Introduction
Metastable intermolecular composites, which include multilayer thermite materials, have received a second life in recent years due to new unique applications in a wide variety of engineering and technology fields. Such materials are capable of storing thermal energy for a long time and releasing it in fractions of a second if necessary. However, researchers are usually interested in everything that happens in energetic materials from formation/synthesis until the end of combustion (unless we are talking about self-propagating high-temperature synthesis). Various methods of forming energetic materials are considered - direct ink writing [1, 2], electrospraying [
3], spray drying [
4], mechanical alloying [
5], aerosol jet printing [
6], electrophoretic deposition [
7], magnetron sputtering [8, 9], for various applications - pyro-MEMS [
1], underwater wet welding [
10], gas-generating energetic materials [
11], joining and welding [
12]. The results of experimental studies and mathematical modeling of combustion initiation processes [13, 14], phase transformations [15, 16], gas evolution [
17], and long-term storage performance [18, 19] have been described many times. In this case, all possible types of thermite and not only energy pairs are considered – multilayer [8, 20], powder [
21], core-shell [
22], bimetallic [
15], as well as multicomponent systems [23-26].
Multilayer thermite materials Al-CuO are a bright representative of metastable intermolecular composite, which is characterized by outstanding energy and combustion properties. In this paper, the influence of the total thickness of the multilayer thermite material Al-CuO, formed on the surface of sitall substrates by magnetron sputtering of targets in vacuum, on the combustion mode and morphology of the reaction products is considered. Based on the obtained experimental results, three combustion modes of multilayer structures will be proposed and substantiated from the point of view of the combustion front temperature.
Experimental details
Multilayer Al-CuO structures were formed on the surface of ST-50 sitall substrates by sequential magnetron sputtering of Al and CuO targets in vacuum. Deposition was performed on a URM-026 setup at a residual gas pressure of 7*10-5 Torr. The substrate holders were mounted on a carousel with planetary rotation. The substrate surface was pre-bombarded with argon ions using an ion source (ion current 40 mA, processing time 120 seconds). To form aluminum and copper oxide layers, Al (diameter 100 mm, Al 99.995%) and CuO (diameter 100 mm, copper II oxide 99.995%) targets were used, respectively. The argon pressure during sputtering was 3*10-3 Torr. The sputtering process was controlled using PlasmaTech IVE 141 power supplies operating in the power stabilization mode with the ability to measure the current values of voltage and current. The sputtering power of the Al and CuO targets was 500 and 350 W, respectively. The thicknesses of the Al and CuO layers were 50 nm, and the total thickness of the multilayer structure was 2, 3, and 4 μm (20, 30, and 40 bilayers, respectively). Sputtering of the structures was performed with pauses to exclude the possibility of combustion initiation during the formation process due to heating of the substrates.
The thickness of individual layers and the overall thickness of the multilayer structure were controlled using a Jeol JSM–6010 Plus/LA scanning electron microscope (SEM). The reaction products, including cross-sections formed using a focused ion beam, were examined using an FEI Helios G4 CX equipped with an energy-dispersive X-ray spectroscopy attachment (EDX).
The combustion process of multilayer thermite materials was monitored using a NAC MEMRECAM HX-7 high-speed video camera with a video recording speed of 15,000 frames per second. The combustion front propagation velocity was determined based on the distance it traveled per unit time.
The adiabatic reaction temperature was calculated using "THERMO" - a package of computer programs designed for calculating thermodynamic equilibrium in the complicated multicomponent heterophase systems at the Institute of Structural Macrokinetics of the Russian Academy of Science. It includes the database of thermodynamic information and the computer program for equilibrium calculations. Calculations were made for combustion at atmospheric pressure.
Results
In this study, samples with different total structure thickness were considered, where the bilayer thickness (the combined thickness of one aluminum layer and one copper oxide layer) was 100 nm. The multilayer thermite structure (MTS) consisted of 20, 30, and 40 layers, which corresponded to a total thickness of 2 μm, 3 μm, and 4 μm, respectively.
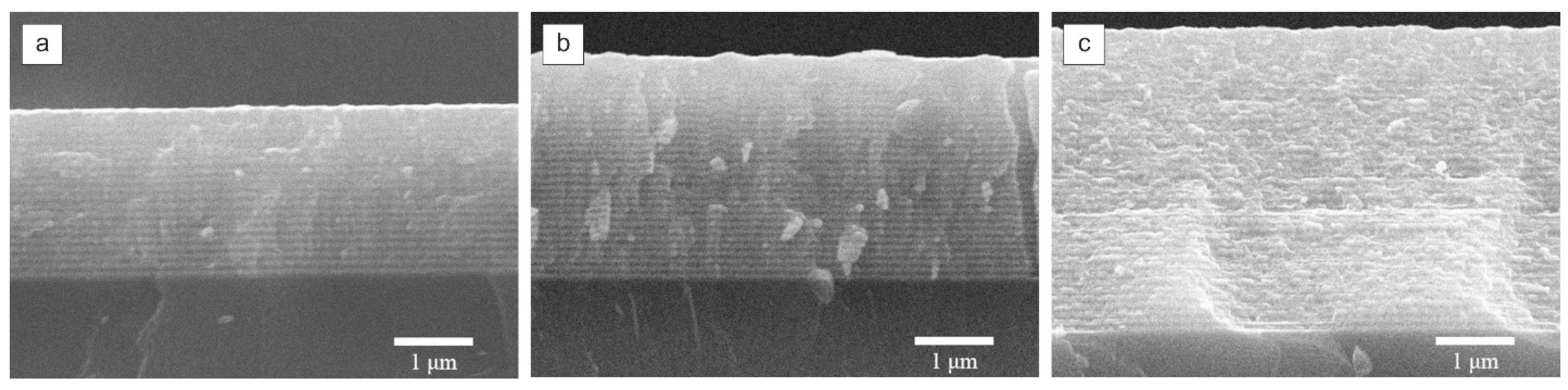
Figure 1 shows the SEM images of the cross sections of the formed multilayer structures of different thicknesses.
SEM images indicate the preservation of the multilayer structure and clear boundaries of individual layers throughout the thickness. The presence of heterogeneities on the cleavage is due to the peculiarities of sample preparation - cross-sections were formed as a result of mechanical fracture of the substrate.
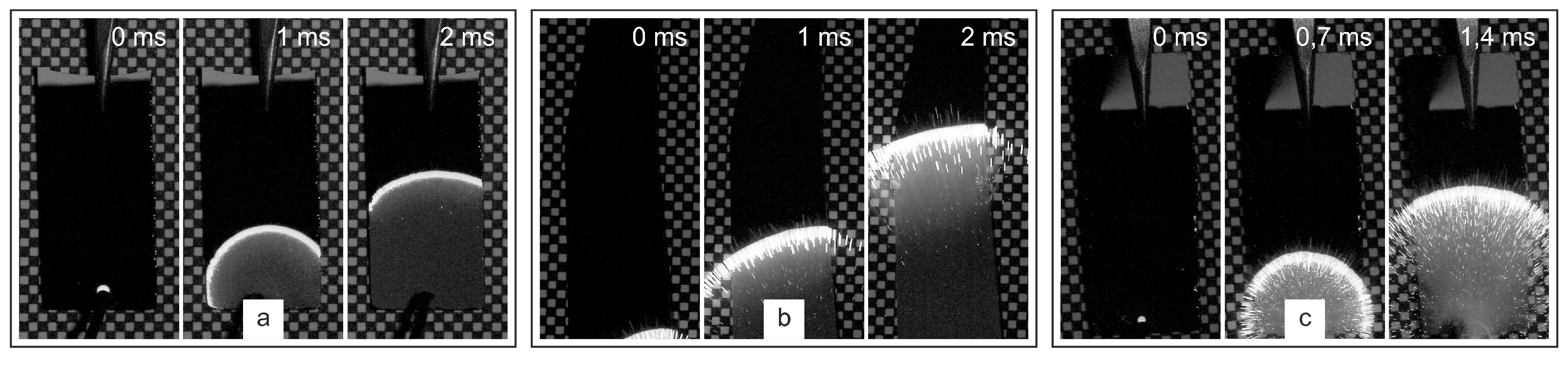
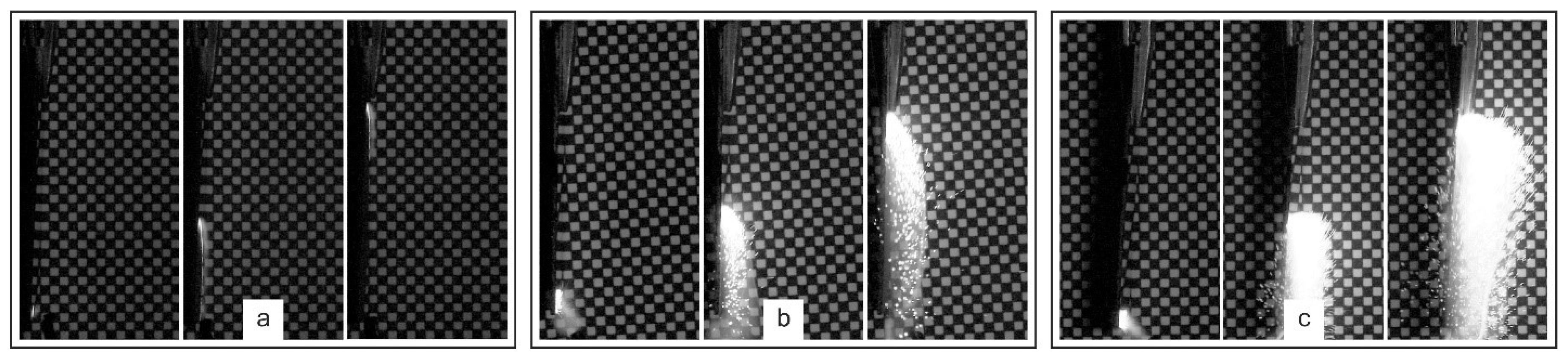
The combustion features of the formed multilayer Al-CuO structures were studied using a high-speed video camera with a shooting speed of 15,000 frames per second. Combustion was initiated using a spark generated by a low-power piezoelectric element. During shooting, the samples were placed on a millimeter dimensional scale for ease of determining the speed of combustion front propagation. The combustion storyboards of multilayer structures of different thicknesses are shown in
Figure 2.
In all cases, the combustion front was clearly visible and spread uniformly from the initiation point over the entire surface of the sample. However, the combustion pattern for different total thicknesses of the structures differed significantly. Thus, for samples with 20 bilayers (total thickness of 2 μm), combustion occurred without visually noticeable gas evolution and separation from the substrate surface and scattering of reaction products.
Figure 3 shows a frame chart of the combustion process of multilayer thermite materials Al-CuO with different total thicknesses, with the shooting performed from the side.
The velocity of the front propagation was 6.5±0.2 m/s. The combustion of structures with a total thickness of 3 μm was already accompanied by visually observable gas evolution and separation of heated reaction products from the substrate surface in the form of sparks. However, the expansion of hot particles in this case occurred along the substrate surface, as was observed earlier [
27]. The velocity of the combustion front propagation increased and was 11.0±0.3 m/s. The combustion of multilayer structures with a total thickness of 4 μm was accompanied by even greater gas evolution and the expansion of heated reaction products occurred, including at an angle to the substrate surface. At the same time, a slight decrease in the velocity of the combustion front propagation was recorded, which was 10.7±0.3 m/s.
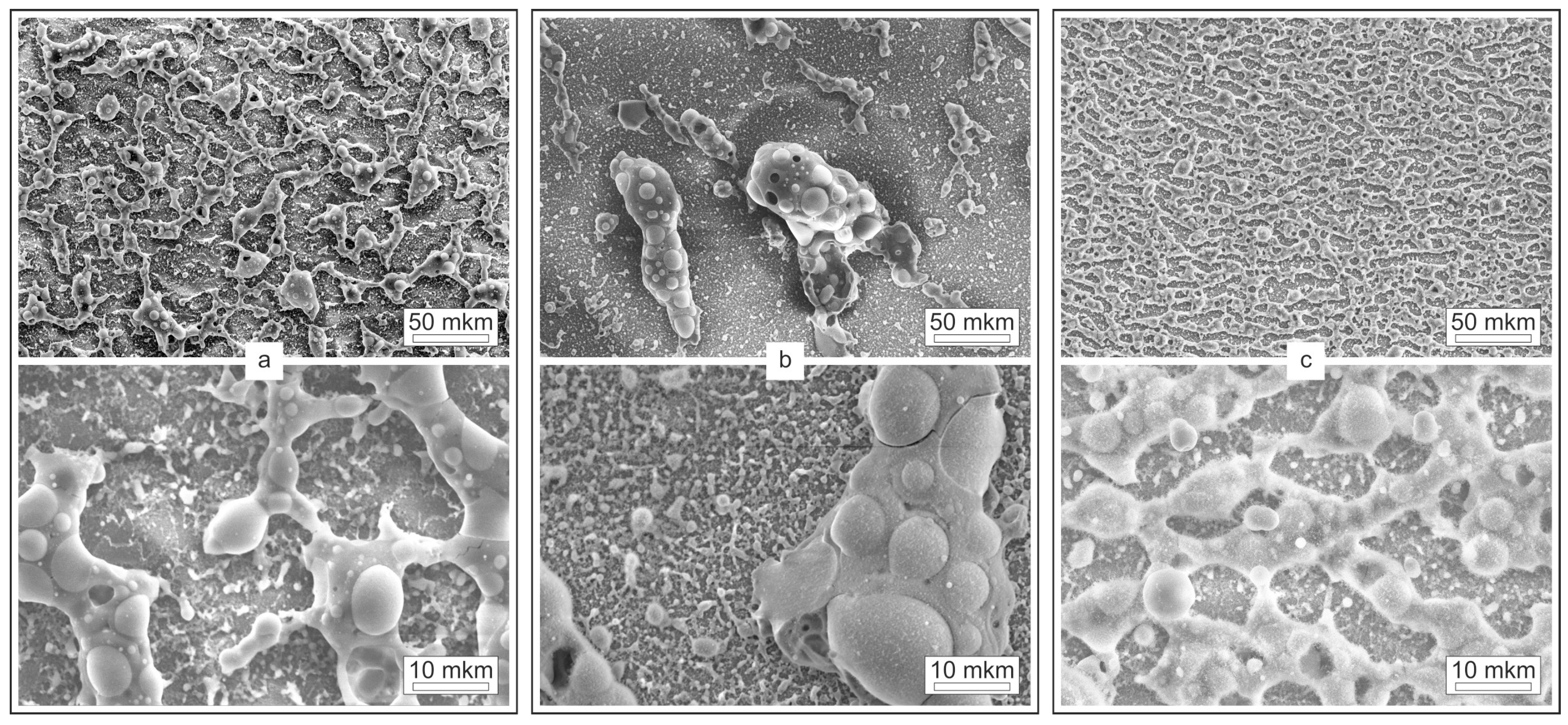
The morphology and elemental composition of the combustion reaction products of multilayer thermite materials with different total thicknesses were studied using SEM and EDX. SEM images with different magnifications are shown in
Figure 4.
The morphology of combustion products differs for different total thicknesses of the multilayer structure. The combustion products of multilayer structures with a thickness of 2 and 4 μm are a network of island structures of complex elongated shape, uniformly distributed over the surface of the substrate. At the same time, the size of the objects for a thinner multilayer structure is significantly larger. The combustion products of a multilayer structure with a thickness of 3 μm are spatially separated large drop-shaped objects with overall dimensions of up to several tens of micrometers. Nevertheless, despite the difference in the characteristic sizes of the reaction products for different total thicknesses of the original multilayer structures, in all cases the presence of a complex-shaped matrix with spherical inclusions was observed.
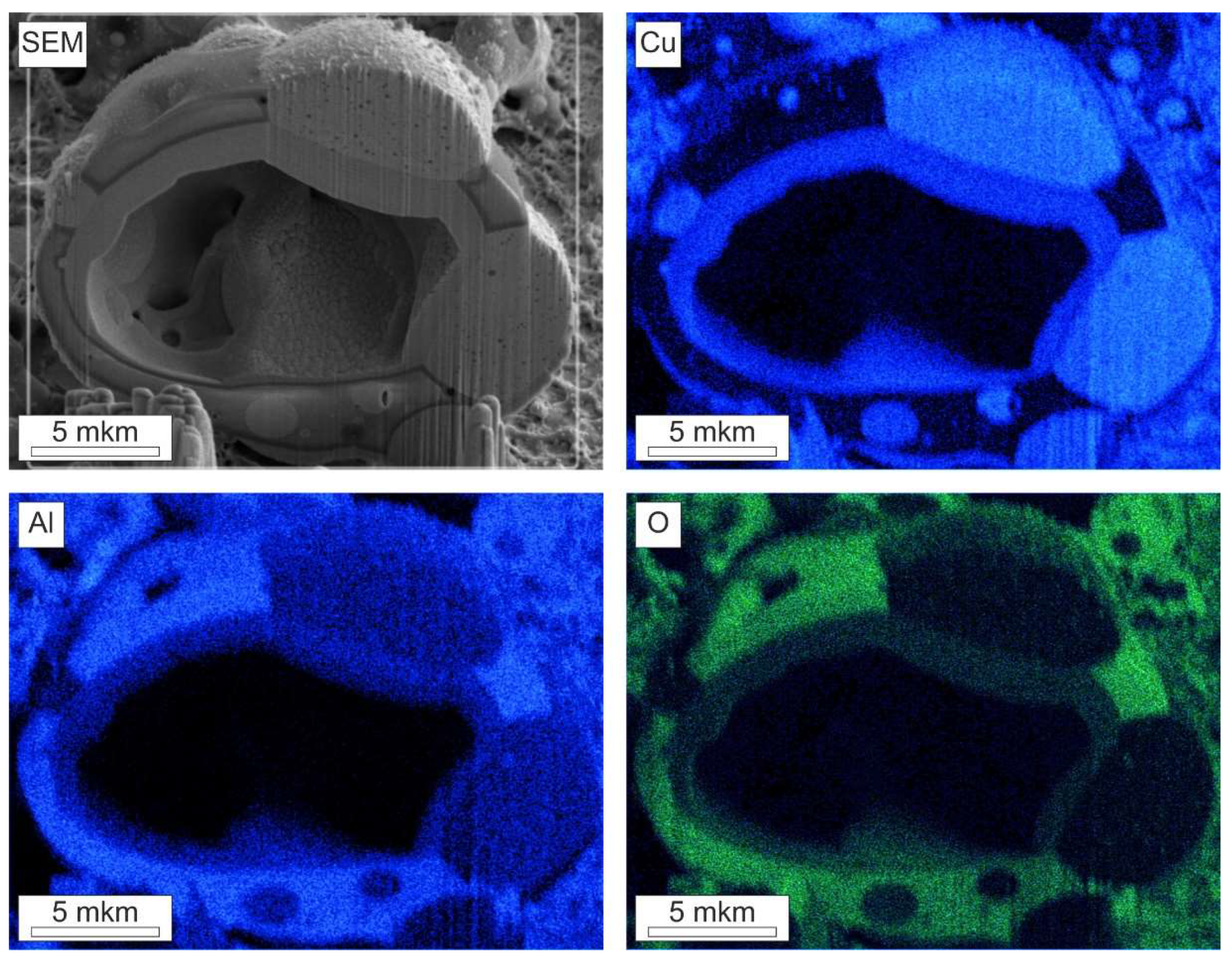
The composition of the combustion products was determined using energy-dispersive X-ray spectroscopy. Using a focused ion beam, cross-sections of the objects of study were prepared.
Figure 5 shows the distribution maps of elements on the surface of the combustion product of a 3-μm-thick Al-CuO multilayer structure. Similar results were obtained for the combustion products of 2- and 4-μm-thick multilayer structures.
For the presented results, the largest combustion product of a multilayer structure with a thickness of 3 μm was chosen as the object of study. The combustion product has a spherical shape with a cavity inside. The matrix of the structure consists of a chemical compound of aluminum and oxygen, since the distribution maps of these elements coincide. Drop-shaped inclusions consist of copper. The sizes of these inclusions are in a fairly large range - from hundreds of nanometers to units of micrometers. Some inclusions are on the surface of the matrix, and some are in its volume. In copper inclusions, one can see the presence of cavities in the form of bubbles with sizes from 10 to 200 nm in diameter. On the inner surface of the large cavity of the combustion product, one can observe the presence of a layered structure with a number of bilayers up to 10 pieces, which is obviously a partially unreacted original multilayer structure of Al-CuO. In this paper, the mechanism of formation of the internal cavity of the combustion products will not be discussed, since this requires additional research. However, it should also be noted that not all reaction products have a cavity inside. The combustion products visible in the background of the images have a similar distribution of elements over the surface.
Discussion
Generalization of the obtained experimental results allows us to make an assumption about the combustion modes of multilayer thermite materials formed on the surface of the substrate. The heat released as a result of the chemical reaction Q
r is dissipated by the atmosphere Q
atm and the substrate Q
wafer, and the rest of the energy is transferred to the unreacted region and is spent on initiating the chemical reaction in it (initiation energy Q
ini).
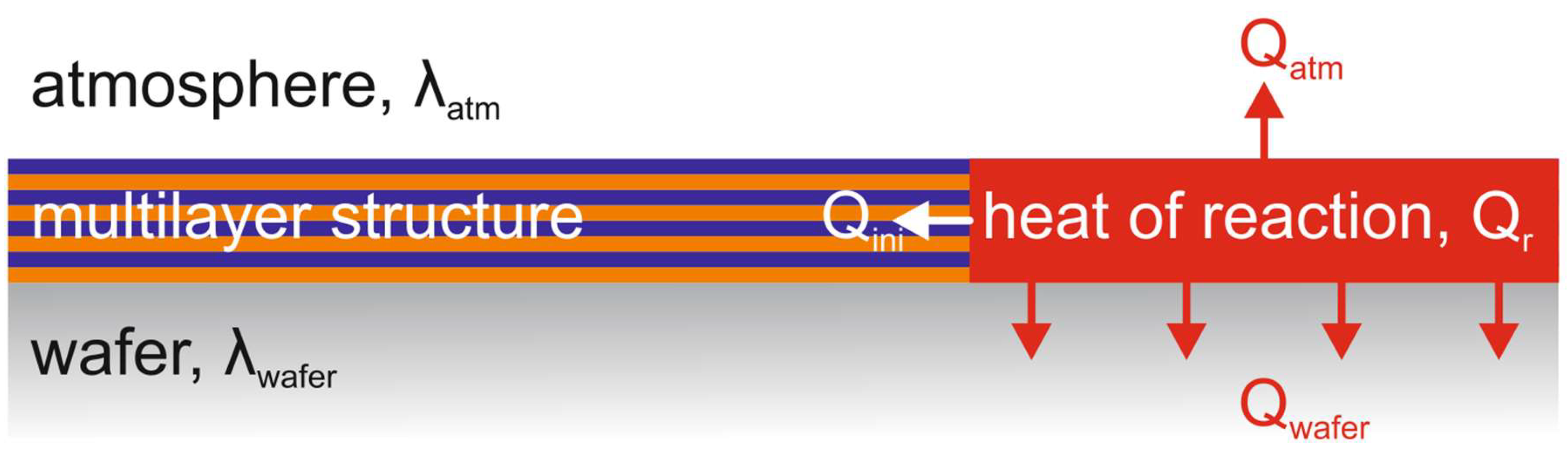
Figure 7 schematically shows the image of the heat balance in the "multilayer structure - substrate" system. In the framework of this work, no assessment of heat losses was made, however, for further discussions we will assume that the values of these quantities are constant and do not depend on the thickness of the multilayer structure.
Figure 6.
– Schematic representation of the heat balance in the “multilayer structure – substrate” system.
Figure 6.
– Schematic representation of the heat balance in the “multilayer structure – substrate” system.
The condition for the combustion front propagation after the reaction initiation can be considered the predominance of the released energy over the heat losses: Qr > Qatm + Qwafer + Qini. Depending on the total thickness of the multilayer structure, the absolute value of the released energy Qr will also change - more energy for thicker structures. At the same time, the share of heat losses to the atmosphere and the substrate will decrease with an increase in the total thickness of the multilayer structure.
The reason for the change in the combustion character of multilayer thermite structures with a change in their overall thickness may be associated with a change in the temperature in the reaction front due to the influence of the substrate and the atmosphere - the smaller the overall thickness, the lower the temperature of the front. As a result, the intensity of gas evolution, which largely determines the combustion mode and which depends on the aggregate state of the reagents and reaction products (the aggregate state of substances is determined by the temperature of the front), can vary and change in a wide range.
The maximum value of the combustion front temperature corresponds to the adiabatic reaction temperature. For the stoichiometric ratio of 2Al + 3CuO, the adiabatic temperature is 2828 K [
28]. In this work, aluminum-rich multilayer thermite structures were considered, for which the stoichiometric ratio corresponded to 5Al + 2CuO, and T
adiab = 2666 K, which was calculated using the THERMO software package. This value exceeds the melting temperatures of aluminum (T
melt_Al = 933 K), copper oxide (T
melt_CuO = 1720 K), copper (T
melt_Cu = 1358 K), aluminum oxide (T
melt_Al2O3 = 2317 K) and the evaporation temperature of copper oxide (T
vapor_CuO = 2273 K). In other words, both the initial reagents and the reaction products will be in the liquid phase as a result of the thermite reaction. The reason for gas generation during combustion of multilayer thermite materials is the evaporation of copper oxide or its decomposition (CuO → Cu
2O + O
2↑), which occurs at 1373 K and is energetically more favorable than other phenomena. The T
adiab temperature is lower than the boiling point of aluminum (2743 K), copper (2835 K) and aluminum oxide (3250 K), which excludes their contribution to gas evolution. The values of melting and evaporation temperatures, as well as latent heats of vaporization / decomposition at atmospheric pressure [
17] are presented in
Table 1.
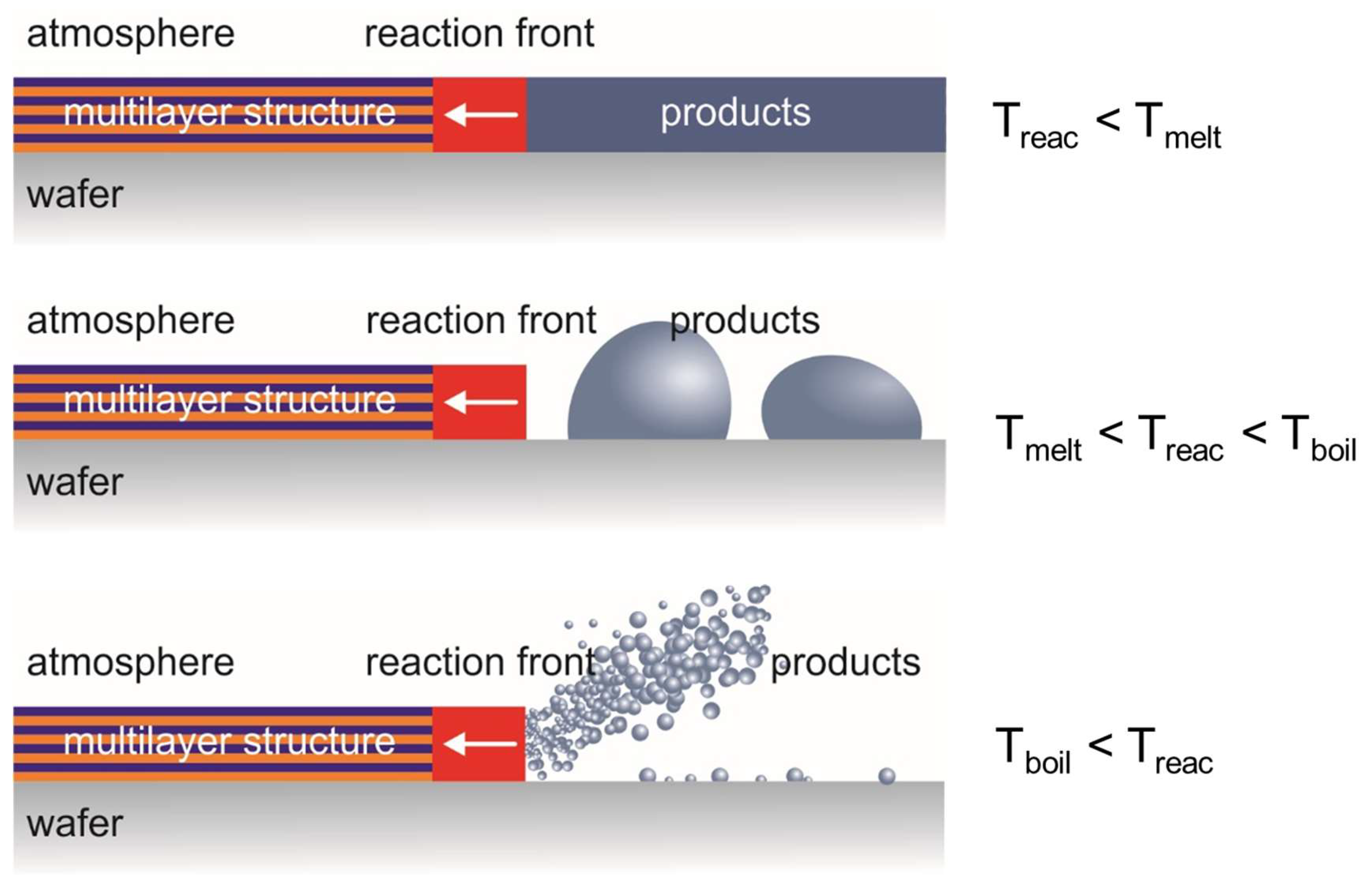
Summarizing the obtained experimental results of the study of the combustion features of multilayer thermite materials formed on the substrate surface, we can conditionally distinguish three combustion modes, which are schematically shown in
Figure 7. The implementation of one or another combustion mode will be determined by the front temperature (Treac), which in turn depends not only on the composition and stoichiometric ratio of the components of the multilayer structure, but also on its total thickness, as was shown experimentally in this work. The first combustion mode is realized when the reaction temperature does not exceed the melting temperatures of the reactants and reaction products - in this case, a solid-state reaction occurs and the combustion products will be in the form of a continuous film, like the original multilayer structure. In the second case, if the reaction temperature is in the range between Tmelt and Tboil of the reactants and products, then as a result of chemical interaction, liquid combustion products will be formed, which can collect in drops or structures of a more complex shape depending on the viscosity of the solution and the wettability of the substrate surface. Gas evolution in the first and second combustion modes is possible and can be caused by the decomposition of reagents or combustion products of the multilayer structure. Finally, in the third combustion mode, the reaction temperature exceeds the evaporation temperature of one or more reagents and combustion products, which can result in intense gas evolution, which leads to the fact that part of the combustion products can be removed from the surface of the substrate in the form of heated droplets (which was observed during high-speed video recording) or gas.
Figure 7.
- Schematic representation of combustion modes.
Figure 7.
- Schematic representation of combustion modes.
In practice, the implementation of only one combustion mode seems unlikely, since the melting and evaporation temperature values of reagents and combustion products of multilayer structures are in a fairly wide range. For example, for a pair of Al and CuO, the difference in aluminum melting and aluminum oxide evaporation temperatures is more than 2000 degrees, and as a result, a combination of two combustion modes is implemented in practice.
Based on the described combustion mechanisms, it is possible to explain the change in the morphology and size of the combustion products of the Al-CuO multilayer structures of different thicknesses considered in this work. The shape and composition of the combustion products indicate that the reaction temperature exceeded the melting point of Al2O3 (2317 K) for all considered total thicknesses. As shown in
Figure 4, an increase in the total thickness of the multilayer structure from 2 to 3 μm led to an increase in the characteristic sizes of the reaction products. This may be due to an increase in the combustion front temperature, which led to a decrease in the viscosity of copper and aluminum oxide melts, due to which larger objects were formed. On the other hand, an increase in the total thickness of the multilayer structure to 4 μm led to an even greater increase in the combustion front temperature, which increased the intensity of gas evolution as a result of evaporation and decomposition of copper oxide. This ultimately led to the fact that a significant part of the combustion products was removed from the substrate surface, and the remaining material formed smaller structures. As a result, for the total thicknesses of the multilayer structure of 2 and 3 μm, we can talk about the prevalence of the 2nd combustion mode, and for 4 μm, combustion was carried out mostly in the third mode. In this context, we can also justify the change in the combustion front propagation velocity with a change in the total thickness of the multilayer structure - an increase in the velocity with an increase in the total thickness of the structure from 2 to 3 μm is associated with an increase in the amount of energy released in the combustion front. On the other hand, with a further increase in the total thickness of the multilayer structure to 4 μm, an increase in the front velocity was not only not observed, but also an insignificant decrease from 11.0 to 10.7 m/s was recorded. This may be due to the fact that some of the thermal energy was removed from the front with heated reaction products.
Conclusions
The combustion features of multilayer thermite structures Al-CuO with different total thickness, formed on the surface of the substrate, are studied. It is found that with a change in the total thickness of the structure, the combustion mode changes - the intensity of gas evolution and dispersion of heated combustion products increases. As a result of the study of the morphology and composition of the combustion products, it was found that for all thicknesses, the combustion products were formed in the form of drop-shaped structures of complex shape, consisting of a matrix based on aluminum oxide with copper inclusions. With a change in the total thickness of the multilayer structure, the characteristic size of the reaction products changes nonlinearly. Based on the experimental results obtained, three combustion modes of multilayer structures are proposed and substantiated from the point of view of the combustion front temperature.
Acknowledgments
Research carried out in the laboratory "Photonic Sensorics and Plasma Materials founded in the framework of the State Assignment No. FSMR-2024-0012.
References
- Xiao, F., Chen, C., & An, C. (2024). Fine-Tuning Combustion Behavior in Microscale Energetic Lines Fabricated by Direct Ink Writing Using Nano Thermite Microspheres for Applications in Pyro-MEMS. ACS Applied Nano Materials, 7(16), 19514–19526. [CrossRef]
- Shu, Y., Zhang, W., Fan, Z., Yue, S., Yin, B., Li, L. K. B., Liu, P., Ren, P., & Ao, W. (2024). Improving the combustion efficiency and agglomeration of aluminum-water propellants via n-Al/CuO metastable intermolecular composites. Combustion and Flame, 260, 113246. [CrossRef]
- Bao, F., Yamashita, S., Daki, H., Nakagawa, K., & Kita, H. (2024). Microstructure modification of alumina prepared by water-stabilized plasma spraying method using Al-Cu-O reaction. Journal of the European Ceramic Society, 44(10), 6113–6123. [CrossRef]
- Li, F., Wang, Q., Cheng, J., Zhang, Z., Zhou, Y., Ouyang, K., Xu, J., Ye, Y., & Shen, R. (2024). Mitigating the negative catalytic effect of CuO by FAS-17 coated Al nanopowder: Isothermal ageing of Al/CuO nanothermite at 71 °C and 60% relative humidity. Defence Technology, 34, 156–167. [CrossRef]
- Xiong, K., Wang, Z., Liu, R., Nie, H., & Yan, Q.-L. (2023). Probing on Mutual Interaction Mechanisms of the Ingredients of Al/CuO/PVDF Nanocomposites. Langmuir, 39(39), 13850–13862. [CrossRef]
- Song, Z., Liu, W., Xian, M., & Jin, M. (2023). Facile Fabrication of Energetic Nanocomposite Materials by Polydopamine. International Journal of Molecular Sciences, 24(22), 16199. [CrossRef]
- Gandhi, P. M., Schoenitz, M., & Dreizin, E. L. (2023). Evaluation and design of metal-based gas-generating energetic materials. Combustion and Flame, 249, 112615. [CrossRef]
- Petrantoni, M., Rossi, C., Salvagnac, L., Conédéra, V., Estève, A., Tenailleau, C., Alphonse, P., & Chabal, Y. J. (2010). Multilayered Al/CuO thermite formation by reactive magnetron sputtering: Nano versus micro. Journal of Applied Physics, 108(8). [CrossRef]
- Dulmaa, A., & Depla, D. (2021). Influence of Impurities on the Front Velocity of Sputter Deposited Al/CuO Thermite Multilayers. Materials, 14(23), 7224. [CrossRef]
- Mao, D., Ma, X., Xie, Y., Meng, X., Wang, N., Zhang, Z., Sun, X., & Huang, Y. (2024). In-situ solid-state deformation-driven rapid reaction towards higher strength-ductility Al-CuO composites. Composites Part A: Applied Science and Manufacturing, 182, 108174. [CrossRef]
- Kim, H. S., & Kim, S. H. (2023). Additive Manufacturing and Combustion Characteristics of Polyethylene Oxide/Aluminum/Copper Oxide-Based Energetic Nanocomposites for Enhancing the Propulsion of Small Projectiles. Nanomaterials, 13(6), 1052. [CrossRef]
- Dombroski, D. M. B., Wang, A., Wen, J. Z., & Alfano, M. (2022). Joining and welding with a nanothermite and exothermic bonding using reactive multi-nanolayers – A review. Journal of Manufacturing Processes, 75, 280–300. [CrossRef]
- Zhou, Y., Wang, Y., Zhang, Z., Li, F., Cheng, J., Ye, Y., Xu, J., & Shen, R. (2023). Low air pressure self-sustaining combustion performances of 3D direct writing Al/CuO film. Chemical Engineering Journal, 473, 145031. [CrossRef]
- Tichtchenko, E., Folliet, V., Simonin, O., Bédat, B., Glavier, L., Esteve, A., & Rossi, C. (2023). Combustion model for thermite materials integrating explicit and coupled treatment of condensed and gas phase kinetics. Proceedings of the Combustion Institute, 39(3), 3637–3645. [CrossRef]
- Rogachev, A. S., Vadchenko, S. G., Baras, F., Politano, O., Rouvimov, S., Sachkova, N. V., Grapes, M. D., Weihs, T. P., & Mukasyan, A. S. (2016). Combustion in reactive multilayer Ni/Al nanofoils: Experiments and molecular dynamic simulation. Combustion and Flame, 166, 158–169. [CrossRef]
- Politano, O., Rogachev, A. S., & Baras, F. (2021). Molecular Dynamics Studies in Nanojoining: Self-Propagating Reaction in Ni/Al Nanocomposites. Journal of Materials Engineering and Performance, 30(5), 3160–3166. [CrossRef]
- Baijot, V., Glavier, L., Ducéré, J., Djafari Rouhani, M., Rossi, C., & Estève, A. (2015). Modeling the Pressure Generation in Aluminum-Based Thermites. Propellants, Explosives, Pyrotechnics, 40(3), 402–412. Portico. [CrossRef]
- Wang, D., Dong, C., Yi, Z., Qi, X., Li, Y., Zhang, L., & Zhu, C. (2024). Preparation of Activated Carbon Nanostructured Al@CuO with Low Static Sensitivity and High Reactivity. ACS Omega, 9(14), 15854–15860. [CrossRef]
- Singh, V., Wu, T., Salvagnac, L., Estève, A., & Rossi, C. (2024). Ignition and Combustion Characteristics of Al/TiB2-Based Nanothermites: Effect of Bifuel Distribution. ACS Applied Nano Materials, 7(4), 3977–3987. [CrossRef]
- Rogachev, A. S. (2008). Exothermic reaction waves in multilayer nanofilms. Russian Chemical Reviews, 77(1), 21–37. [CrossRef]
- Jabraoui, H., Estève, A., Hong, S., & Rossi, C. (2023). Initial stage of titanium oxidation in Ti/CuO thermites: a molecular dynamics study using ReaxFF forcefields. Physical Chemistry Chemical Physics, 25(16), 11268–11277. [CrossRef]
- He, X., Feng, T., Cheng, Y., Hu, P., Le, Z., Liu, Z., & Cheng, Y. (2023). Fast formation of a black inner α-Al2O3 layer doped with CuO on Al–Cu–Li alloy by soft sparking PEO process. Journal of the American Ceramic Society, 106(11), 7019–7042. Portico. [CrossRef]
- Mikhailov, Yu. M., Aleshin, V. V., Vershinnikov, V. I., Ignat’eva, T. I., & Kovalev, D. Yu. (2021). Combustion Modes of Mixtures of Copper (II) Oxide with Aluminum and Titanium. Combustion, Explosion, and Shock Waves, 57(5), 570–575. [CrossRef]
- Li, H., Hu, C., Hu, J., Han, K., Wang, Z., Yang, R., & Liu, D. (2024). Underwater wet welding of high-strength low-alloy steel using self-shielded flux-cored wire with highly exothermic Al/CuO mixture. Journal of Materials Processing Technology, 328, 118404. [CrossRef]
- Li, C.-M., Wang, K.-B., Ji, X.-G., Dong, X.-F., & Wang, D. (2024). Effect of copper in the stabilization of Al/CuO energetic semiconductor bridge. Journal of Applied Physics, 135(18). [CrossRef]
- Wu, T., Hagen, E., Wang, H., Kline, D. J., Zachariah, M. R., & Rossi, C. (2024). Achieving superior ignition and combustion performance of Al/I2O5 biocidal nanoenergetic materials by CuO addition. Combustion and Flame, 259, 113190. [CrossRef]
- Lebedev, E. A., Rogachev, A. S., Vadchenko, S. G., Gromov, D. G., & Alymov, M. I. (2022). A “riding” combustion mode in CuO/Al reactive multilayer nano-foils. Applied Physics Letters, 121(13). [CrossRef]
- Stamatis, D., Jiang, X., Beloni, E., & Dreizin, E. L. (2010). Aluminum Burn Rate Modifiers Based on Reactive Nanocomposite Powders. Propellants, Explosives, Pyrotechnics, 35(3), 260–267. Portico. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).