Submitted:
22 January 2025
Posted:
22 January 2025
You are already at the latest version
Abstract
Compositing and hybridizing compounds is a generally accepted route to making new materials that perform better than the individual components taken separately. Herein, we describe a new electrochemical route for constructing hybrid materials from Polypyrrole (PPy). In this context, thin films of silica layer (Si) deposited by electro-assisted on a flexible ITO (f-ITO) surface modified by diazonium salt (f-ITO-NH2) were covered with an adhesive layer of polypyrroles. Deposition periods ranging from 20 to 45 seconds produce more electroactive silica layers. The electrochemical method confirmed the growth of a silica layer on the surface of the f-ITO-NH2 electrode. The different flexible electrodes were characterized by XPS, by electrochemical and scanning electron microscopy (SEM), which showed the central role of the diazonium chemical interface in the development of PPy on the silica layer. According to cyclic voltammetric studies, altering the f-ITO surface with diazonium salts produces PPy-Si polymers with more conductivity than a comparable coating without integrated treatment. This work demonstrates the power of a subtle combination of diazonium coupling agents on f-ITO, silica layer, and conductive polymers (f-ITO-NH2-Si-PPy) to design high-performance electrochemical materials.
Keywords:
1. Introduction
2. Experimental
2.1. Chemicals
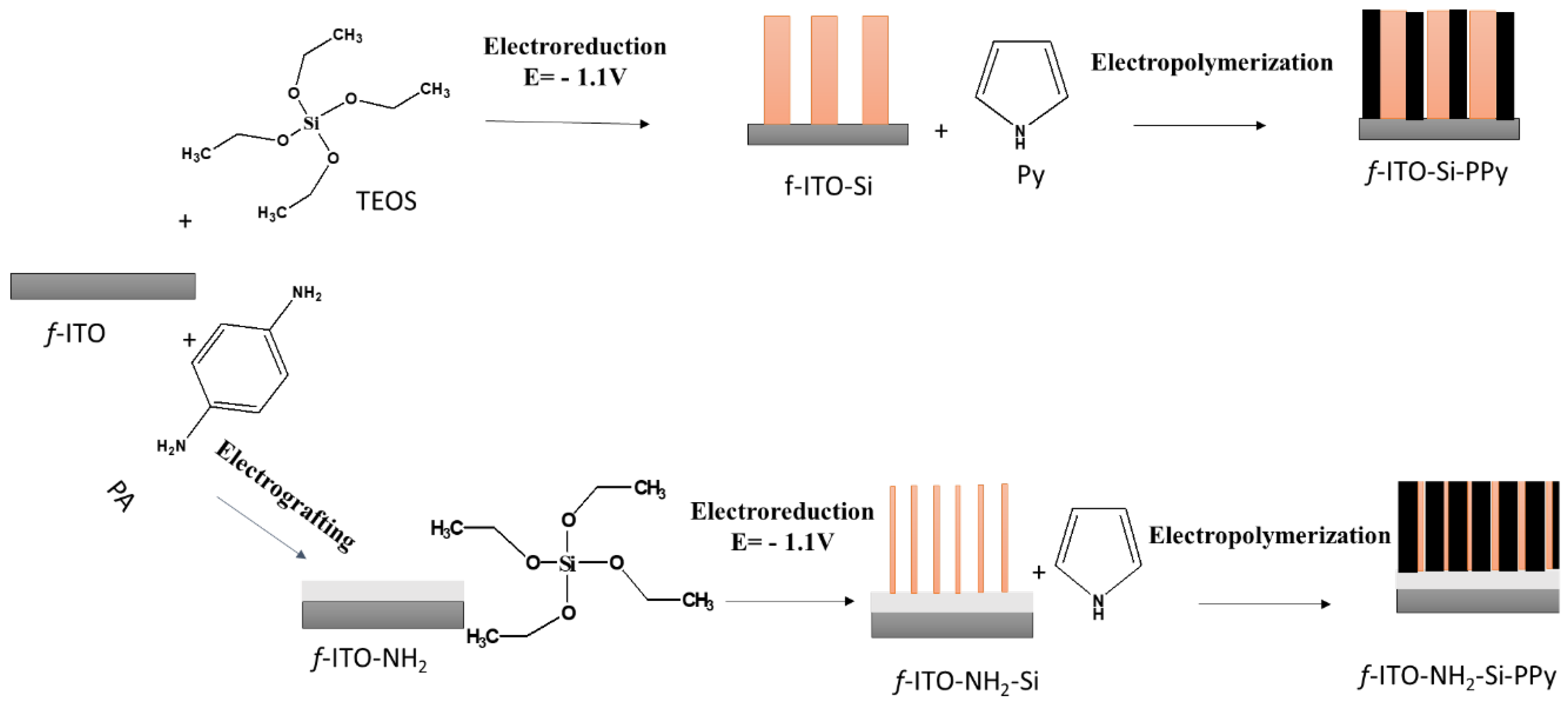
2.2. Preparation of the Electrode f-ITO-NH2-Si(t)
2.3. Electropolymerization and Characterization of Polypyrrole on f-ITO-NH2-Si
3. Results and discussions
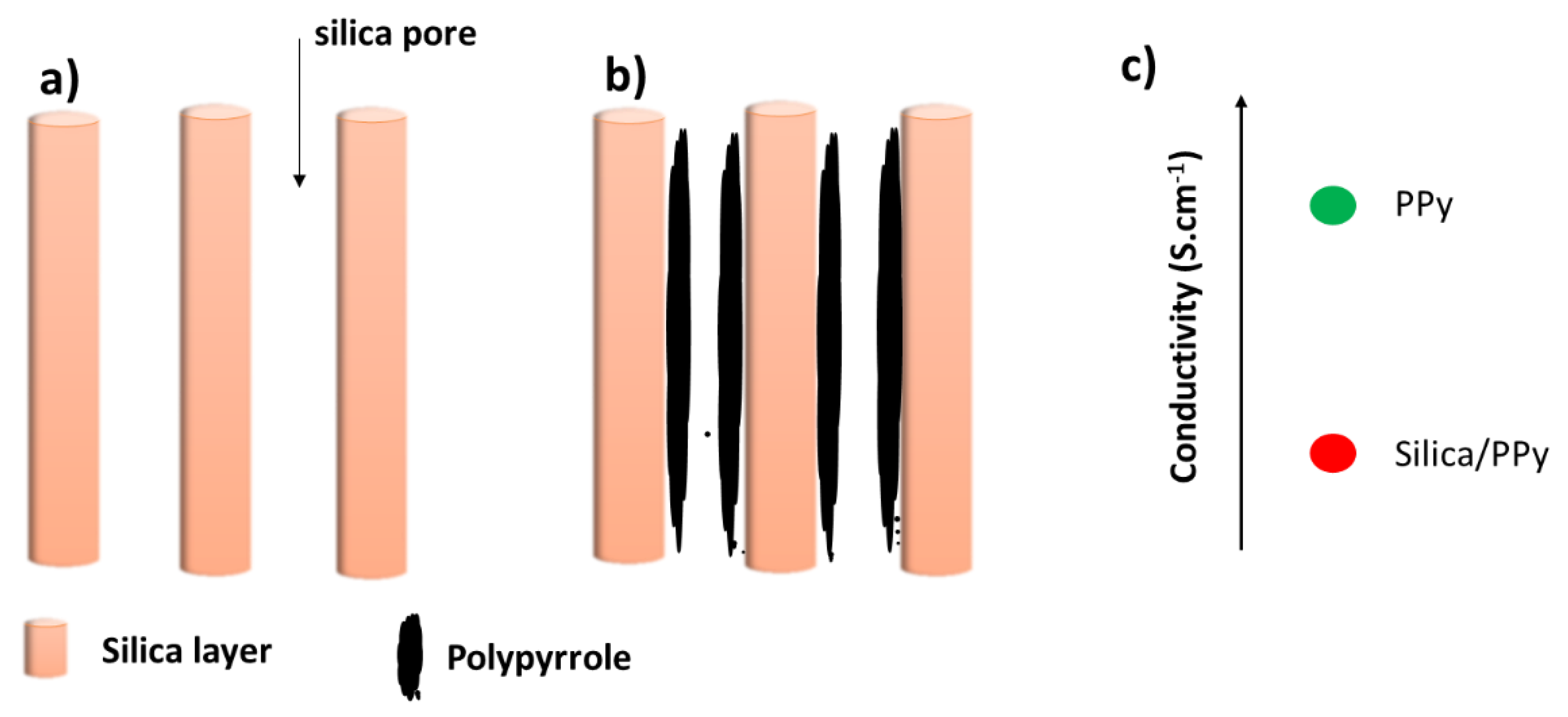
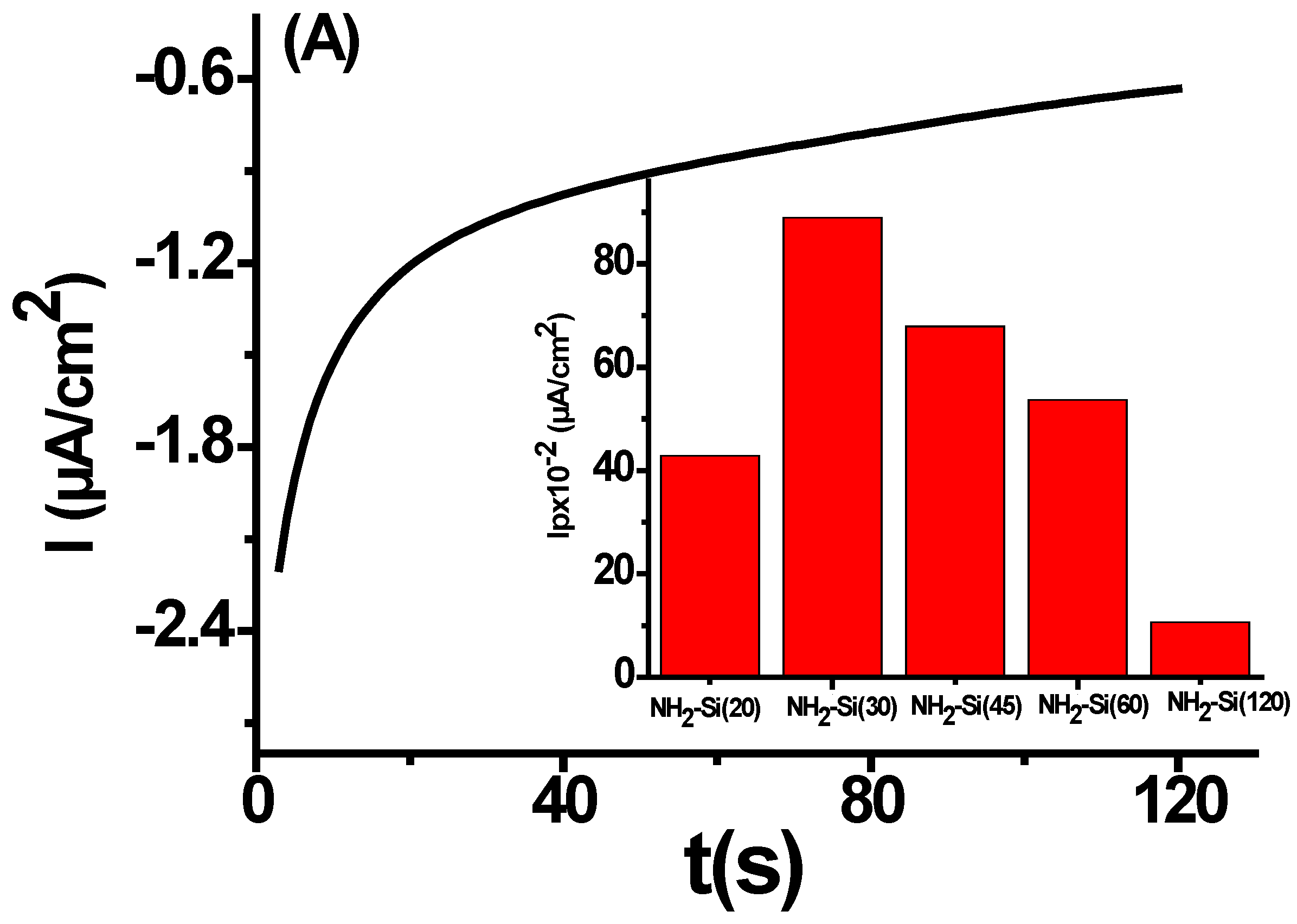
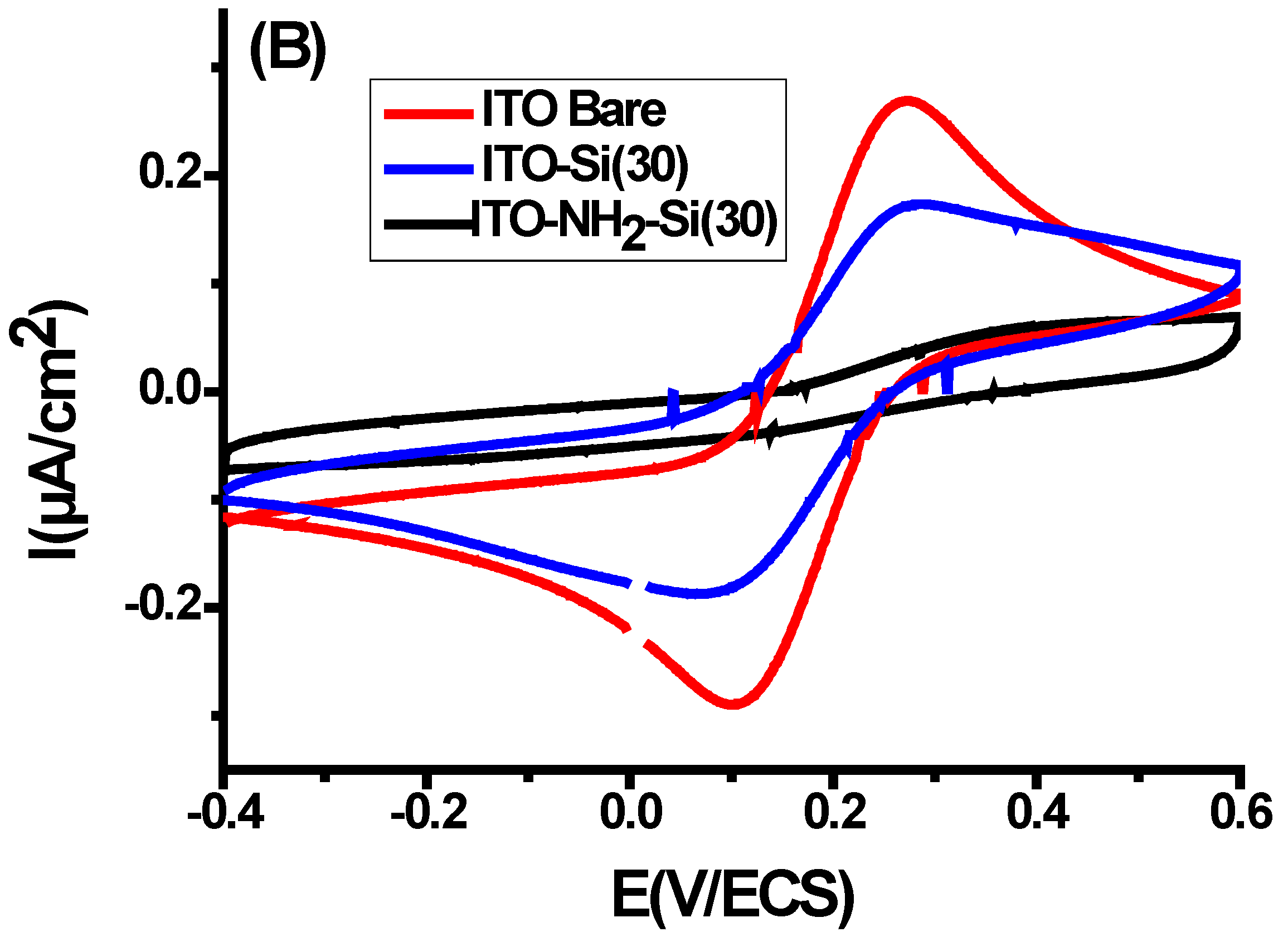
3.1. Electro la Chemical Assisted Self-Assisted of Silica Layer on f-ITO-NH2
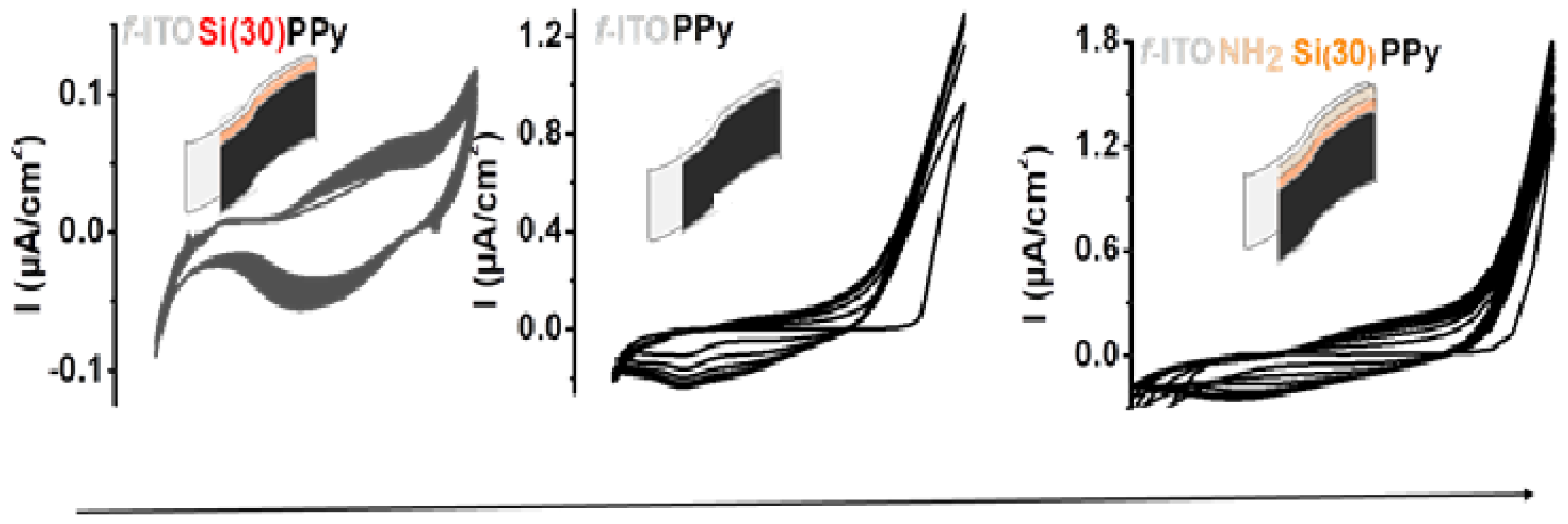
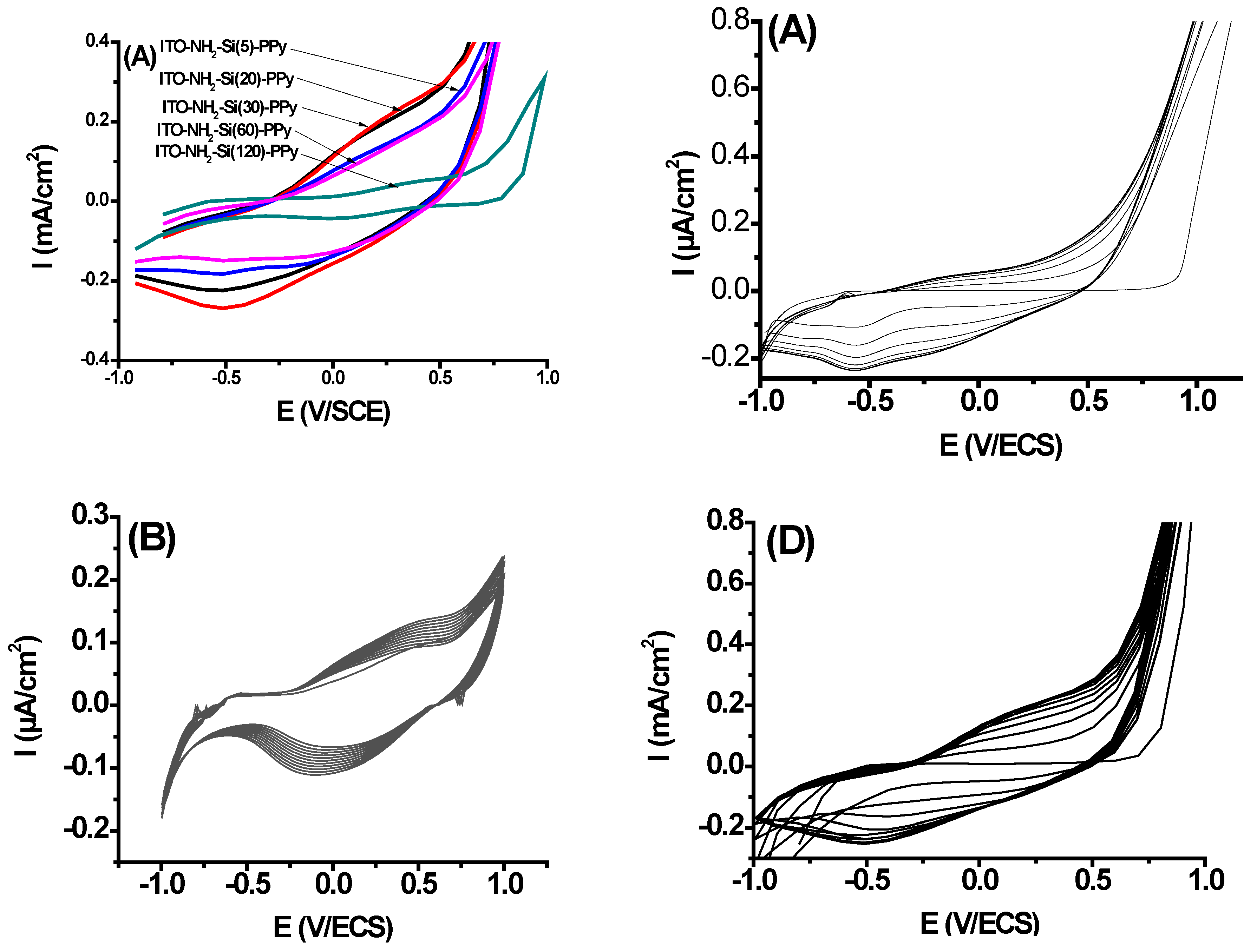
3.2. Electropolymerization of PPy of Flexible ITO Sheets
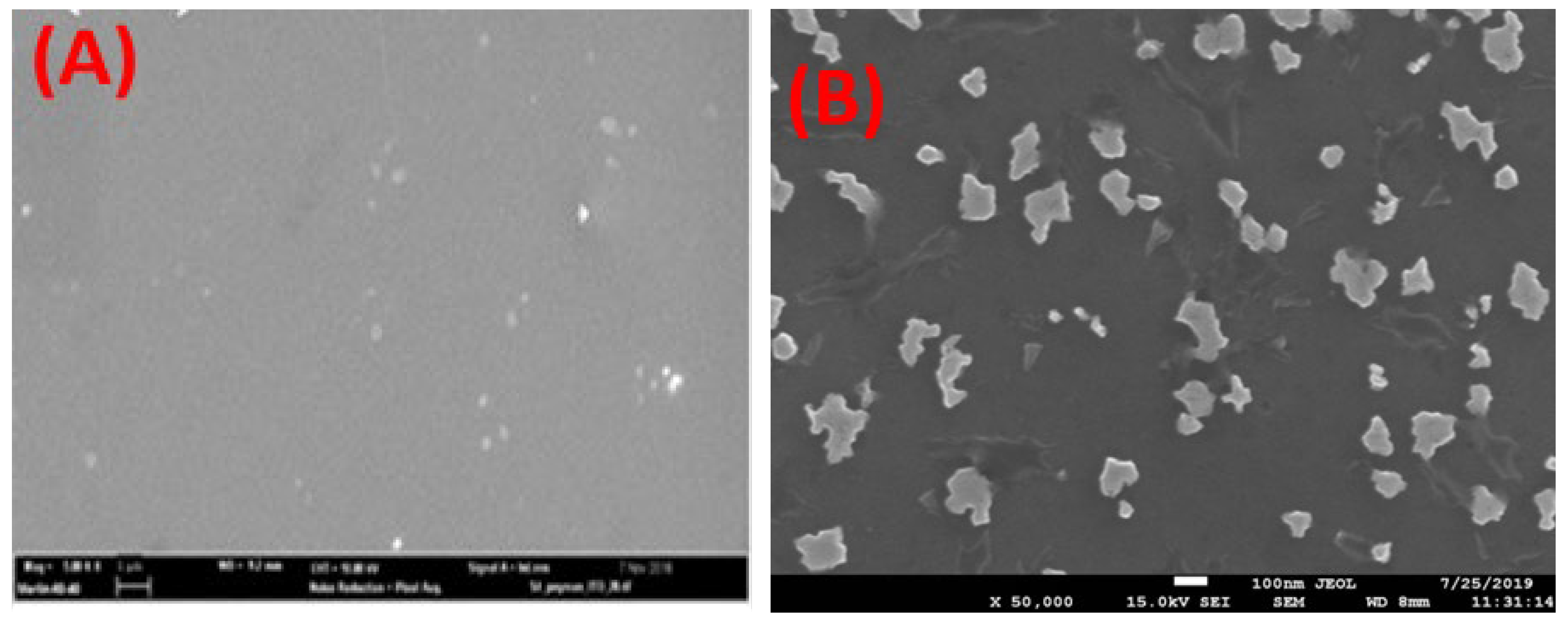
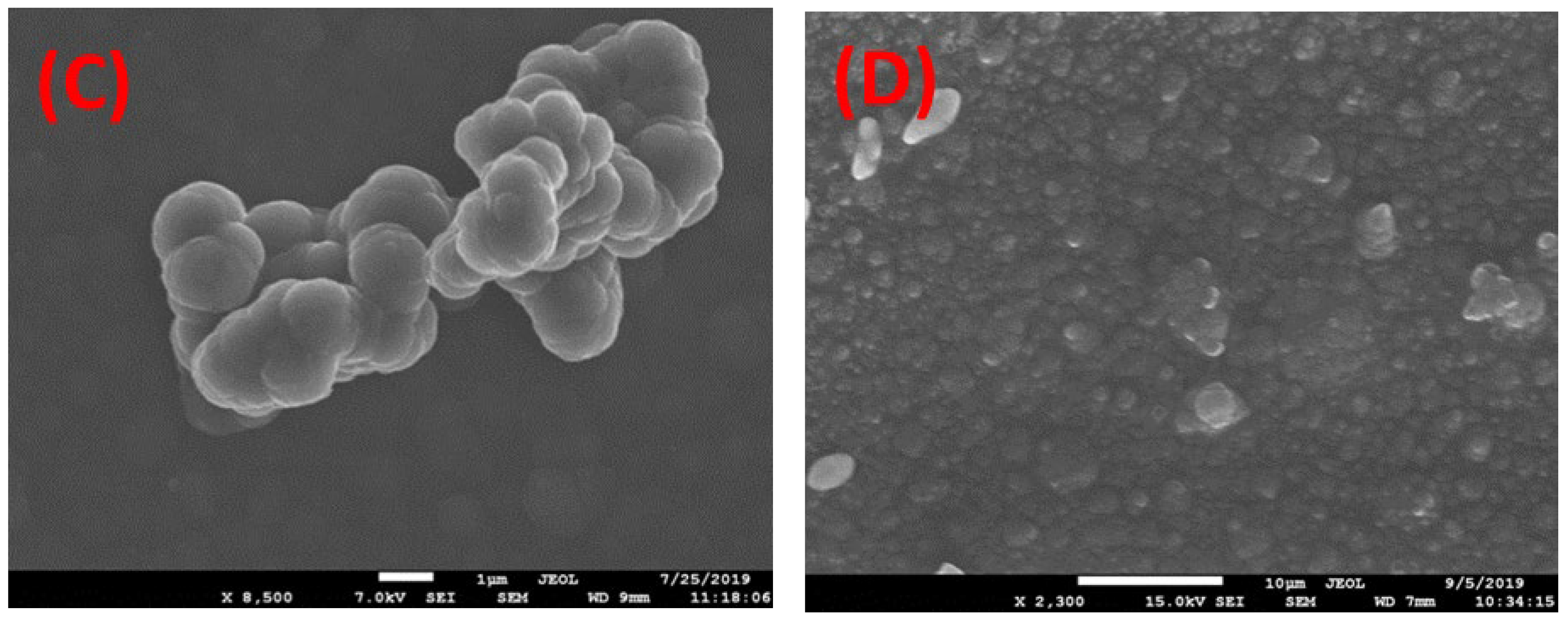
3.3. SEM Analysis
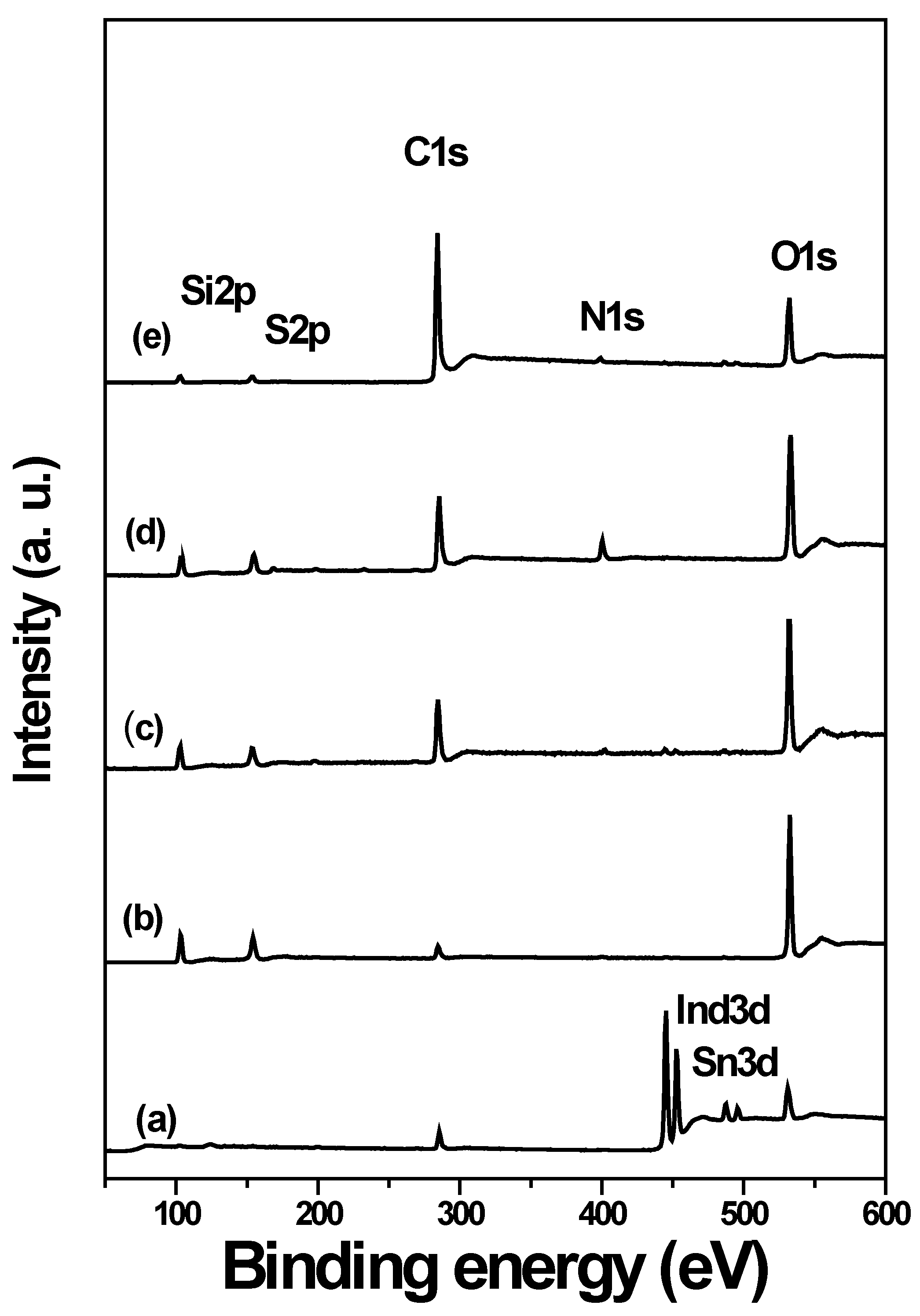
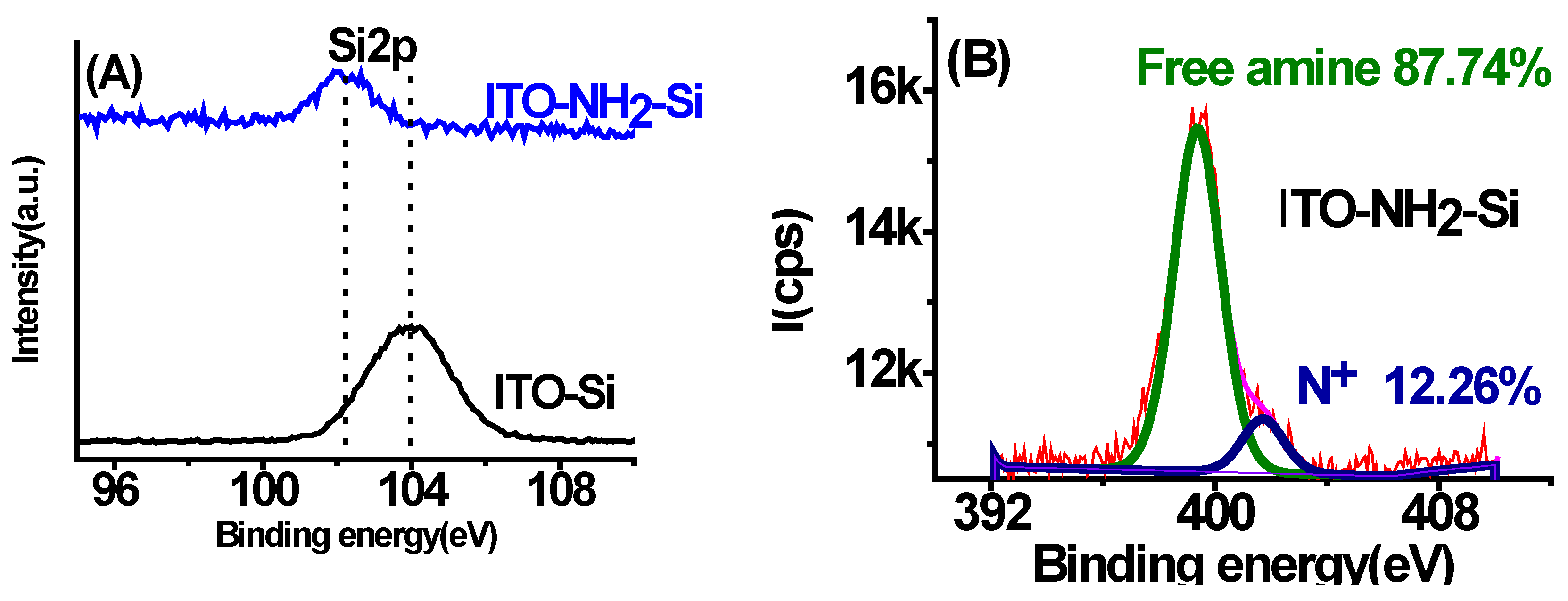
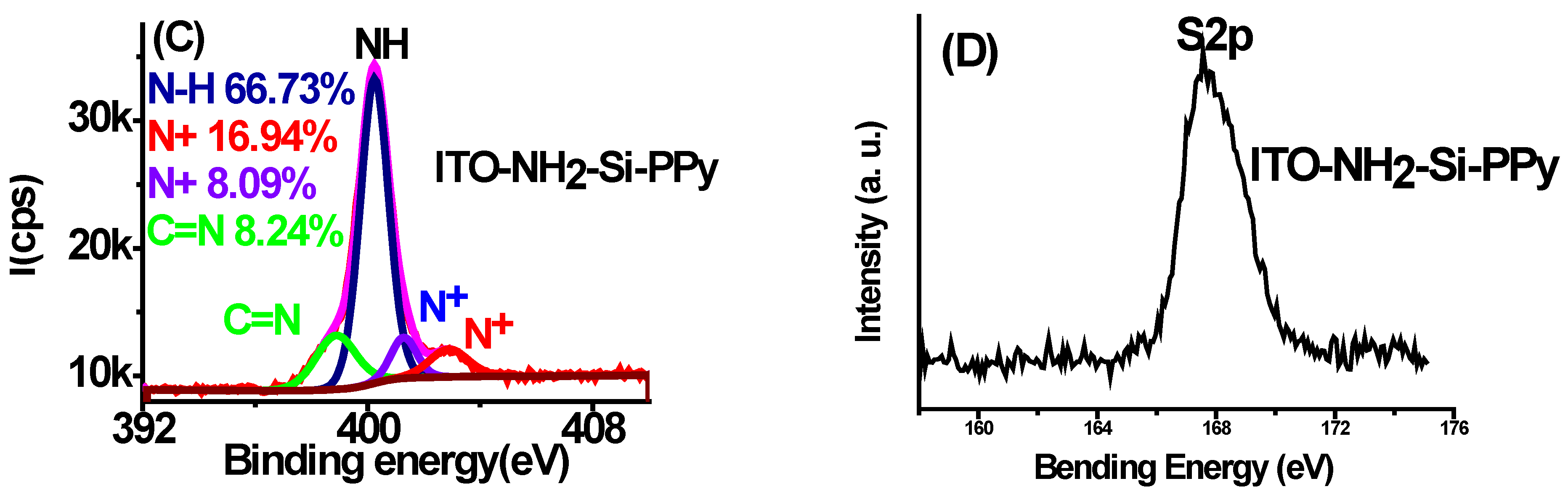
3.4. Surface Chemical Composition (XPS)
4. Conclusion
References
- Ngo, T.T.T.; Besson, E.; Bloch, E.; Bourrelly, S.; Llewellyn, R.; Gastaldi, S.; Llewellyn, P.L.; Gigmes, D.; Phan, T.N.T. T. One-pot synthesis of organic polymer functionalized mesoporous silicas. Microporous and Mesoporous Mater. 2021, 319, 111036. [Google Scholar] [CrossRef]
- Ghattavi, S.; Homaei, A. Synthesis and characterization of ZnO-SiO2 hybrid nanoparticles as an effective inhibitor for marine biofilm and biofouling. J. Mol. Liq. 2024, 396, 123974. [Google Scholar] [CrossRef]
- Al-Gharram, M.; AlZoubi, T. Electrochemical synthesis of a novel hybrid nanocomposite based on Co3O4 nanoparticles embedded in PANI- camphor sulfonic Acid matrix for optoelectronic applications. Ceram. Int. 2024, 50, 5473. [Google Scholar] [CrossRef]
- Saad, A.; Cabet, E.; Lilienbaum, A.; Hamadi, S.; Abderrabba, M.; Chehimi, M.M. Polypyrrole/Ag/mesoporous silica nanocomposite particles: Design by photopolymerization in aqueous medium and antibacterial activity. Journal Taiwan Inst. Chem. Eng. 2017, 80, 1022. [Google Scholar] [CrossRef]
- Diop, M.G.; Gueye, M.; Lo, M.; Faye, D.; Cisse, M.; Guene, M. Development of a hybrid material based on Zn-Mg spinel ferrites covered with polypyrrole: structural, morphological, and electrical characteristics. Digest, J. Nanomater. Biostruct. 2024, 3, 1215. [Google Scholar] [CrossRef]
- Perruchot, C.; Chehimi, M.M.; Delamar, M.; Dardoize, F. Characterisation of the chromatographic properties of a silica–polypyrrole composite stationary phase by inverse liquid chromatography. J. Chromatogr. A 2002, 969, 167. [Google Scholar] [CrossRef]
- Gan, T.; Li, J.; Zhao, A.; Xu, J.; Zheng, D.; Wang, H.; Liu, Y. Detection of theophylline using molecularly imprinted mesoporous silica spheres. Food Chemistry 2018, 268, 1. [Google Scholar] [CrossRef]
- Snoussi, Y.; Bastide, S.; Abderrabba, M.; Chehimi, M.M. Sonochemical synthesis of Fe3O4@NH2-mesoporous silica@Polypyrrole/Pd: a core/double shell nanocomposite for catalytic application. Ultrason. Sonochem. 2018, 41, 551. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Pavlinek, V.; Li, C.; Lengalova, A.; He; Ying; Saha, P. Synthesis and characterization of new mesoporous material with conducting polypyrrole confined in mesoporous silica Materials. Chem. and Phys. 2006, 98, 504. [Google Scholar] [CrossRef]
- Richard, J.; Vashishtha, A.; Phimphachanh, A.; Rydzek, G.; Lacroix-Desmazes, P.; Marcotte, N.; Gérardin, C. Mesoporous silica for sustainable dye removal: fast and reversible adsorption from ordered mesopores densely functionalized with polymers. Microporous Mesoporous Mater. 2024, 379, 113254. [Google Scholar] [CrossRef]
- Humelnicu, D.; Ghiorghita, C.A.; Humelnicu, I.; Dragan, E.S. Experimental and theoretical investigations on Hg(II) removal by recyclable composite sorbents comprised of polymers bearing thiourea or amidoxime functional groups and mesoporous silica. Chem. Eng. J. 2024, 479, 147690. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Govindaraj, Y.; Prabhakar, M.; Ganapathi, A.; Sakairi, M.; Rohwerder, M.; Neelakantan, L. ; Smart release of turmeric as a potential corrosion inhibitor from a pH-responsive polymer encapsulated highly ordered mesoporous silica containers. Surf. Interfaces. 2024, 45, 103883. [Google Scholar] [CrossRef]
- Kovačević, M.; Pobirk, A.Z.; Ilić, I.G. ; The effect of polymeric binder type and concentration on flow and dissolution properties of SMEDDS loaded mesoporous silica-based granules. Eur. J. Pharm. Sci. 2024, 193, 78. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Asadzadeh, F. ; Synthesis of Fe3O4/SiO2/Polypyrrole magnetic nanocomposite polymer powder: Investigation of structural properties and ability to purify of edible sea salts. Adv. Powder Technol 2021, 32, 1233. [Google Scholar] [CrossRef]
- Kang, H.; Lee, H.; Kwak, J. ; Electrodeposition of Polypyrrole Nanowires within Vertically Oriented Mesoporous Silica Template. J. Korean Electrochem. Soc. 2011, 14, 22. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Tao, J.; Zhang, H.; Hu, S.; Liu, Q.; Jiao, J. Mesoporous silica@polypyrrole and its epoxy composites with ultra-broadband electromagnetic wave absorption properties. Chemical Engineering Journal 2024, 491, 152051. [Google Scholar] [CrossRef]
- Wysocka-Zolopa, M., Zablocka, I., Basa, A. Formation and characterization of mesoporous silica MCM-48 and polypyrrole composite. Chem. Heterocycl. Comp.
- Wysocka-Żołopa, M.; Zabłocka, I.; Grądzka, E.; Goclon, J.; Winkler, K. Synthesis and characterization of polypyrrole@MCM-41 nanocomposites, Microporous Mesoporous Mater. 2021, 317, 1387.
- Cho, M.S.; Choi, H.J.; Kim, K.Y.; Ahn, W.S. Synthesis and Characterization of Polyaniline/Mesoporous SBA-15 Nanocomposite. Macromol. Rapid. Commun. 2002, 23, 713. [Google Scholar] [CrossRef]
- Coutinho, D.; Yang, Z.; Ferraris, J.P.; Balkus, K.J. ; Proton conducting polyaniline molecular sieve composites. Microporous Mesoporous Mater. 2005, 81, 321. [Google Scholar] [CrossRef]
- Kuliček, J.; Gemeiner, Pavol. ; Omastová, M.; Mičušík, M. Preparation of polypyrrole/multi-walled carbon nanotube hybrids by electropolymerization combined with a coating method for counter electrodes in dye-sensitized solar cells. Chem. Pap. 2018, 72, 1651. [Google Scholar] [CrossRef]
- Elayappan, V.; Murugadoss, V.; Fei, Z.; Dyson, P.J.; Angaiah, S. Influence of Polypyrrole Incorporated Electrospun Poly (vinylidene fluoride-co-hexafluoropropylene) Nanofibrous Composite Membrane Electrolyte on the Photovoltaic Performance of Dye Sensitized Solar Cell. Eng. Sci. 2020, 10, 78–84. [Google Scholar] [CrossRef]
- Angélica del Valle, M.; Gacitúa, M.; Díaz, F.R.; Armijo, F.; del Río, R. Electrosynthesis of polythiophene nanowires via mesoporous silica thin film templates. Electrochem. Commun 2009, 11, 2117. [Google Scholar] [CrossRef]
- Sandomierski, M.; Poniedziałek, K.; Bielicka-Daszkiewicz, K.; Voelkel, A. Infuence of diazonium and surfactant modification of the mesoporous material on its adsorption properties. Chem. Pap. 2020, 74, 929. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. Diazonium Salts: Versatile Molecular Glues for Sticking Conductive Polymers to Flexible Electrodes. Surface 2018, 1, 43. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. The role of diazonium interface chemistry in the design of high performance polypyrrole-coated flexible ITO sensing electrodes. Electrochem. Commun. 2017, 77, 14. [Google Scholar] [CrossRef]
- Datson, Z.; Darwish, N. Electrochemical grafting of diazonium salts on silica-terminated versus H-terminated silicon. Electrochim. Acta. 2024, 507, 145183. [Google Scholar] [CrossRef]
- Giordano, G.; Vilà, N.; Aubert, E.; Ghanbaja, J.; Walcariusa, A. Multi-layered, vertically-aligned and functionalized mesoporous silica films generated by sequential electrochemically assisted self-assembly. Electrochim. Acta. 2017, 237, 227. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. nature mater. 2007, 6, 602. [Google Scholar] [CrossRef]
- Xie, Z.; Qu, Wei. ; Fisher, E.A.; Fahlman, J.; Asazawa, K.; Hayashi, T.; Shirataki, H.; Murase, H. Hideaki Murase Capacitance Determination for the Evaluation of Electrochemically Active Surface Area in a Catalyst Layer of NiFe-Layered Double Hydroxides for Anion Exchange Membrane Water Electrolyser. Mater. 2024, 17, 556. [Google Scholar]
- Fall, B.; Diaw, A.K.D.; Fall, M.; Sall, M.L.; Lo, M.; Gningue-Sall, D.; Thotiyl, M.O.; Maria, H.J.; Kalarikkal, N.; Thomas, S. Synthesis of highly sensitive rGO@CNT@Fe2O3/polypyrrole nanocomposite for the electrochemical detection of Pb2+. Mater. Today Commun. 2021, 26, 102005. [Google Scholar] [CrossRef]
- Mariusz Sandomierski, Beata Strzemiecka, Mohamed, M. Chehimi, and Adam Voelkel, Reactive Diazonium-Modified Silica Fillers for High-Performance Polymers. Langmuir 2016, 32, 44–11646. [Google Scholar]
- Shen, J.; Zhang, S.; Zeng, Z.; Huang, J.; Shen, Y.; Guo, Y. ; Synthesis of Magnetic Short-Channel Mesoporous Silica SBA-15 Modified with a Polypyrrole/Polyaniline Copolymer for the Removal of Mercury Ions from Aqueous Solution. ACS Omega 2021, 6, 25791. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Pires, R.; Diaw, A.K.D.; Gningue-Sall, D.; Oturan, M.A.; Aaron, J.-J.; Chehimi, M.M. Diazonium Salts: Versatile Molecular Glues for Sticking Conductive Polymers to Flexible Electrodes. Surfaces 2018, 1, 43. [Google Scholar] [CrossRef]
| Electrodes | (µA/cm2) | (µA/cm2) | (mV) | (mV) | ||
|---|---|---|---|---|---|---|
| ITO-NH2-Si(20) | 42.86 | 33.96 | 1.26 | 322.73 | 133.43 | 189.3 |
| ITO-NH2-Si(30) | 88.92 | 95.63 | 0.92 | 278.22 | 84.12 | 194.1 |
| ITO-NH2-Si(45) | 67.91 | 58.9 | 5.43 | 365.36 | 6.81 | 340.51 |
| ITO-NH2-Si(60) | 53.65 | 81.47 | 0.65 | 368.28 | -30.58 | 398.86 |
| ITO-NH2-Si(120) | 10.67 | - | - | - | - | - |
| Materials | Si | O | C | N | S | In | Sn |
|---|---|---|---|---|---|---|---|
| f-ITO | - | 39.1 | 32.5 | - | - | 25.4 | 3.06 |
| f-ITO-Si(30) | 27.90 | 56.41 | 14.16 | - | - | - | - |
| f-ITO-NH2-Si(30) | 8.9 | 42.44 | 30.16 | 3.6 | - | 11.28 | 5.31 |
| f-ITO-Si(30)-PPy | 12.54 | 30.40 | 41.98 | 7.26 | 7.81 | - | - |
| f-ITO-NH2-Si(30)-PPy | 3.82 | 15.33 | 77.22 | 12.21 | 2.22 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





