crude enzyme was extracted to transform several major saponins in PNR for 72 hours. The enzyme was found to be capable of transforming four major saponins (Rb1, Rg1, R1, and Re) in PNR into 13 minor saponins, of which ginsenoside CK had the highest yield at 11.88%, followed by ginsenoside Rd with a yield of 13.03%. To the best of our knowledge, this biotransformation characteristic of P. fimorum is reported here for the first time in fungi. In addition, commercial β-glucosidase was purchased for comparison, and it was found to transform the four major saponins in PNR into 15 minor saponins, of which ginsenoside Rd had the highest yield at 53.87%, with the yields of the remaining products being similar to those obtained from the transformation by the extracellular crude enzyme of P. fimorum.
By analyzing two comparative experiments, we found that the extracellular crude enzyme of P. fimorum and commercial β-glucosidase exhibited the following transformation capacities: deglycosylation, epimerization, and dehydration. The main difference is that the extracellular crude enzyme of P. fimorum specifically hydrolyzes glucose at the C-3 position of ginsenoside Rb1, efficiently producing ginsenoside CK, whereas β-glucosidase specifically hydrolyzes glucose at the C-20 position of ginsenoside Rb1, producing ginsenosides Rd, 20(S/R)-Rg3, Rk1, and Rg5. Analysis via TLC and HPLC, we have hypothesized that the biotransformation pathway of P. fimorum extracellular crude enzyme and commercial β-glucosidase for transforming four major saponins in PNR, along with the yields of the substrates and products involved. This study is expected to facilitate the conversion of total saponins from PNR into minor saponins, thereby greatly enhancing the economic and functional value of P. notoginseng.
2. Materials and Methods
2.1. Materials
Authentic standards of ginsenosides Rb1, Rd, F2, CK, 20(S/R)-Rg3, Rk1, Rg5, 20(S/R)-Rh1, Rh4, Rk3, Re, 20(S/R)-Rg2, F4, Rg6 and notoginsenosides R1, 20(S/R)-R2 were purchased from the Sichuan Victory. The commercial β-glucosidase was purchased from the Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China). The solvents methanol and acetonitrile for HPLC were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The HSGF254 silica gel TLC plate was purchased from Yantai Jiangyou Silicone Development Co., Ltd. (Shangdong, China). A Welchrom C18 column (4.6 ×250 mm, 5 µm) was sourced from Yuexu Technology Co., Ltd. (Sichuan, China). An Agilent 1260 high-performance liquid chromatography instrument was purchased from Agilent (Grand Island, NY, USA).
2.2. Medium
PDA medium: potato extract powder 6 g/L, glucose 25 g/L, and agar 25 g/L. PDB medium: potato extract powder 6 g/L and glucose 25 g/L.
2.3. Isolation and Identification of Plant Endophytic Fungi
A single colony, S62, was isolated from fresh
Gastrodia elata using the isolation and purification method of plant endophytic fungi [
21,
22]. The amplification and sequencing of the ITS rDNA gene was performed by Kunming Branch of Tsingke Biotechnology Co., Ltd. The ITS rDNA gene was submitted to the National Centre for Biotechnology Information (NCBI) to obtain the NCBI-recorded RSA registry number OR958833. Phylogenetic tree construction by neighbour-joining (NJ) method using MEGA 11.0 software. And the morphologies of conidiophores and ascospores were observed by optical microscopy. A voucher specimen (No. Li20221001) was deposited at the Faculty of Life Science and Technology, Kunming University of Science and Technology.
2.4. Screening of Fungi for Its Ability to Biotransform Saponins
Microbial fermentation of the fungus was carried out using PDB medium. After 5 days of fermentation, a solution of total saponins from PNR was added to the fermentation broth to continue microbial transformation for an additional 18 days. The total saponins solution of PNR was dissolved in 75% ethanol and filtered through a 0.22 μm filter before being added to the fermentation broth, achieving a final concentration of 4 mg/mL in the broth. After 18 days of fermentation, the fermentation broth was extracted with an equal volume (1:1) of aqueous saturated n-BuOH. The upper phase was dried by evaporation and then analyzed by TLC to assess its saponin conversion capacity [
23,
24].
2.5. Preparation of Crude Enzyme from Microorganism
The extracellular crude enzymes of the strain were extracted using supersaturated ammonium sulfate [
4,
25]. The fermentation broth from the 18-day fermentation was extracted with aqueous saturated n-BuOH. Ammonium sulfate was then added to the lower phase of the fermentation broth to form a saturated solution, followed by the extraction of the crude enzyme.
2.6. Determination of Optimal pH and Optimal Temperature
Optimization of the optimal conditions for the enzyme reaction was carried out using ginsenoside Rb1. A 0.5 mL aliquot of 1 mg/mL ginsenoside Rb1 and 1 mL of 4 mg/mL enzyme solution were mixed well, and the reaction was then carried out. The buffer solution was prepared using phosphate and citrate with a pH range of 3–8. The reaction temperature was varied between 30–70 °C.
2.7. Biotransformation of Monomer Saponins by Enzymes
Enzymatic biotransformation of four monomeric saponins (Rb1, Rg1, Re, and R1) from PNR was carried out for 72 hours under optimal reaction conditions. At the end of the reaction, the solution was extracted with 2 times volume of aqueous saturated n-BuOH. The upper solution was then evaporated to dryness and analyzed by TLC and HPLC.
2.8. Monitoring of Substrate Conversion and Product Yields
To monitor changes in the substrate and products during biotransformation by the enzyme, samples from different time points were collected. Enzyme-substrate conversion reactions were performed at 3, 6, 12, 24, 48, and 72 hours, respectively. At the end of the reaction, the solution was extracted with 2 times volume of aqueous saturated n-BuOH. The upper solution was then evaporated to dryness and analyzed by TLC and HPLC.
where C
s is the initial concentration of the substrate, C
fs is the final concentration of the substrate, C
p is the final concentration of the product ginsenoside.
2.9. Analytical Methods of TLC and HPLC
Experimental samples were extracted using water saturated n-butanol, and the upper solution was dried up and used for subsequent experiments.
For the TLC analysis, CHCl3−CH3COOH−CH3OH−H2O (6.75:0.25:2.5:0.5, v/v/v) was used as a developing solvent, and 10% (v/v) H2SO4−ethanol was used as a chromogenic solvent.
For the HPLC analysis, the samples were analyzed by HPLC, using a Agilent 1260 system (NY, USA) connected to a Welchrom C18 chromatography column (4.6 ×250 mm, 5 µm). The mobile phase entailed a mixture of water (A) and acetonitrile (B). The gradient elution program was set as follows: 0−15 min (20% B), 15−20 min (20−30% B), 20−45 min (30−36% B), 45−52 min (36−38% B), 52−55 min (38−40% B), 55−63 min (40−42% B), 63−69 min (42−44% B), 69−75 min (44−51% B), 75−85 min (51−53% B), 85−90 min (53−60% B), 90−91 min (60−63% B), 91−100 min (63−66% B), 100−105 min (66−100% B). The system maintained a flow rate of 1.0 mL/min. Absorbance measurements were taken at 203 nm with an injection volume of 30 μL and the column temperature set at 25 °C.
3. Results
3.1. Screening and Characterisation of Strain for the Transformation of Saponins
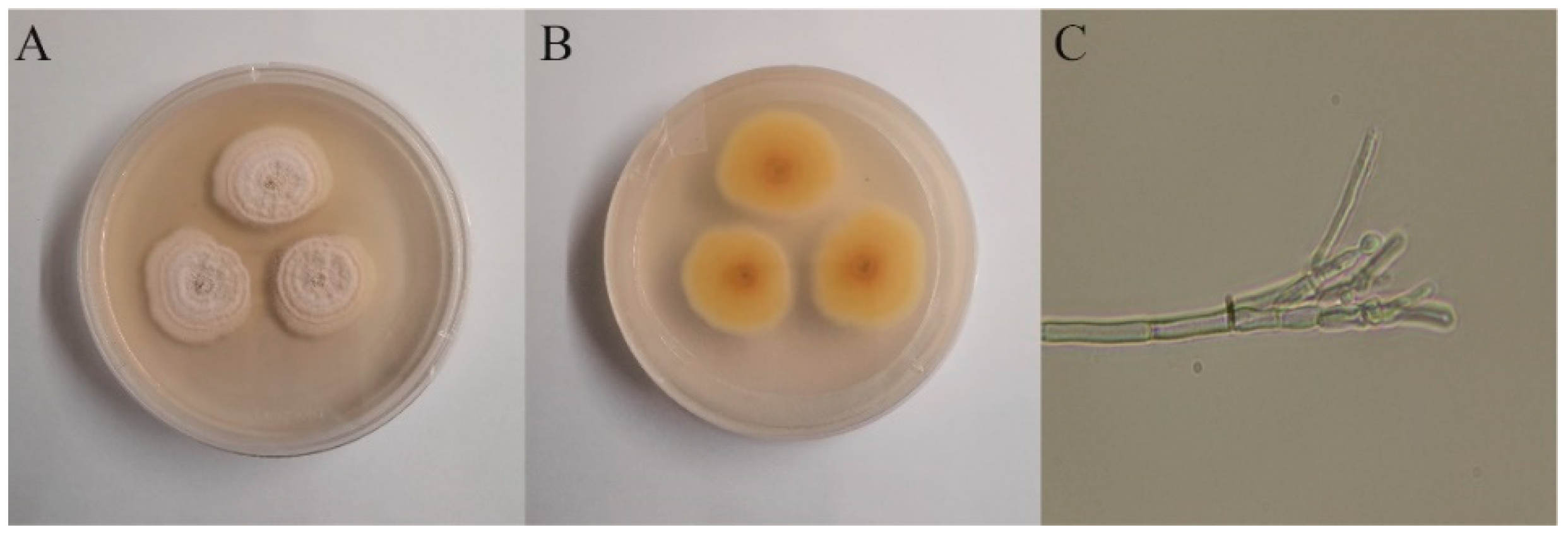
The plant endophytic fungus S62 was isolated from fresh
Gastrodia elata. The morphology of the colonies after 5 days of culture on PDA medium is shown in
Figure 1A and B. The surface appeared white, fluffy, or flocculent, while the back side was light yellow. Under optical microscopy, asymmetrical broom-like branches and subglobose conidia were observed.
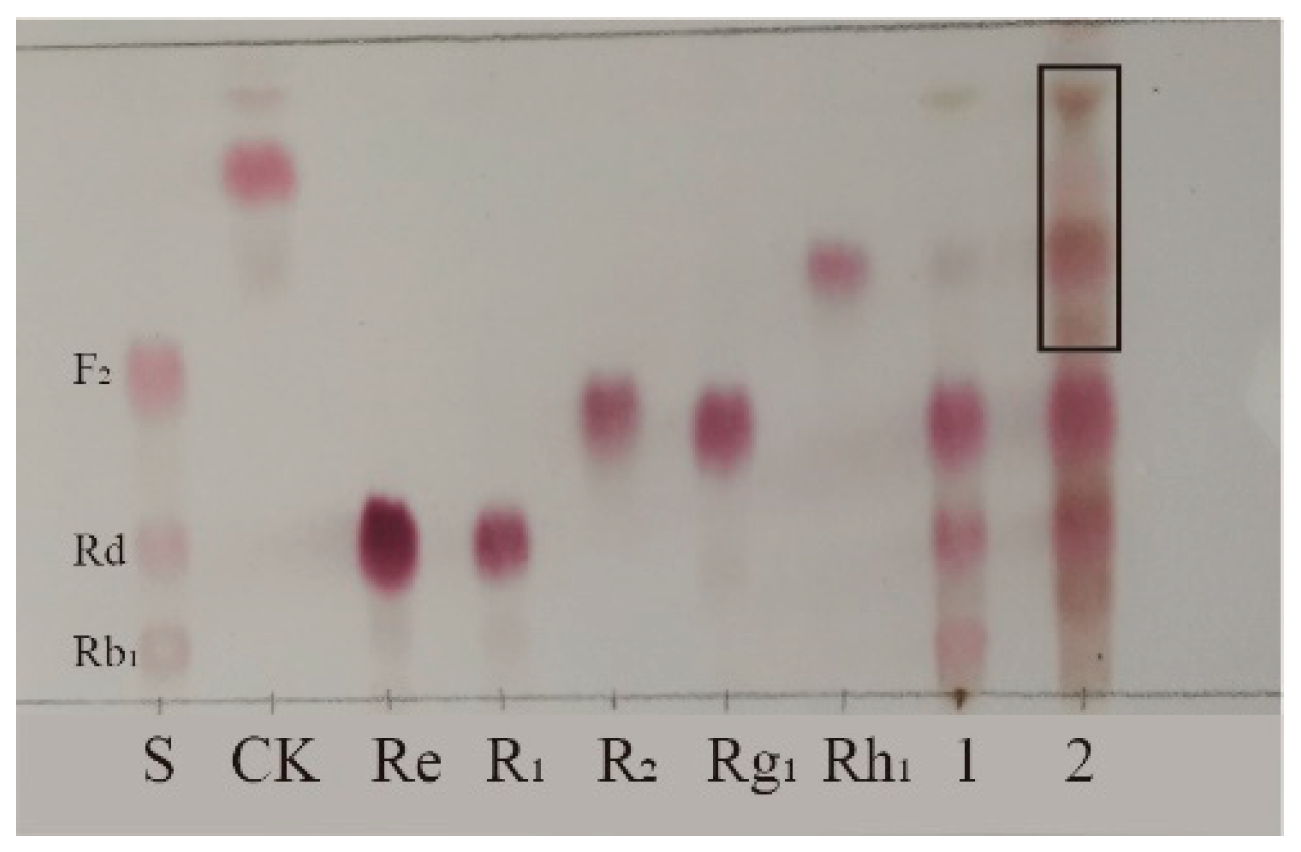
Biotransformation of total saponins from PNR was carried out for 18 days using strain S62 in PDB medium. TLC analysis of the results shown in
Figure 2 reveals new spots with a larger Rf value above the original substrate, indicating that strain S62 has better saponin conversion ability.
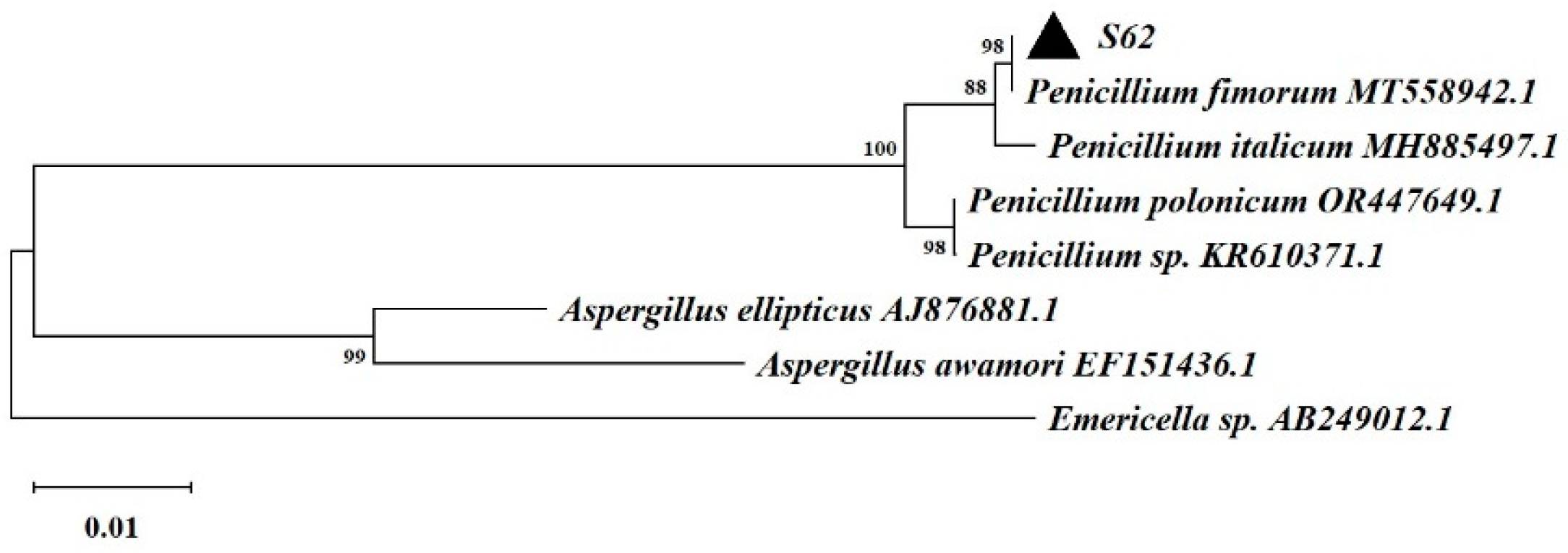
Based on ITS rDNA gene sequencing and comparison with the GenBank database, strain S62 was found to belong to the genus Penicillium and exhibited remarkable similarity to Penicillium fimorum, as shown in
Figure 3.
3.2. Results of Optimal pH and Temperature for Enzyme Transformation of Monomeric Saponin
Ginsenoside Rb
1, the major saponin in PNR, the content of Rb
1 is the highest in common ginsenosides, it could be used to determine the optimal conditions for biotransformation [
26].
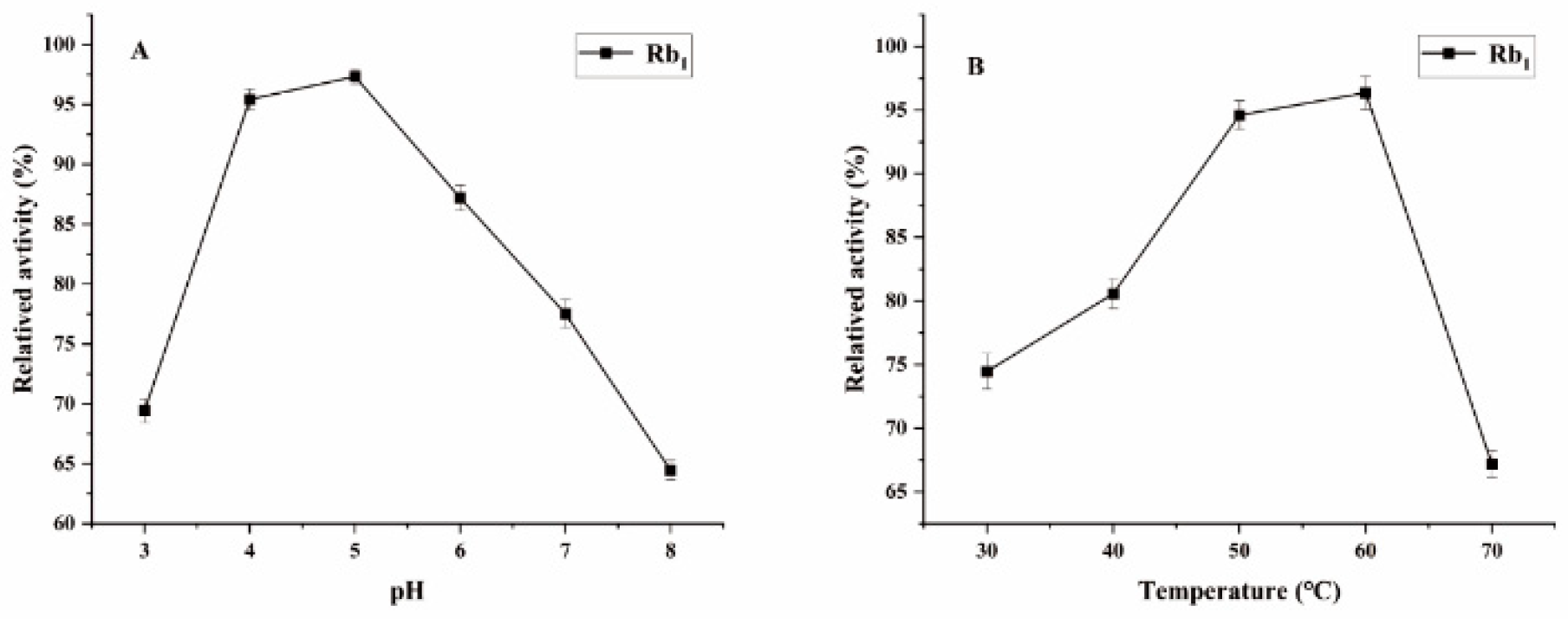
Figure 4 shows the optimal conditions for the conversion by
P. fimorum crude enzyme, with an optimal pH of 5 and an optimal temperature of 60 °C. Similarly, the optimal reaction conditions for commercial
β-glucosidase are also pH 5 and 60 °C, as shown in
Figure 5. These results indicate that both enzymes exhibit optimal activity under weakly acidic conditions, which is similar to the enzyme reaction conditions reported for other strains with saponin conversion ability [
27,
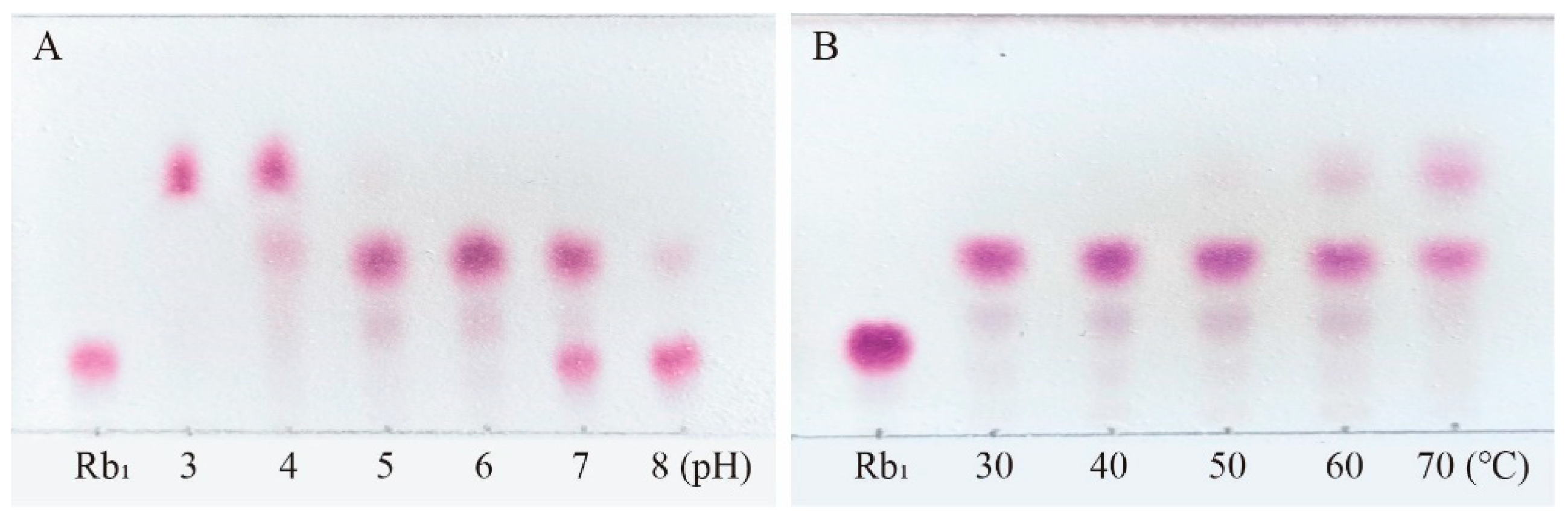
28]. Both enzymes also exhibit good temperature tolerance, with activity remaining effective up to 60 °C. Additionally, a control experiment was conducted to examine the effects of pH and temperature on the reaction conditions. It was found that acidic hydrolysis of ginsenoside Rb
1 occurred at both pH 3 and pH 4, while high-temperature pyrolysis was observed at 70 °C, as shown in
Figure S1.
3.3. Transformatiom Ability of Enzymes on Monomer Ginsenosides Rb1, Rg1, Re and Notoginsenoside R1
Among the total saponins of PNR, Rb
1 is PPD-type saponin, Rg
1 is PPT-type saponin, and R
1 is also PPT-type saponin which is a kind of saponin mainly contained in PNR [
29]. Therefore, the enzyme's ability to transform the total saponins of PNR can be indirectly inferred from its conversion of these three saponins.
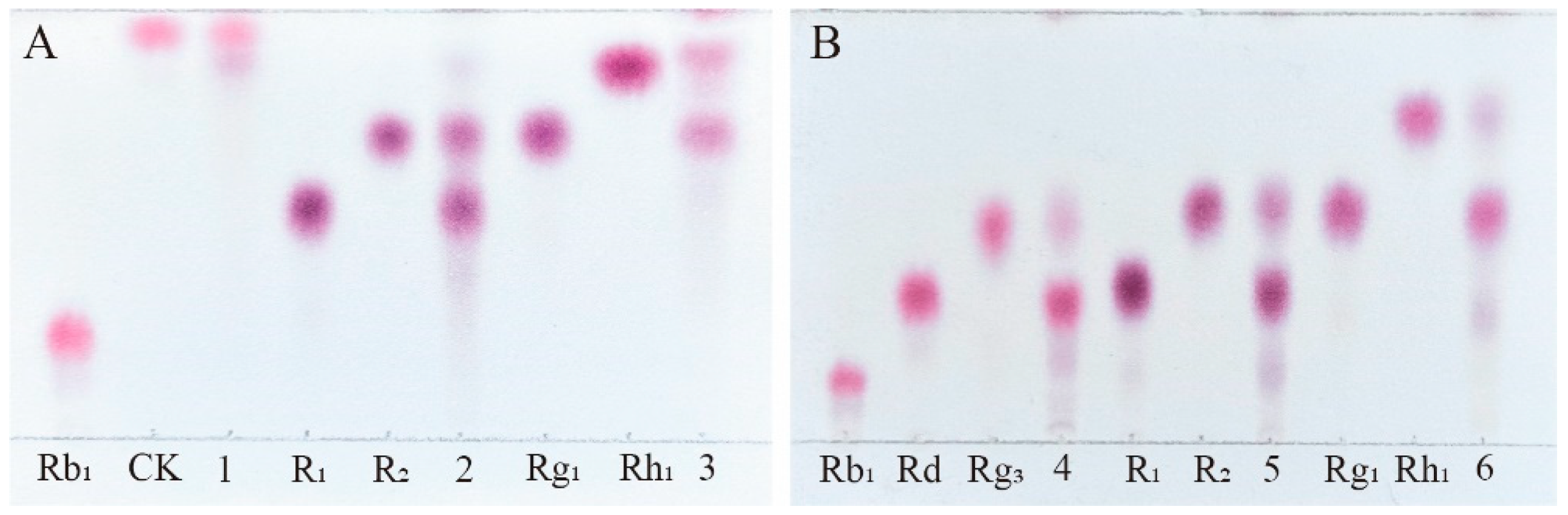
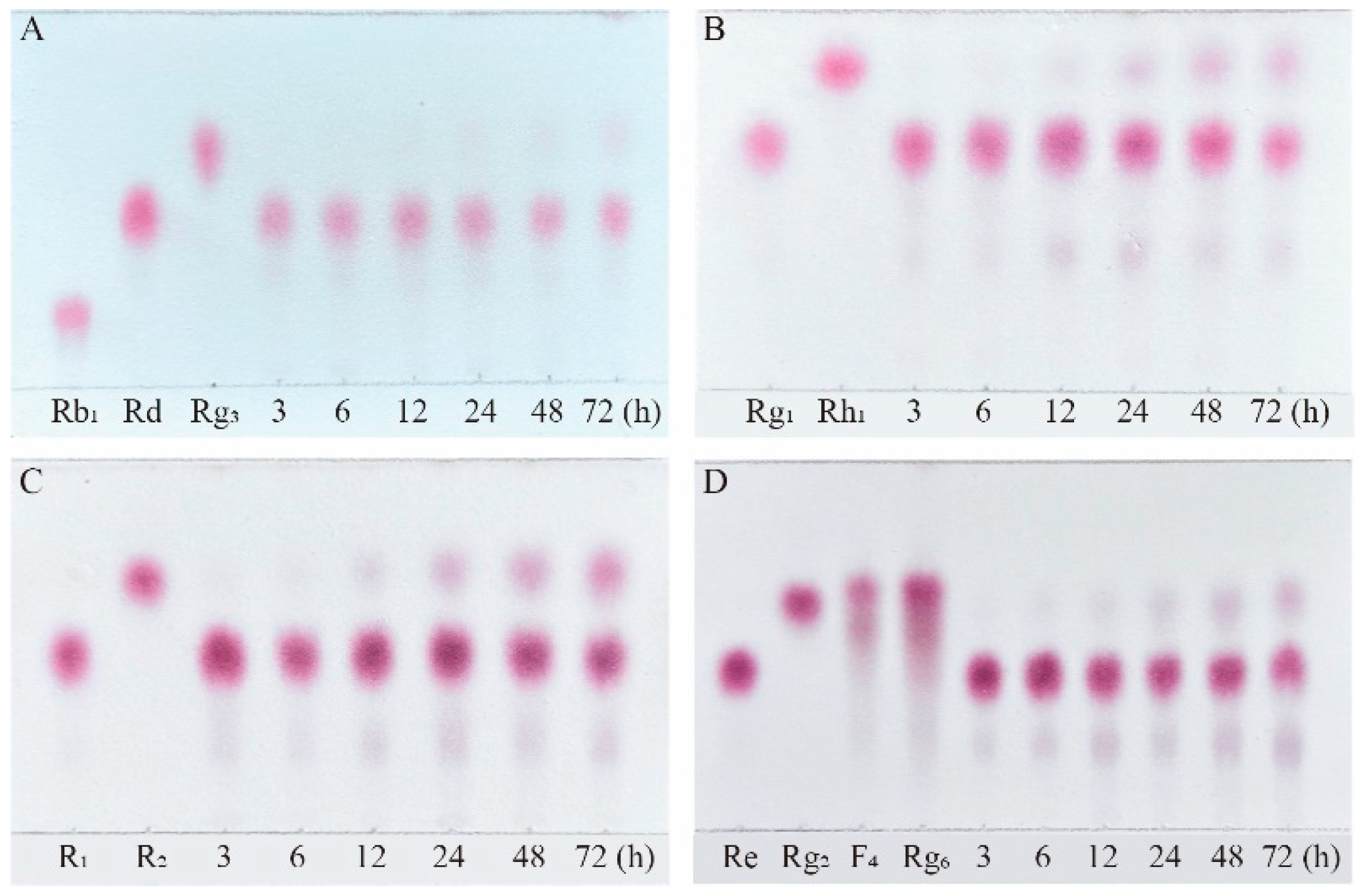
Figure 6A shows the TLC analysis of the crude enzyme transformation of these three types of saponins by
P. fimorum, where ginsenoside Rb
1 was almost completely transformeded to ginsenoside CK, while ginsenoside Rg
1 and notoginseng R
1 were transformeded to other minor saponins.
Figure 6B presents the TLC results of the transformation of these three saponins by commercial
β-glucosidase, where ginsenoside Rb
1 was predominantly transformeded into ginsenosides Rd and Rg
3, while commercial
β-glucosidase also transformeded ginsenoside Rg
1 and notoginseng R
1, similar to the crude enzyme of
P. fimorum.
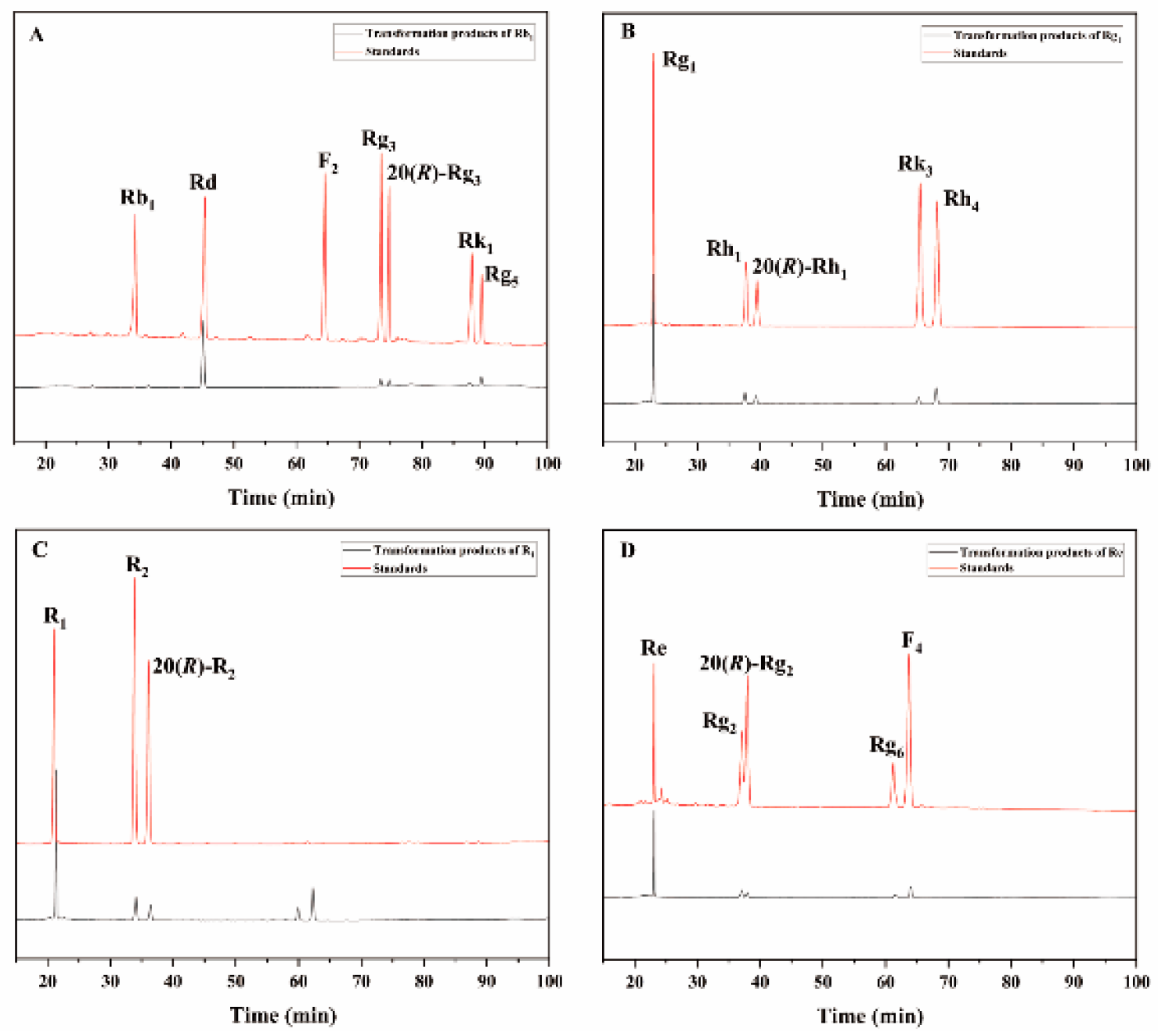
To further evaluate the enzyme's ability to transform the total saponins of PNR, we qualitatively analyzed the products of enzyme-mediated conversion of ginsenosides Rb
1, Rg
1, Re and notoginseng R
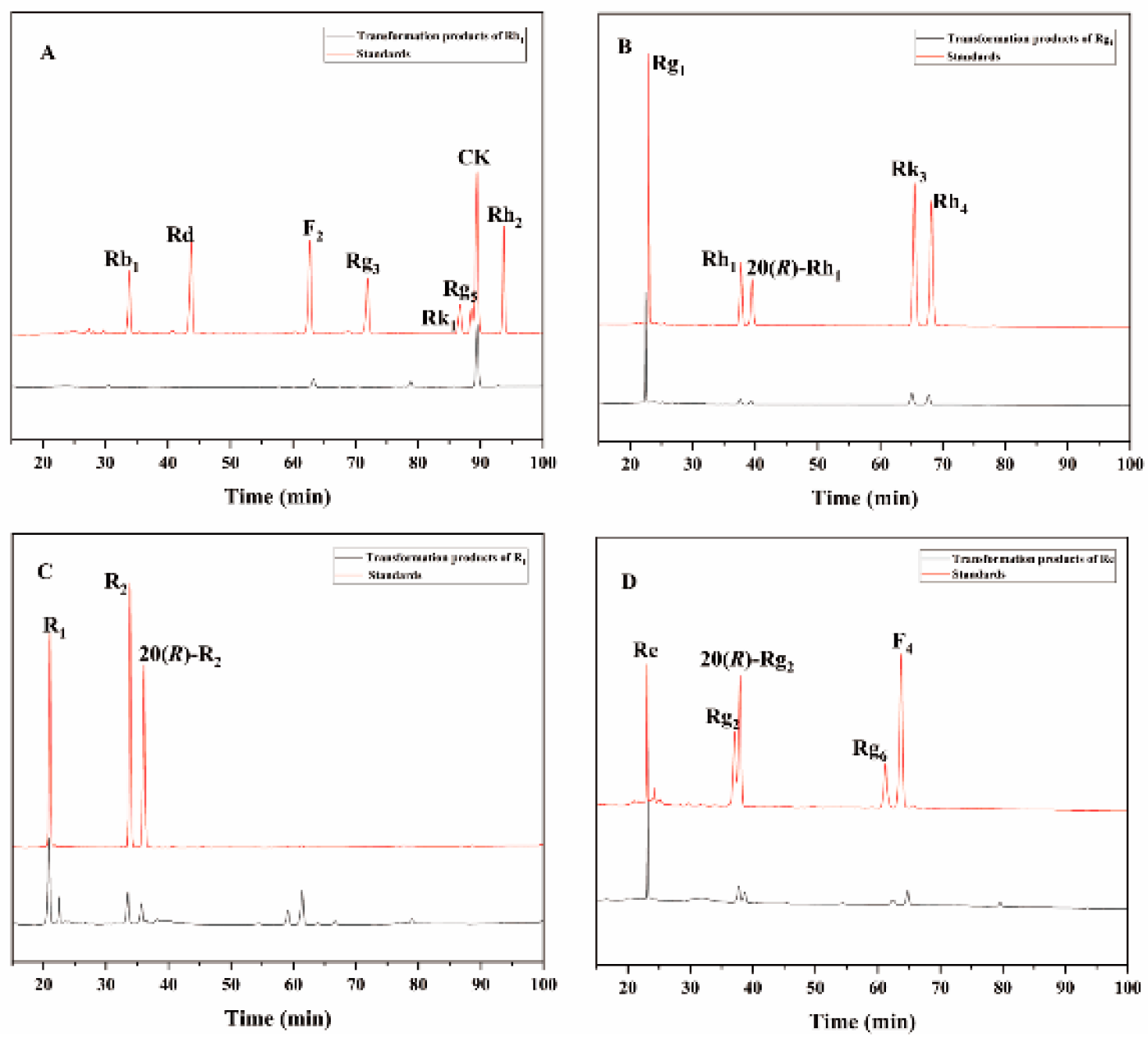
1 over 72 hours by HPLC. The 72 hours transformation products of
P. fimorum crude enzyme transforming ginsenoside Rb
1 were found to have F
2 and CK and an unknown product (
Figure 7A). The transformation products of ginsenoside Rg
1 included 20(
S/
R)-Rh
1, Rk
3, and Rh
4 (
Figure 7B). The transformation of notoginseng R
1 yielded 20(
S/
R)-R
2 (
Figure 7C), while the transformation of ginsenoside Re produced 20(
S/
R)-Rg
2, Rg
6, and F
4 (
Figure 7D).
The 72 hours conversion products of commercial
β-glucosidase transforminging ginsenoside Rb
1 had Rd, 20(
S/
R)-Rg
3, Rk
1 and Rg
5 (
Figure 8A); the 72 hours conversion products of transforminging ginsenoside Rg
1 had 20(
S/
R)-Rh
1, Rk
3 and Rh
4 (
Figure 8B); and the 72 hours conversion products of transforminging notoginseng R
1 had 20(
S/
R)-R
2 (
Figure 8C); The 72 hours conversion products of transforminging ginsenoside Re had 20(
S/
R)-Rg
2, Rg
6 and F
4 (
Figure 8D). The HPLC results of these two enzymes were in general consistent with the results of TLC analyses.
3.4. Propose Possible Biotransformation Pathways of Major Ginsenosides Rg1, Re, Rb1 and Notoginsenoside R1 of PNR
Based on qualitative and quantitative analyses by TLC and HPLC, as well as dynamic monitoring of the conversion reactions of several major saponins using linear regression equations (
Table S1), we have proposed a pathway analysis for the conversion of the four major saponins in
P. notoginseng by the crude enzyme from
P. fimorum and commercial
β-glucosidase. We also calculated the substrate conversion rates and product yields.
Figure 9 presents the results of the dynamically monitored TLC analysis of the conversion of four major saponins by the
P. fimorum crude enzyme, while
Figure 10 shows the corresponding results for
β-glucosidase.
Table 1 summarizes the substrate conversion rates, and
Figure 11 illustrates the proposed transformation pathway along with the yield analysis for each product.
3.5. Dynamic Change of Substrate Conversion of Major Ginsenosides and the Yield in the Transformation Products
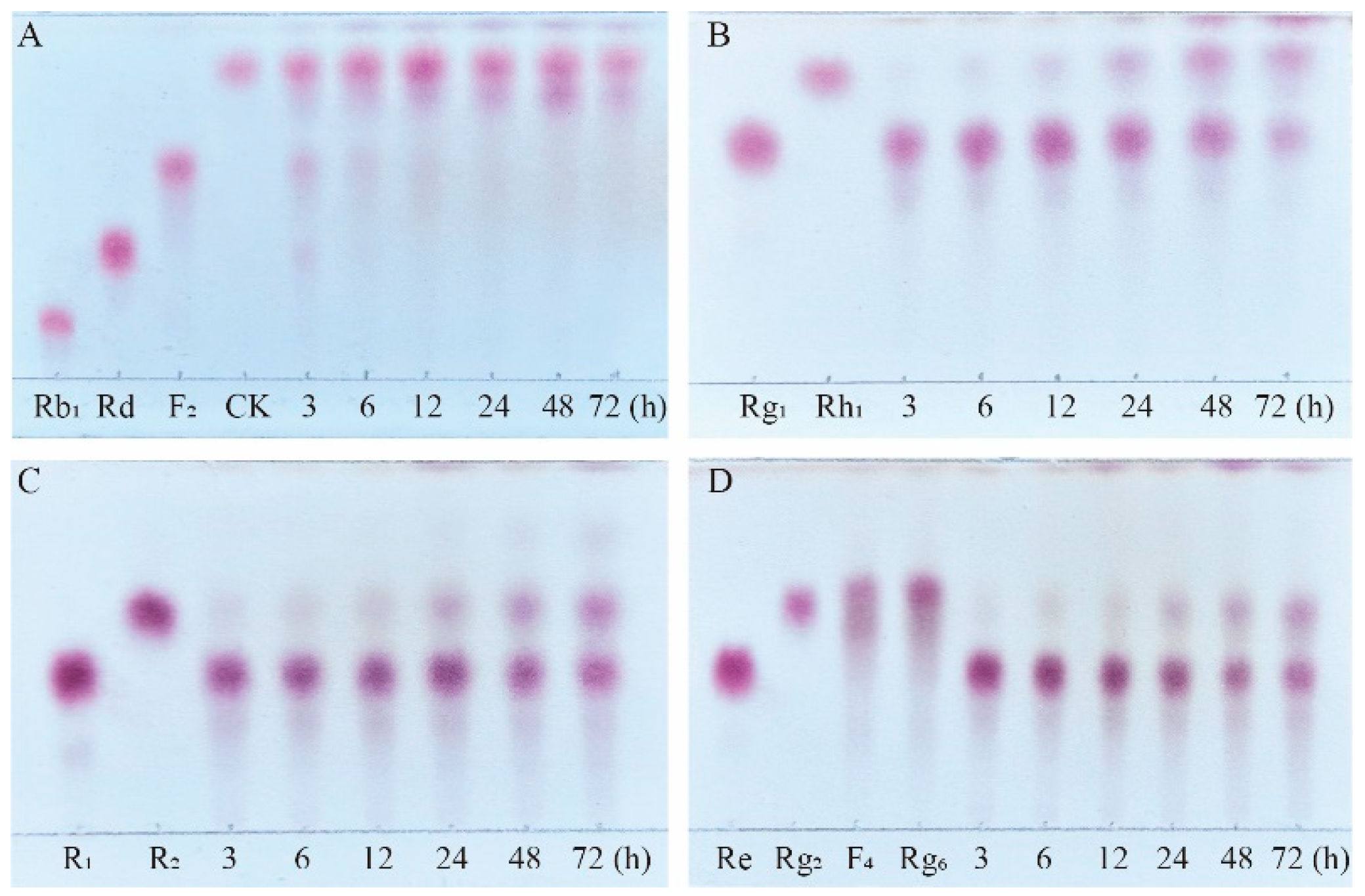
The transformation analysis of several major saponins and dynamic monitoring of product generation were performed. As shown in
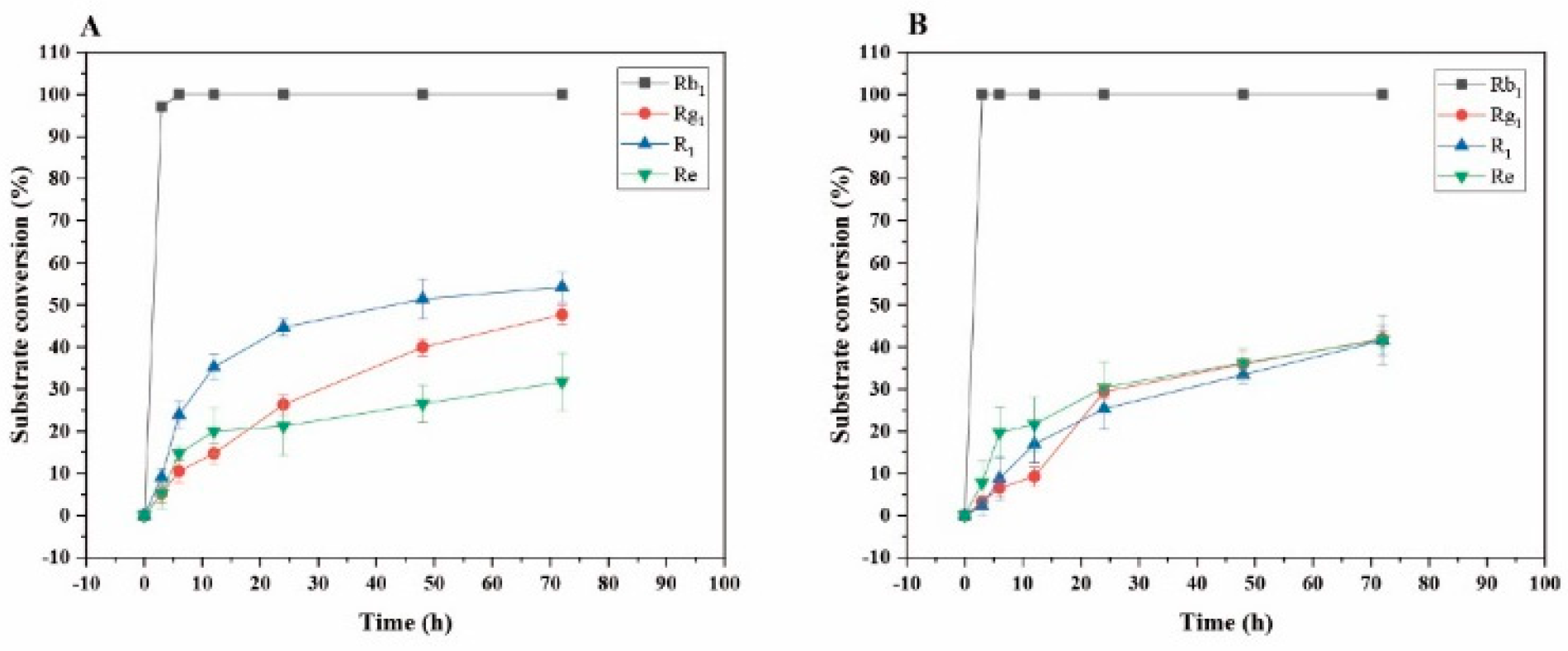
Figure 12A, for the transformation of ginsenoside Rb
1 by
P. fimorum crude enzyme, the substrate conversion rate reached its highest value of 100% at 6 hours. For the transformation of ginsenoside Rg
1, the highest transformation rate was 47.66% at 72 hours; for notoginseng R
1, the highest transformation rate was 54.23% at 72 hours; and for ginsenoside Re, the highest transformation rate was 31.68% at 72 hours.
As shown in
Figure 12B, for the transformation of ginsenoside Rb
1 by commercial
β-glucosidase, the substrate transformation rate reached its highest value of 100% at 3 hours. For the transformation of ginsenoside Rg
1, the highest transformation rate was 41.94% at 72 hours; for notoginseng R
1, the highest transformation rate was 41.58% at 72 hours; and for ginsenoside Re, the highest transformation rate was 41.72% at 72 hours.
Figure 13 shows the dynamic analysis results of the transformation product yields of four major saponins in
P. notoginseng transformed by
P. fimorum crude enzyme, while
Figure 14 presents the corresponding results for the transformation by commercial
β-glucosidase.
4. Discussion
In recent years, microbial transformation or botransformation using enzymes have become a popular method for preparing minor ginsenosides, and many studies used these methods to transform the main ginsenosides for the preparation of minor ginsenosides [
30,
31,
32,
33].
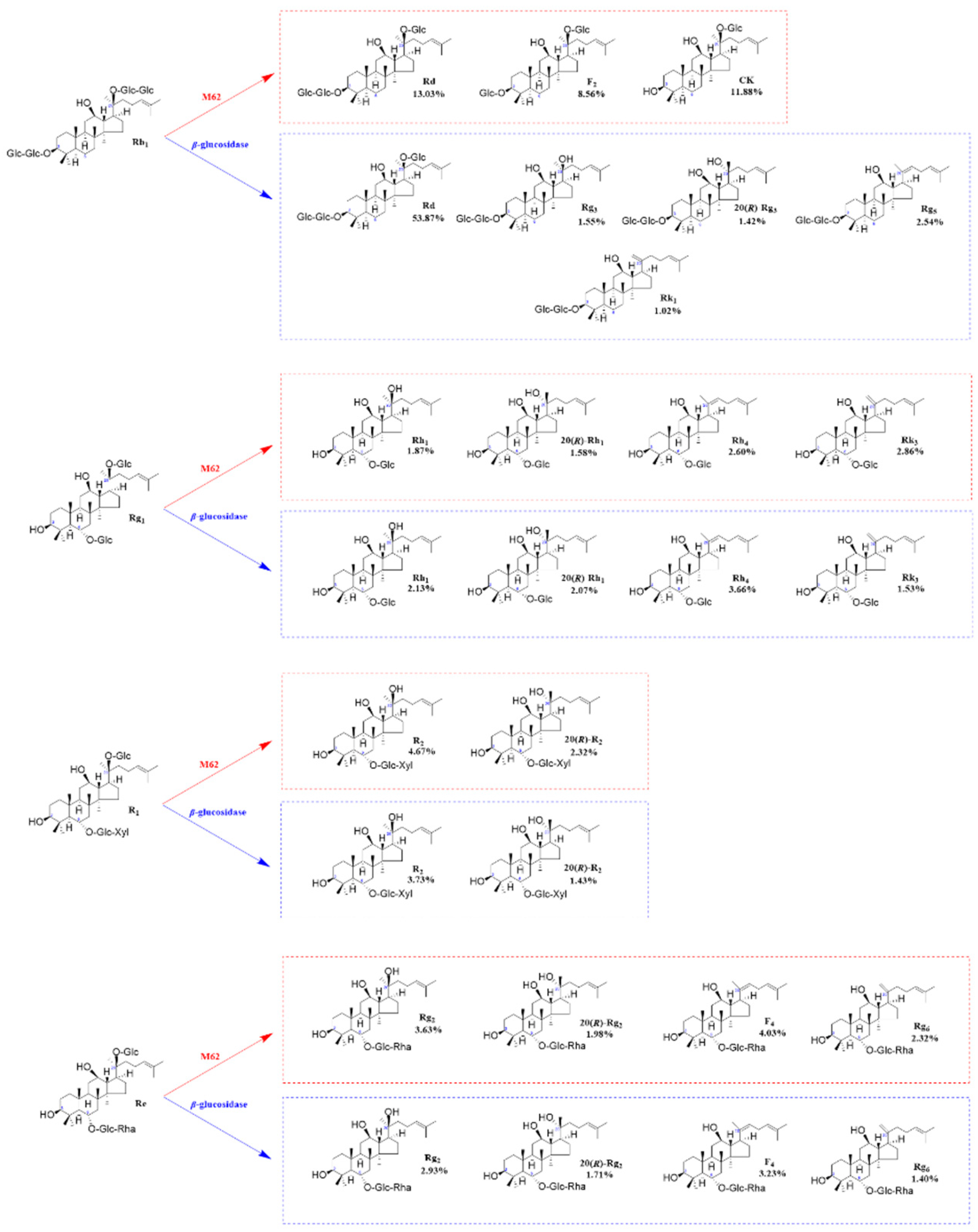
In this study, P. fimorum was isolated from fresh Gastrodia elata and demonstrated the ability to transform the total saponins of PNR. The four major saponins (Rb1, Rg1, Re and R1) from PNR were transformed using extracellular crude enzymes extracted from P. fimorum. The transformation products were thoroughly analyzed by TLC and HPLC. The transforming abilities of this fungus were found to include deglycosylation, epimerization, and dehydration, which, to the best of our knowledge, have not been previously reported for P. fimorum. The crude enzyme effectively hydrolyzed glucose attached at the C-3 and C-20 positions of the major saponins, forming 20(S/R)-epimers at C-20 via isomerization, as well as double-bonded isomers at C-20 through dehydration.
According to most of the studies, it is known that
β-glucosidase plays an important role in the conversion process against ginsenosides [
27,
28,
30,
31,
32,
33,
34]. In this study, biotransformation experiments were conducted using commercial
β-glucosidase to compare its transformation ability with that of
P. fimorum crude enzyme. The results revealed significant differences in their transformation of ginsenoside Rb
1. The
P. fimorum crude enzyme first hydrolyzed the glucose at the C-20 position of ginsenoside Rb
1 to form ginsenoside Rd, and then sequentially hydrolyzed the glucose at the C-3 position of ginsenoside Rd, ultimately efficiently transformating it into ginsenoside CK. In contrast,
β-glucosidase also formed ginsenoside Rd by hydrolyzing glucose at C-20 of ginsenoside Rb
1, but then continued to hydrolyze it into 20(
S/
R)-Rg
3, which further underwent dehydration to produce double-bonded isomers Rg
5 and Rk
1 at C-20. For the other three major saponins (Rg
1, Re and R
1) the transformation abilities of commercial
β-glucosidase and
P. fimorum crude enzyme were nearly identical, as both enzymes efficiently transformed the major saponins into minor saponins.
Based on the analysis of transformation products by HPLC, several uncharacterized products were observed during the transformation of major saponins by P. fimorum crude enzyme. For example, for the transformation products F2 and CK of ginsenoside Rb1 there is an unknown product in the middle of them, while the transformation product 20(S/R)-R2 from notoginseng R1 was followed by several unknown products. Similarly, the transformation product F4 from ginsenoside Re was followed by an unknown product. These uncharacterized products may be new saponin derivatives, as their retention times closely resemble those of known minor saponins. If monomeric saponins can be efficiently transformed by P. fimorum crude enzyme in large quantities through fermentation and subsequently purified via column chromatography, there is potential to obtain novel saponin derivatives.
For the HPLC analysis of the conversion products of commercial
β-glucosidase, we found that two unknown products were also present in the conversion products of notoginseng R
1. By analysing the transformation products of other major saponins, we can conclude the regularity that ginsenosides Rb
1, Rg
1 and Re can be transformed by
β-glucosidase to form 20(
S/
R)-epimers, C-20(21) and C-20(22) double-bond isomers. Therefore, we hypothesise that notoginseng R
1 can not only be transformeded to 20(
S/
R)-R
2 only, but it can be further transformed to the notoginsenoside T
5 with a C-20(21) double bond but also to 3
β, 12
β-Dihydroxydammarane-(
E)-20(22), 24-diene-6-O-
β-D-xylopyranosyl-(1→2)-
β-D-glucopyranoside with a C-20(22) double bond [
4].
In addition, in this study, the transformation of various major saponins by P. fimorum crude enzyme and commercial β-glucosidase were monitored dynamically in terms of the conversion of the substrates and the yield of the conversion products. The results of the dynamic monitoring can thus be used for the qualitative production of a particular conversion product with a higher yield. In summary, this study investigated the transformation of major saponins from PNR using two enzymes, which is expected to improve the utilisation of PNR and increase the methods of producing minor saponins.
5. Conclusions
In this experiment, biotransformation of the major saponins in PNR was carried out using the extracellular crude enzyme of the plant endophytic fungus P. fimorum and commercial β-glucosidase. The extracellular crude enzyme, extracted in our laboratory, could be further purified to enhance transformation efficiency. Nevertheless, the biotransformation experiments demonstrated that the crude enzyme efficiently transformed ginsenoside Rb1 with a 100% substrate conversion rate, producing ginsenoside CK. While commercial β-glucosidase also achieved 100% conversion of ginsenoside Rb1, it generated multiple products, including Rd, 20(S/R)-Rg3, Rk1, and Rg5, which followed a different transformation pathway compared to P. fimorum crude enzyme. For the total saponins in PNR, including notoginseng R1, ginsenoside Rg1, and Re, both P. fimorum extracellular crude enzyme and commercial β-glucosidase exhibited similar transformation abilities, transforming the major saponins into various minor saponins. This study also proposed a transformation pathway and analyzed the transformation rates and product yields through dynamic monitoring. Overall, the study introduces two methods for transforming major saponins in P. notoginseng into minor saponins, thereby improving the utilization of PNR and expanding the production pathways of minor saponins. These findings may be applicable to other parts of P. notoginseng and lay the groundwork for the development of genetically engineered strains and enzyme immobilization technologies for large-scale production of minor saponins.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: The control experiment of the condition optimization process; Table S1: Linear regression equation of various saponins.
Author Contributions
Conceptualization, F.L.; methodology, F.L. and R.Z.; software, J.Y. and D.L.; validation, X.C., Y.Y. and X.Y.; formal analysis, F.L., R.Z. and X.Y.; investigation, F.L.; resources, X.C., Y.Y. and X.Y.; data curation, F.L.; writing—original draft preparation, F.L.; writing—review and editing, F.L., R.Z. and X.Y.; visualization, F.L.; supervision, X.Y.; project administration, F.L. and X.Y.; funding acquisition, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China, grant number 32460114.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohanan, P.; Yang, T.J.; Song, Y.H. Genes and regulatory mechanisms for ginsenoside biosynthesis. J. Plant Biol. 2023, 66, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, N.; Yan, X.; Yuan, Y.; Luo, F.; Jiang, Z.; Zhou, Y. Ginsenoside Re impacts on biotransformation products of ginsenoside Rb1 by Cellulosimicrobium Cellulans Sp. 21 and its mechanisms. Process Biochem. 2019, 77, 57–62. [Google Scholar] [CrossRef]
- Cong, L.; Ma, J.; Zhang, Y.; Zhou, Y.; Cong, X.; Hao, M. Effect of anti-skin disorders of ginsenosides-A Systematic Review. J. Ginseng Res. 2023, 47, 605–614. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Guo, M.; Li, Y.F.; Shao, L.J.; Cui, X.M.; Yang, X.Y. Highly regioselective biotransformation of protopanaxadiol-type and protopanaxatriol-type ginsenosides in the underground parts of Panax Notoginseng to 18 minor ginsenosides by Talaromyces Flavus. ACS Omega 2022, 7, 14910–14919. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Mathur, A.K.; Mathur, A. A literature update elucidating production of Panax ginsenosides with a special focus on strategies enriching the anti-neoplastic minor ginsenosides in ginseng preparations. Appl. Microbiol. Biotechnol. 2017, 101, 4009–4032. [Google Scholar] [CrossRef]
- Guo, Y.P.; Chen, M.Y.; Shao, L.; Zhang, W.; Rao, T.; Zhou, H.H.; Huang, W.H. Quantification of Panax notoginseng saponins metabolites in rat plasma with in vivo gut microbiota-mediated biotransformation by HPLC-MS/MS. Chin. J. Nat. Med. 2019, 17, 231–240. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, M.; Ai, Z.; Huang, J.; Wang, Y.; Xiao, S.; Wang, Y. Highly regioselective hydrolysis of the glycosidic bonds in ginsenosides catalyzed by snailase. Process Biochem. 2021, 103, 114–122. [Google Scholar] [CrossRef]
- Ke, Y.; Huang, L.; Song, Y.; Liu, Z.; Liang, L.; Wang, L.; Wang, T. Preparation and pharmacological effects of minor ginsenoside nanoparticles: A Review. Front. Pharmacol. 2022, 13, 974274. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ling, X.; Zhao, S.; Xu, L.; Wang, R. Diversity and isolation of endophytic fungi in Panax japonicus and biotransformation activity on saponins. Curr. Pharm. Biotechnol. 2024, 25, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, R.; Hou, G.; Wang, Q.; Zhao, F.; Liu, Z.; Meng, Q. Stereoscopic differences in the identification, bioactivity, and metabolismof C-20 and C-24 epimeric ginseng saponins. Mini. Rev. Med. Chem. 2023, 23, 804–820. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent advances in biotransformation of saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Dai, Z.; Liang, Y.; Zhu, C.; Su, C.; Song, L.; Wang, K.; Li, J.; Wei, X. Gypenoside biotransformation into ginsenoside F2 by endophytic Aspergillus Niger from Gynostemma Pentaphyllum. Nat. Prod. Res. 2024, 38, 3086–3092. [Google Scholar] [CrossRef]
- Yang, F.; Wu, Z.; Cao, S.; Tao, Z.; Fan, D.; Liu, X. Simultaneous transformation of ginsenoside Rb1 into rare ginsenoside F2 and compound K by the extracellular enzyme from Aspergillus Niger Wu-16. Bioresour. Technol. Rep. 2023, 22, 101419. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Fan, D. Biotransformation of ginsenoside Rb1 to ginsenoside CK by strain XD101: A safe bioconversion strategy. Appl. Biochem. Biotechnol. 2021, 193, 2110–2127. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhou, R.X.; Sun, C.K.; Jin, Y.H.; Yu, H.S.; Zhang, T.Y.; Xu, L.Q.; Jin, F.X. Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American Ginseng PPD-ginsenoside using special ginsenosidase Type-I from Aspergillus Niger g.848. J. Ginseng Res. 2015, 39, 221–229. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, C.; Im, W.T.; Chen, S.; Zuo, K.; Yu, H.; Song, J.; Xu, L.; Yi, T.H.; Jin, F. Dynamic changes of multi-notoginseng stem-leaf ginsenosides in reaction with ginsenosidase Type-I. J. Ginseng Res. 2019, 43, 186–195. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Q.; Zhang, C.; Lu, M.; Fu, Y.; Im, W.T.; Lee, S.T.; Jin, F. A new ginsenosidase from Aspergillus strain hydrolyzing 20-O-multi-glycoside of PPD ginsenoside. Process Biochem. 2009, 44, 772–775. [Google Scholar] [CrossRef]
- Jin, X.F.; Yu, H.S.; Wang, D.M.; Liu, T.Q.; Liu, C,Y. ; An, D.S.; Im, W.T.; Kim, S.G.; Jin F.X. Kinetics of a cloned special ginsenosidase hydrolyzing 3-O-Glucoside of multi-protopanaxadiol-type ginsenosides, named ginsenosidase Type III. J. Microbiol. Biotechnol. 2012, 22, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Yu, H.S.; Song, J.G.; Xu, Y.F.; Liu, C.Y.; Jin, F.X. A novel ginsenosidase from an Aspergillus strain hydrolyzing 6-O-multi-glycosides of protopanaxatriol-type ginsenosides, named ginsenosidase Type IV. J. Microbiol. Biotechnol. 2011, 21, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Yu, H.S.; Song, J.G.; Xu, Y.F.; Jin, F.X. Enzyme kinetics of ginsenosidase Type IV hydrolyzing 6-O-multi-glycosides of protopanaxatriol type ginsenosides. Process Biochem. 2012, 47, 133–138. [Google Scholar] [CrossRef]
- Selim, K. Biodiversity and antimicrobial activity of endophytes associated with egyptian medicinal plants. Mycosphere 2011, 2, 669–678. [Google Scholar] [CrossRef]
- Selim, K.A.; Elkhateeb, W.A.; Tawila, A.M.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I.; Ahmed, E.F. Antiviral and antioxidant potential of fungal endophytes of Egyptian medicinal plants. Fermentation 2018, 4, 49. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Shen, Y.; Zhang, B.; Liu, J.; Cao, Y.; Zhao, J. Targeted preparation of six rare ginsenosides using two β-glucosidases from Bifidobacterium Adolescentis. Food Biosci. 2024, 59, 104192. [Google Scholar] [CrossRef]
- Diao, M.; Chen, Y.; Meng, L.; Li, J.; Xie, N. Biotransformation approach to produce rare ginsenosides F1, Compound Mc1, and Rd2 from major ginsenosides. Arch. Microbiol. 2024, 206, 176. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, H.; Yang, L.; Li, F.X.; Cui, X.M.; Lin, D.M.; Lou, D.J.; Yang, X.Y. Preparation of minor ginsenosides from Panax notoginseng root and flower by the extracted enzyme of Mucor Abundans. Process Biochem. 2024, 137, 134–142. [Google Scholar] [CrossRef]
- Yang, F.; Wu, Z.; Cao, S.; Tao, Z.; Fan, D.; Liu, X. Simultaneous transformation of ginsenoside Rb1 into rare ginsenoside F2 and compound K by the extracellular enzyme from Aspergillus Niger Wu-16. Bioresour. Technol. 2022, 22, 101419. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, R.; Huang, Z.; Zhou, J. Progress in the conversion of ginsenoside Rb1 into minor ginsenosides using β-glucosidases. Foods 2023, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, Y.; Zhou, Y.; Xu, M.; Yu, S. Production of gypenoside XVII from ginsenoside Rb1 by enzymatic transformation and their anti-inflammatory activity In vitro and In vivo. Molecules 2023, 28, 7001. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, M.; Li, X.; Li, J.; Xu, M.; Wei, D.; Wu, D.; Wan, J.; Mei, S.; Cui, T.; et al. New dammarane-type triterpenoid saponins from Panax notoginseng saponins. J. Ginseng Res. 2020, 44, 673–679. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, W.J.; Gebru, Y.A.; Upadhyaya, J.; Ko, S.R.; Kim, Y.H.; Kim, M.K. Production of minor ginsenosides C-K and C-Y from naturally occurring major ginsenosides using crude β-glucosidase preparation from submerged culture of Fomitella Fraxinea. Molecules 2021, 26, 4820. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.M.; Sung, B.H.; An, D.S.; Lee, H.G.; Kim, S.G.; Kim, S.C.; Lee, S.T.; Im, W.T. Bioconversion of ginsenosides Rb1, Rb2, Rc and Rd by novel β-glucosidase hydrolyzing outer 3-O glycoside from Sphingomonas sp. 2F2: cloning, expression, and enzyme characterization. J. Biotechnol. 2011, 156, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Kim, T.H.; Choi, J.H.; Oh, D.K. Complete biotransformation of protopanaxadiol-type ginsenosides to 20-O-β-glucopyranosyl-20(S)-protopanaxadiol using a novel and thermostable β-glucosidase. J. Agric. Food Chem. 2018, 66, 2822–2829. [Google Scholar] [CrossRef]
- Liu, C.; Zuo, K.; Yu, H.; Sun, C.; Zhang, T.; Xu, L.; Jin, Y.; Im, W.T.; Jin, F. Preparation of minor ginsenosides C-Mx and C-K from notoginseng leaf ginsenosides by a special ginsenosidase Type-I. Process Biochem. 2015, 50, 2158–2167. [Google Scholar] [CrossRef]
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.H.; Nam, M.S. Identification of β-glucosidase activity of Lentilactobacillus Buchneri URN103L and its potential to convert ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Morphology identification of strain-S62. (A) Front view of morphology of strain-S62. (B) Back view of morphology of strain-S62. (C) Mycelium spore morphology at 10×40 times microscope of strain-S62.
Figure 1.
Morphology identification of strain-S62. (A) Front view of morphology of strain-S62. (B) Back view of morphology of strain-S62. (C) Mycelium spore morphology at 10×40 times microscope of strain-S62.
Figure 2.
TLC analysis of the transformation products of total saponins from P. notoginseng root by P. fimorum for 18 days. S: Standards; 1: Substrate; 2: Transformation products after 18 days of biotransformation.
Figure 2.
TLC analysis of the transformation products of total saponins from P. notoginseng root by P. fimorum for 18 days. S: Standards; 1: Substrate; 2: Transformation products after 18 days of biotransformation.
Figure 3.
The neighbor-joining tree based on ITS rDNA gene sequences of strain-S62.
Figure 3.
The neighbor-joining tree based on ITS rDNA gene sequences of strain-S62.
Figure 4.
The optimum reaction temperature and pH for saponins transformed by the crude enzyme from P. fimorum. (A) pH effect on the transformation of Rb1. (B) Temperature effect on the transformation of Rb1.
Figure 4.
The optimum reaction temperature and pH for saponins transformed by the crude enzyme from P. fimorum. (A) pH effect on the transformation of Rb1. (B) Temperature effect on the transformation of Rb1.
Figure 5.
The optimum reaction temperature and pH for saponins transformed by β-glucosidase. (A) pH effect on the transformation of Rb1. (B) Temperature effect on the transformation of Rb1.
Figure 5.
The optimum reaction temperature and pH for saponins transformed by β-glucosidase. (A) pH effect on the transformation of Rb1. (B) Temperature effect on the transformation of Rb1.
Figure 6.
TLC analysis of the transformation products of different types of saponins (Rb1, R1 and Rg1) by crude enzyme from P. fimorum and β-glucosidase, respectively. (A) TLC analysis of the transformation products by the crude enzyme from P. fimorum. (B) TLC analysis of the transformation products by β-glucosidase. 1, 2, 3: The transformation products of Rb1, R1 and Rg1 by crude enzyme from P. fimorum, respectively. 4, 5, 6: The transformation products of Rb1, R1 and Rg1 by β-glucosidase, respectively.
Figure 6.
TLC analysis of the transformation products of different types of saponins (Rb1, R1 and Rg1) by crude enzyme from P. fimorum and β-glucosidase, respectively. (A) TLC analysis of the transformation products by the crude enzyme from P. fimorum. (B) TLC analysis of the transformation products by β-glucosidase. 1, 2, 3: The transformation products of Rb1, R1 and Rg1 by crude enzyme from P. fimorum, respectively. 4, 5, 6: The transformation products of Rb1, R1 and Rg1 by β-glucosidase, respectively.
Figure 7.
HPLC analysis of the transformation products of different types of saponins for 72 hours by crude enzyme from P. fimorum. (A) The transformation products of Rb1. (B) The transformation products of Rg1. (C) The transformation products of R1. (D) The transformation products of Re.
Figure 7.
HPLC analysis of the transformation products of different types of saponins for 72 hours by crude enzyme from P. fimorum. (A) The transformation products of Rb1. (B) The transformation products of Rg1. (C) The transformation products of R1. (D) The transformation products of Re.
Figure 8.
HPLC analysis of the transformation products of different types of saponins for 72 hours by β-glucosidase. (A) The transformation products of Rb1. (B) The transformation products of Rg1. (C) The transformation products of R1. (D) The transformation products of Re.
Figure 8.
HPLC analysis of the transformation products of different types of saponins for 72 hours by β-glucosidase. (A) The transformation products of Rb1. (B) The transformation products of Rg1. (C) The transformation products of R1. (D) The transformation products of Re.
Figure 9.
TLC analysis of the time-course variation in different types of saponins and their transformation products during the biotransformation process by the crude enzyme from P. fimorum. (A) Transformation products analysis of Rb1 dynamic monitoring. (B) Transformation products analysis of Rg1 dynamic monitoring. (C) Transformation products analysis of R1 dynamic monitoring. (D) Transformation products analysis of Re dynamic monitoring.
Figure 9.
TLC analysis of the time-course variation in different types of saponins and their transformation products during the biotransformation process by the crude enzyme from P. fimorum. (A) Transformation products analysis of Rb1 dynamic monitoring. (B) Transformation products analysis of Rg1 dynamic monitoring. (C) Transformation products analysis of R1 dynamic monitoring. (D) Transformation products analysis of Re dynamic monitoring.
Figure 10.
TLC analysis of the time-course variation in different types of saponins and their transformation products during the biotransformation process by β-glucosidase. (A) Transformation products analysis of Rb1 dynamic monitoring. (B) Transformation products analysis of Rg1 dynamic monitoring. (C) Transformation products analysis of R1 dynamic monitoring. (D) Transformation products analysis of Re dynamic monitoring.
Figure 10.
TLC analysis of the time-course variation in different types of saponins and their transformation products during the biotransformation process by β-glucosidase. (A) Transformation products analysis of Rb1 dynamic monitoring. (B) Transformation products analysis of Rg1 dynamic monitoring. (C) Transformation products analysis of R1 dynamic monitoring. (D) Transformation products analysis of Re dynamic monitoring.
Figure 11.
Structures and productivity of minor ginsenosides from the biotransformation of ginsenosides Rb1, Rg1, Re and notoginseng R1 by crude enzyme from P. fimorum and β-glucosidase, respectively. Red dotted box: Products and yields of minor ginsenosides from biotransformation by crude enzyme from P. fimorum; Blue dotted box: Products and yields of minor ginsenosides from biotransformation by β-glucosidase.
Figure 11.
Structures and productivity of minor ginsenosides from the biotransformation of ginsenosides Rb1, Rg1, Re and notoginseng R1 by crude enzyme from P. fimorum and β-glucosidase, respectively. Red dotted box: Products and yields of minor ginsenosides from biotransformation by crude enzyme from P. fimorum; Blue dotted box: Products and yields of minor ginsenosides from biotransformation by β-glucosidase.
Figure 12.
Dynamic changes in the substrate conversion of ginsenosides Rb1, Rg1, Re and notoginsenoside R1 at different reaction time by crude enzyme from P. fimorum (A) and β-glucosidase (B), respectively.
Figure 12.
Dynamic changes in the substrate conversion of ginsenosides Rb1, Rg1, Re and notoginsenoside R1 at different reaction time by crude enzyme from P. fimorum (A) and β-glucosidase (B), respectively.
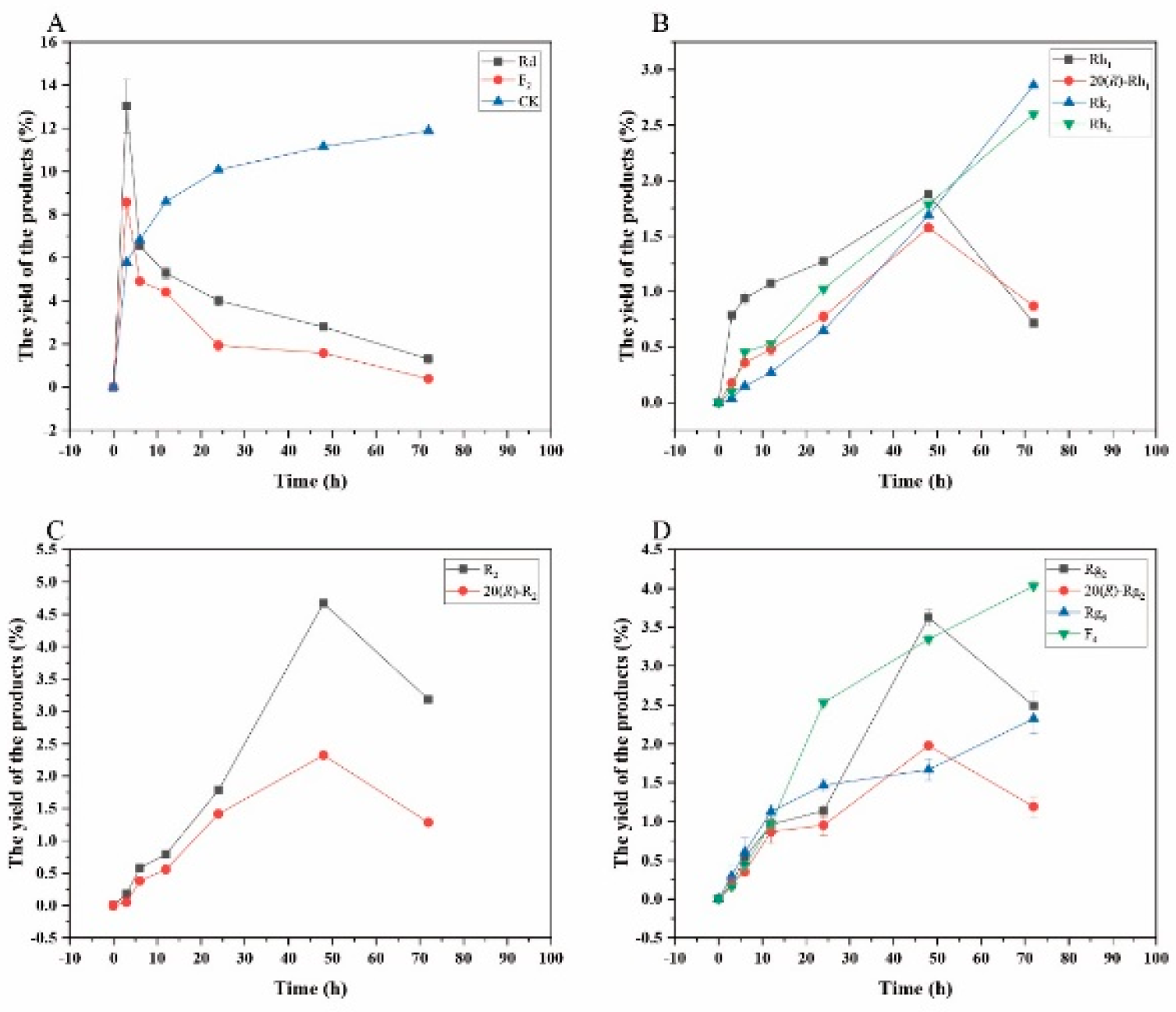
Figure 13.
Dynamic changes in the yield of the transformation products of ginsenosides Rb1 (A), Rg1 (B), Re (D) and notoginsenoside R1 (C) at different reaction time by crude enzyme from P. fimorum.
Figure 13.
Dynamic changes in the yield of the transformation products of ginsenosides Rb1 (A), Rg1 (B), Re (D) and notoginsenoside R1 (C) at different reaction time by crude enzyme from P. fimorum.
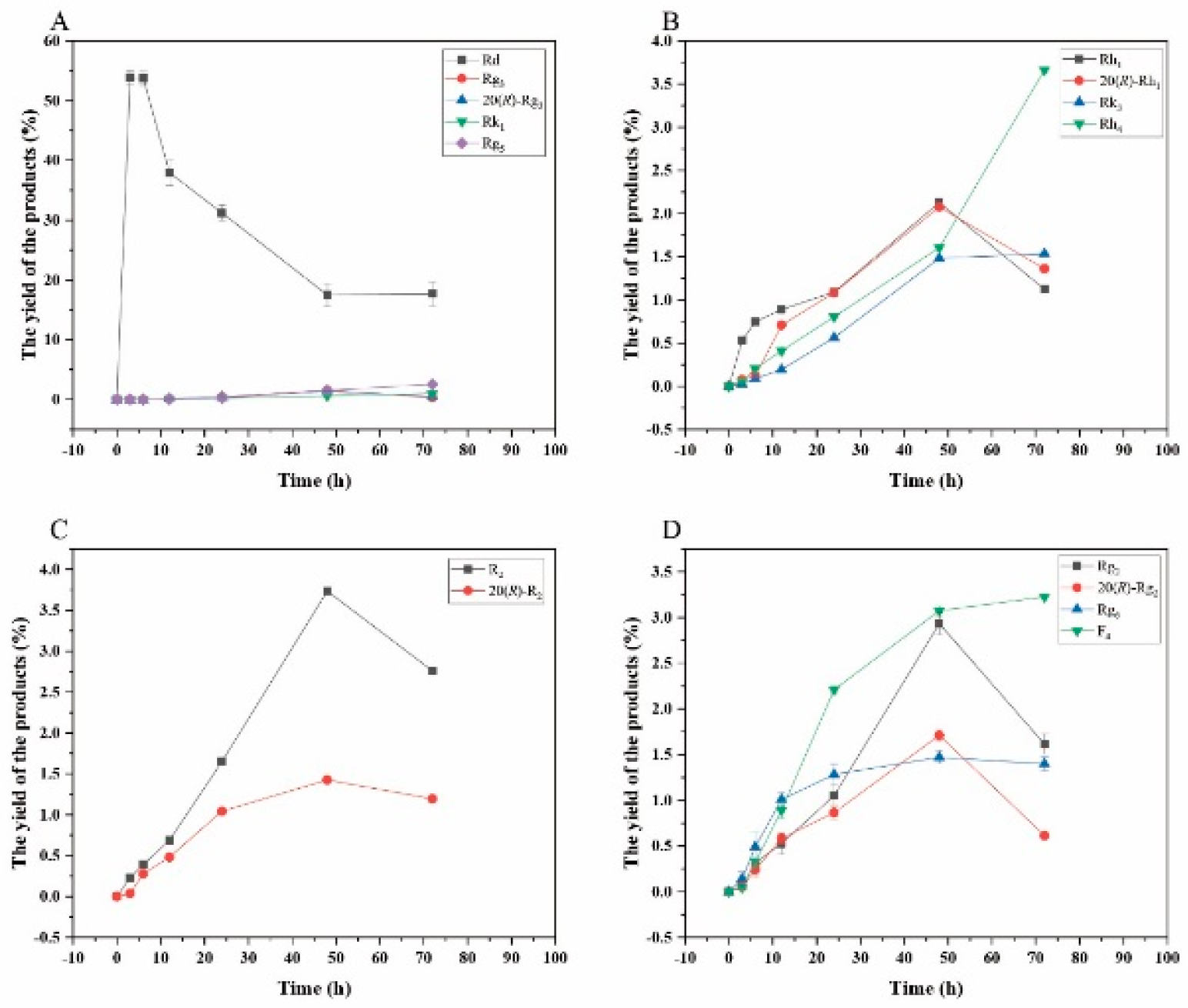
Figure 14.
Dynamic changes in the yield of the transformation products of ginsenosides Rb1 (A), Rg1 (B), Re (D) and notoginsenoside R1 (C) at different reaction time by β-glucosidase.
Figure 14.
Dynamic changes in the yield of the transformation products of ginsenosides Rb1 (A), Rg1 (B), Re (D) and notoginsenoside R1 (C) at different reaction time by β-glucosidase.
Table 1.
The substrate conversion of four main saponins from the roots of P. notoginseng during biotransformation.
Table 1.
The substrate conversion of four main saponins from the roots of P. notoginseng during biotransformation.
| The types of enzymes |
Substrates |
Substrate conversion (%) |
Crude enzyme from
P. fimorum
|
Rb1
|
100 |
| Rg1
|
47.66 |
| R1
|
54.23 |
| Re |
31.68 |
|
β-glucosidase |
Rb1
|
100 |
| Rg1
|
41.94 |
| R1
|
41.58 |
| Re |
41.72 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).