Submitted:
26 January 2025
Posted:
28 January 2025
You are already at the latest version
Abstract
Objective: To analyze the efficacy of transcutaneous electrical nerve stimulation (TENS) on parotid glands in patients with xerostomia. Material and methods: A simple, blinded, randomized, longitudinal, and prospective study was performed with 50 patients with xerostomia, divided into two groups: 25 patients received TENS and 25 received a sham treatment. The treatment group took part in three sessions with TENS that lasted 15 minutes each for three weeks. The production of saliva was measured through sialometry at the start and end of the treatment. The perception of xerostomia was assessed with the Visual Analog Scale (VAS-X) and the Xerostomia Inventory (XI), also at the beginning and the end. The impact on oral health (OHIP-14) was assessed in the first and third sessions. Results: A progressive improvement was observed after three treatment sessions. With respect to the initial VAS scores, these significantly decreased from the start 7.52 ± 1.92 to the end 6.84 ± 1.84 (p < 0.001). The sialometry values significantly increased, showing an increase in the salivary fluid from the start to the end of the treatment (p<0.001). With respect to the Xerostomia Inventory (XI) (Thomson), it was reduced from the initial 36.88 ± 7.78 to 35.60 ± 7.42 after the sessions (p = 0.01). No significant changes were observed in the OHIP-14 in patients treated with TENS. Conclusion: The TENS therapy is presented as a promising and non-invasive alternative for the management of xerostomia, as it is able to objectively increase the salivary flow and improve the subjective perception of mouth dryness.
Keywords:
Introduction
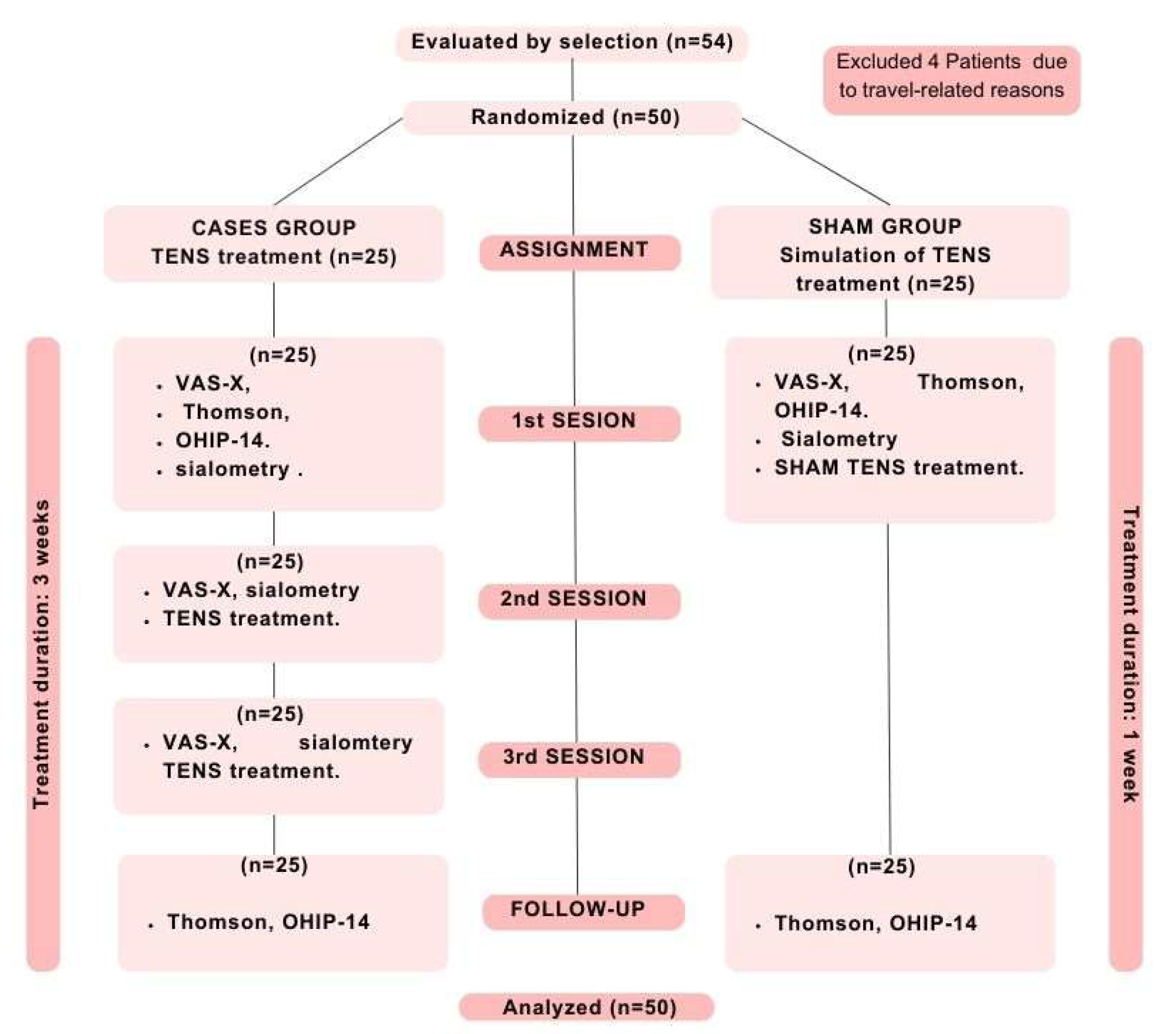
Materials and Methods
- ∘
- Age ≥ 18 years with xerostomía of any etiology
- ∘
- Signed informed consent and commitment to attend the three study sessions.
- ∘
- Patients with resection of major salivary glands.
- ∘
- Decompensated systemic disease.
- ∘
- Medical conditions such as pacemaker implants, active skin infections, vertigo, continuous headaches, hearing problems, neuralgia or pregnancy. Motor problems or inability to follow instructions.
- A-
- B-
-
Questionnaires:
- Visual Analog Scale for Xerostomia (VAS-X): It measures the intensity of xerostomia perceived by the patient [0-10].
- Xerostomia Inventory (XI): It assesses xerostomia with a scale of 11 items. The total score can vary between 11 and 55, representing the severity of the xerostomia. A score of 11 indicates a very slight or non-existent xerostomia, while a value of 55 indicates severe xerostomia; it is considered that scores of 14 or higher indicate intense xerostomia [18].
- Oral Health Impact Profile (OHIP-14): It assesses the quality of life related with oral health. The total score varies from 0 to 70, where a higher score indicates a worse quality of life. It was given at the start and the end of the last session [19].
Results
| Group | Test | Baseline (Mean ± SD) | Post-session (Mean ± SD) | p-value (Baseline vs Post) |
|---|---|---|---|---|
| Treatment | VAS-X | 7.52 ± 1.92 points | 6.84 ± 1.84 points | < 0.001 |
| Sialometry mm | 21.00 ± 16.38 mm | 27.68 ± 29.44 mm | < 0.001 | |
| Xerostomia Inventory (XI) | 36.88 ± 7.78 points | 35.60 ± 7.42 points | 0.01 | |
| Sham | VAS-X | 5.24 ± 2.13 points | 5.04 ± 2.15 points | 0.04 |
| Sialometry mm | 29.56 ± 16.55 mm | 29.80 ± 16.51 mm | 0.68 | |
| Xerostomia Inventory (XI) | 28.74 ± 9.30 points | 28.74 ± 9.30 points | Not significant |
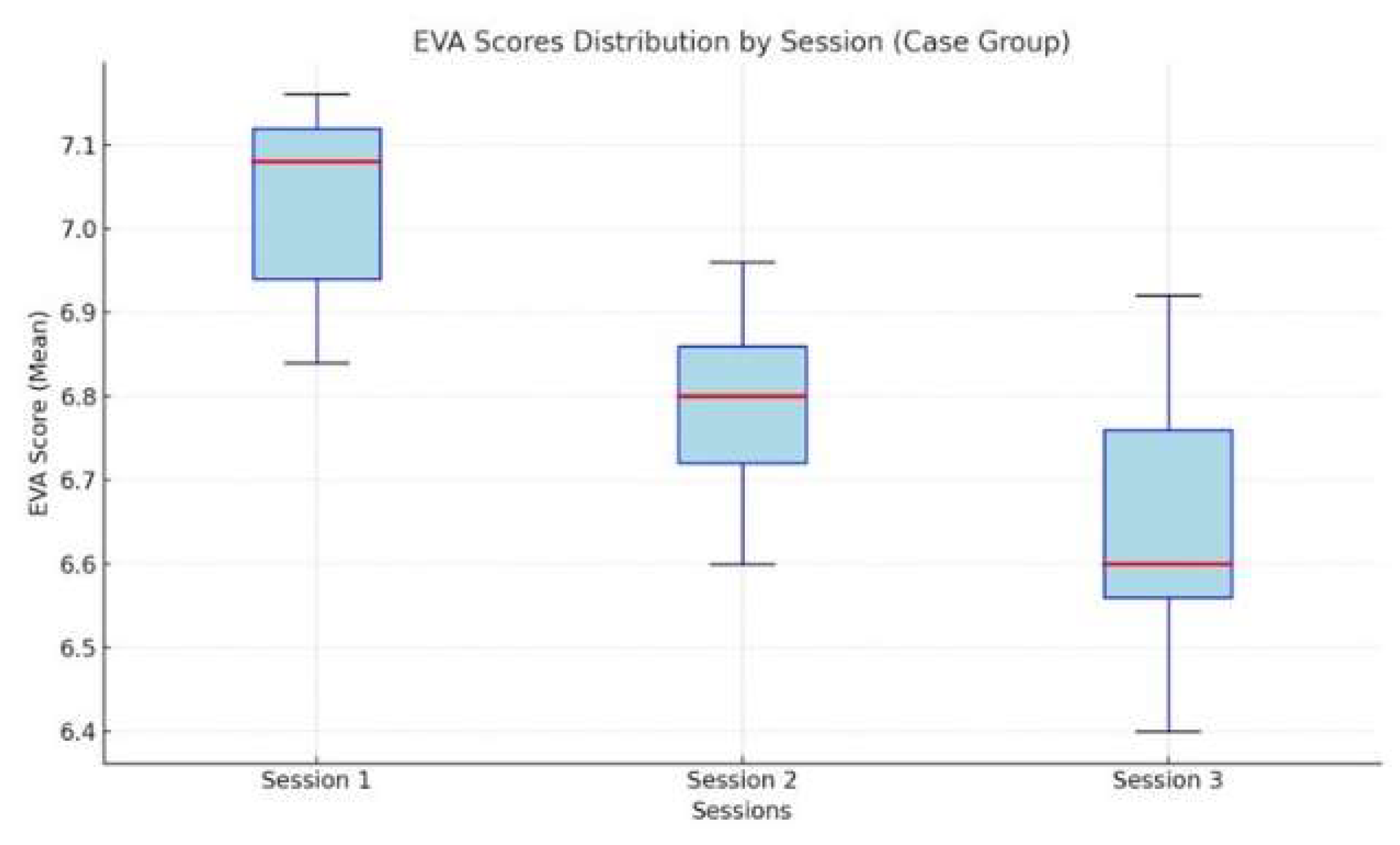
Conclusions
References
- López Jornet P, Hernandez L, Gomez García F, Galera Molero F, Pons-Fuster López E, Tvarijonaviciute A. A Clinical Study on the Efficacy and Tolerability of a New Topical Gel and Toothpaste in Patients with Xerostomia: A Randomized Controlled Trial. J Clin Med. 2021 Nov 29;10(23):5641. [CrossRef]
- Silvestre-Donat FJ, Miralles-Jordá L, Martinez-Mihi V. Tratamiento de la boca seca: puesta al día. Med Oral. 2004;9:273-9.
- López-López J, Jané Salas E, Chimenos Küstner E. Pronóstico y tratamiento de la boca seca. Revisión sistemática [Prognosis and treatment of dry mouth. Systematic review]. Med Clin (Barc). 2014 Feb 4;142(3):119-24. [CrossRef]
- Hosseini MS, Sanaie S, Mahmoodpoor A, Jabbari Beyrami S, Jabbari Beyrami H, Fattahi S, et al. Cancer treatment-related xerostomia: basics, therapeutics, and future perspectives. Eur J Med Res. 2024 Nov 30;29(1):571. [CrossRef]
- Łysik D, Niemirowicz-Laskowska K, Bucki R, Tokajuk G, Mystkowska J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int J Mol Sci. 2019 Jun 29;20(13):3199. [CrossRef]
- Gil-Montoya JA, Silvestre FJ, Barrios R, Silvestre-Rangil J. Treatment of xerostomia and hyposalivation in the elderly: A systematic review. Med Oral Patol Oral Cir Bucal. 2016 May 1;21(3):e355-66. [CrossRef]
- Khamdi S, Matangkasombut O, Lam-Ubol A. Non-pharmacologic interventions for management of radiation-induced dry mouth: A systematic review. Oral Dis. 2024 Jul;30(5):2876-2893. [CrossRef]
- López-Jornet MP, García-Teresa G, Viñas M, Vinuesa T. Clinical and antimicrobial evaluation of a mouthwash and toothpaste for xerostomia: a randomized, double-blind, crossover study. J Dent. 2011 Nov;39(11):757-63. [CrossRef]
- Sivaramakrishnan G, Sridharan K. Electrical nerve stimulation for xerostomia: A meta-analysis of randomised controlled trials. J Tradit Complement Med. 2017 Feb 14;7(4):409-13. [CrossRef]
- Salimi F, Saavedra F, Andrews B, FitzGerald J, Winter SC. Trans-cutaneous electrical nerve stimulation to treat dry mouth (xerostomia) following radiotherapy for head and neck cancer. A systematic review. Ann Med Surg (Lond). 2021 Feb 3;63:102146. [CrossRef]
- Khamdi S, Matangkasombut O, Lam-Ubol A. Non-pharmacologic interventions for management of radiation-induced dry mouth: A systematic review. Oral Dis. 2024 Jul;30(5):2876-2893. [CrossRef]
- Melo JLMA, Coelho CPES, Nunes FPES, Heller D, Grisi DC, Guimarães MDCM, et al. A scoping review on hyposalivation associated with systemic conditions: the role of physical stimulation in the treatment approaches. BMC Oral Health. 2023 Jul 21;23(1):505. [CrossRef]
- Aggarwal H, Pal-Singh M, Mathur H, Astekar S, Gulati P, Lakhani S. Evaluation of the effect of transcutaneous electrical nerve stimulation (TENS) on whole salivary flow rate. J Clin Exp Dent. 2015 Feb 1;7(1):e13-7. [CrossRef]
- Konidena A, Sharma D, Puri G, Dixit A, Jatti D, Gupta R. Effect of TENS on stimulation of saliva in postmenopausal women with or without oral dryness - An interventional study. J Oral Biol Craniofac Res. 2016 Nov;6(Suppl 1):S44-S50. [CrossRef]
- Dyasnoor S, Kamath S, Khader NFA. Effectiveness of Electrostimulation on Whole Salivary Flow Among Patients with Type 2 Diabetes Mellitus. Perm J. 2017;21:15-164. [CrossRef]
- Yang LY, Chen HM, Su YC, Chin CC. The effect of transcutaneous electrical nerve stimulation on increasing salivary flow rate in hemodialysis patients. Oral Dis. 2019 Jan;25(1):133-41. [CrossRef]
- López-Jornet P, Camacho-Alonso F, Bermejo-Fenoll A. A simple test for salivary gland hypofunction using Oral Schirmer’s test. J Oral Pathol Med. 2006 Apr;35(4):244-8. [CrossRef]
- Schoppmeier CM, Janson M, Höfer K, Graf I, Wicht MJ, Barbe AG. Use of the modified Schirmer test to measure salivary gland hypofunction/hyposalivation: Systematic review and meta-analysis. Eur J Oral Sci. 2024 Apr;132(2):e12977. [CrossRef]
- Serrano C, Fariña MP, Pérez C, Fernández M, Forman K, Carrasco M. Translation and validation of a Spanish version of the xerostomia inventory. Gerodontology. 2016 Dec;33(4):506-12. [CrossRef]
- Montero J, López JF, Vicente MP, Galindo MP, Albaladejo A, Bravo M. Comparative validity of the OIDP and OHIP-14 in describing the impact of oral health on quality of life in a cross-sectional study performed in Spanish adults. Med Oral Patol Oral Cir Bucal. 2011 Sep 1;16(6):e816-21. [CrossRef]
- Tabrez S, Patil N, Sareen M, Meena M, Tyagi N, Kaswan S. Efficacy of Transcutaneous Electric Nerve Stimulation (TENS) on Whole Salivary Flow Rate: A Descriptive Observational Study. J Dent (Shiraz). 2022 Jun;23(1 Suppl):214-21. [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2024. Available from: https://www.R-project.org/.
- Talal N, Quinn JH, Daniels TE. The clinical effects of electrostimulation on salivary function of Sjogren’s syndrome patients. Rheumatol Int. 1992;12:43-5. [CrossRef]
- Wong RKW, Deshmukh S, Wyatt G, Sagar S, Singh AK, Sultanem K, et al. Acupuncture-like transcutaneous electrical nerve stimulation versus pilocarpine in treating radiation-induced xerostomia: results of RTOG 0537 phase 3 study. Int J Radiat Oncol Biol Phys. 2015;92(2):220-7. [CrossRef]
- Wong RKW, Jones GW, Sagar SM, Babjak AF, Whelan T. Phase I-II study in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57(2):472-80. [CrossRef]
- Vijayan A, Asha ML, Babu S, Chakraborty S. Prospective phase II study of the efficacy of transcutaneous electrical nerve stimulation in post-radiation patients. Clin Oncol. 2014;26(12):743-7. [CrossRef]
- Lakshman AR, Subhas Babu G, Rao S. Evaluation of effect of transcutaneous electrical nerve stimulation on salivary flow rate in radiation-induced xerostomia patients: a pilot study. J Cancer Res Ther. 2015;11(1):229-33. [CrossRef]
- Paim ED, Zanella VG, Martins VB, Macagnan FE, Berbert MCB. Effects of transcutaneous electrical nerve stimulation on the salivary flow of patients with hyposalivation induced by radiotherapy in the head and neck region—a randomized clinical trial. J Oral Rehabil. 2019;46(12):1142-50. [CrossRef]
- Vilas SK, Shashikant MC, Ali IM. Evaluation of the effects of transcutaneous electrical nerve stimulation on whole saliva flow: A clinical study. J Indian Acad Oral Med Radiol. 2009;21(1):7-11. [CrossRef]
| Variable | Treatment group (n=25) | Sham Group (n=25) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 61.92 ± 12.35 | 54.76 ± 12.44 | 0.06 |
| Sex | 0.17 | ||
| - Men | 3 (12%) | 8 (32%) | |
| - Women | 22 (88%) | 17 (68%) | |
| Tobacco | 0.17 | ||
| - Yes | 3 (12%) | 8 (32%) | |
| - No | 22 (88%) | 17 (68%) | |
| Alcohol | 0.49 | ||
| - Yes | 0 (0%) | 2 (8%) | |
| - No | 25 (100%) | 23 (92%) | |
| Quality of hygiene | 0.14 | ||
| - Good | 15 (60%) | 10 (40%) | |
| - Average | 7 (28%) | 14 (56%) | |
| - Bad | 3 (12%) | 1 (4%) | |
| Number of medications | 3.28 ± 2.19 | 3.80 ± 4.25 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





