Submitted:
10 March 2025
Posted:
11 March 2025
You are already at the latest version
Abstract
Exploring cognitive abilities is necessary in educational contexts, where such insights shape decisions about student placement and teaching methods. Traditionally, educational assessments have been leaned on academic performance to guide decisions related to grading and student placement. This study examines the relationships among specific neuropsychological measures, namely the Event Related Potentials (ERPs), P300 waveform, reaction time, and fluid intelligence in children. Raven’s Standard Progressive Matrices (RSPM) was utilized to assess intelligence levels. Based on their RSPM scores, participants were grouped into two categories: those with "high mental abilities" and those with "average mental abilities." It was hypothesized that children with higher RSPM scores might display reduced P300 latencies and quicker reaction times, potentially reflecting greater neural efficiency. Electrophysiological data collected using ERPs, focusing on the P300 component. The results suggest a possible association between higher intelligence scores and shorter P300 latencies and quicker reaction times, which could support the concept of neural efficiency and the significance of cognitive speed in understanding intelligence. This investigation into the neuropsychological foundations of cognitive ability in children is in the same line with studies supporting how brain activity, connectivity, and processing efficiency vary. These differences could help develop educational strategies that are more tailored to individual cognitive processing styles.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
| Step | Procedure | Description |
| Step 1: Informed Consent | 1.1 Ethical Briefing | Parents/guardians receive a detailed explanation of the study’s aims, procedures, and potential risks. |
| 1.2 Consent Form Signing | Written informed consent is obtained from parents/guardians in accordance with ethical guidelines. | |
| 2.3 Clinical interview | Children, parents/guardians and educators | |
| Step 2: Cognitive Assessment | 2.1 Instruction Phase | Children are given instructions and sample items to familiarize them with RSPM format. |
| 2.2 RSPM Test Completion | Children complete the RSPM to assess fluid intelligence and abstract reasoning. | |
| 2.3 Break (if needed) | A short break is provided to ensure sustained attention and optimal performance. | |
| Step 3: EEG Data Acquisition | 3.1 EEG Preparation | Electrode placement, impedance checks, and EEG system calibration are conducted. |
| 3.2 Auditory Oddball Paradigm (ERP Task) | Children perform an auditory oddball task to elicit the P300 component while EEG data are recorded. | |
| 3.3 Reaction Time Recording | Behavioral responses (button presses) are recorded concurrently with EEG to measure reaction time. | |
| 3.4 Data Quality Check | EEG data undergoes visual inspection to ensure artifact-free, high-quality recordings. |
2.2. Implementation
2.3. Electrophysiological Assessment
2.3.1. Electrode Placement and Data Recording
2.3.2. P300 Component Detection
2.3.3. Auditory Stimuli
2.3.4. Data Preprocessing and Artifact Removal
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Psychoeducational implications of the study using ERPs and RSPM results in identifying children’s mental abilities.
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, D.J.; Mousley, A.L.; Shafto, M.A.; Duncan, J. Neural contributions to reduced fluid intelligence across the adult lifespan. Journal of Neuroscience 2023, 43, 293–307. [Google Scholar] [PubMed]
- Scherrer, V.; Breit, M.; Preckel, F. Crystallized Intelligence, Fluid Intelligence, and Need for Cognition: Their Longi-tudinal Relations in Adolescence. Journal of Intelligence 2024, 12, 104. [Google Scholar] [PubMed]
- Wang, T.; Ren, X.; Altmeyer, M.; Schweizer, K. An account of the relationship between fluid intelligence and complex learning in considering storage capacity and executive attention. Intelligence 2013, 41, 537–545. [Google Scholar]
- Amin, H.U.; Malik, A.S.; Kamel, N.; Chooi, W.T.; Hussain, M. P300 correlates with learning & memory abilities and fluid intelligence. Journal of neuroengineering and rehabilitation 2015, 12, 1–14. [Google Scholar]
- Neubauer, A.C.; Fink, A. Intelligence and neural efficiency. Neuroscience & Biobehavioral Reviews 2009, 33, 1004–1023. [Google Scholar]
- Liu, T.; Xiao, T.; Shi, J.; Zhao, D.; Liu, J. Conflict control of children with different intellectual levels: an ERP study. Neuroscience Letters 2011, 490, 101–106. [Google Scholar]
- Raven, J.C. (1938). Raven standard progressive matrices. Journal of Cognition and Development.
- Raven, J. (2008). The Raven progressive matrices tests: their theoretical basis and measurement model. Uses and abuses of Intelligence. Studies advancing Spearman and Raven’s quest for non-arbitrary metrics, (Part I).
- Zurrin, R.; Wong, S.T. S.; Roes, M.M.; Percival, C.M.; Chinchani, A.; et al. , Functional brain networks involved in the Raven's standard progressive matrices task and their relation to theories of fluid intelligence. Intelligence 2024, 103, 101807. [Google Scholar]
- Duncan, J.; Chylinski, D.; Mitchell, D.J.; Bhandari, A. Complexity and compositionality in fluid intelligence. Pro-ceedings of the National Academy of Sciences 2017, 114, 5295–5299. [Google Scholar] [CrossRef]
- Tanaka, F.; Kachi, T.; Yamada, T.; Sobue, G. Auditory and visual event-related potentials and flash visual evoked potentials in Alzheimer's disease: correlations with Mini-Mental State Examination and Raven's Coloured Progressive Matrices. Journal of the neurological sciences 1998, 156, 83–88. [Google Scholar]
- Jung, R.E.; Haier, R.J. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral and brain sciences 2007, 30, 135–154. [Google Scholar]
- Zacks, J.M. Neuroimaging studies of mental rotation: a meta-analysis and review. Journal of cognitive neuroscience 2008, 20, 1–19. [Google Scholar] [PubMed]
- Haier, R.J.; Siegel, B.; Tang, C.; Abel, L.; Buchsbaum, M.S. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence 1992, 16, 415–426. [Google Scholar] [CrossRef]
- Neubauer, A.C.; Grabner, R.H.; Fink, A.; Neuper, C. Intelligence and neural efficiency: Further evidence of the influence of task content and sex on the brain–IQ relationship. Cognitive Brain Research 2005, 25, 217–225. [Google Scholar]
- Zygouris, N.C. Differences in children and adolescents with depression before and after a remediation program: an event-related potential study. Brain sciences 2024, 14, 660. [Google Scholar]
- Karapetsas, A.V.; Zygouris, N.C. Event Related Potentials (ERPs) in prognosis, diagnosis and rehabilitation of children with dyslexia. Encephalos 2011, 48, 118–127. [Google Scholar]
- Howe, A.S.; Bani-Fatemi, A.; De Luca, V. The clinical utility of the auditory P300 latency subcomponent event-related potential in preclinical diagnosis of patients with mild cognitive impairment and Alzheimer’s disease. Brain and cognition 2014, 86, 64–74. [Google Scholar]
- Demirayak, P.; Kıyı, İ.; İşbitiren, Y.Ö.; Yener, G. Cognitive load associates prolonged P300 latency during target stimulus processing in individuals with mild cognitive impairment. Scientific Reports 2023, 13, 15956. [Google Scholar]
- Zhong, R.; Li, M.; Chen, Q.; Li, J.; Li, G.; Lin, W. The P300 event-related potential component and cognitive im-pairment in epilepsy: a systematic review and meta-analysis. Frontiers in neurology 2019, 10, 943. [Google Scholar]
- Sternberg, R.J. (Ed.). (2020). The Cambridge handbook of intelligence. Cambridge University Press.
- Walhovd, K.B.; Nyberg, L.; Lindenberger, U.; Amlien, I.K.; Sørensen, Ø.; et al. Brain aging differs with cognitive ability regardless of education. Scientific reports 2022, 12, 13886. [Google Scholar] [CrossRef]
- Warchoł, Ł.; Zając-Lamparska, L. The Relationship of N200 and P300 Amplitudes With Intelligence, Working Memory, and Attentional Control Behavioral Measures In Young Healthy Individuals. Advances in Cognitive Psychology 2023, 19. [Google Scholar]
- Walhovd, K.B.; Fjell, A.M. One-year test–retest reliability of auditory ERPs in young and old adults. International Journal of Psychophysiology 2002, 46, 29–40. [Google Scholar] [PubMed]
- Polich, J. (2011). Neuropsychology of P300. In E. S. Kappenman & S. J. Luck (Eds.), The Oxford handbook of event-related potential components (pp. 160–188). Oxford University Press.
- Sternberg, R.J. A theory of adaptive intelligence and its relation to general intelligence. Journal of Intelligence 2019, 7, 23. [Google Scholar] [PubMed]
- Lozano-Blasco, R.; Quílez-Robres, A.; Usán, P.; Salavera, C.; Casanovas-López, R. Types of intelligence and academic performance: A systematic review and meta-analysis. Journal of Intelligence 2022, 10, 123. [Google Scholar]
- Ren, X.; Schweizer, K.; Wang, T.; Chu, P.; Gong, Q. On the relationship between executive functions of working memory and components derived from fluid intelligence measures. Acta Psychologica 2017, 180, 79–87. [Google Scholar]
- Ren, X.; Wang, T.; Sun, S.; Deng, M.; Schweizer, K. Speeded testing in the assessment of intelligence gives rise to a speed factor. Intelligence 2018, 66, 64–71. [Google Scholar]
- Bazana, P.G.; Stelmack, R.M. Intelligence and information processing during an auditory discrimination task with backward masking: an event-related potential analysis. Journal of personality and social psychology 2002, 83, 998. [Google Scholar]
- De Pascalis, V.A.; Varriale, V.; Matteoli, A. Intelligence and P3 components of the event-related potential elicited during an auditory discrimination task with masking. Intelligence 2008, 36, 35–47. [Google Scholar]
- Troche, S.J.; Houlihan, M.E.; Stelmack, R.M.; Rammsayer, T.H. Mental ability, P300, and mismatch negativity: Analysis of frequency and duration discrimination. Intelligence 2009, 37, 365–373. [Google Scholar] [CrossRef]
- Teixeira-Santos, A.C.; Pinal, D.; Pereira, D.R.; Leite, J.; Carvalho, S.; Sampaio, A. Probing the relationship between late endogenous ERP components with fluid intelligence in healthy older adults. Scientific Reports 2020, 10, 11167. [Google Scholar]
- Polich, J. Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology 2007, 118, 2128–2148. [Google Scholar]
- Der, G.; Deary, I.J. The relationship between intelligence and reaction time varies with age: Results from three rep-resentative narrow-age age cohorts at 30, 50 and 69 years. Intelligence 2017, 64, 89–97. [Google Scholar] [PubMed]
- Kannen, K.; Aslan, B.; Boetzel, C.; Herrmann, C.S.; Lux, S.; et al. P300 modulation via transcranial alternating current stimulation in adult attention-deficit/hyperactivity disorder: a crossover study. Frontiers in Psychiatry 2022, 13, 928145. [Google Scholar] [PubMed]
- Jungeblut, H.M.; Hagemann, D.; Löffler, C.; Schubert, A.L. An investigation of the slope parameters of reaction times and P3 latencies in the Sternberg memory scanning task–A fixed-links model approach. Journal of Cognition 2021, 4. [Google Scholar]
- Schubert, A.L.; Löffler, C.; Hagemann, D.; Sadus, K. How robust is the relationship between neural processing speed and cognitive abilities? Psychophysiology 2023, 60, e14165. [Google Scholar]
- Schubert, A.L. A meta-analysis of the worst performance rule. Intelligence 2019, 73, 88–100. [Google Scholar]
- Regel, S.; Meyer, L.; Gunter, T.C. Distinguishing neurocognitive processes reflected by P600 effects: Evidence from ERPs and neural oscillations. PloS one 2014, 9, e96840. [Google Scholar]
- Beldzik, E.; Ullsperger, M. A thin line between conflict and reaction time effects on EEG and fMRI brain signals. Imaging Neuroscience 2024, 2, 1–17. [Google Scholar]
- Raven, J. (2003). Raven progressive matrices. In Handbook of nonverbal assessment (pp. 223–237). Boston, MA: Springer US.
- Jasper, H.H. Ten-twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol 1958, 10, 371–375. [Google Scholar]
- Sadus, K.; Schubert, A.L.; Löffler, C.; Hagemann, D. An explorative multiverse study for extracting differences in P3 latencies between young and old adults. Psychophysiology 2024, 61, e14459. [Google Scholar]
- Picton, T.W.; Bentin, S.; Berg, P.; Donchin, E.; Hillyard, S.A.; Johnson, R.; et al. , Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology 2000, 37, 127–152. [Google Scholar]
- Zygouris, N.C.; Avramidis, E.; Karapetsas, A.V.; Stamoulis, G.I. Differences in dyslexic students before and after a remediation program: A clinical neuropsychological and event related potential study. Applied Neuropsychology: Child 2018, 7, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zygouris, N.C.; Vlachos, F.; Stamoulis, G.I. ERPs in Children and Adolescents with Generalized Anxiety Disorder: Before and after an Intervention Program. Brain Sciences 2022, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- OpenAI. (2024). ChatGPT [Large Language Model]. Retrieved from https://openai.com.
- Cohen, J. Set correlation and contingency tables. Applied psychological measurement 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Beauchamp, C.M.; Stelmack, R.M. The chronometry of mental ability: An event-related potential analysis of an auditory oddball discrimination task. Intelligence 2006, 34, 571–586. [Google Scholar] [CrossRef]
- Wongupparaj, P.; Sumich, A.; Wickens, M.; Kumari, V.; Morris, R.G. Individual differences in working memory and general intelligence indexed by P200 and P300: A latent variable model. Biological psychology 2018, 139, 96–105. [Google Scholar] [CrossRef]
- McGarry-Roberts, P.A.; Stelmack, R.M.; Campbell, K.B. Intelligence, reaction time, and event-related potentials. Intelligence 1992, 16, 289–313. [Google Scholar] [CrossRef]
- Sur, S.; Sinha, V.K. Event-related potential: An overview. Industrial psychiatry journal 2009, 18, 70–73. [Google Scholar] [CrossRef]
- Gmaj, B.; Januszko, P.; Kamiński, J.; Drozdowicz, E.; Kopera, M.; Wołyńczyk-Gmaj, D.; Wojnar, M. EEG source activity during processing of neutral stimuli in subjects with anxiety disorders. Acta Neurobiologiae Experimentalis 2016, 76, 75–85. [Google Scholar] [CrossRef]
- Doebler, P.; Scheffler, B. The relationship of choice reaction time variability and intelligence: A meta-analysis. Learning and Individual Differences 2016, 52, 157–166. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lu, H.J.; Chang, C.F.; Liang, W.K.; Muggleton, N.G.; Juan, C.H. Electrophysiological and behavioral evidence reveals the effects of trait anxiety on contingent attentional capture. Cognitive, Affective, & Behavioral Neuroscience 2017, 17, 973–983. [Google Scholar]
- Jensen, A.R. (1998). The factor. Westport, CT: Prager.
- Sanz, M.; Molina, V.; Martin-Loeches, M.; Calcedo, A.; Rubia, F.J. Auditory P300 event related potential and sero-tonin reuptake inhibitor treatment in obsessive-compulsive disorder patients. Psychiatry research 2001, 101, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Cox, S.R.; Okely, J.A. Inspection time and intelligence: A five-wave longitudinal study from age 70 to age 82 in the Lothian Birth Cohort 1936. Intelligence 2024, 105, 101844. [Google Scholar] [CrossRef]
- Coles, M.G. H.; Smid, H.G. O. M.; Scheffers, M.K.; Otten, L.J. (1995). Mental Chronometry and the study of human in-formation processing. In Electrophysiology of Mind. (pp. 86–127). Oxford University Press.
- Shaw, P.; Greenstein, D.; Lerch, J.; Clasen, L.; Lenroot, R.; et al. Intellectual ability and cortical development in children and adolescents. Nature 2006, 440, 676–679. [Google Scholar] [CrossRef]
- Kutas, M.; Federmeier, K.D. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Annual review of psychology 2011, 62, 621–647. [Google Scholar] [CrossRef]
- Wronka, E.; Kaiser, J.; Coenen, A.M. Psychometric intelligence and P3 of the event-related potentials studied with a 3-stimulus auditory oddball task. Neuroscience Letters 2013, 535, 110–115. [Google Scholar] [CrossRef]
- Luck, S.J. (2014). An introduction to the event-related potential technique. MIT press.
- Zygouris, N.C.; Dermitzaki, I.; Karapetsas, A.V. Differences in brain activity of children with higher mental abilities. An Event Related Potentials study using the latency of P300 and N100 waveforms. International Journal of Developmental Neuroscience 2015, 118–119. [Google Scholar] [CrossRef]
- Gagné, F. (2005). From gifts to talents: The DMGT as a devalopmental model. U: RJ Sternberg & JE Davidson (Eds.), Concep-tions of giftedness (str. 98-120).
- Greer, K. Neural Assemblies as Precursors for Brain Function. NeuroSci 2022, 3, 645–655. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Liu, Z. Predicting Fluid Intelligence via Naturalistic Functional Connectivity Using Weighted En-semble Model and Network Analysis. NeuroSci 2021, 2, 427–442. [Google Scholar] [CrossRef]
- Merks, S. (2016). Elucidating different aspects of speed of information processing: comparison of behavioral response latency and P300 latency in a modified Hick reaction time task (Doctoral dissertation, Universität Bern).
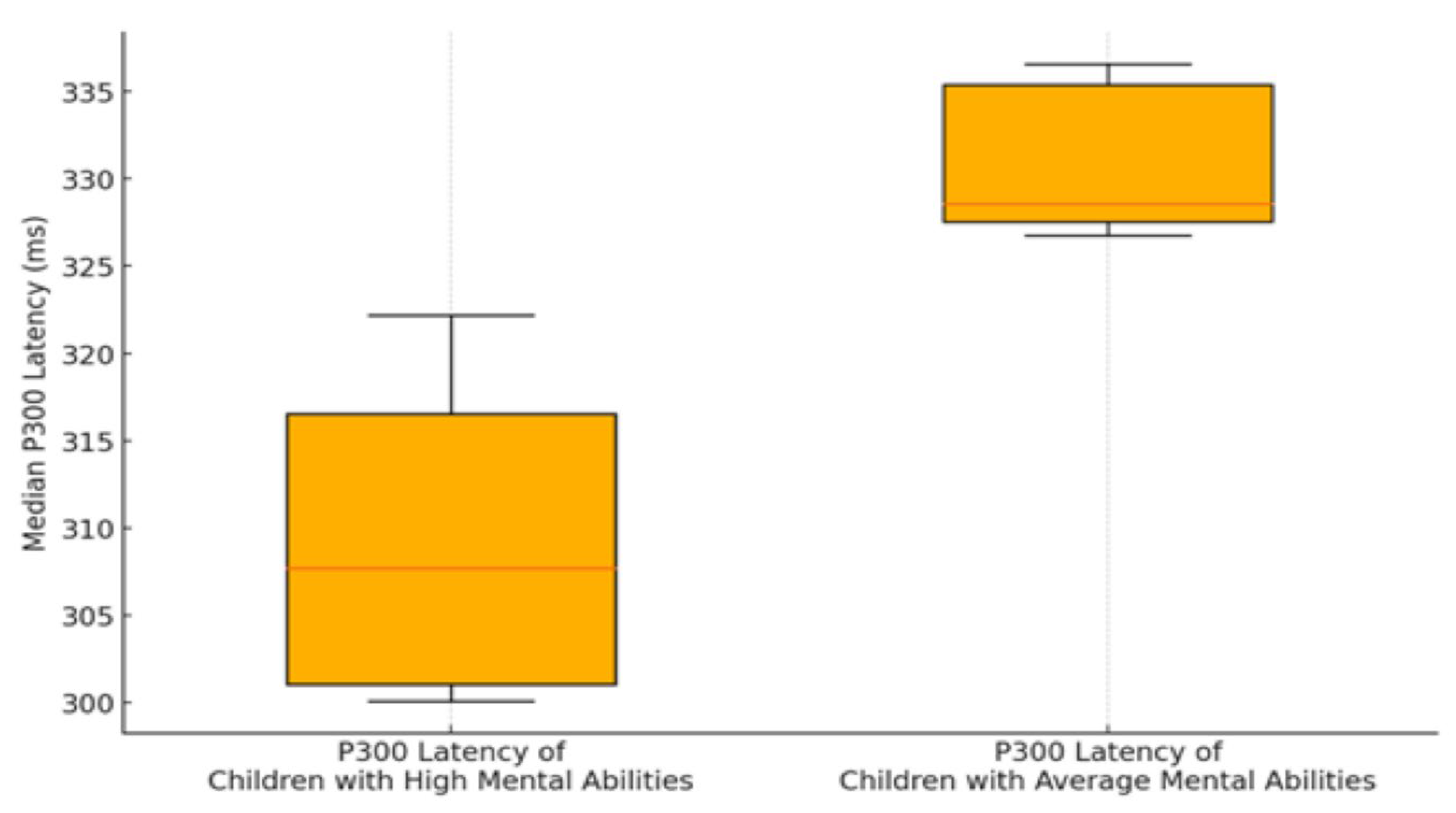
| Electro/Encephalographic Sites | P300 Latency ofChildren with high mental abilities | SD | P300 Latency ofChildren with average mental abilities | SD | t | p | Cohen's d |
| Fp1 | 304.48 | 6.21 | 316.58 | 3.46 | -5.89 | <0.001 | 2.41 |
| FPz | 305.44 | 7.64 | 318.41 | 4.63 | -5.89 | <0.001 | 2.05 |
| Fp2 | 307.55 | 6.33 | 320.53 | 5.92 | -5.03 | <0.001 | 2.12 |
| F3 | 307.00 | 7.14 | 326.65 | 7.56 | -5.03 | <0.001 | 2.67 |
| Fz | 307.87 | 6.05 | 325.53 | 2.21 | -5.18 | <0.001 | 3.88 |
| F4 | 313.15 | 10.31 | 336.38 | 1.99 | -5.18 | <0.001 | 3.13 |
| T3 | 306.28 | 10.03 | 329.00 | 4.82 | -6.54 | <0.001 | 2.89 |
| T4 | 308.32 | 11.85 | 325.85 | 2.45 | -6.54 | <0.001 | 2.05 |
| C3 | 306.78 | 13.10 | 330.45 | 6.37 | -9.49 | <0.001 | 2.30 |
| Cz | 311.69 | 15.84 | 337.53 | 3.22 | -9.49 | <0.001 | 2.26 |
| C4 | 317.77 | 9.41 | 338.19 | 2.89 | -7.66 | <0.001 | 2.93 |
| P3 | 309.51 | 10.04 | 329.96 | 5.21 | -7.66 | <0.001 | 2.56 |
| Pz | 311.98 | 14.98 | 336.37 | 5.45 | -7.07 | <0.001 | 2.16 |
| P4 | 313.39 | 17.56 | 339.68 | 3.67 | -7.07 | <0.001 | 2.07 |
| Oz | 316.91 | 10.38 | 337.87 | 5.71 | -5.01 | <0.001 | 2.50 |
| Electro/Encephalographic Sites | P-Value | BH Critical Value |
| Fp1 | <0.05 | 0.02 |
| FPz | <0.05 | 0.01 |
| Fp2 | <0.05 | 0.03 |
| F3 | <0.05 | 0.01 |
| Fz | <0.05 | 0.01 |
| F4 | <0.05 | 0.01 |
| T3 | <0.05 | 0.01 |
| T4 | <0.05 | 0.05 |
| C3 | <0.05 | 0.03 |
| Cz | <0.05 | 0.04 |
| C4 | <0.05 | 0.01 |
| P3 | <0.05 | 0.02 |
| Pz | <0.05 | 0.04 |
| P4 | <0.05 | 0.04 |
| Oz | <0.05 | 0.02 |
| Reaction Time | High Mental Abilities | Average Mental Abilities | |||||
| M | SD | M | SD | t | p | Cohen’s d | |
| 319.70 | 6.54 | 352.30 | 11.76 | -8.39 | <0.001 | 3.43 | |
| Electro/encephalographic sites | Correlation ρ | Sign |
| FP1 | -0.844 | 0.001 |
| FPZ | -0.804 | 0.001 |
| FP2 | -0.742 | 0.001 |
| F3 | -0.862 | 0.001 |
| FZ | -0.889 | 0.001 |
| F4 | -0.813 | 0.001 |
| T3 | -0.818 | 0.001 |
| T4 | -0.893 | 0.001 |
| C3 | -0.844 | 0.001 |
| CZ | -0.865 | 0.001 |
| C4 | -0.770 | 0.001 |
| P3 | -0.853 | 0.001 |
| PZ | -0.803 | 0.001 |
| P4 | -0.790 | 0.001 |
| OZ | -0.781 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





