Introduction
Radiosurgery (SRS) is a mainstay treatment modality today for intracranial metastatic disease [
1]. Cerebral radiation necrosis (RN), a delayed side effect that can develop months to years after treatment, occurs in about 25% of cases. Unlike post-SRS tumor progression (TP), which is largely indicative of limited to no response to treatment, RN is thought to represent a subset of SRS-responders. Mechanistically, it is thought to be an autoreactive immune process, albeit initiated by radiation injury to blood vessels and cerebral tissue within the targeted field [
2,
3,
4]. Even so, while there are clear, histologic differences between RN and TP lesions, they are not readily distinguishable using standard radiological imaging thus often requiring surgical tissue biopsy [
5,
6]. Given the divergent treatment approaches to RN and TP, improving the reliability of radiographic diagnosis is crucial.
Recent studies have explored alternate, accessible radiographic modalities to delineate RN lesions. Current MRI sequences used to differentiate RN and TP include MR spectroscopy and MR perfusion both of which are limited by interobserver variability and overlapping radiographic findings between RN and TP [
5,
6,
7,
8,
9]. The central vein sign (CVS) is a novel MRI biomarker that has not yet been utilized in this capacity. In practice, CVS – which correlates with immune infiltrate-rich perivascular spaces – has been reported as an effective tool in delineating multiple sclerosis (MS) lesions [
10,
11]. In our practice, we had already anecdotally noted that a number of our RN patients had lesions with large, associated venous structures (Supplementary Figure S1). In applying our understanding of RN as an autoreactive process associated with an immune infiltrate, we hypothesized that patients with radiation necrosis would have a similar CVS seen on MRI that could potentially be predictive of this diagnosis.
Brief Report
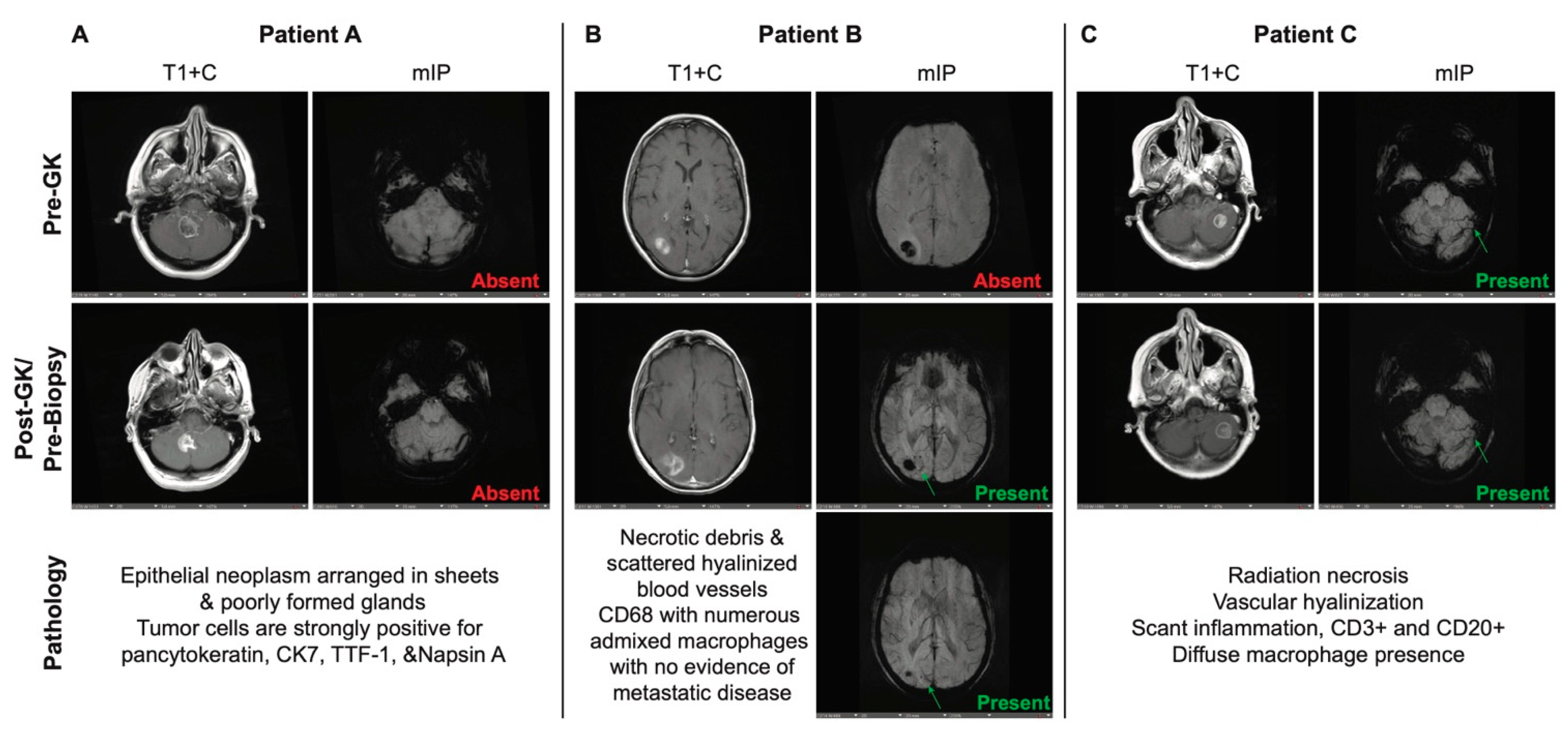
We retrospectively identified 163 patients with lung, melanoma, breast, or other metastatic cancer brain metastases treated at our institution who presented between July 2018 and July 2019 with progressive lesions suggestive of RN or TP after undergoing stereotactic radiosurgery (SRS), necessitating biopsy for definitive diagnosis. Of these, 80 patients were selected that had a full complement of pre- and post-SRS, in addition to pre-biopsy, imaging. We then compiled imaging, histologic and clinical data from each of these patients (Supplemental Table S1, Supplemental Figure S1a-c). TP was defined as post-biopsy histology showing any evidence of metastatic disease with or without necrosis. RN was defined as histology showing reactive changes in the absence of metastatic disease, including hyalinized blood vessels, necrotic debris, and immune infiltrate.
Susceptibility-weighted images using minimum intensity projection (mIP) were evaluated by two independent researchers to look for and identify CVS (
Figure 1a-p). The findings were validated by an independent neuroradiologist. Of the 52 patients with post-biopsy diagnosis of RN alone across all tumor subtypes, 31 (60%) were found to have a pre-biopsy CVS on MRI. There were 14 patients that were found to have predominantly radiation necrosis with few residual tumor cells on histology; of these, 12 (86%) had a pre-biopsy CVS present as well. Of the 14 patients with a post-biopsy diagnosis of TP, only 3 (21%) had CVS on MRI. We performed Fisher exact tests and multivariate logistic regression analysis to determine whether these findings were predictive and found that there was a strong correlation between presence of CVS and diagnosis of RN (
Supplemental Figure S2a-c).
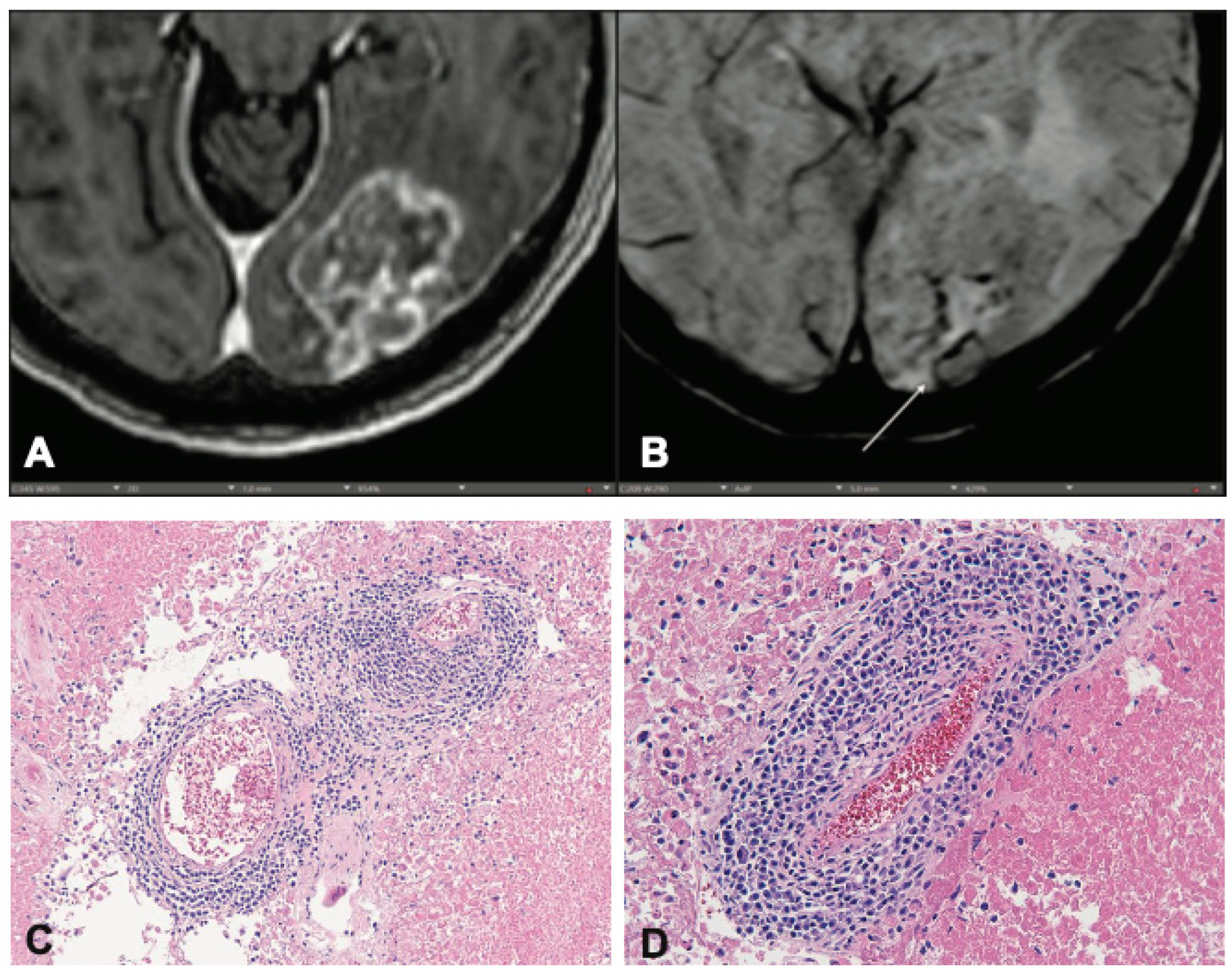
To understand the biologic relevance of the CVS in the context of post-RT disease, we retrospectively identified patients whose records indicated that there was residual fixed tissue available for additional histologic staining. Of these, 9 had sufficient tissue for processing and staining steps as determined by our Pathology Department. We found that, regardless of diagnosis of RN or TP, patients with a CVS (
Figure 2a, b) had a corresponding presence of perivenular immune infiltrate (
Figure 2c, d). This immune infiltrate was not present in patients without CVS. Hematoxylin & Eosin-stained slides showed stigmata of radiation induced changes, such as vascular hyalinization, necrosis and/or the presence of few residual tumor cells. Immunohistochemical stains highlighted individual cell types and demonstrated the presence of leukocytes (CD45) (
Supplemental Figure S3a), T-lymphocytes (CD3) (
Supplemental Figure S3b), and macrophages (CD68) (
Supplemental Figure S3c), or in a diffuse intraparenchymal distribution pattern and occasionally as small perivascular accumulation (
Supplemental Figure S3d).
Discussion
The use of SRS for the treatment of brain metastases is growing exponentially. As a result there is an increasing need not only to be able to differentiate RN from TP using non-invasive methods but to also better understand the pathophysiology underlying the development of RN and TP after SRS. Given the proposed auto-reactive immunologically driven nature of RN, and drawing parallels with multiple sclerosis where CVS has been shown to likely indicate perivascular inflammation, this study speculated that CVS in metastatic cancer patients might predict an effective inflammatory anti-tumor response to radiation. Histologic analysis of tissues from patients with MRI identified CVS showed significant inflammatory infiltrates, in comparison to those without identified CVS. In addition, a significantly larger percentage of RN patients had CVS identified on their pre-SRS imaging compared with those who had TP.
We propose therefore that SRS triggers an immunologically driven treatment-response in metastatic lesions, that results in outcomes ranging from TP to RN. In those patients with evidence of pre-treatment perivascular inflammation, SRS seems more likely to result in RN whereas in those patients without evidence of pre-treatment perivascular inflammation, SRS is more likely to fail resulting in TP. The idea that SRS response is driven by an immune mechanism is not a new concept [12]. The existence of CVS in the pre-SRS images may suggest that these patients already have a more robust intrinsic immune cell population within the brain. This primed immune system may then result in not only a better tumor response but also a higher likelihood of developing post-SRS RN. The radiographic and histology findings in this study support this hypothesis.
Given the findings in this study, in conjunction with other imaging sequences, the addition of the finding of CVS pre-SRS might help better distinguish between TP and RN in post-SRS patients, potentially offering an additional non-invasive method to differentiate these clinical scenarios. In addition, CVS may also emerge as an indicator of a patient's likelihood of responding to SRS. While promising, these findings prompt further research. Future studies should focus on validating these observations in larger patient cohorts and investigating the potential of CVS as a predictive marker for cancer therapy response.
References
- Nieder, C.; Grosu, A.L.; E Gaspar, L. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat. Oncol. 2014, 9, 155–155. [CrossRef]
- Yoritsune, E.; Furuse, M.; Kuwabara, H.; Miyata, T.; Nonoguchi, N.; Kawabata, S.; Hayasaki, H.; Kuroiwa, T.; Ono, K.; Shibayama, Y.; et al. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J. Radiat. Res. 2014, 55, 803–811. [CrossRef]
- Ali, F.S.; Arevalo, O.; Zorofchian, S.; Patrizz, A.; Riascos, R.; Tandon, N.; Blanco, A.; Ballester, L.Y.; Esquenazi, Y. Cerebral Radiation Necrosis: Incidence, Pathogenesis, Diagnostic Challenges, and Future Opportunities. Curr. Oncol. Rep. 2019, 21, 66. [CrossRef]
- Abdulla, S.; Saada, J.; Johnson, G.; Jefferies, S.; Ajithkumar, T. Tumour progression or pseudoprogression? A review of post-treatment radiological appearances of glioblastoma. Clin. Radiol. 2015, 70, 1299–1312. [CrossRef]
- Mayo, Z.S.; Halima, A.; Broughman, J.R.; Smile, T.D.; Tom, M.C.; Murphy, E.S.; Suh, J.H.; Lo, S.S.; Barnett, G.H.; Wu, G.; et al. Radiation necrosis or tumor progression? A review of the radiographic modalities used in the diagnosis of cerebral radiation necrosis. J. Neuro-Oncology 2023, 161, 23–31. [CrossRef]
- Romano, A., et al., Radiosurgery for Brain Metastases: Challenges in Imaging Interpretation after Treatment. Cancers, 2023. 15(20): p. 5092.
- Lasocki, A.; Sia, J.; Stuckey, S.L. Improving the diagnosis of radiation necrosis after stereotactic radiosurgery to intracranial metastases with conventional MRI features: a case series. Cancer Imaging 2022, 22, 1–8. [CrossRef]
- Hainc, N.; Alsafwani, N.; Gao, A.; O’halloran, P.J.; Kongkham, P.; Zadeh, G.; Gutierrez, E.; Shultz, D.; Krings, T.; Alcaide-Leon, P. The centrally restricted diffusion sign on MRI for assessment of radiation necrosis in metastases treated with stereotactic radiosurgery. J. Neuro-Oncology 2021, 155, 325–333. [CrossRef]
- Caulfield, J.I. and H.M. Kluger, Emerging studies of melanoma brain metastasis. Current Oncology Reports, 2022. 24(5): p. 585-594. Sati, P., et al., The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nature Reviews Neurology, 2016. 12(12): p. 714-722.
- Sinnecker, T.; Clarke, M.A.; Meier, D.; Enzinger, C.; Calabrese, M.; De Stefano, N.; Pitiot, A.; Giorgio, A.; Schoonheim, M.M.; Paul, F.; et al. Evaluation of the Central Vein Sign as a Diagnostic Imaging Biomarker in Multiple Sclerosis. JAMA Neurol. 2019, 76, 1446–1456. [CrossRef]
- Matsui, J.K.; Perlow, H.K.; Raj, R.K.; Nalin, A.P.; Lehrer, E.J.; Kotecha, R.; Trifiletti, D.M.; McClelland, S.; Kendra, K.; Williams, N.; et al. Treatment of Brain Metastases: The Synergy of Radiotherapy and Immune Checkpoint Inhibitors. Biomedicines 2022, 10, 2211. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).