Introduction
In modern literature, different histological types of esophagitis are considered as unrelated diseases. For example, eosinophilic esophagitis (EoE) is considered a special disease, not related to other problems of the digestive tract. There is a group of authors who devote their numerous articles to this problem. However, neither the etiology nor the pathogenesis of EoE is known yet, because the authors are afraid to go beyond the established boundaries. For example, it is known that the use of PPIs leads to an improvement in the clinical picture and a decrease in eosinophils in biopsies. Nevertheless, the authors suggest that PPIs are better classified as a treatment for esophageal eosinophilia that may be due to EoE than as a diagnostic criterion [
1]. In other words, they do not even consider the possibility that EoE may be a particular histological picture of GERD. Other authors devote their articles to lymphocytic esophagitis as a special disease [
2,
3]. However, in most patients with esophagitis, who did not undergo histological examination of the esophageal mucosa, esophagitis is diagnosed based on visual signs of inflammation (erosion, ulcers, stenosis) during endoscopic examination. Such findings are considered evidence of gastroesophageal reflux disease (GERD). It is known that endoscopic examination reveals only complications of GERD. In the so-called non-erosive form of GERD, the endoscopic picture of the pathology does not reveal [
4]. To confirm the inflammatory process, some researchers recommend determining the width of the intercellular space, which increases with the inflammatory process [
5,
6]. Chandrasoma et al believe that the appearance of cardiac epithelium over the lower esophageal sphincter (LES), which occurs because of cardiac metaplasia of the squamous epithelium due to exposure to gastric juice results in cephalad movement of the squamo-columnar junction (SCJ) is evidence of GERD [
7]. Since different histologic patterns suggest different treatments, it would be logical to perform histologic examination during routine endoscopy. However, neither Lyon consensus 2.0, nor the Chicago Classification 4 recommends doing this [
8,
9]. Practitioners, following the decisions of the conferences, do not recommend even performing endoscopic examinations for so-called functional disorders of the digestive tract, if organic damage is not suspected [
10,
11].
Since esophagitis is an inflammation of the esophagus wall, it is obvious that the accurate diagnosis is histological examination, especially in the presence of a specific reaction (eosinophilia, lymphocytosis). For practicing doctors, recommendations are needed on what stage treatment can be carried out without histological examination. However, for solving scientific problems, histological examination is a defining scientific document. Before moving on to histological examination, it is necessary to evaluate other diagnostic methods.
To what extent can we trust the clinical symptoms of GERD and how do they compare with objective methods?
A). It is known that the inflammatory process in the esophagus can occur without clinical manifestations. For example, endoscopic examinations of individuals who consider themselves healthy revealed GERD in 16% among 6,683 health examinees [
12]. Similar results were obtained by Stål et al, who noted that “Histologic abnormalities are poorly related to acid reflux in healthy volunteers” [
13]. Shieh et al showed that after POEM, 41.9% had erosive esophagitis, but only 12% had GERD symptoms [
14]. Often, GERD hides behind non-esophageal symptoms [
15]. If we consider that endoscopic examination based on visual data determines only complications of GERD, it becomes obvious that the number of patients with GERD among individuals without clinical symptoms is significantly higher than shown above. This reliable data shows that the absence of clinical symptoms does not allow us to exclude GERD. Secondly, the absence of complaints in patients with reflux esophagitis can be explained by damage to sensitive nerve elements by hydrochloric acid and pepsin. Thirdly, endoscopic examination without histology does not allow GERD to be ruled out.
B). In 2006, the Montreal Consensus, using a modified Delphi process, adopted the following definition of GERD: - «GERD was defined as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications» [
16]. From the point of view of a practicing physician, such a definition seems logical, since people without complaints do not seek medical help. However, from a scientific point of view, as shown above, it is not correct. Secondly, in a scientific definition of a disease, the etiology or pathogenesis of the disease is always recorded, and not the symptoms. A vote of selectively selected physicians led to a change in the name of the disease, which was previously defined as "GER", and began to be called "GERD". This meant that reflux can be physiological, i.e., not requiring treatment, or a disease if it is confirmed by a pH study in the esophagus.
С). In 1974 and 1976 DeMeester et al published an articles proposing a normal range for esophageal pH monitoring. It was defined as pH < 4 for 4% of the 24 hours of monitoring 5 cm proximal to the LES. The authors examined 15 individuals who believed that they had no problems with their digestive system. Since then, this boundary has been called the "DeMeester score", and the proposed method of pH monitoring has long been considered the gold standard for diagnosing gastroesophageal reflux disease [
17,
18]. First, these studies contradicted the existing scientific facts that repeated reflux causes reflux esophagitis and cannot be physiological. Second, the assumption that acid and pepsin, being in the lumen of the esophagus for about an hour a day, may not damage the mucosa is contrary to common sense. Thirdly, the authors examined 15 people as the norm, considering them healthy, based on the absence of typical complaints. In other studies, they used endoscopic, manometric and radiographic studies, but for an unknown reason did not use them to determine the norm. As a result, 24-hour esophageal pH measurement has a false negative rate of 15% to 30%” [
12,
13,
19]. In addition, some patients have only atypical, including extraesophageal symptoms [
15].
Since 1999 [
20], T.R. DeMeester, in collaboration with histologists, published numerous studies showing that GER begins with acid damage to the intra-abdominal portion of the LES. Cardiac epithelium, which occurs because of metaplasia of the esophageal squamous epithelium, appears over the LES at a later stage. They consider the recommendation not to perform histological examination during endoscopy to be erroneous, since timely detection of cardiac epithelium in the esophagus will allow treatment to begin at an early stage and avoid the development of Barrett's esophagus [
7,
20,
21]. Thus, it was proven that changes in the esophageal mucosa occur when acid enters the esophagus, which excludes the possibility of functional reflux, which DeMeester stated in his first articles. However, I did not find DeMeester's statement that his articles about the supposedly objective method of diagnosing GER (pH monitoring) were just advertising for equipment.
Analysis of the situation in modern gastroenterology indicates that:
a) The hypothesis about the high reliability of clinical symptoms in the diagnosis of GER is erroneous.
b) pH monitoring, proposed based on the determining role of clinical symptoms, detects only severe forms of GER. Its use is dangerous, since about 30% of patients with GER are not diagnosed, which means they remain without treatment.
c) The hypothesis based on pH monitoring, about the possibility of physiological reflux does not correspond to scientific facts.
d) The Lyon Consensus 2.0 (2023) provides a modern definition of actionable GERD, where evidence from esophageal testing (prolonged wireless pH monitoring or catheter-based pH or pH-monitoring off antisecretory medication; pH-impedance monitoring) guides diagnosis and treatment [
22]. All decisions determined by voting (Lyon Consensus 2.0, Rome IV criteria, Montreal definition GERD, Los Angeles classification GERD, and Chicago Classification version 4) are not based on reliable scientific facts and are therefore not scientific. Unlike scientific hypotheses, false hypotheses create chaos.
For example, the article by Gorgulu et al presents the results of a study of Turkish patients who had their esophageal biopsies examined under high magnification [
5]. Daniel Sifrim, who works in London, is listed as a co-author with the Turkish doctors. His role was critical revision and supervision. Patients who had typical GERD symptoms (heartburn and/or regurgitation) at least once a week were included. After taking the biopsies, high-resolution 36-channel solid-state esophageal manometry was performed to exclude motility disorders, with the exception of pathologies associated with GERD. Then, 24-hour pH-multichannel monitoring of intraluminal esophageal impedance was carried out.
The patients who had acid exposure time (AET) > 6% without erosion were classified as conclusive NERD patients according to Lyon Consensus. The patients who had typical GERD symptoms and AET < 4% without erosion were divided according to symptom association probability (SAP) and symptom index (SI). Patients who had both positive SAP (≥ 95%) and SI (≥ 50%) were classified as reflux hypersensitivity (RH). Patients who had both negative SAP (< 95%) and negative SI (< 50%) were classified as functional heartburn (FH). Healthy controls (HC) had normal UGE, 24-hour pH-impedance monitoring and high-resolution manometry while having no gastrointestinal symptoms or surgical history.
The authors found that only mild and severe ERD patients had increased mean intercellular spaces (IS) values compared to other groups. There was no significant difference between NERD, RH, FH, and HC. They would not recommend the practical use of mean IS length measurement for conclusive diagnostic purposes.
The analysis of the article by Gorgulu et al reveals many contradictions.
A retrospective study of 149 patients who had typical GERD symptoms (heartburn and/or regurgitation) at least once a week were included. Fourteen of them had "normal UGE, 24-hour pH-impedance monitoring and high-resolution manometry while having no gastrointestinal symptoms" [
5]. Firstly, on what basis were patients with heartburn and/or regurgitation at least once a week assessed as having no gastrointestinal symptoms? Secondly, patients with typical GERD symptoms cannot be considered healthy and could not serve as a control group. It is known that endoscopic examination without histology does not reveal pathology in most patients with GERD (Nonerosive reflux disease). As shown above, the absence of pathological changes in manometry does not allow GER to be excluded, since with an AET index of < 6%, GER is not diagnosed in more than 30% of patients. It follows that dilated intercellular spaces (DIS) in obvious patients were compared not with the control group, but with the same patients, and therefore no significant difference was obtained with all other so-called phenotypes. And since DIS, which indicates damage to the mucous membrane, was the same in patients with FH and RH as in typical GER, it follows from this that FH and RH are organic diseases (reflux esophagitis), and not functional disorders. Interestingly, in 14 articles published by Sifrim on DIS, including with Italian and Chinese doctors, where he also acted as a corrector, other equally important but more convincing signs of inflammation (impaired barrier integrity and immune cell infiltration, often combined with eosinophilic and lymphocytic infiltration) were not studied in biopsies. For 17 years (2007 - 2024), the reason for the study was articles about the feasibility of the DIS study for the accurate diagnosis of GERD. And most of the articles ended with an advertisement for HRM and pH monitoring.
The article states that after taking the biopsies, HRM was performed "with the exception of pathologies associated with GERD" [
5]. This statement contradicts the description of the "Material", since GERD was suspected in all patients. Therefore, in accordance with the recommendation of the Chicago Classification, HRM was not indicated in all cases. The described contradictions reveal the true purpose of this study, as well as all the other 13 articles by Daniel Sifrim. (1) The authors advertise equipment for HRM and impedance pH monitoring, claiming that past is the only objective method for diagnosing GERD. To this end, they discriminate against histological examination as such, which is easiest to do by attacking DIS. Histological diagnostics that reveal esophagitis is very simple, cheap and impeccable in accuracy, especially using the method of determining the cardiac epithelium over the LES [
7]. But its use will not only destroy the thriving industry of unnecessary equipment but also destroy all the false ideas that were based on pH monitoring. Therefore, all consensuses on GERD recommend not to perform histological examination of the esophagus if there is no visual pathology during endoscopy [
7,
22]. (2) It is not clear why the authors of the article violated the Chicago Classification recommendation not to perform HRM in GERD. But it is important to understand why such a recommendation exists. I analyzed 29 radiographic studies of children diagnosed with esophageal achalasia (EA), including those using HRM. Radiographic evidence of true EA, but without histological confirmation, was detected in only one observation. In 4 cases, there was congenital stenosis at the level of the LES. In all other cases, the radiographic appearance, clinical symptoms, and anamnesis were consistent with reflux esophagitis, including stenosis at the level of the LES in 4 observations [
23]. Since reflux esophagitis leads to significant changes in esophageal motility, knowledge of this pattern would destroy the doctrine of EA, which was adopted by vote in Chicago (Chicago Classification). Unreasonable substitution of the diagnosis of GER for EA led to an increase in the frequency of EA from 0.03 to 32.58 per 100,000 population (in one of the districts of Chicago) i.e. increased more than 1000 times [
24]. Daniel Sifrim is one of a large group of physicians who stand guard over the well-being of diagnostic equipment manufacturers, voting that impedance pH monitoring is superior to histology for diagnosis GERD, and HRM for diagnosing EA. They receive grants for publishing lectures and review articles and are paid consultants for companies that create diagnostic equipment and drugs. All this data is drawn from open sources.
Eosinophilic esophagitis (EoE)
EoE represents a chronic, local immune-mediated esophageal disease, characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation. ЕoЕ manifested by dysphagia, intermitted food impactions and symptoms like gastroesophageal reflux disease, that predominantly affects young adults [1,27]. The diagnosis of EoE is established by an esophageal biopsy demonstrating at least 15 eosinophils per high-power field in the absence of other conditions associated with esophageal eosinophilia such as gastroesophageal reflux disease or achalasia [
1]. Current therapies include proton pump inhibitors; topical steroid preparations, such as fluticasone and budesonide and dietary. Proton pump inhibitor therapy is associated with a histologic response, defined as less than 15 eosinophils per high-power field on endoscopic biopsy, in 41.7% of patients, while placebo was associated with a 13.3% response rate [
1]. The authors avoid deciding why PPIs have a therapeutic effect. They suggest that PPIs are better classified as a treatment for esophageal eosinophilia that may be due to EoE than as a diagnostic criterion [
1]. Currently, EoE is defined as antigen-mediated chronic disease distinct from GERD [28]. This explains why instrumental research proponents have taken it beyond their interests, since histological examination is a necessary diagnostic method, unlike GERD, in which it allegedly has no advantage over pH monitoring. However, an analysis of the literature reveals many contradictions in the theoretical opposition of EoE to GERD.
1. Eosinophilic gastrointestinal diseases (EGIDs) comprise a group of chronic, inflammatory diseases of the gastrointestinal (GI) tract, that are characterized, clinically, by symptoms related to the dysfunction of the involved segment(s) of the GI tract, and histologically, by dense eosinophilic inflammation, in the absence of an identifiable secondary cause. The group of EGIDs comprises EoE, eosinophilic gastritis, eosinophilic gastroenteritis, and eosinophilic colitis. Eosinophilic infiltration can be found in several parts of the digestive tract in one patient [29]. Mahendra et al showed that duodenal eosinophilia was associated with symptomatic erosive GERD [30].
These data indicate that EoE is one of the sites of inflammatory reaction of the digestive tract against the background of allergic disposition of the organism.
2. It is well known that GERD and EoE may be accompanied by eosinophilia in mucosal biopsies. Therefore, the question of a possible connection between these diseases has repeatedly arisen [26]. Monnerat and Lemme, using pH monitoring, found pathological reflux in 25% of patients with EoE [27]. Pesce et al found that Higher esophageal acid exposure time and lower baseline impedance values were significantly associated with eosinophilic infiltration (P < .05 and P < .01, respectively) [31]. Frazzoni et al, using impedance-pH monitoring, concluded that reflux plays a role in the pathogenesis of EoE [32]. Surprisingly, all authors believe that pH monitoring cannot predict whether PPI treatment will be effective, as if pH monitoring is used for such predictions. If pH monitoring is the main diagnostic method for GERD, then why can't it be considered a diagnostic method for EoE? We should not be confused by the low percentage of reflux detection (25%), because it is known that pH monitoring detects only severe forms of GERD. Thus, in the above articles the difference between EoE and GERD is only in the size of the eosinophilic infiltrate, which determines the more severe clinical picture in EoE.
From these studies it follows that reflux (acidic, slightly acidic and bile) plays a role in the pathogenesis of EoE.
3. Normally, i.e., in healthy people, there are no inflammatory cells, including eosinophils, in the wall of the esophagus [33]. Eosinophils arise during an inflammatory reaction, the more so, the more severe the allergy. This means that the limit of the norm is the absence of eosinophils. There are no limits for pathology. The limit of "at least 15 eosinophils per high-power field" was proposed empirically, since dysphagia is more common under these conditions. However, not always, and especially after PPI treatment. Secondly, even with greater eosinophilia, typical symptoms are not always detected. It is not surprising that a higher barrier (>35 eo/HPF) are phenotypically indistinguishable from EoE patients [34].
It follows that GERD and EoE differ only in the number of eosinophilic cells and the degree of allergic inflammation, which causes differences in symptoms.
4. PPI treatment for EoE has a positive effect, alleviating symptoms and reducing the number of eosinophils. Its effect reaches 33 to 70% [26,27,32,34], almost the same as for GERD. Why do the authors suggest that PPIs are better classified as a treatment for esophageal eosinophilia that may be due to EoE than as a diagnostic criterion? [1]. In other words, ЕoE should supposedly be treated with PPIs, but it cannot be assumed that their effect is due to a decrease in the acidity of the refluxant, i.e., to assume the presence of reflux. In 2018, 66 doctors in the proceedings of the AGREE conference stated that gastric acid inhibition is not the only important effect of PPIs, hinting, but without evidence, at the possibility of an anti-inflammatory effect of PPIs. However, what "may be" but has no scientific evidence should not be considered.
It follows that only a decrease in the pH of gastric secretion can explain the therapeutic effect of PPI, which confirms the role of reflux in the pathophysiology of EoE.
5. Some authors argue that EoE is a chronic allergic disease associated with type 2 inflammation and epithelial barrier dysfunction. For example, Rank et al, in an experiment on mice, discovered disrupted epithelial integrity was noted in (detergent) SDS-treated esophageal organoids. They showed increased esophageal width, increased IL-33 protein expression, basal zone hyperplasia, CD4+ cell infiltration, and esophageal eosinophilia [35]. The impaired barrier may develop as result of acid injury, from trauma, or infection. In this circumstance, food or aeroallergens may then contact the damaged epithelium and sensitized microenvironment in the esophageal mucosa, leading to activation of type 2 inflammatory pathway [33]. A study by Markey et al revealed a previously unappreciated role for miR-155 in mediating epithelial barrier dysfunction in esophageal inflammation in EoE [36]. It is known that with GERD, hydrochloric acid and pepsin cause damage to the esophageal mucosa, which manifests itself in ulcers, erosions, stenosis, Barrett's esophagus. Wei-Yi Lei et al found that all patients with GERD had changes related to GERD on histology [37]. It is known that histological changes in GERD occur because of damage to the mucosa during reflux of gastric contents.
Should other causes be sought damaging the mucosa in EoE, if there is no doubt: (1) in the presence of acid reflux, and (2) in the effectiveness of treatment that reduces the secretion of hydrochloric acid?
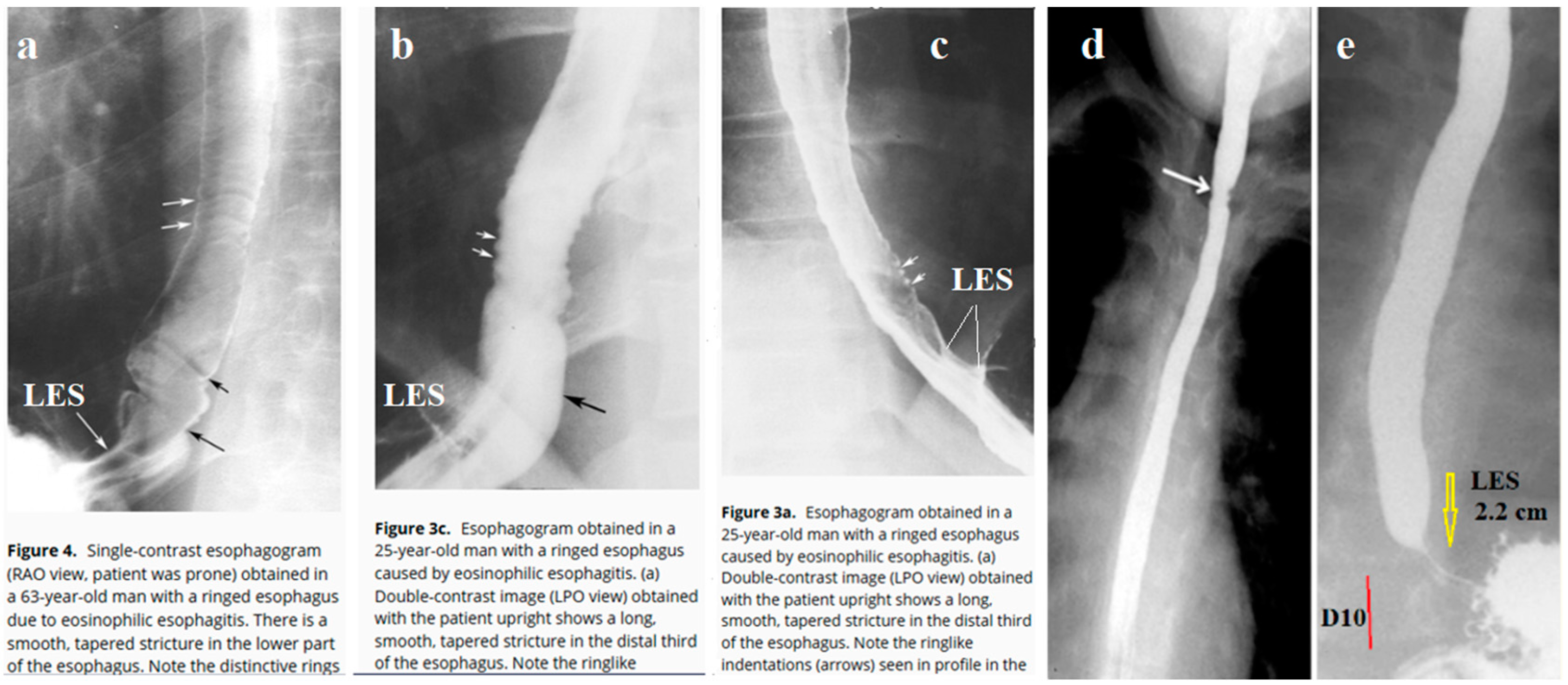
6. In the available literature, I found 10 cases of radiographic examination in patients with EoE, where the radiograph, in addition to the esophagus, captured the esophagogastric junction. All authors drew attention to the narrow width of the esophagus and the presence of erosions. Meanwhile, in all cases, I discovered the pathological function of the lower esophageal sphincter (LES).
Figure 1 shows the most demonstrative radiographs.
Barium swallow study is frequently normal in pediatric EoE except for narrow-caliber esophagus [39]. In adults at barium studies in seven patients (50%), the strictures contained multiple fixed ringlike indentations that produced a ringed esophagus. The ringlike indentations appeared as multiple, fixed, closely spaced, concentric rings traversing the stricture. Ten (77%) had hiatal hernias and nine (69%) had reflux during X-ray examination [38]. The analysis of radiographs found in published articles confirms the hypothesis that EoE is in most cases accompanied by reflux of barium into the esophagus. This is evidenced by both the detection of the episodes of reflux and the shortening of the dilated and sometimes gaping LES.
Thus, radiological studies confirm the role of reflux in the pathogenesis of EoE.
Hypothesis of the pathogenesis of EoE.
If we discard assumptions that have no scientific evidence, as well as statements that contradict known scientific facts, then the pathophysiology of EoE is as follows. Eosinophilic esophagitis is a reflux esophagitis in individuals with an allergic reaction to various allergens, including food ones. The trigger is hypersecretion of hydrochloric acid, which disrupts the integrity of the esophageal mucosa and causes an inflammatory reaction. In addition to eosinophils, basal cell hyperplasia, intercellular edema, elongation of the epithelial papillae all occurs in EoE and GERD. There is no absolute histologic criterion allowing distinction between EoE and GERD, and cutoff values for numbers of eosinophils vary according to studies and authors [40]. Elimination of foods that cause allergies from the diet leads to a decrease in eosinophilia and clinical improvement in more than 75% of patients [41]. Clinical and histological improvement after refusal to drink milk is explained by the cessation of lactose intake, which in individuals with lactose intolerance causes hypersecretion of hydrochloric acid [
24]. Analysis of the literature and our observations indicate that EoE is gastroesophageal reflux due to an allergic reaction. The narrowing of the esophagus that leads to dysphagia does not occur suddenly but is the culmination of a process that occurred long before and is manifested by symptoms of GERD. Since hypersecretion of hydrochloric acid affects all parts of the digestive tract, it can lead to eosinophilic infiltration of other parts and cause a violation of their functions [1,29,30].
What does the new view on the pathogenesis of EoE change?
A) Since EoE is reflux esophagitis, which occurs as a result of hypersecretion of hydrochloric acid, then the treatment of gastroesophageal reflux should be an indispensable and constant condition. It should include the rejection of provocateurs of hypersecretion of hydrochloric acid, i.e., products containing lactose; taking drugs that suppress the secretion of hydrochloric acid (PPI); drugs that neutralize acid; protectors of the esophageal mucosa from contact with the refluxant; as well as a lifestyle change (take a horizontal position on an empty stomach, eat small portions) and do not provoke reflux by increasing the pressure in the stomach with a tight belt, physical exercises after meals, etc.
B) Identify and eliminate from food the allergen that causes the eosinophilia in the inflamed wall of the esophagus, both by testing and by temporarily refusing to eat foods that are often involved in the allergic process.
С) This treatment is effective and allows to improve the clinical picture up to the disappearance of dysphagia. This will allow in most cases to refuse balloon dilation of the esophagus, which leads to ruptures of the mucosa with subsequent development of fibrous tissue and resumption of dysphagia.
D) This tactic is applicable to all causes of dysphagia that are caused by hypersecretion of hydrochloric acid (GERD, EoE, so-called achalasia of the esophagus [
23] and Schatzki ring [42]), as well as eosinophilia of the stomach and duodenum.
The following example shows whose interests are protected by doctors who advertise expensive diagnostic equipment and drugs. A 19-year-old girl complained of frequent heartburn and abdominal pain. About a year ago, she weighed 104 kg, but within six months, using vomiting after eating, she lost weight to 70 kg. Gastroscopy diagnosed GERD. No pathology was found in the stomach and duodenum and, in accordance with the recommendations of all consensuses, histological examination was not performed. It is known that the patient has lactose intolerance, since drinking milk causes abdominal pain and loose stools. She has an allergy to chicken feathers and dust. Her father died of stomach cancer. Taking Esomeprazole 40 mg x 2 times a day for 4 weeks slightly reduced the frequency of symptoms, she gained 6 kg, but the heartburn did not go away.
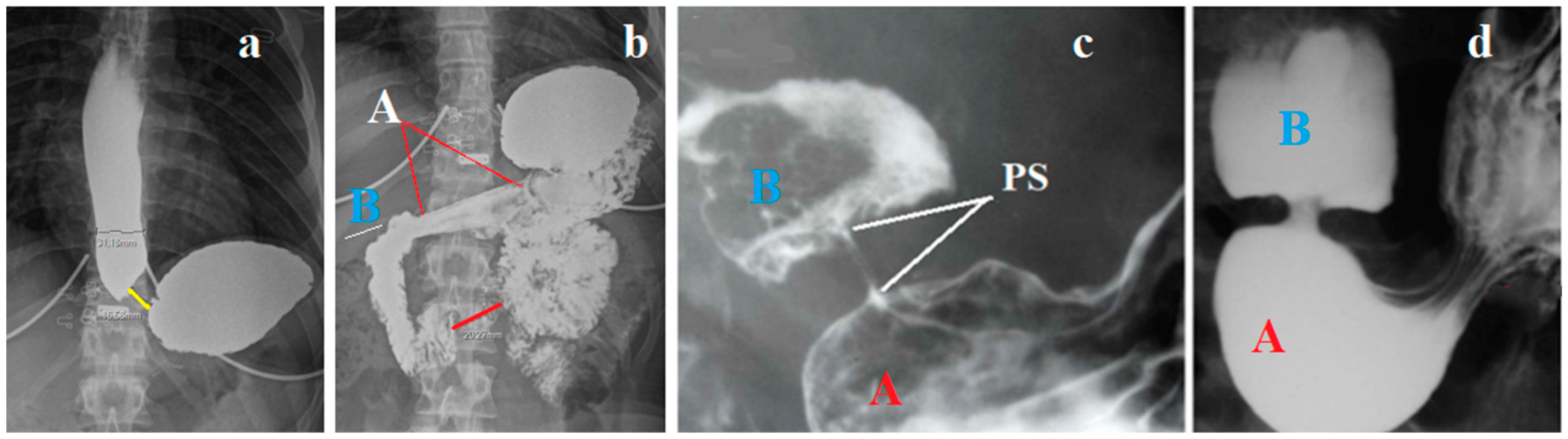
An X-ray examination of the esophagus was performed under high pressure in the stomach. Radiograph (a) was taken during straight legs raise at the time, when the patient stopped drinking 200 ml of barium. The second radiograph (b) was taken 5 minutes later in a state of rest (
Figure 2).
Conclusion: Shortening of the LES and dilation of the esophagus confirm the endoscopy data on GERD. A sharp thickening of the antral wall of the stomach and a decrease in its lumen indicate an inflammatory process. Rapid weight loss in combination with vomiting confirms the assumption of a narrowing of the outlet of the stomach as the cause of the clinical picture. Given the presence of an allergic predisposition of the organism, there is a high probability of eosinophilic infiltration of the stomach wall. A biopsy of the mucosa of the esophagus, stomach and duodenum was not taken, since there is a clear recommendation of all consensuses not to perform a biopsy if there are no signs of pathology during endoscopy due to the allegedly low reliability compared to pH monitoring. Before contacting me, an X-ray examination was not performed, because there is a clear recommendation not to perform an X-ray examination due to its allegedly low reliability. Gupta and Grinman in their lecture, which they call a review, stated that: - "The gold standard for diagnosis of a motility disorder is esophageal manometry, as endoscopic changes may be absent.32". [44] First, This contradicts another clear recommendation not to use HRM to diagnose GERD. Since dysphagia can occur with GERD, EoE and so-called EA, then without knowing the diagnosis, it is impossible to decide which recommendation to apply, since all recommendations contradict each other. Biosy is not recommended to perform, since after this the diagnosis will become obvious and there will be no need for pH monitoring and HRM. X-ray examination is not recommended since it has low diagnostic accuracy compared to pH monitoring, which in fact diagnoses only very severe forms of reflux. Manometric examination is not recommended to perform for the diagnosis of GERD, because then it will be impossible to differentiate GERD from EA, since they are one and the same disease. In their recommendation to apply HRM, Gupta and Grinman cite an article by Leiman et al [45] that contains no scientific evidence and the recommendations are based on a vote of 9 experts who considered the recommendations correct if 80% of the indicators were agreed upon.
Below are the details of whose interests defended by experts:
D.A.L. (Consultant: Allakos Pharmaceuticals, Astra Zeneca; Research Grant: Takeda Pharmaceuticals; and Advisory Board: Sanofi); A.N.K. (Advisory board: Castle Bioscience); F.O. (none to report); A.J.B. (Nutricia, Norgine, DrFalkPharma, Thelial, and SST: research; Laborie, Medtronic, Dr Falk Pharma, Alimentiv, Sanofi/Regeneron and AstraZeneca: consulting); E.S.D. (Abbott, Abbvie, Adare/Ellodi, Aimmune, Akesobio, Alfasigma, ALK, Allakos, Amgen, Arena, Aslan, AstraZeneca, Avir, Biorasi, Calypso, Celgene/Receptos/BMS, Celldex, Eli Lilly, EsoCap, Eupraxia, Ferring, GSK, Gossamer Bio, Invena, Landos, LucidDx, Morphic, Nextstone Immunology, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda, Target RWE, and Upstream Bio: consulting; Adare/Ellodi, Allakos, Arena, AstraZeneca, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Revolo, and Shire/Takeda: grant/research support; Allakos, Banner, and Holoclara: educational grant); G.W.F. (Arena: grant/research support; Allakos: grant/research support and consulting; Celgene/Bristol Myers Squibb: grant/research support and consulting; Astra Zeneca: consulting; Lucid: grant/research support and consulting; Nexstone: consulting; Phathom: consulting; Regeneron/Sanofi: grant research support and consulting; Takeda: grant/research support and consulting; Upstream Bio: consulting; N.Q.F.-B. (none to report); N.G. (consulting: Astra-Zeneca, Sanofi-Regeneron, Abbvie, BMS, Invea, and Allakos; Speakers bureau: Sanofi-Regeneron and Takeda; Royalties: Up-to-date); I.H. (Arena: consulting, grant/research support; AstraZeneca: consulting, grant/research support; BMS/Receptos: consulting; Calypso/Parexel: consulting; Ellodi/Adare: consulting and grant/research support; Esocap: consulting; Lilly: consulting; Phathom: consulting; Sanofi/Regeneron: consulting and grant/research support; Takeda/Shire: consulting, grant/research support); D.A.K. (Celgene: consulting; Regeneron: consulting; Takeda: consulting); K.P. (Alladapt: consulting; Allakos: speaking and teaching and grant/research support; AstraZeneca: advisory committees or review panels; Bristol Meyers Squibb: advisory committees or review panels and consulting; Chobani: grant/research support; Ellodi: advisory committees or review panels; Lucid: advisory committees or review panels; Medscape: advisory committees or review panels, speaking, and teaching; NexeosBio: patent held/filed; peerview: speaking and teaching; Regeneron: advisory committees or review panels, speaking, and teaching; Takeda: speaking and teaching); R.Y. (Ironwood Pharmaceuticals: consulting, grant/research support; Medtronic: consulting; Phathom Pharmaceuticals [45].