1. Introduction
One of the problems that are present nowadays is the pollution caused by domestic residual waters, as a consequence of the growing world population. This problem affects human health and the environment [
1]. Due to this, it is necessary to develop innovating, effective and economical techniques which solve this phenomenon [
2]. The human excreta (stool and urine) are catalogued between the main pollutants present in the domestic residual waters [
3]. One of the alternatives to solve this problem is to use human excreta to produce energy, because it has molecules with low oxidation potentials that can be utilized to generate hydrogen in a reduction process. The urea is the most abundant organic substance in the urine (human excretion product), SIFC (patent register N° NC2017 / 0012602 SIC, PCT/IB2018/058596)[
4]. They are electrochemical systems developed to produce hydrogen from the reduction of hydronium ions and the oxidation of organic matter decaying (excrements). These cells are formed by two semicells with stainless steel electrodes: an anodic semicell where the reaction of oxidation of organic molecules (present in the human excrements) is produced. Then, applying the electrolysis principle and a cathode semicell where the hydronium ions are reduced (H3O+) applying the chemical balance law. These two half cells are separated by a membrane system: a protonic exchange membrane and another one of anionic exchange. This allows the electric transportation and a selective form of masses. The technology SIFC has shown a hydrogen production with a purity of 96% (w/v) [
4,
5,
6].
The technology SIFC has the following advantages: the produced hydrogen is a clean energy source, thus in the combustion process it produces water vapor and high energy quantities [
7], and it also degrades organic matter through electrolysis in high potentials (12V) providing an alternative treatment in matrices which contain big quantities of organic matter, such as home residual waters, the conditions allow to obtain the electric energy for the reactions of photovoltaic cells (solar energy), the gaseous hydrogen can replace several conventional energy sources (gasoline, diesel, home gas and hydroelectricity) [
8]. Accordingly, the technology SIFC points out directly to the reduction of atmospheric emissions and the prevention of the greenhouse effect and acid rain.
The idea of using electrochemical techniques for water treatment is not a new idea. Currently, there exist two techniques, electrolysis and electrocoagulation. The former is an electrochemical technique which is based on the organic matter oxidation utilizing inert electrodes, such as diamond electrodes doped with boron (DDB) or stainless steel. The electrolysis complements commonly with electrocoagulation techniques in order to improve the elimination of pollutant domestic residual waters [
9].
On the other hand, the electrocoagulation consists on the induction of electric current in the residual water through parallel electrodes. The commonly used electrodes are iron and aluminum; this is to generate iron and aluminum coagulants (Fe2+ and Al3+). The respective electrodes, the metallic ions are produced in the anode and in the cathode, there exist hydrogen carbonated bubbles generation. And they help the floccule to float without particles. When this process occurs, the pollutants create hydrophobic particles which can generally precipitate or float. As they are formed, they can be eliminated easily through a common physic separation method [
10,
11,
12].
In this research work, the utilization of the SIFC technology with solar energy is proposed as an innovating, effective and economical alternative for the treatment of domestic residual waters. The ionic flow selective cells were proved using urea at 10% of (w/w) in a real residual water sample.
2. Materials and Methods
2.1. General Assembly of the System
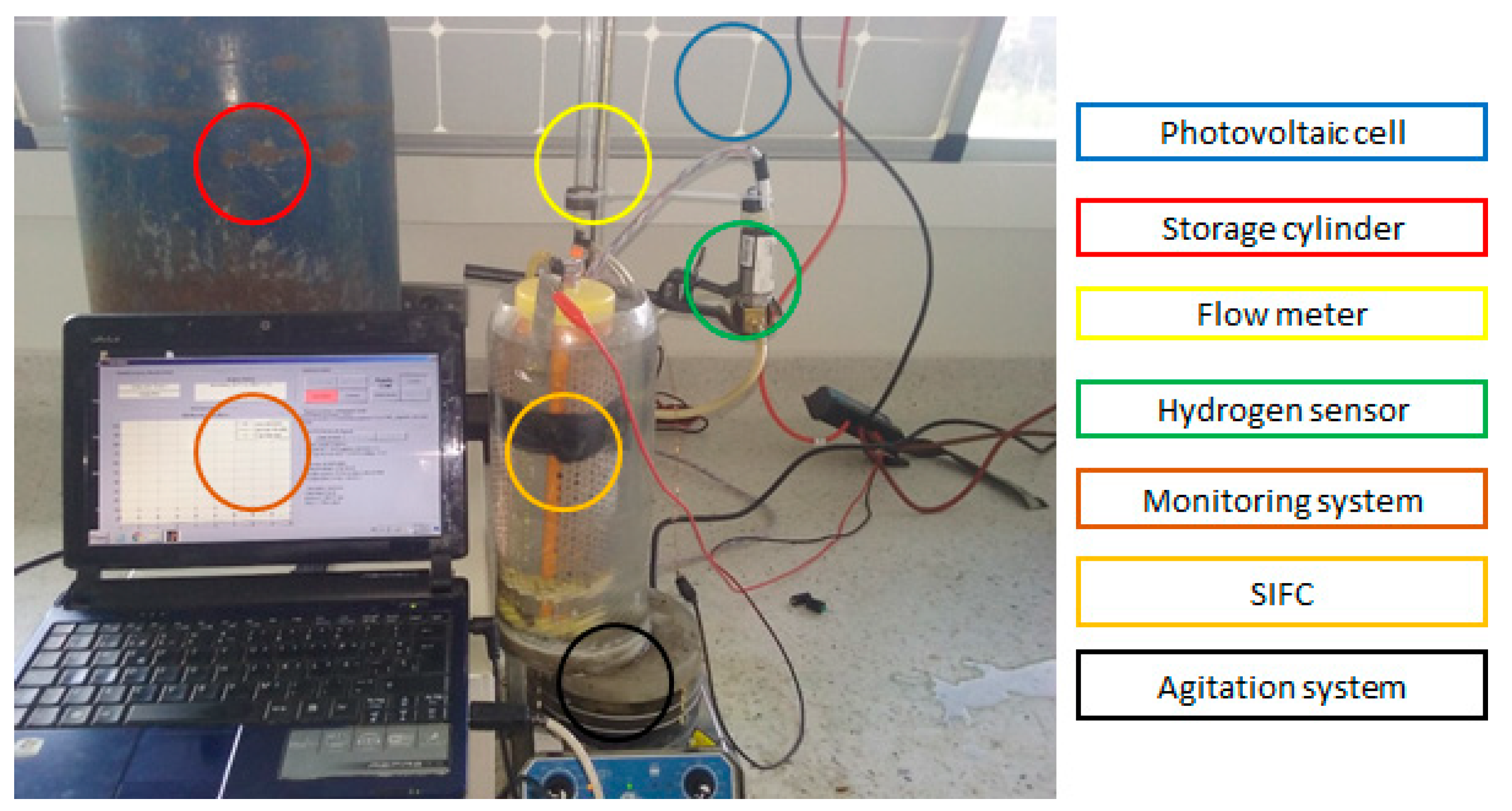
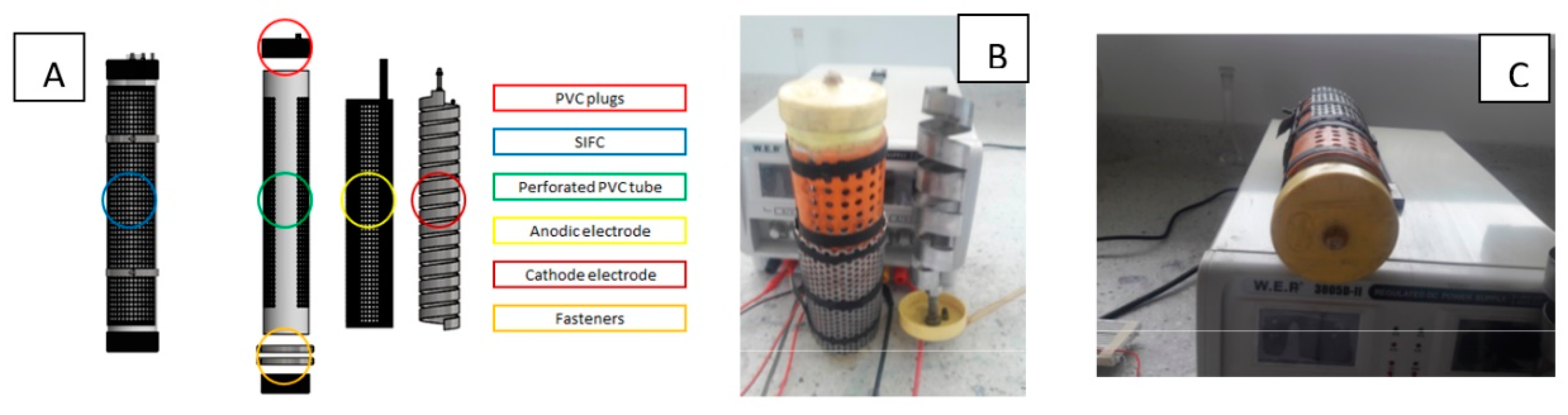
The chosen system to degrade the organic matter and produce hydrogen consists on the parts as shown in
Figure 1. Photovoltaic cell, storage cylinder, flow meter, hydrogen sensor, monitoring system, SIFC system and agitation system.
2.2. Selective ion Flow Cell (SIFC)
The ionic selective flow cells are compound from the indicated parts as shown in
Figure 2: PVC plugs, SIFC, perforated PVC tube, anodic electrode, cathode electrode and fasteners. The electrodes and fasteners are made of stainless steel 316 L, an electric bridge (elder plug) located in the lower cover of the tube.
The membrane system is located on the surface of the perforated tube. The membranes are located in such a way that generate two transportation ionic routes. Through the anionic membrane located in the upper part, go past from the cathode to ions hydroxyl anode, and through the protonic exchange membrane located in the lower space, go past from the anode to the hydronium ions cathode, see
Figure 3 [
4,
13].
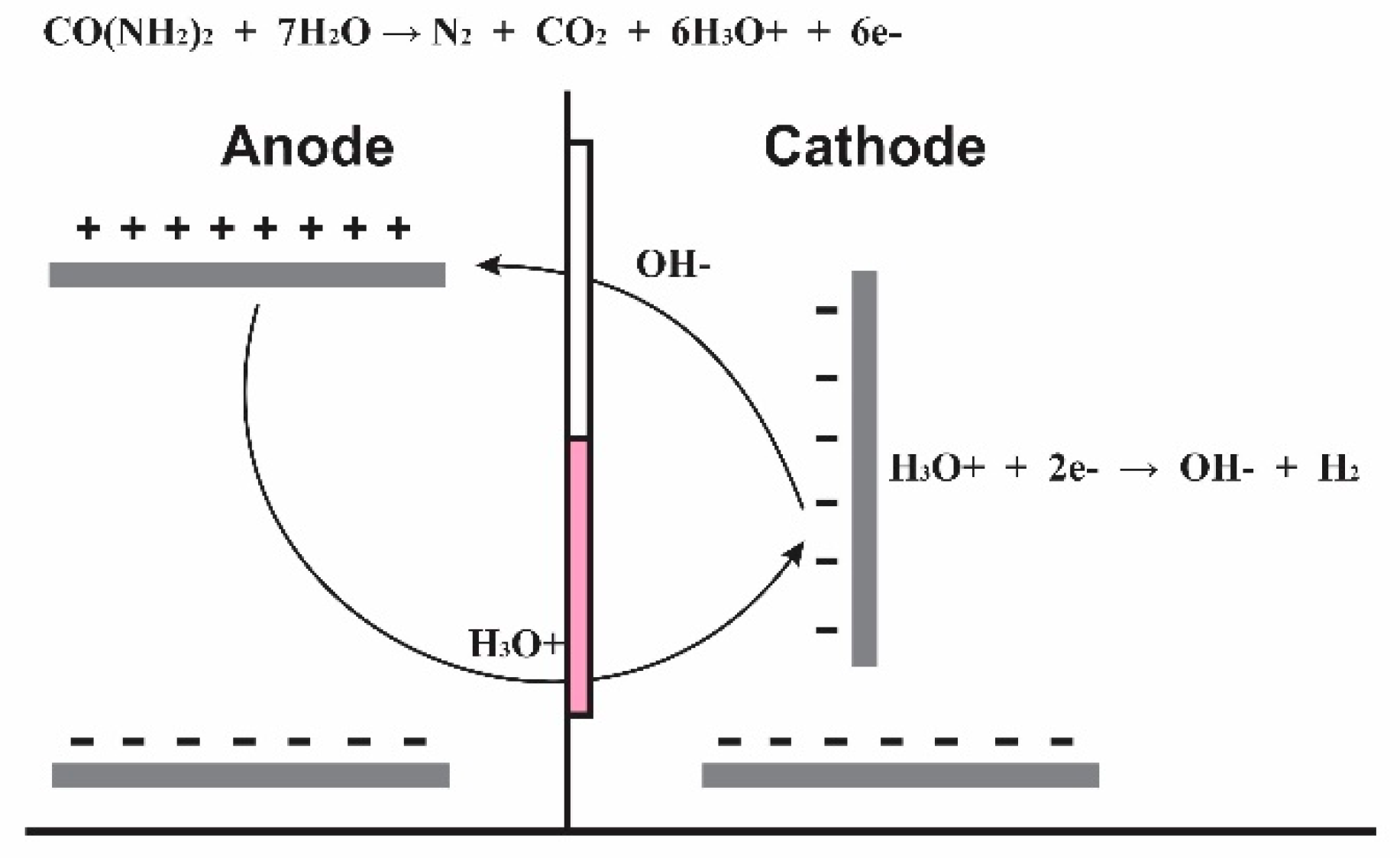
Taking urea as a model molecule, the urea oxidation occurs in the anode in water presence, producing: nitrogen gas, carbon dioxide, hydronium ions and electrons (half reaction 1a). The formed ions in the anode go through protonic exchange membrane and are reduced in the cathode forming gaseous nitrogen (average reaction 1b). Those reactions are shown then.
Taking into account that the half cells are separated the gases formed N
2 and CO
2 are released into the atmosphere, the electrons are taken advantage of which are transported by the electrical contacts, electric bridges and the ions, in a few words the organic matter takes advantage of the electrons and hydronium ions.
The hydronium ions produced at the anode and transported through the membrane of proton exchange by migration, are reduced in the cathode plates and produce hydrogen and water, the hydrogen is used as a clean fuel.
As the amount of hydronium ions in the cathode decreases, the balance of reaction 3 shifts to the right by Lechatelier's law and forms an excess of OH
- ions, which are neutralized by those entering through the proton exchange membrane and the excess is transferred through the anion exchange membrane, establishing the charge and mass balance in the system.
The selective flows for positive and negative charges generate an electric current due to the phenomena of load transport within the solution, which eliminates energy barriers that occur in conventional systems and allows working at high potentials 12 V, in this case a high percentage of electrical energy applied to the system is used to oxidize the organic matter and produce hydrogen by reduction of hydronium ions.
2.3. Experimental Conditions of the SIFC Using a Urea Pattern
Anodic semicell: 1L of urea 10% (w/w), sodium carbonate as supporting electrolyte to get a pH of 9, continuous agitation of 250 rpm.
Cathodic semicell: it is added phosphoric acid solution at 10% (w/w) to the cathode mean.
The system worked at a temperature of 22° C and 0.74 Atm of pressure. The energy source used was a solar panel which generated a potential of 12V and 0.386 A. The experiment was carried out during two hours to analyze COD. The samples taken were of 10 mL, before starting the experiment and each 30 minutes after having started the experiment. The hydrogen production was monitored during the whole experiment by the Myhidros system.
2.4. Experimental Conditions of the SIFC Using a Real Sample of Residual Water
Anodic semicell: 1L of a sample of residual water, taken from a characteristic flow of water in the Colombian city, Pasto. Sodium carbonate as supporting electrolyte to reach a pH of 9, continuous agitation at 250 rpm.
Cathodic semicell: it is added phosphoric acid solution at 10% (w/w) to the cathode mean.
The system worked at a temperature of 22° C and 0.74 Atm of pressure. The energy source used was a solar panel which generated a potential of 12V and 0.4 A. The experiment was carried out during two hours, for the analysis of COD 10 mL samples were taken. COD was measured before starting the experiment, after an hour of having started the experiment and when the experiment was finished. The hydrogen production was monitored the whole experiment by Myhidros system.
The analysis of COD was done by the accredited laboratory of water of the University of Nariño.
2.5. Hydrogen Measuring
The H
2 produced by the SIFC is quantified by a monitoring system that allows to measure the hydrogen percentage (w/v) linearly and continuously. The H
2 produced in the cathode is monitored by the Mhydros system, which is compound by a concentration sensor HPS-AMS-100 that measures the hydrogen percentage. A flow meter calibrated for H
2 and it measures the total volumetric flow and a sensor that measures the applied current to the system [
6]. Knowing that the combustion heat of H
2 is 286 kJ/mol, the generated energy by the SIFC is stablished, and comparing with the applied energy, it allows to calculate the energetic efficiency of the system. It is necessary to emphasize that through this process electrical efficiency is measured by taking as a quantity of electrical energy applied to the system, the product between electric potential and electric current and the amount of output energy produced by hydrogen in a combustion process.
3. Results and Discussion
3.1. Demonstration of the Organic Matter Degradation Using SIFC and a Urea Pattern
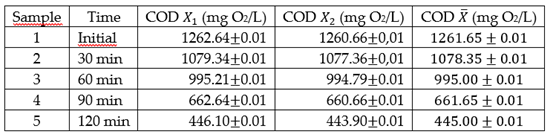
The organic matter degradation utilizing SIFC was proved applying the technology SIFC on a urea pattern at 10%. The results of COD, measured in the time for each sample, are shown in the
Table 1. Each sample was analyzed twice.
The results are shown in
Table 1, the ANOVA analysis was done from a factor for COD, the null hypothesis (H
0) was: “the measurements of COD between the taken samples in different moments do not differ meaningfully and due to that the taken samples in the time represent the same measurements of COD”.
According to the ANOVA summary, the value P of the trial F is 1.012x10-12 that is less than 0.05. Thus, the null hypothesis is rejected, which shows that the COD is meaningfully different in the different taken samples at time in a level of α = 0.05.
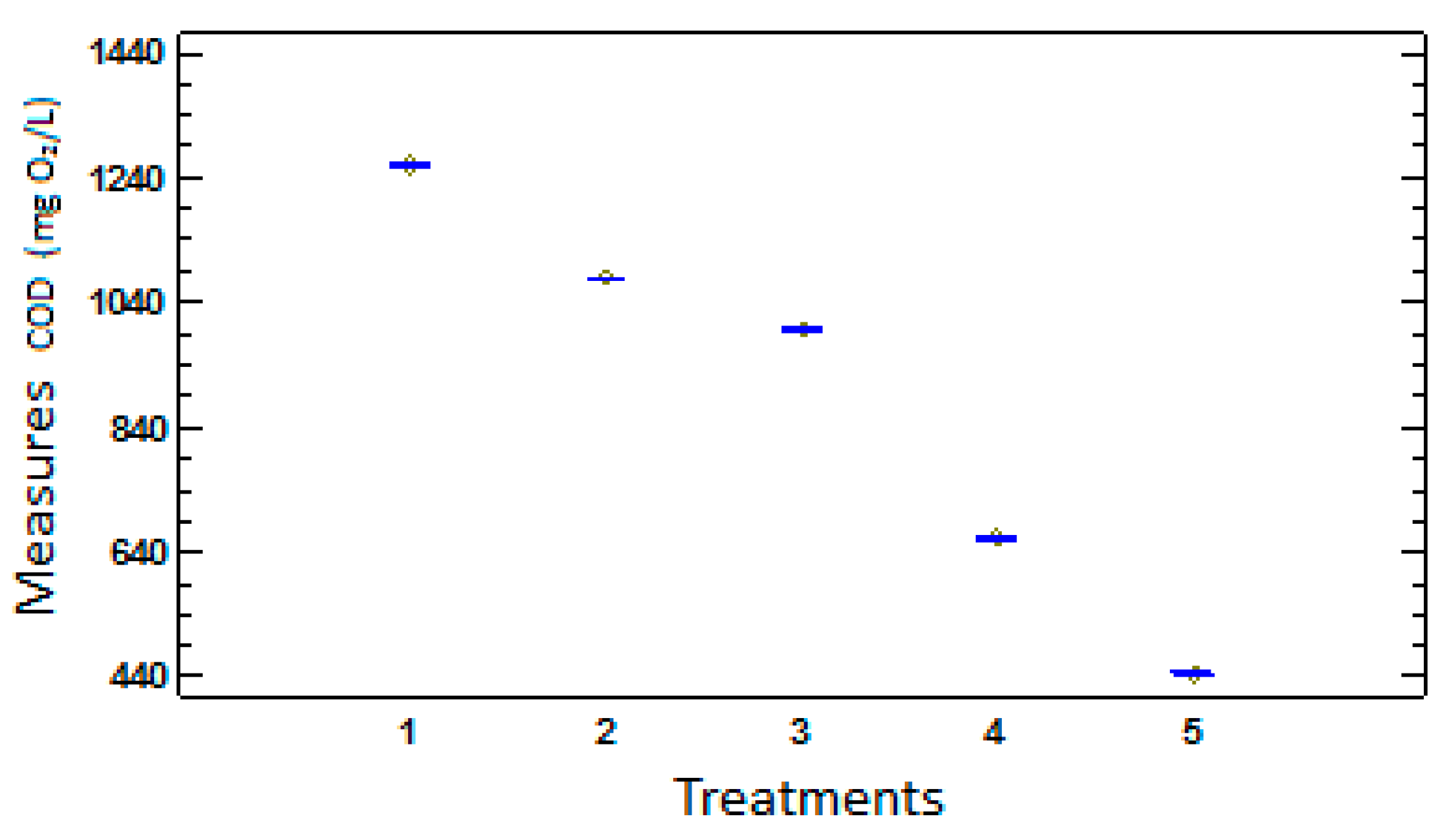
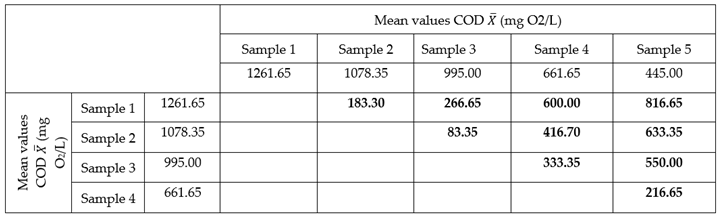
When it is discovered that the COD varies with time, it is deduced directly that the SIFC is degrading the organic matter. Fisher's Least Significant Difference (LSD) was calculated to compare all the possible differences between the averages of SIFC measured in different moments and to define whether all the taken samples in different times present degradation or only some of them. The LSD in this case is of LSD = 3.33.
In the following table, all the possible differences between the averages of COD measured in different times, are shown.
There is not any difference higher than a value of 3.33 of LSD which shows that all the intermediate values of COD through the time, are statistically different to a level of α = 0.05 (95% of reliability), which was confirmed by the graphic mediums for the measurement of COD.
Figure 4.
Graphic of mediums for the measurements of LSD.
Figure 4.
Graphic of mediums for the measurements of LSD.
According to the results of LSD and the previous figure, it is obtained that all the measured values in different times show significant differences. That means that the organic matter degradation through the technology SIFC is continuous in the time because as the time goes by the content of urea decreases continuously in order to analyze the effectiveness of SIFC in the organic matter degradation, and the degradation percentage is calculated based on the initial average value (CODi) and the final (CODf).
Calculation of the percentage of the organic degradation of organic matter:
% organic matter degradation= (CODi-CODf)/CODi x 100%
% organic matter degradation= (1261.65-445.00)/1261.65 x 100% = 64.73%
Which means that the SIFC in a duration of two hours degrade more than a half of the organic matter. This percentage of degradation is low in comparison with other electrochemical techniques; however, it is justified if analyzing the profitableness of the implementation of the technology SIFC in the production of clean matter (hydrogen). It should be taken into consideration that to obtain higher oxidation values, the time has to be longer.
Some research reports up a 99% of elimination of the chemical demand of oxygen (COD), using combined techniques of electrocoagulation with iron electrodes and direct anodic electrolysis with inert diamond electrodes doped with boron (DDB). The disadvantage of utilizing these techniques in an industrial scale is the implementation cost, it means they are not economically profitable techniques [
14]. Other authors report an electrochemical combined treatment of irradiation for the organic matter degradation in industrial residual waters, pointing out that the best results of elimination are obtained using the electrochemical techniques, eliminating 78% of COD [
15]. It should be taken into account that the SIFC make the processes of electrolysis and electrocoagulation when they are applied to more complex matrices such as the residual waters.
3.2. Production of Hydrogen Utilizing the SIFC in a Urea Pattern
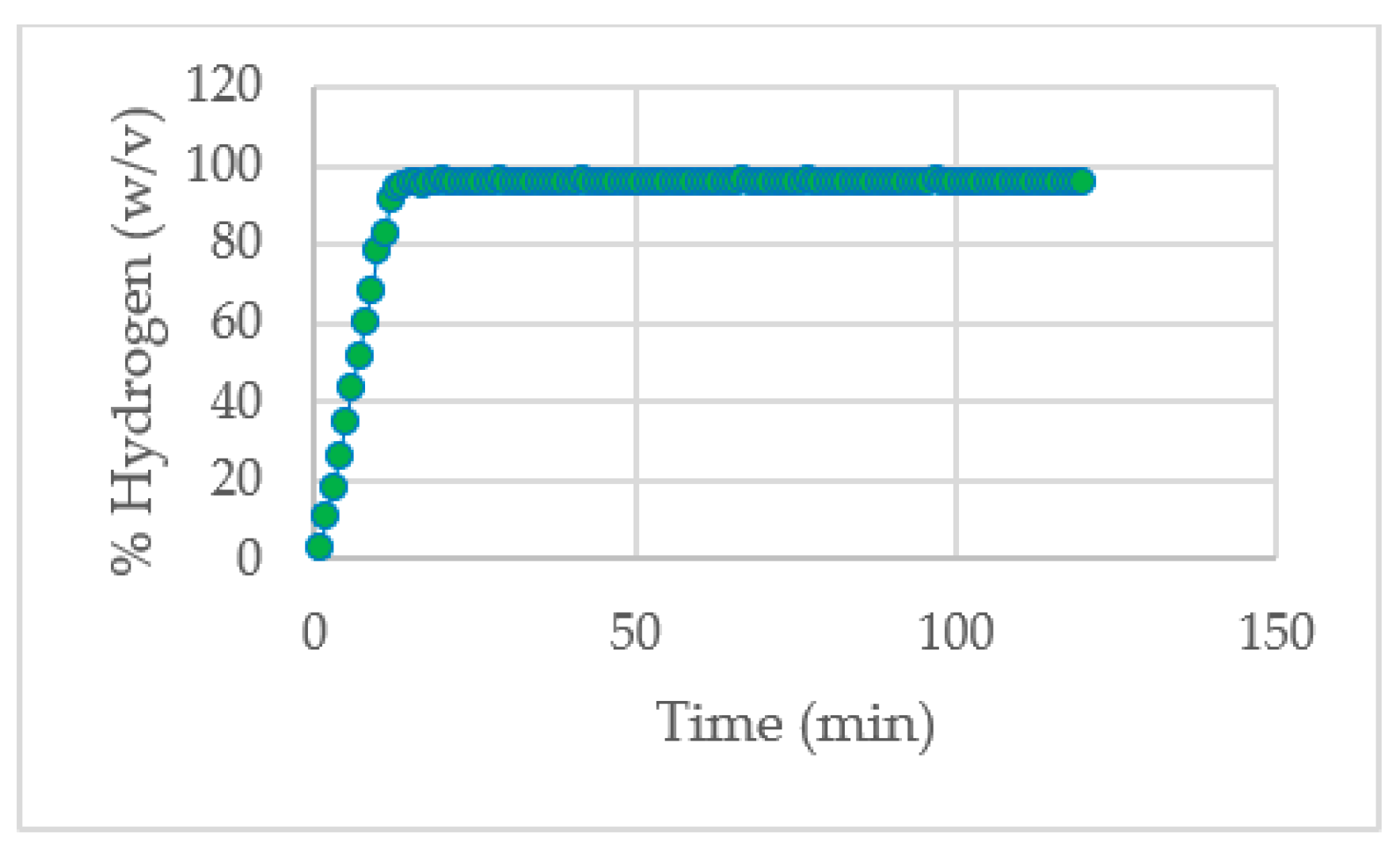
The production of hydrogen is represented in the
Figure 5, which shows the percentage of concentration (w/v) of hydrogen during the whole experiment. It is found that the highest percentage of hydrogen concentration (96%) is reached approximately in 20 minutes and from that it stays constant until the end of experiment.
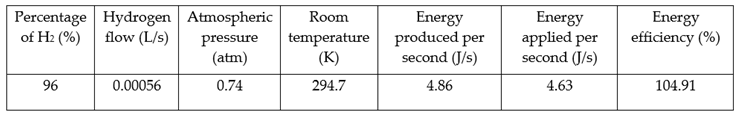
To calculate the electric efficiency of the hydrogen production using the technology SIFC, it was taken into account the produced energy by the hydrogen system, which is related to the electric energy applied to the system. The following table shows the results.
Table 3.
Energetic efficiency calculated from the relation between the produced energy by the system in a hydrogen form and the electric energy applied to the system.
Table 3.
Energetic efficiency calculated from the relation between the produced energy by the system in a hydrogen form and the electric energy applied to the system.
Using 12V and 0.386 amperes, it was applied an energy of 4.63 J / s to the SIFC and the energy that the SIFC is producing is 4.86 J / s, with a flow of 0.00056 L / s, in a hydrogen concentration of 96%, it was found that the efficiency percentage is 104.91%. Which shows that there is a gain of energy, it means, the energy in a heat form that would be obtained when burning the produced hydrogen in such conditions is higher that the energy which was applied to produce hydrogen.
3.3. Degradation of Organic Matter in Residual Waters Utilizing the Technology SIFC
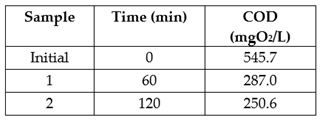
The
Table 4 shows that after a two-hour analysis, the COD decreased from 545.7 to 250.6 mg O
2 / L, which shows that the SIFC degrade in two hours 54.07% of the organic matter present in the residual waters.
Table 4.
decrease of COD in residual waters.
Table 4.
decrease of COD in residual waters.
3.4. Production of Hydrogen Utilizing SIFC in Residual Waters
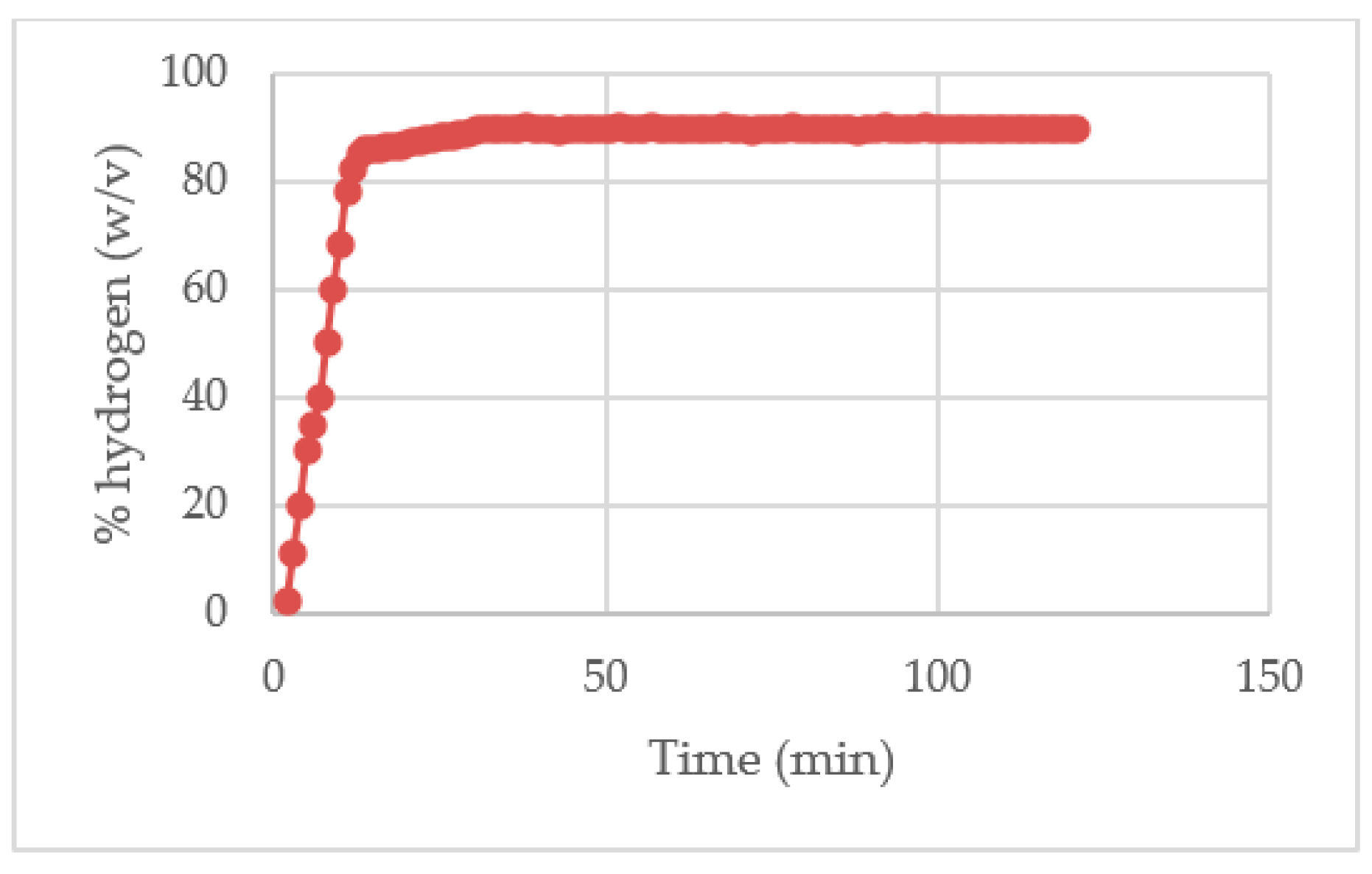
In the
Figure 6 it can be seen that the highest concentration of hydrogen obtained using a matrix of residual water was of 90%.
The highest concentration of hydrogen was of 90%, this concentration was reached in 30 minutes, from this point the concentration of H2 remained constant with a volumetric flow of 31.2 mL /min (0.00052 L / s), which means that after 30 minutes the system keeps on with a percentage energetic efficiency of hydrogen production of 94.89%.
4. Conclusions
When analyzing the organic matter degradation through the analysis of variance (ANOVA) and the Fisher's Least Significant Difference (LSD), it is shown that the organic matter degradation is continuous along the time.
There are differences in the degradation percentages (COD) with a urea pattern and residual water (64.73% y 54.07% respectively) because when the urea pattern is used the electrolytic reactions appear in a more efficient way due to the patterns, as well as the energetic efficiencies of the hydrogen production (104.91% y 94.89% respectively).
The selective ionic flow cells (SIFC) are innovative, effective and economical in the organic matter degradation because in a two-hour duration in real residual water samples degrade 54.07% of the organic matter, with an advantage, that the degradation basically is free-cost, as in the general balance of hydrogen production, it is produced an efficiency of 94.89%.
It is important to use the technology SIFC for the residual water treatment, in comparison with other electrochemical techniques, as the technology SIFC is an economically sustainable and energetically viable technique to produce gaseous hydrogen.
Author Contributions
Conceptualization, Lozada-Castro, Juan José.; methodology, Cueltan-Solarte, Jhon David; formal analysis, Cueltan-Solarte, Jhon David and Lozada-Castro, Juan José; investigation, Cueltan-Solarte, Jhon David and Guerrero-Fajardo, Carlos Alberto.; resources, Guerrero-Fajardo, Carlos Alberto.; data curation, Cueltan-Solarte, Jhon David; writing—original draft preparation, Cueltan-Solarte, Jhon David; writing—review and editing, Cueltan-Solarte, Jhon David and Guerrero-Fajardo, Carlos Alberto; visualization, Cueltan-Solarte, Jhon David and Lozada Castro, Juan José; supervision, Guerrero-Fajardo, Carlos Alberto; project administration, Lozada-Castro, Juan José and Guerrero-Fajardo, Carlos Alberto; funding acquisition, Lozada-Castro, Juan José and Guerrero-Fajardo, Carlos Alberto. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank the Universidad de Nariño y al grupo de investigación en Estudio de Sistemas Contaminantes and the Universidad Nacional de Colombia Departamento de Química Facultad de Ciencias for their support and the possibility of using equipment and techniques that allowed the development of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Von, M.; de Lemos, C. Biological wastewater treatment in warm climate regions; IWA Publishing, 2017; ISBN 1843390027. [Google Scholar]
- Butler, E.; Hung, Y.; Yeh, R.; Suleiman, M. Electrocoagulation in wastewater treatment. Water 2011, 495–525. [Google Scholar] [CrossRef]
- Sharma, M.; Tyagi, V.; Saini, G.; Kazmi, A. On-site treatment of source separated domestic wastewater employing anaerobic package system. Jour. of Env. Chem. Eng. 2016, 1209–1216. [Google Scholar] [CrossRef]
- Lozada, C.J. Colombia Patente N° NC2017/0012602. 2017. Available online: https://sipi.sic.gov.co/sipi/Extra/IP/Mutual/Browse.aspx?sid=638623657950260889.
- Boggs, B.; King, R.; Botte, G. Urea electrolysis: direct hydrogen production from urine. Chem. Comm. 2009, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Lozada, J.; Casanova, C.; Arturo, D. Electronic Monitoring System for Hydrogen Produced from the Oxidation of Human Urine (Mhydros). Jour. of Scien. and Eng. Res. 2021, 52–57. [Google Scholar]
- Singh, S. et al. Hydrogen: A sustainable fuel for future of the transport sector. Renewable and Sustainable Energy Reviews 2015, 623–633. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Intern. Jour. of hydrogen energy 2015, 11094–11111. [Google Scholar] [CrossRef]
- Migliorini, F. , et al. Efficiency study and mechanistic aspects in the Brilliant Green dye degradation using BDD/Ti electrodes. Diamond and Related Materials 2016, 5–12. [Google Scholar] [CrossRef]
- Eckenfelder, W.; Cecil, L. Applications of New Concepts of Physical-Chemical Wastewater Treatment; Elsevier, 2013; Volume 1, pp. 18–22. [Google Scholar]
- Gupta, V.; Mazumdar, B.; Acharya, N. COD and colour reduction of sugar industry effluent by electrochemical treatment. Intern. Jour. of Energy Tech. and Policy 2017, 177–187. [Google Scholar] [CrossRef]
- Bassyouni, D. et al. Comparative performance of anodic oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. Jour. of hazardous mat. 2017, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Lozada, J.; Delacruz, C.; Cueltan, J. Hydrogen Production from the Oxidation of Human Excretion Products. Jour. of Scien. and Engin. Res. 2018, 79–86. [Google Scholar]
- Linares, I. et. al. Oxidación de materia orgánica persistente en aguas residuales industriales mediante tratamientos electroquímicos. Avances en Ciencias e Ingeniería. 2011, 2. Available online: https://dialnet.unirioja.es/descarga/articulo/3624174.pdf.
- Barrera, D. et al. A combined electrochemicalirradiation treatment of highly colored and polluted industrial wastewater. Rad. Phys. and Chem. 2003, 657–663. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).