Submitted:
20 January 2025
Posted:
20 January 2025
Read the latest preprint version here
Abstract
Keywords:
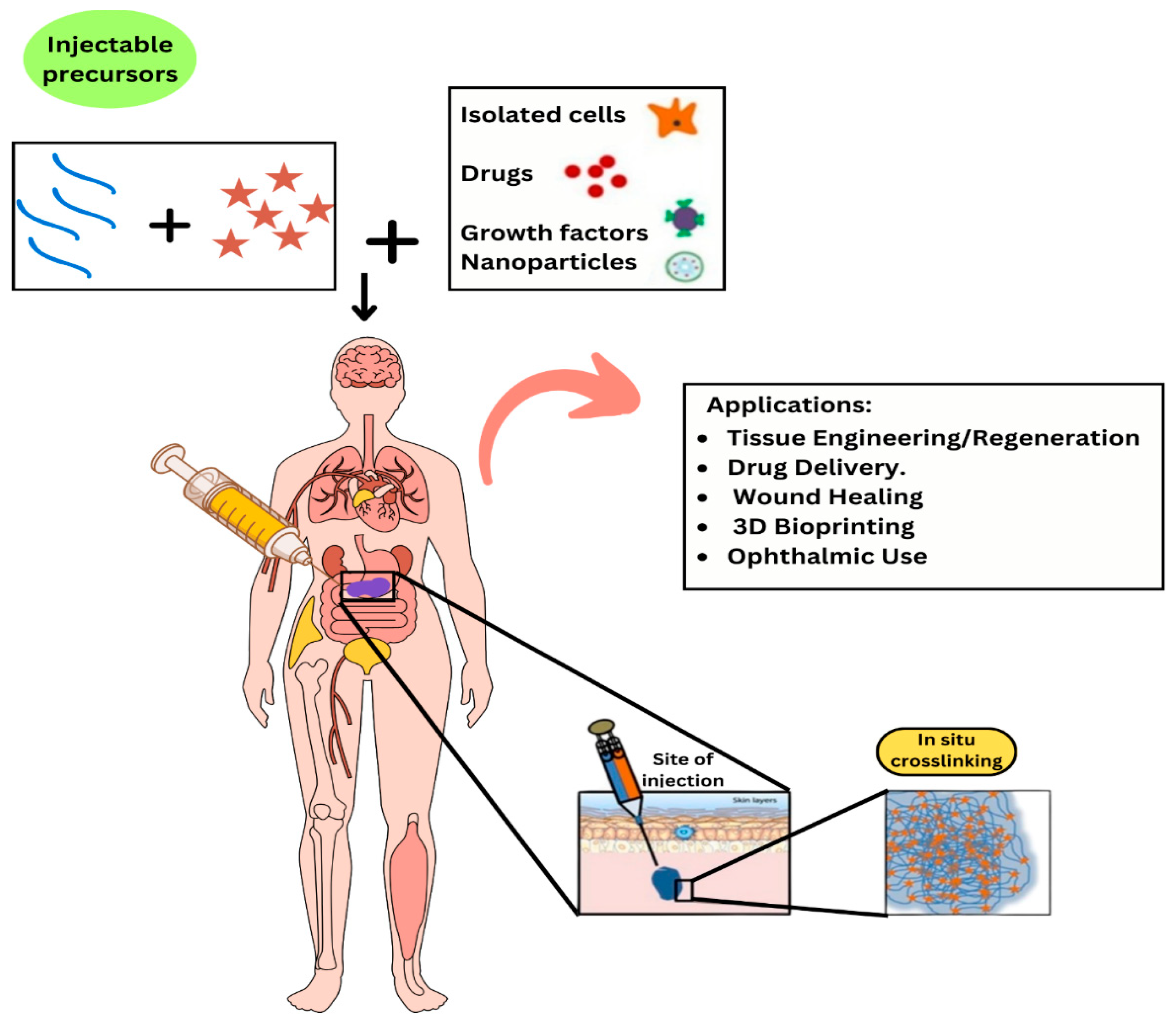
1. Introduction
2. Material Properties of the Injectable Hydrogels
2.1. Specific Natural Polymer Types
2.2. Synthetic Hydrogels
2.3. Hybrid Hydrogels
3. Desired Characteristics for Tissue Engineering
3.1. Mechanical Strength
3.2. Biocompatibility
3.3. Degradation Profiles
3.4. Injectability
4. Delivery Strategies for Enhanced Tissue Regeneration
4.1. Delivery Strategies Overview
4.1.1. Cell Encapsulation
4.1.2. Controlled Release of Growth Factors
4.1.3. Incorporation of Mineralizing Agents
4.1.4. Drug Delivery for Infection Control
5. Major Applications of Injectable Hydrogels
5.1. Tissue Engineering and Regeneration
| Classification | Material | Advantages | Disadvantages | Ref. |
| Natural | Collagen | Biocompatible, biodegradable, promotes cell adhesion, intrinsic RGD sequences, readily available | Weak mechanical properties, rapid degradation, batch-to-batch variability, potential immunogenicity | [91,92] |
| Hyaluronic Acid (HA) | Biocompatible, biodegradable, promotes cell proliferation and migration, natural component of cartilage | Poor mechanical properties, rapid degradation, difficult to crosslink stably, limited bioactivity without modification | [93,94] | |
| Agarose | Inert, easily processable, good diffusion properties, low cost | Weak mechanical properties, lacks cell adhesion sites, requires chemical modification for bioactivity | [95] | |
| Alginate | Biocompatible, relatively easy to process, mild gelation conditions, good diffusion properties | Weak mechanical properties, limited cell adhesion, prone to degradation, susceptible to bacterial degradation | [96] | |
| Chitosan | Biocompatible, biodegradable, promotes cell adhesion, antibacterial properties, natural component of ECM | Variable degradation rate, limited mechanical strength, difficulty in achieving uniform pore size | [97] | |
| Fibrin | Biocompatible, biodegradable, promotes cell adhesion, contains cell adhesion sites, fast gelation | Weak mechanical properties, rapid degradation, can shrink during culture, poor long-term stability | [98] | |
| Synthetic | Polyethylene Glycol (PEG) | Biocompatible, tunable degradation, easy to modify chemically, precisely controlled crosslinking | Inert biologically, limited cell adhesion without modification, potential for non-specific protein binding | [99] |
| Poly (vinyl alcohol) (PVA) | Good mechanical properties, non-toxic, relatively low cost, easy to process | Limited biodegradability, lacks cell adhesion sites, often requires physical crosslinking, swelling | [100] | |
| Poly (caprolactone) (PCL) based | Biodegradable, tunable degradation rates, good mechanical properties, can be fabricated into various geometries | Hydrophobic, slow degradation, limited cell adhesion, requires organic solvents for processing | [101] | |
| Poly (methacrylic acid) (PMAA) | High water content, pH sensitive, tunable mechanical properties | Can be cytotoxic at high concentrations, lacks cell adhesion sites, requires crosslinking | [102] | |
| Poly(N-isopropylacrylamide) (PNIPAAm) | Thermo-responsive, tunable swelling properties, cell sheet harvest capability | Limited mechanical properties, lack of cell adhesion without modification, non-biodegradable | [103] |
5.2. Drug Delivery Systems
5.3. Wound Healing Applications
5.5. Ophthalmic Applications
6. Limitations and Future Directions
7. Conclusion
Funding
Acknowledgments
Declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Abbreviations
| MSDs | Musculoskeletal diseases |
| ECM | Extracellular matrix |
| PEG | Poly(ethylene glycol) |
| PVA | Poly(vinyl alcohol) |
| TGF-β | Transforming growth factor beta |
| BMPs | Bone morphogenetic proteins |
| BTE | Bone tissue engineering |
| NFC | Nanofibrillated cellulose |
| SF | Silk fibroin |
| OA | Oxidized alginate |
| HA | Hyaluronic acid |
| GE | Gelatin-ECM |
| GelMA | Gelatin methacryloyl |
| PFS | Peptide sequence |
| SEM | Scanning electron microscopy |
| BMSCs | Bone marrow-derived mesenchymal stem cells |
| CECM | Cell-derived extracellular matrix |
| DBM | Decellurized Bone Matri |
| PLGA | Poly(lactic-co-glycolic acid) |
| nHAp | nanohydroxyapatite |
| bFGF | basic fibroblast growth factor |
| DFO | Deferoxamine |
| ZIF-8 | Zeolitic imidazolate framework-8 |
| PDGF-BB | Platelet-derived growth factor BB |
| PAG/AG | Poly(acrylic acid-co-acrylamide)/agarose |
References
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sànchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56 Suppl 2, S243–255. [Google Scholar] [CrossRef]
- Musculoskeletal health. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on Jan 2, 2025).
- Focsa, M.A.; Florescu, S.; Gogulescu, A. Emerging Strategies in Cartilage Repair and Joint Preservation. Medicina 2025, 61, 24. [Google Scholar] [CrossRef] [PubMed]
- Solovev, I.; Sergeeva, A.; Geraskina, A.; Shaposhnikov, M.; Vedunova, M.; Borysova, O.; Moskalev, A. Aging and physiological barriers: mechanisms of barrier integrity changes and implications for age-related diseases. Mol Biol Rep 2024, 51, 917. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manshaii, F.; Chen, J.; Wang, X.; Wang, S.; Yin, J.; Yang, M.; Chen, X.; Yin, X.; Zhou, Y. Unleashing the Potential of Electroactive Hybrid Biomaterials and Self-Powered Systems for Bone Therapeutics. Nano-Micro Lett. 2024, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Lin, X.; Xu, R.; Liu, L.; Zhang, Y.; Tian, F.; Li, J.J.; Xue, J. Advances in the Development of Gradient Scaffolds Made of Nano-Micromaterials for Musculoskeletal Tissue Regeneration. Nano-Micro Lett. 2024, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Damme, L.V.; Blondeel, P.; Vlierberghe, S.V. Injectable biomaterials as minimal invasive strategy towards soft tissue regeneration—an overview. J. Phys. Mater. 2021, 4, 022001. [Google Scholar] [CrossRef]
- Hou, M.; Wang, X.; Yue, O.; Zheng, M.; Zhang, H.; Liu, X. Development of a multifunctional injectable temperature-sensitive gelatin-based adhesive double-network hydrogel. Biomaterials Advances 2022, 134, 112556. [Google Scholar] [CrossRef]
- Karami, P.; Laurent, A.; Philippe, V.; Applegate, L.A.; Pioletti, D.P.; Martin, R. Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels. International Journal of Molecular Sciences 2024, 25, 9984. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. International Journal of Molecular Sciences 2021, 22, 1128. [Google Scholar] [CrossRef]
- Alkhursani, S.A.; Ghobashy, M.M.; Al-Gahtany, S.A.; Meganid, A.S.; Abd El-Halim, S.M.; Ahmad, Z.; Khan, F.S.; Atia, G.A.N.; Cavalu, S. Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers 2022, 14, 3791. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Aslam, M.A.; Bin Abdullah, M.F.; Stojanović, G.M. Current Perspectives of Protein in Bone Tissue Engineering: Bone Structure, Ideal Scaffolds, Fabrication Techniques, Applications, Scopes, and Future Advances. ACS Appl. Bio Mater. 2024, 7, 5082–5106. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Materials Science and Engineering: C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Mredha, Md.T.I.; Jeon, I. Biomimetic anisotropic hydrogels: Advanced fabrication strategies, extraordinary functionalities, and broad applications. Progress in Materials Science 2022, 124, 100870. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12. [Google Scholar] [CrossRef]
- Gholap, A.D.; Rojekar, S.; Kapare, H.S.; Vishwakarma, N.; Raikwar, S.; Garkal, A.; Mehta, T.A.; Jadhav, H.; Prajapati, M.K.; Annapure, U. Chitosan scaffolds: Expanding horizons in biomedical applications. Carbohydrate Polymers 2024, 323, 121394. [Google Scholar] [CrossRef]
- Baniasadi, H.; Abidnejad, R.; Fazeli, M.; Lipponen, J.; Niskanen, J.; Kontturi, E.; Seppälä, J.; Rojas, O.J. Innovations in hydrogel-based manufacturing: A comprehensive review of direct ink writing technique for biomedical applications. Advances in Colloid and Interface Science 2024, 324, 103095. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Wu, Y.; Xu, L. Recent advancements in hydrogels as novel tissue engineering scaffolds for dental pulp regeneration. International Journal of Biological Macromolecules 2024, 264, 130708. [Google Scholar] [CrossRef]
- Yao, M.-X.; Zhang, Y.-F.; Liu, W.; Wang, H.-C.; Ren, C.; Zhang, Y.-Q.; Shi, T.-L.; Chen, W. Cartilage tissue healing and regeneration based on biocompatible materials: a systematic review and bibliometric analysis from 1993 to 2022. Front. Pharmacol. 2024, 14. [Google Scholar] [CrossRef]
- Yang, X.; Huang, C.; Wang, H.; Yang, K.; Huang, M.; Zhang, W.; Yu, Q.; Wang, H.; Zhang, L.; Zhao, Y.; et al. Multifunctional Nanoparticle-Loaded Injectable Alginate Hydrogels with Deep Tumor Penetration for Enhanced Chemo-Immunotherapy of Cancer. ACS Nano 2024, 18, 18604–18621. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Cui, J.; Zhu, Y.; Sun, M.; Hamley, I.W.; Xiao, C.; Chen, L. An injectable antibacterial hydrogel with bacterial-targeting properties for subcutaneous suppuration treatment. Chemical Engineering Journal 2024, 488, 151137. [Google Scholar] [CrossRef]
- Li, A.; Ma, B.; Hua, S.; Ping, R.; Ding, L.; Tian, B.; Zhang, X. Chitosan-based injectable hydrogel with multifunction for wound healing: A critical review. Carbohydrate Polymers 2024, 333, 121952. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Xu, Y.; Fan, C.; Li, Z.A.; Zheng, L.; Luo, B.; Li, Z.-P.; Lin, B.; Zha, Z.-G.; et al. Collagen fibril-like injectable hydrogels from self-assembled nanoparticles for promoting wound healing. Bioactive Materials 2024, 32, 149–163. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Wei, H.; Yu, C.-Y. Injectable hydrogels as emerging drug-delivery platforms for tumor therapy. Biomater. Sci. 2024, 12, 1151–1170. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Sun, Y.; Wang, M.; Liu, H.; Zhang, W. Endogenous Tissue Engineering for Chondral and Osteochondral Regeneration: Strategies and Mechanisms. ACS Biomater. Sci. Eng. 2024, 10, 4716–4739. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Paiva-Santos, A.C.; Sarwar, H.S.; Jamshaid, M. A Comprehensive Review of Hydrogel-Based Drug Delivery Systems: Classification, Properties, Recent Trends, and Applications. AAPS PharmSciTech 2024, 25, 64. [Google Scholar] [CrossRef]
- Mohammadi, A.T.; Taheri, S.A. mohammad; Karamouz, M.; Sarhaddi, R. Rising Innovations: Revolutionary Medical and Dental Breakthroughs Revolutionizing the Healthcare Field; Nobel Sciences, 2024.
- García-Fernández, L.; Olmeda-Lozano, M.; Benito-Garzón, L.; Pérez-Caballer, A.; San Román, J.; Vázquez-Lasa, B. Injectable hydrogel-based drug delivery system for cartilage regeneration. Materials Science and Engineering: C 2020, 110, 110702. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Maji, S.; Lee, H. Engineering Hydrogels for the Development of Three-Dimensional In Vitro Models. International Journal of Molecular Sciences 2022, 23, 2662. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Advanced Drug Delivery Reviews 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Zhang, J.; Luo, H. Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polymer Reviews 2023, 63, 574–612. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Advanced Materials 2021, 33, 2006362. [Google Scholar] [CrossRef]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Advanced Healthcare Materials 2021, 10, 2001341. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Sig Transduct Target Ther 2021, 6, 1–31. [Google Scholar] [CrossRef]
- Correia, C.R.; Moreira-Teixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Chitosan Scaffolds Containing Hyaluronic Acid for Cartilage Tissue Engineering. Tissue Engineering Part C: Methods 2011, 17, 717–730. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Fan, D.; Liu, Y.; Wang, Y.; Wang, Q.; Guo, H.; Cai, Y.; Song, R.; Wang, X.; Wang, W. 3D printing of bone and cartilage with polymer materials. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front Bioeng Biotechnol 2021, 9, 603444. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Laurent, A.; Philippe, V.; Applegate, L.A.; Pioletti, D.P.; Martin, R. Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels. International Journal of Molecular Sciences 2024, 25, 9984. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact Mater 2020, 6, 998–1011. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite design of injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 2017, 5, 1–20. [Google Scholar] [CrossRef]
- Liu, X.; Sun, S.; Wang, N.; Kang, R.; Xie, L.; Liu, X. Therapeutic application of hydrogels for bone-related diseases. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- De Giorgio, G.; Matera, B.; Vurro, D.; Manfredi, E.; Galstyan, V.; Tarabella, G.; Ghezzi, B.; D’Angelo, P. Silk Fibroin Materials: Biomedical Applications and Perspectives. Bioengineering 2024, 11, 167. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioactive Materials 2023, 20, 137–163. [Google Scholar] [CrossRef]
- Deng, J.; Song, Q.; Liu, S.; Pei, W.; Wang, P.; Zheng, L.; Huang, C.; Ma, M.; Jiang, Q.; Zhang, K. Advanced applications of cellulose-based composites in fighting bone diseases. Composites Part B: Engineering 2022, 245, 110221. [Google Scholar] [CrossRef]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioactive Materials 2021, 6, 666–683. [Google Scholar] [CrossRef]
- Kazemian, A. Evaluation of the effectiveness of 3D bone matrices osteoinduction by using in vitro and in vivo models. thesis, University of Leicester, 2024.
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Annals of Joint 2016, 1. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front Bioeng Biotechnol 2020, 8, 474. [Google Scholar] [CrossRef]
- Huang, B.; Li, P.; Chen, M.; Peng, L.; Luo, X.; Tian, G.; Wang, H.; Wu, L.; Tian, Q.; Li, H.; et al. Hydrogel composite scaffolds achieve recruitment and chondrogenesis in cartilage tissue engineering applications. J Nanobiotechnol 2022, 20, 1–17. [Google Scholar] [CrossRef]
- Fu, L.; Li, L.; Bian, Q.; Xue, B.; Jin, J.; Li, J.; Cao, Y.; Jiang, Q.; Li, H. Cartilage-like protein hydrogels engineered via entanglement. Nature 2023, 618, 740–747. [Google Scholar] [CrossRef]
- Shan, B.; Wu, F. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Advanced Materials 2024, 36, 2210707. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater Res 2018, 22, 27. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat Commun 2019, 10, 3523. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 2017, 5, 17014. [Google Scholar] [CrossRef]
- Utech, S.; Boccaccini, A.R. A review of hydrogel-based composites for biomedical applications: enhancement of hydrogel properties by addition of rigid inorganic fillers. J Mater Sci 2016, 51, 271–310. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Kong, M.; Li, Z.; Yang, T.; Wang, Q.; Teng, W. Self-healing hybrid hydrogels with sustained bioactive components release for guided bone regeneration. Journal of Nanobiotechnology 2023, 21, 62. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yang, J.; Lin, J.; Zhu, Y.; Xu, X.; Cui, W. Living and Injectable Porous Hydrogel Microsphere with Paracrine Activity for Cartilage Regeneration. Small 2023, 19, 2207211. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat Commun 2023, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Li, D.; Zhu, Y.; Wu, P.; Chen, Z.; Chen, F.; Chen, Y.; Deng, Z.; Cai, L. Smart-Responsive Multifunctional Therapeutic System for Improved Regenerative Microenvironment and Accelerated Bone Regeneration via Mild Photothermal Therapy. Advanced Science 2024, 11, 2304641. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Huebsch, N.; Lippens, E.; Lee, K.; Mehta, M.; Koshy, S.T.; Darnell, M.C.; Desai, R.M.; Madl, C.M.; Xu, M.; Zhao, X.; et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nature Mater 2015, 14, 1269–1277. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv Funct Materials 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnology Advances 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res 2022, 10, 17. [Google Scholar] [CrossRef]
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart Polymeric Hydrogels for Cartilage Tissue Engineering: A Review on the Chemistry and Biological Functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioengineering & Transla Med 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Wang, M.O.; Vorwald, C.E.; Dreher, M.L.; Mott, E.J.; Cheng, M.; Cinar, A.; Mehdizadeh, H.; Somo, S.; Dean, D.; Brey, E.M.; et al. Evaluating 3D-Printed Biomaterials as Scaffolds for Vascularized Bone Tissue Engineering. Advanced Materials 2015, 27, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Engineering Part B: Reviews 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Wang, H.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Advanced Materials 2015, 27, 3717–3736. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int J Oral Sci 2020, 12, 6. [Google Scholar] [CrossRef]
- Hwang, C.M.; Sant, S.; Masaeli, M.; Kachouie, N.N.; Zamanian, B.; Lee, S.-H.; Khademhosseini, A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2010, 2, 035003. [Google Scholar] [CrossRef]
- Shan, B.; Wu, F. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Advanced Materials 2024, 36, 2210707. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. IJMS 2023, 24, 1291. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Z.; Liu, W.; Piao, M.; Li, J.; Zhang, H. Controlled Release of Growth Factor from Heparin Embedded Poly(aldehyde guluronate) Hydrogels and Its Effect on Vascularization. Gels 2023, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Berry, D.; Moran, A.; He, F.; Tam, T.; Chen, L.; Chen, S. Controlled Growth Factor Release in 3D-Printed Hydrogels. Adv Healthc Mater 2020, 9, e1900977. [Google Scholar] [CrossRef] [PubMed]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Zhang, X.; Li, Y.; Zhang, S.; Yang, L.; Li, R.; Wan, Q.; Pei, X.; Chen, J.; et al. Drug-Delivery Nanoplatform with Synergistic Regulation of Angiogenesis–Osteogenesis Coupling for Promoting Vascularized Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 17543–17561. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, J.; Fu, Y.; Pan, H.; He, H.; Gan, Q.; Liu, C. Smart Biomaterials for Articular Cartilage Repair and Regeneration. Adv Funct Materials 2023, 33, 2212561. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Dong, J.; Li, D.; Dong, W.; Li, H.; Zhou, Y.; Liu, Q.; Deng, B. Mussel-Inspired Multifunctional Hydrogels with Adhesive, Self-Healing, Antioxidative, and Antibacterial Activity for Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 16515–16525. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: identification, retention and assessment of biological properties. Sig Transduct Target Ther 2021, 6, 1–28. [Google Scholar] [CrossRef]
- Mano, J. f; Silva, G. a; Azevedo, H. s; Malafaya, P. b; Sousa, R. a; Silva, S. s; Boesel, L. f; Oliveira, J. m; Santos, T. c; Marques, A. p; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. Journal of The Royal Society Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, L.; Zhang, X.; Heng, B.C.; Wang, D.-A.; Ge, Z. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioactive Materials 2021, 6, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydrate Polymers 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.; Karim, S.; Aslam, S.; Jahangeer, M.; Nelofer, R.; Nadeem, A.A.; Qamar, S.A.; Jesionowski, T.; Bilal, M. Alginate-Based Bio-Nanohybrids with Unique Properties for Biomedical Applications. Starch - Stärke 2024, 76, 2200100. [Google Scholar] [CrossRef]
- Kudiyarasu, S.; Karuppan Perumal, M.K.; Rajan Renuka, R.; Manickam Natrajan, P. Chitosan composite with mesenchymal stem cells: Properties, mechanism, and its application in bone regeneration. International Journal of Biological Macromolecules 2024, 275, 133502. [Google Scholar] [CrossRef]
- Li, S.; Dan, X.; Chen, H.; Li, T.; Liu, B.; Ju, Y.; Li, Y.; Lei, L.; Fan, X. Developing fibrin-based biomaterials/scaffolds in tissue engineering. Bioactive Materials 2024, 40, 597–623. [Google Scholar] [CrossRef]
- Hacker, M.C.; Nawaz, H.A. Multi-Functional Macromers for Hydrogel Design in Biomedical Engineering and Regenerative Medicine. International Journal of Molecular Sciences 2015, 16, 27677–27706. [Google Scholar] [CrossRef]
- Bercea, M. Recent Advances in Poly(vinyl alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. Journal of Biomaterials Science, Polymer Edition 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Luckanagul, J.A.; Alcantara, K.P.; Bulatao, B.P.I.; Wong, T.W.; Rojsitthisak, P.; Rojsitthisak, P. Kim, J.-C., Alle, M., Husen, A., Eds.; Thermo-Responsive Polymers and Their Application as Smart Biomaterials. In Smart Nanomaterials in Biomedical Applications; Springer International Publishing: Cham, 2021; ISBN 978-3-030-84262-8. [Google Scholar]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Dong, R. Physical dynamic double-network hydrogels as dressings to facilitate tissue repair. Nat Protoc 2023, 18, 3322–3354. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Advanced Science 2023, 10, 2303326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv Healthcare Materials 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Du, R.; Yang, Y.; Liu, Y.; Wan, Z.; Yang, X. Injectable Self-Healing Adhesive Natural Glycyrrhizic Acid Bioactive Hydrogel for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17562–17576. [Google Scholar] [CrossRef]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. JFB 2023, 14, 55. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirouz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable hydrogels for cartilage and bone tissue regeneration: A review. International Journal of Biological Macromolecules 2023, 246, 125674. [Google Scholar] [CrossRef]
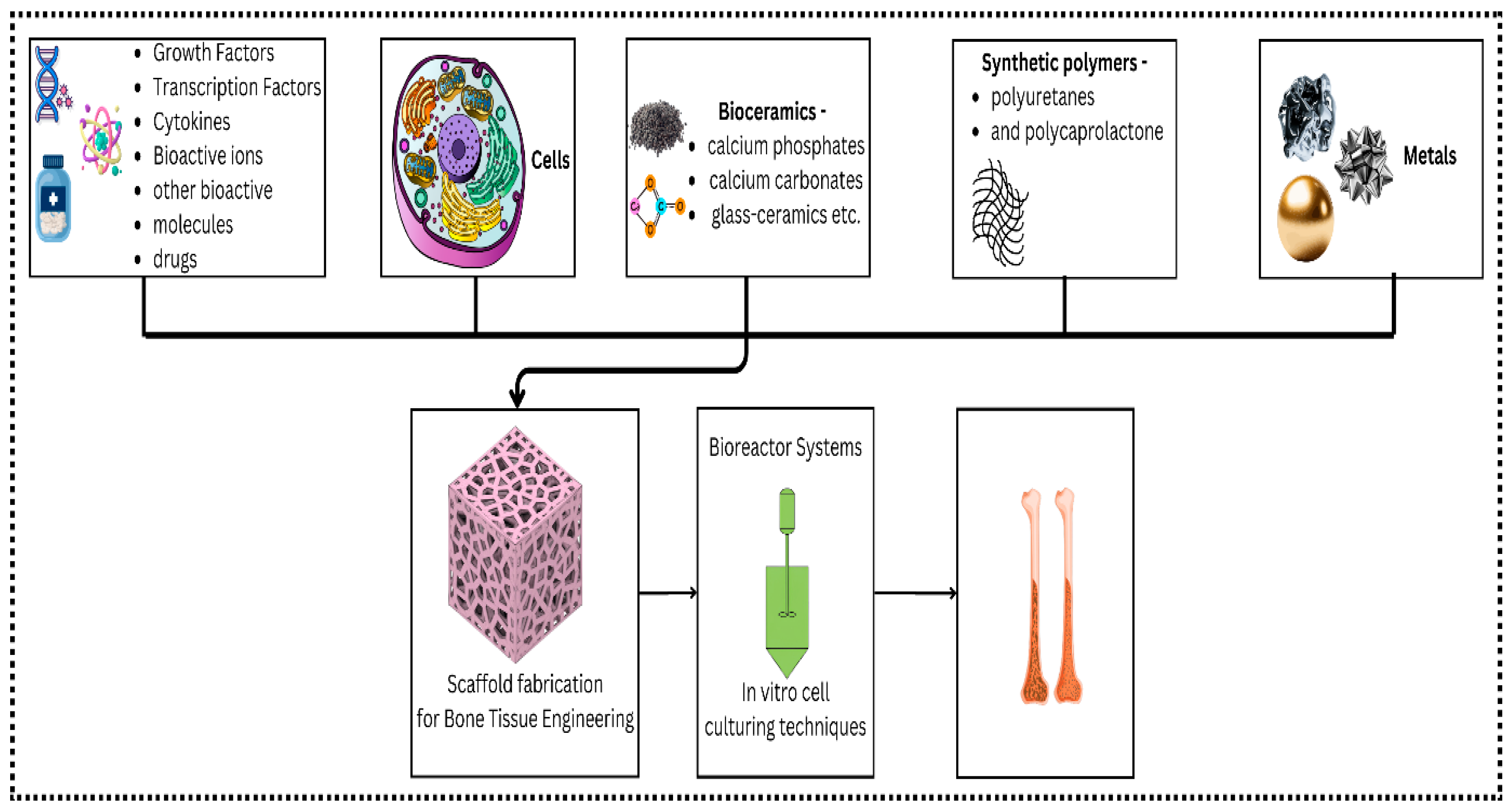
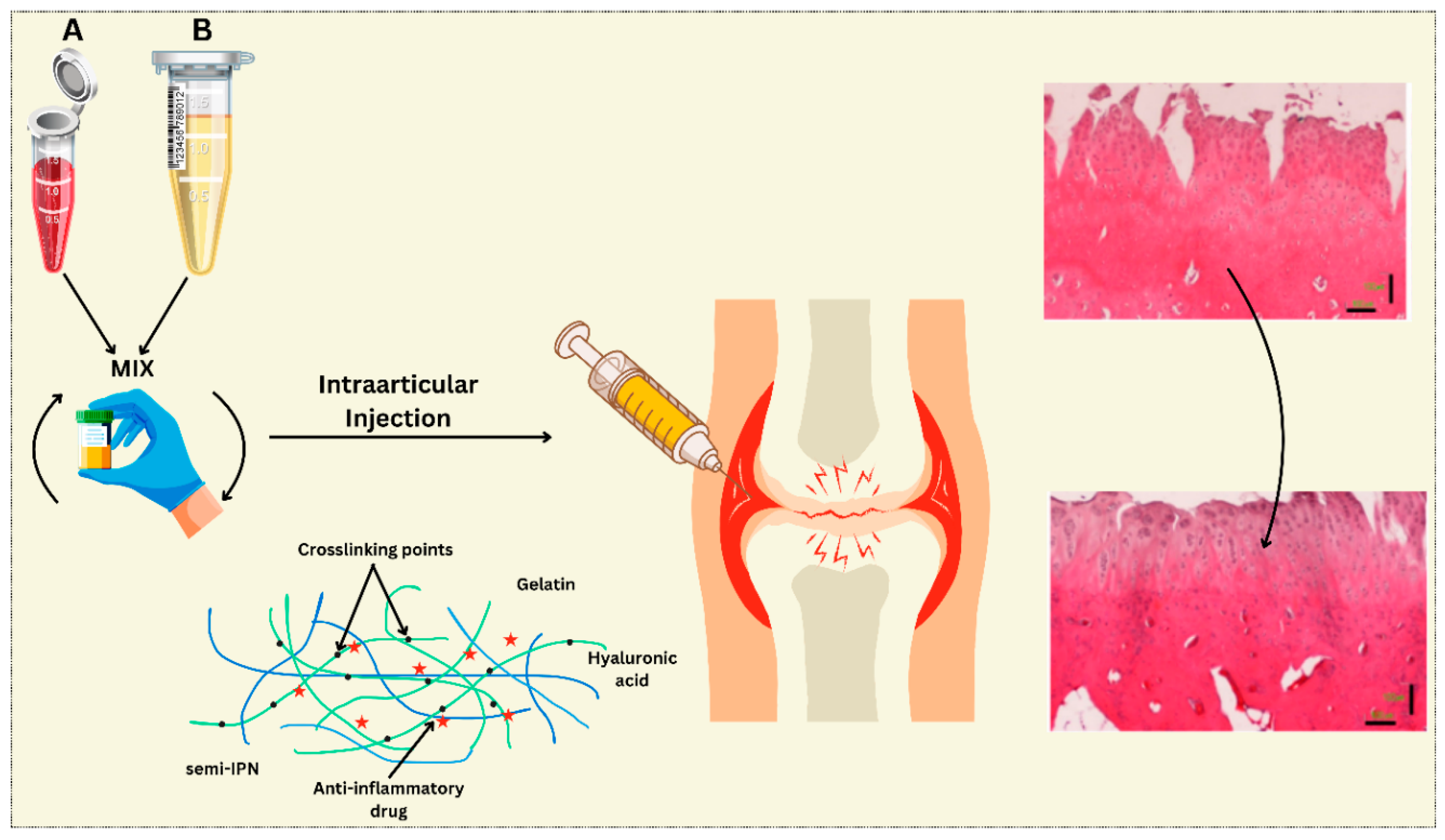
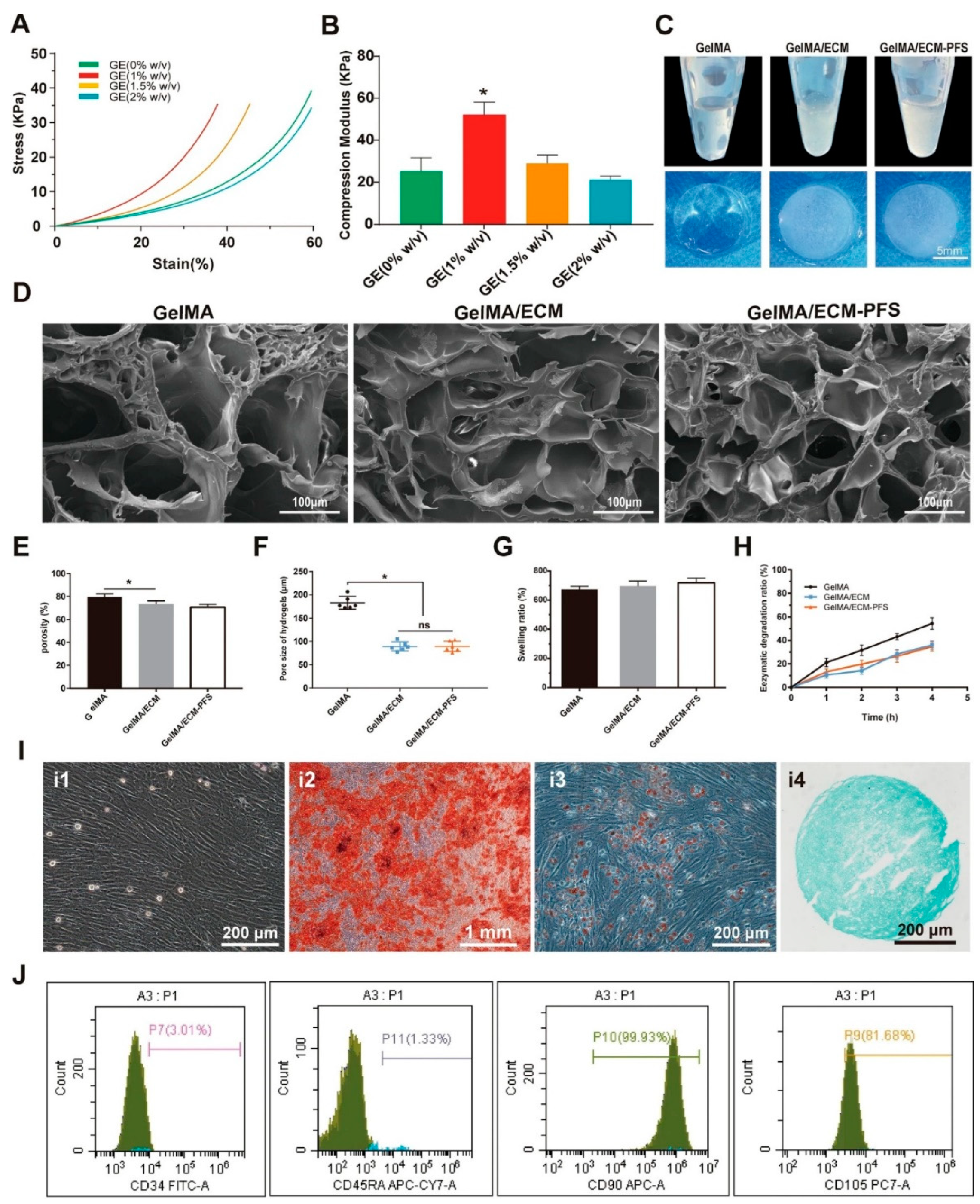
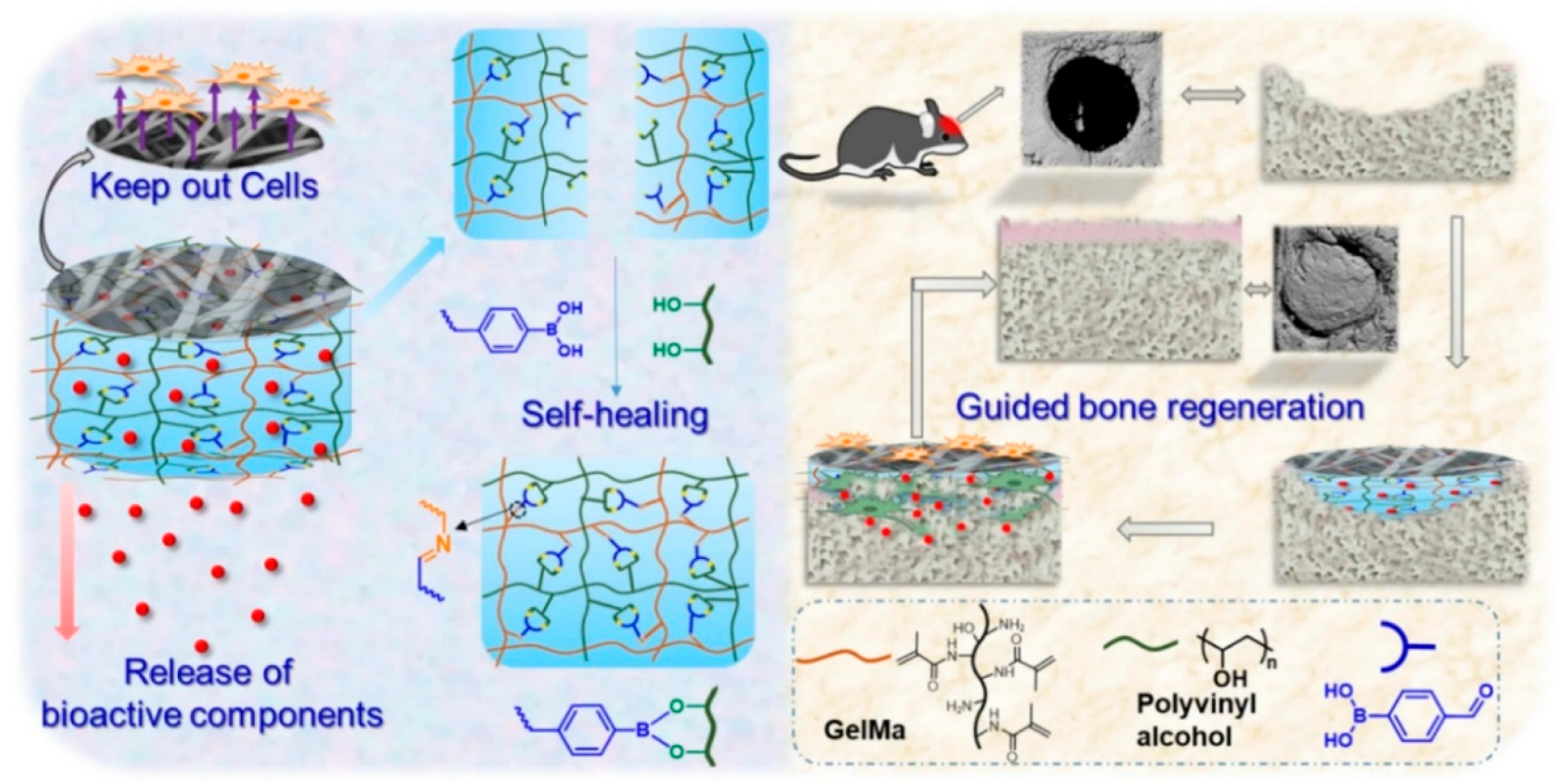
| Polymer Type | Source | Key Properties | Applications (Cartilage) | Applications (Bone) | Pros | Cons | Ref |
| Hyaluronic Acid (HA) | ECM of Cartilage, etc. | Hydrophilic, biocompatible, biodegradable, promotes cell migration & proliferation | Hydrogels, scaffolds, often combined with others | - | Excellent biocompatibility, promotes cell growth, readily available | Mechanically weak on its own, requires crosslinking or reinforcement | [40,41] |
| Chitosan | Crustacean shells, fungi | Positively charged, antibacterial, biodegradable, forms hydrogels/scaffolds | Scaffolds, combined with others | Scaffolds, bone graft substitutes | Antibacterial, promotes cell adhesion, readily available | Limited mechanical strength, variable quality, rapid degradation | [42,43] |
| Collagen | Connective tissues | Highly biocompatible, promotes cell adhesion & ECM deposition, biodegradable | Scaffolds, membranes, hydrogels | Scaffolds, membranes, coatings, combined with minerals | Naturally, present in tissue, promotes cell attachment, high biocompatibility | Variable mechanical strength, source-dependent properties, can degrade rapidly | [44,45,46] |
| Alginate | Brown seaweed | Forms hydrogels, biocompatible, readily available | Cell encapsulation, injectable hydrogels, scaffolds | - | Easily formed into hydrogels, versatile, biocompatible, low cost | Limited mechanical strength, can degrade rapidly, less supportive of cell attachment without modification | [44,47,48] |
| Fibrin | Blood plasma | Biodegradable matrix, promotes wound healing | Injectable hydrogels, tissue adhesives | - | Supports cell growth & proliferation, biodegradable, readily available | Degradation rate can be difficult to control, can be fragile | [45,46] |
| Silk Fibroin | Silkworm cocoons | Biocompatible, biodegradable, high mechanical strength | - | Porous scaffolds, films, fibers | Good mechanical strength, high biocompatibility, biodegradable, versatile | Slower degradation compared to some, requires processing | [49] |
| Cellulose | Plants | Biocompatible, readily available, porous structures | - | Scaffolds, reinforcement in composites | Abundant, low cost, good mechanical strength | Not inherently bioactive for bone, needs modifications | [50,51] |
| Decellularized Bone Matrix (DBM) | Natural Bone | Biocompatible, osteoconductive, retains some osteoinductive potential | - | Bone graft substitutes | Retains natural structure & signals, promotes regeneration | Specialized processing, potential for immune response | [52,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




