1. Introduction
Marine sponges (Porifera) have been a primary focus of research aimed at discovering biologically active secondary metabolites. The Red Sea sponges’ biodiversity is characterized by a wide variety of morphologies and secondary metabolites, some of which play critical roles in marine ecosystems, including symbiosis with microorganisms and the production of bioactive compounds with potential pharmaceutical applications. Marine sponges of the genus
Agelas (class Demospongiae, order Agelasida, family Agelasidae) are among the most common sponges found in tropical and subtropical regions worldwide, with 36 valid species currently recognized. The understanding of these species continues to grow. The secondary metabolites isolated from
Agelas sponges, since their initial discovery, represent a fascinating area of research that has driven significant advancements in the field of marine natural products [
1]. Over five decades (1971-2021), more than 355 compounds have been reported from several members of the genus
Agelas [
1]. The most producres of the compounds are
A. oroides (15%),
A. nakamurai (13%) and
A. mauritiana (11%), while the rest was reported from unclassified
Agelas species [
1].
Members of the genus
Agelas exhibit notable structural diversity in their pyrrole and terpenoidal alkaloids [
1]. Following the isolation of specific bromopyrrole derivatives from
Agelas oroides in 1971 [
2] and the identification of agelasine, a quaternary 9-methyladenine derivative of an unidentified diterpene from
Agelas dispar in 1975 [
3], beside to pyrrole and terpenoidal alkaloid, s numerous bioactive metabolites of varying biogenetic origins such as glycosphingolipids, sterols, caretonoids and many others have been discovered within genus
Agelas [
1,
4,
5,
6,
7,
8]. Pyrrole alkaloids of this genus typically possess a backbone consisting of a bromo- or debromo-pyrrole-2-carboxamide structure, which is associated with various side chains and cyclic formations [
9,
10,
11]. In contrast, the less common diterpene alkaloids primarily include those containing a 9-N-methyladeninium group (such as agelines, agelasines, and nemoechines) [
12,
13,
14], as well as diterpenes related to hypotaurocyamine (for example, agelasidines) [
15]. Secondary metabolites of the genus
Agelas show a wide range of biological activities, including antimicrobial [
16,
17], antihistaminic [
18], antimalarial [
19], antileukemic [
20], cytotoxic [
17,
21], antifouling [
21,
22], Na
+,K
+-adenosine triphosphatase (ATPase) inhibitory effects [
12,
15] and antiangiogenic matrixmetalloproteinase inhibitory effect [
23]. In our continuous effort to identify bioactive compounds from Red Sea marine sponges [
24,
25], we investigated the sponge
Agelas sp. aff. marmarica. Bioassay-guided partition of the antimicrobial fraction of the organic extract of the sponge and final HPLC purification afforded three new brominated pyrrole-derived alkaloids, marmaricines A-C. The current study describes the isolation, structural elucidation, and antimicrobial activities of these compounds.
2. Results and Discussion
2.1. Purification of Compounds 1-3.
Fractionation of the antimicrobial fraction of the organic extract of the Red Sea sponge
Agelas sp. aff. Marmarica on normal silica gel, Sephadex LH 20, and purification of active fraction on HPLC afforded compounds
1-
3 (
Figure 1).
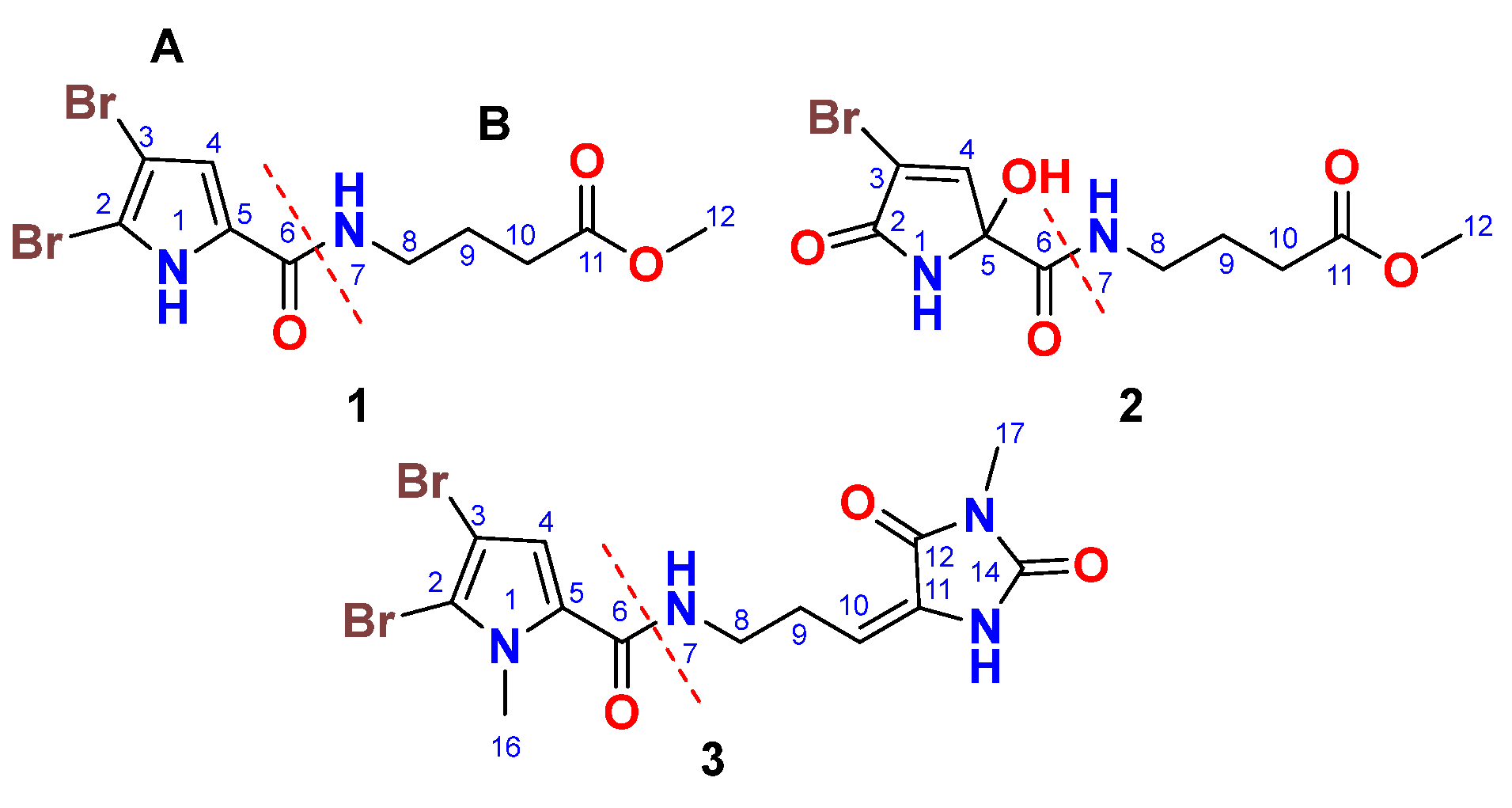
2.2. Structure of Compound 1.
Compound
1 (
Figure 1) was isolated as a yellowish powder with the molecular formula C
10H
12Br
2N
2O
3, determined from the positive HRESIMS pseudomolecular ion peak at
m/z 388.9111 [M + Na]
+. The presence of two bromine atoms in compound
1 was confirmed by the observation of three pseudomolecular ion peaks at
m/z 388.9, 390.9, and 392.9 in a 1:2:1 ratio. The structure of
1 was elucidated through the interpretation of its 1D and 2D NMR spectra. The
13C NMR spectrum showed signals for 10 carbon atoms (
Table 1). Analysis of the
13C NMR spectrum, supported by the HSQC experiment, led to the assignment of five quaternary carbons, one methine, three methylene groups, and one methyl group. The
13C NMR chemical shifts suggested the presence of two distinct parts (A and B) in compound
1 including a 4,5-dibromo-2-carboxylic acid moiety (A) linked to a 4-amino-1-methylbutanoate (B) unit via an amidic linkage (CO-NH) at C-6/NH-7. The
13C NMR spectrum exhibited two carbonyl signals at δ
C 159.4 and 173.6, which were attributed to carboxamide (C-6) and carboxylic acid ester (C-11) groups, respectively. The
1H/
13C signals at δ
H/C 12.65 (s) (N
H-1), 104.9 (C, C-2), 98.2 (C, C-3), 6.90 (s)/112.9 (CH, C-4), and 128.6 (C, C-5) were consistent with the structure of an amidic derivative of 4,5-dibromo-pyrrole-2-carboxylic acid (substructure A).
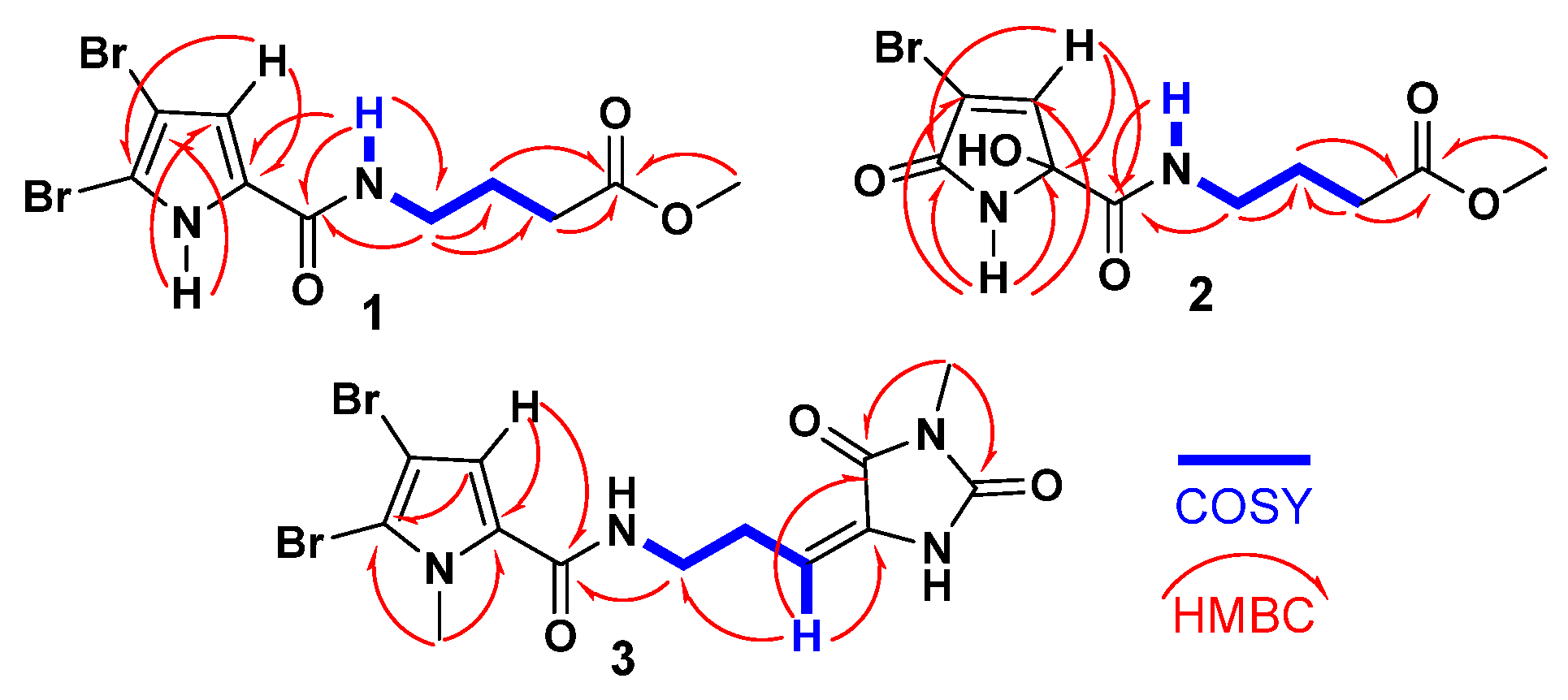
The
1H-
1H COSY spectrum revealed a single spin-coupling system from N
H-7 through H
2-8 to H
2-10 (
Figure 2). The signals at δ
H/C 8.14 (t,
J = 5.6 Hz, N
H-7), 3.21 (q,
J = 6.5 Hz)/38.3 (CH
2, C-8), 1.73 (quin.,
J = 7.0 Hz)/25.0 (CH
2, C-9), and 2.34 (t,
J = 7.4 Hz)/31.1 (CH
2, C-10) confirmed this coupling system and this portion of the molecule.
Further HMBC correlations from the three-proton singlet at δ
H 3.57 (s) to C-11 (δ
C 173.6) supported the presence of a methyl ester group. HMBC couplings from H
2-9 and H
2-10 to C-11 (δC 174.9) further confirmed this assignment. The connectivity between the two parts of the compound was established through HMBC correlations (
Figure 2) from H-4 (δ
H 6.90) to C-6 (δ
C 159.4), from N
H-7 (δ
H 12.65) to C-6 (δ
C 159.4), and from H
2-8 (δ
H 3.21) to C-6 (δ
C 159.4). Additional HMBC correlations from N
H-1 (δ
H 12.65) to C-3 (δ
C 98.2) and C-4 (δ
C 112.9), and from H-4 (δ
H 6.90) to C-2 (δ
C 104.9) and C-5 (δ
C 128.6), confirmed the assignments of these carbons. Therefore, compound
1 was identified as 4-(4,5-dibromo-1H-pyrrole-2-carboxamido)-1-methylbutanoate. This is the first report of its isolation from a natural source, making it a newly identified natural product. The generic name marmaricine A was given to compound
1.
2.3. Structure of Compound 2.
Compound
2 (
Figure 1) was isolated as an optically inactive yellowish powder ([α]
D 0°, c 0.10, MeOH) with the molecular formula C
10H
13BrN
2O
5, as determined from the positive HRESIMS pseudomolecular ion peak at m/z 342.9906 [M + Na]
+. The observation of two pseudomolecular ion peaks at m/z 342.9 and 344.9 in a 1:1 ratio confirmed the presence of a single bromine atom in compound
2. The structure of compound
2 was determined through the analysis of its 1D and 2D NMR spectra. The
13C NMR spectrum showed signals for 10 carbon atoms (
Table 2).
Interpretation of the
13C NMR data, combined with the HSQC experiment, allowed the assignment of the carbons as five quaternary carbons, one methine, three methylene groups, and one methyl group. The COSY,
13C NMR, HSQC, and HMBC data facilitated the identification of two main parts (A and B) in compound
2 including a 4-bromo-2-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxamide moiety (A) linked to a 4-amino-1-methylbutanoate unit (B) via an amidic bond (C-6/NH-7). When compared to compound
1, which contains a 4,5-dibromo-1H-pyrrole-2-carboxamide moiety as part A, compound
2 features a 4-bromo-2-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxamide moiety. The assignment of this substructure in compound
2 was supported by the
1H/
13C NMR signals at δ
H/C 9.05 (NH-1), 167.5 (C, C-2), 120.3 (C, C-3), 7.24 (d, J = 1.6 Hz)/147.0 (CH, C-4), 87.8 (C, C-5), and 167.3 (C, C-6). HMBC correlations from NH-1 to C-2, C-3, C-5, and C-6, as well as from H-4 to C-2 and C-5, supported this assignment. The chemical shifts of the
1H and
13C NMR signals for the second part of compound
2 (B) are similar to those in compound
1, suggesting that the same substructure is present in both molecules. The connection between the two parts of compound
2 was further confirmed by HMBC correlations (
Figure 2) from H-4 to C-6, from NH-7 to C-6, and from H
2-8 to C-6 (δ
C 167.3). The racemic nature of compound
2 was confirmed by the lack of any optical activity and the absence of any Cotton effects (CE) in the experimental ECD spectrum. Therefore, compounds
2 was determined to be a racemic mixture and assigned as (±)-4-(4-bromo-2-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxamido)-1-methylbutanoate. Compound
2 is reported here as a new natural product and named marmaricine B.
2.4. Structure of Compound 3.
Compound
3 (
Figure 1) was purified and obtained as a yellowish powder with the molecular formula C
13H
14Br
2N
4O
3, as indicated by the (+)-HRESIMS pseudomolecular ion peak at m/z 454.9327 [M + Na]
+, which suggests the presence of eight degrees of unsaturation. The detection of three ion peaks at m/z 454.9, 456.9, and 458.9 in a 1:2:1 ratio further corroborates the dibrominated nature of compound
3. The structure for compound
3 was determined through analysis of its 1D (
1H and
13C) and 2D (COSY, HSQC, HMBC, and NOESY) NMR spectra. The NMR data (
Table 3) supports the presence of two substructures (A and B). With the exception of the absence of the NH-1 signal, compound
3 exhibited similar
1H and
13C NMR signals to the substructure A of compound
1. Notably, the
1H/
13C NMR signals at 3.90 (3H, s, H
3-16)/36.4 (CH
3, C-16) in the
1H and
13C NMR spectra of compound
3, along with the HMBC correlations from H
3-16 (δ
H = 3.90) to C-2 (δ
C = 111.9), C-5 (δ
C = 129.4), and from H-4 (δ
H = 6.24) to C-2, C-5, and C-6 (δ
C = 161.4), support the assignment of substructure A as the 4,5-dibromo-1-methyl-1H-pyrrole-2-carboxamide moiety.
The remaining signals for compound
3 were attributed to 5-(3-aminopropylidene)-3-methylimidazolidine-2,4-dione moiety, based on the
1H and
13C NMR signals at δ
H/C 3.45 (t, J = 7.5 Hz)/39.2 (CH
2, C-8), 2.61 (q, J = 7.5 Hz)/28.8 (CH
2, C-9), 6.24 (q, J = 7.5 Hz)/120.5 (CH, C-10), 130.0 (C, C-11), 163.1 (C, C-12), 154.0 (C, C-14), and 3.20 (3H, s, H
3-17)/26.5 (CH
3, C-17). This assignment is further validated by COSY correlations (
Figure 2) from H
2-8 to H-10 and HMBC correlations (
Figure 2) from H-10 to C-8 (δ
C = 39.2), C-11 (δ
C = 130.0), and C-12 (δ
C = 163.1) as well as from CH
3-17 to C-12 (δ
C = 163.1) and C-14 (δ
C = 154.0). The interconnection between the substructures in compound
3 is also supported by an HMBC correlation (
Figure 2) from H
2-8 to C-6. Additionally, the assignment of the
1H and
13C NMR signals for substructure B was confirmed through COSY (
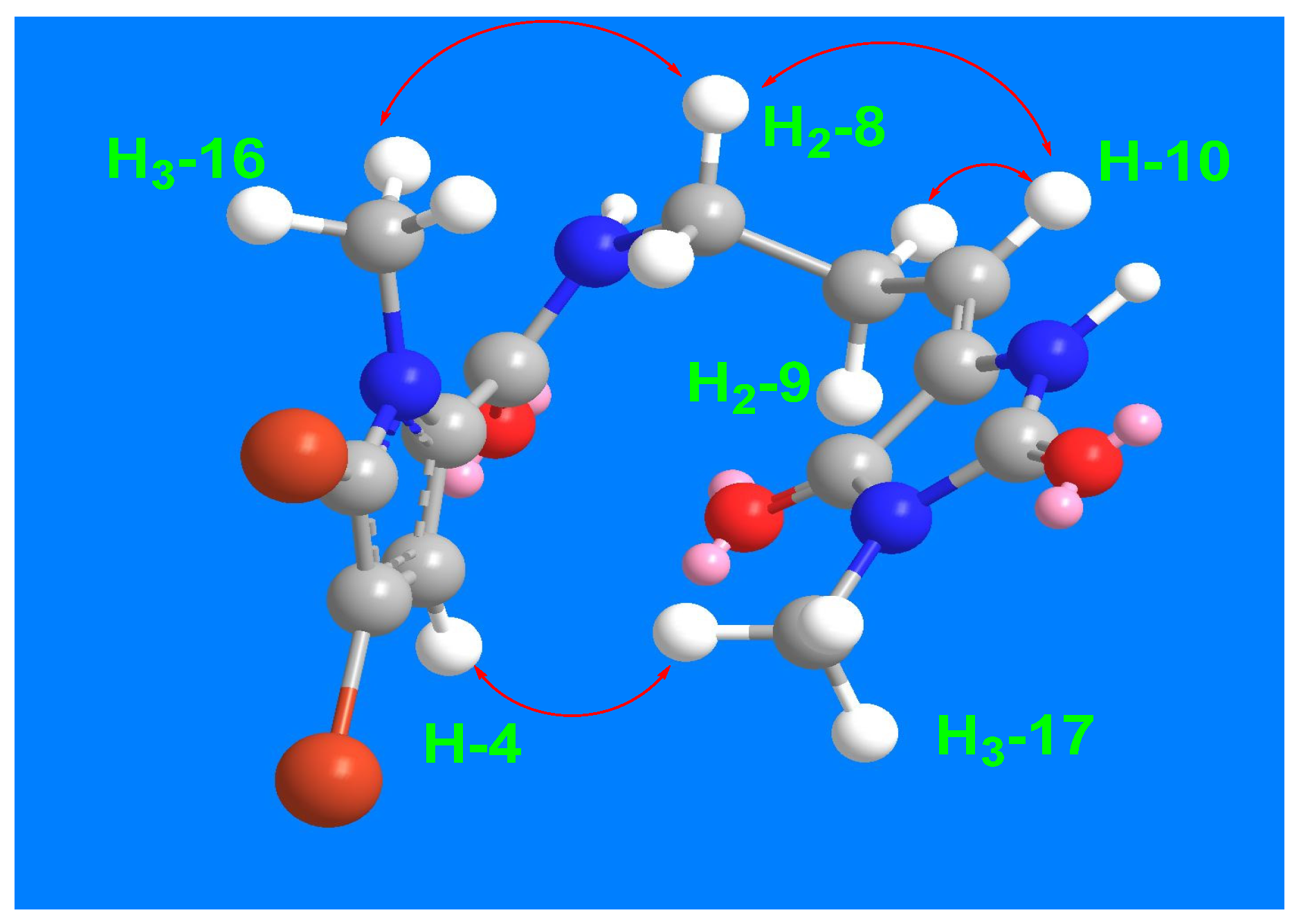
Figure 2) and HMBC correlations. The E configuration at Δ
10,11 is supported by NOESY correlations between H-10 and H
2-8, between H
3-17 and H-4 as well as between H
2-8 and H
3-16 (
Figure 3). Therefore, compound
3 is assigned as (E)-4,5-dibromo-1-methyl-N-(3-(1-methyl-2,5-dioxoimidazolidin-4-ylidene)propyl)-1H-pyrrole-2-carboxamide and is being reported here as a new natural product and named marmaricine C.
2.5. Antimicrobial Activities of the Compounds
Compounds
1-3 were screened for their antimicrobial effects using a disc diffusion assay, testing against
methicillin-resistant Staphylococcus aureus (MRSA),
Escherichia coli, and
Candida albicans at a concentration of 50 µg/disc. Compounds
1-3 displayed significant inhibition zones of 14, 15, and 12-15 mm, respectively, against MRSA (
Table 3). In contrast, compounds
2 and
3 exhibited inhibition zones of 15 and 14 mm, respectively, against
C. albicans, while compound
1 showed no activity (
Table 3). None of the compounds exhibited any effect against
E. coli (
Table 3). These findings indicate a strong selectivity of compounds
1-3 against MRSA and of compounds
2 and
3 against
C. albicans. These results suggest that compounds
1-3 represent promising candidates for the development of new antibiotics.
Table 4.
Antimicrobial activities of compounds 1-3.
Table 4.
Antimicrobial activities of compounds 1-3.
| Compound |
Inhibition zone (mm, 50μg/disc)
|
| MRSA |
E. coli |
C. albicans |
| 1 |
14 |
NI |
NI |
| 2 |
15 |
NI |
15 |
| 3 |
12 |
NI |
14 |
| Ciprofloxacin1
|
3.5 |
19 |
NT |
| Clotrimazole2
|
NT |
NT |
18 |
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were recorded using a JASCO DIP-370 digital polarimeter (Jasco Co., Tokyo, Japan) at 25 °C, with measurements taken at the sodium D line (589 nm). One-dimensional and two-dimensional NMR spectra (chemical shifts in ppm and coupling constants in Hz) were acquired on Bruker Avance DRX 800 MHz (800 MHz for ¹H and 200 MHz for ¹³C) or 500 MHz (500 MHz for 1H and 125 MHz for 13C) spectrometers (Bruker, Rheinstetten, Germany), using DMSO-d6 or CD3OD as the solvent. HPLC separation was carried out on a C18 column (150 × 4.6 mm, 2.5 μm, Waters Atlantis®, Massachusetts, USA), with a CH₃CN:H₂O gradient as the mobile phase, monitored at 220 nm and a flow rate of 2.0 mL/min.
3.2. Biological Materials
The Red Sea sponge Agelas sp. aff. marmarica (
Figure 4) was collected from the Saudi Red Sea coast (N021°39′17.5′′,E038°52′26.3′′). The sponge belongs to Kingdom: Animalia, Phylum: Porifera, Class: Demospongiae, Subclass: Heteroscleromorpha, Order: Agelasida, Family: Agelasidae, Genus: Agelas, Species: Agelas sp. aff. marmarica. The sponge was kindly identified by Rob van Soest. A specimen of the sponge was kept at the collection of the Naturalis Biodiversity Center at Leiden, The Netherlands under registeration number RMNHPOR 9165. Another specimen was stored at the Red Sea Invertebrates Collection at King Abdulaziz University under code No. DY-16.
3.3. Purification of the Compounds
The freeze-dried sponge materials (0.35 Kg) were macerated in a mixture of CH2Cl2:CH3OH (1:1) (3 × 2000 mL) at room temperature. The combined extracts were dried under reduced pressure to give a brown residue. The dried residue (17.5 g) was subjected to partition on VLC silica gel column using n-hexane-CH2Cl2-MeOH gradients affording 12 main fractions (Fr. 1-12). The antimicrobial fraction eluted with 100% CH2Cl2, Fr. 6 (0.41 g) (inhibition zone = 8 mm against C. albicans), was subjected to partition on Sephadex LH-10 using MeOH to afford five factions (Fr. A-E). The antimicrobial fraction (Fr. C) (126 mg) (inhibition zone = 10 mm against C. albicans) was purified on reversed-phase HPLC column (XDB-C18, 250 x 9.4 mm 5 µm, Agilent) using CH3CN:H2O gradients at 2 mL/min starting from 20% CH3CN to 0% CH3CN in 50 min to yield compounds 1 (2.5 mg, tR = 11 min), 2 (3.9 mg, tR = 20.5 min), and 3 (4.7 mg, tR = 17.5 min).
3.4. Spectra Data of 1-3
3.4.1. Marmaricine A (1). Yellowish powder; NMR data: see Table 1; HRESIMS m/z 388.9111 (calcd for C10H12Br2N2O3Na [M + Na]+, 388.9106).3.4.2. Marmaricine B (2). Yellowish powder; NMR data: see Table 2; HRESIMS m/z 342.9906 (calcd for C10H13BrN2O5Na [M + Na]+, 342.9900).3.4.3. Marmaricine C (3): Yellowish powder; [α]D 0° (c 0.1, MeOH); NMR data: see Table 3; HRESIMS m/z 454.9327 (calcd for C13H14Br2N4O3Na [M + Na]+, 454.9324).
3.5. Antimicrobial Activities of the Compounds
The antimicrobial effects of the compounds was performed against methicillin-resistant
Staphylococcus aureus (ATCC 43300) ,
Escherichia coli (ATCC 35218), and
Candida albicans (ATCC 76615) were performed at 50 μg/disc as previously reported in a disk diffusion assay [
26,
27,
28,
29].
4. Conclusions
In this study, the organic extract of the Red Sea sponge Agelas sp. aff. marmarica was studied. Bioassay-guided partition of the antimicrobial-active fraction on SiO₂, Sephadex LH-20, and HPLC purification, led to the isolation of three compounds, marmaricines A-C (1-3). The structures of these compounds were elucidated through spectroscopic analysis, including 1D and 2D NMR and (+)-HRESIMS measurements. The compounds were identified as 4-(4,5-dibromo-1H-pyrrole-2-carboxamido)-1-methylbutanoate (1), (±)-4-(4-bromo-2-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxamido)-1-methylbutanoate (2), and (E)-4,5-dibromo-1-methyl-N-(3-(1-methyl-2,5-dioxoimidazolidin-4-ylidene)propyl)-1H-pyrrole-2-carboxamide (3). Compounds 1-3 exhibited notable antimicrobial activity with inhibition zones of 14, 15, and 12 mm, respectively, against methicillin-resistant Staphylococcus aureus (MRSA). Furthermore, compounds 2 and 3 demonstrated inhibition zones of 15 and 14 mm, respectively, against Candida albicans, while compound 1 showed no activity. These findings highlight the strong selectivity of compounds 1-3 against MRSA and compounds 2 and 3 against C. albicans. The results suggest that compounds 1-3 represent promising scaffolds for further development as therapeutic agents targeting antimicrobial resistance.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figures S1-S16: 1D (1H and 13C) and 2D (COSY, HSQC, HMBC, NOESY) NMR spectra of compounds 1-3.
Author Contributions
Conceptualization, D.T.A.Y. and L.A.S.; methodology, D.T.A.Y, A.S.A., T.A. and L.A.S.; formal analysis, D.T.A.Y, A.S.A., T.A., A.M.A. and L.A.S.; investigation, D.T.A.Y, A.S.A., A.M.A., T.A. and L.A.S.; resources, D.T.A.Y.; data curation, D.T.A.Y. and L.A.S..; writing—D.T.A.Y. and L.A.S.; writing—review and editing, D.T.A.Y.; supervision, D.T.A.Y.; project administration, D.T.A.Y.; funding acquisition, D.T.A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This Project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University under grant no. (GPIP: 1370-166-2024).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data related to this manuscript are available in the manuscript and its related supplementary materials.
Acknowledgments
This Project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, under grant no. (GPIP: 1370-166-2024). The authors, therefore, acknowledge with thanks DSR for technical and financial support. We would like to thank Rob van Soest for the identification of the sponge material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chu, M.-J.; Li, M.; Ma, H.; Li, P.-L.; Li, G.-Q. Secondary metabolites from marine sponges of the Genus Agelas: a Comprehensive Update Insight on Structural Diversity and Bioactivity. RSC Adv. 2022, 12, 7789–7820. [Google Scholar] [CrossRef]
- Forenza, S.; Minale, L.; Riccio, R.; Fattorusso, E. New Bromopyrrole Derivatives from the Sponge Agelas oroides. Chem. Commun. 1971, 18, 1129–1130. [Google Scholar] [CrossRef]
- Cullen, E.; Devlin, J.P. Agelasine: A Novel Quaternary 9-Methyladenine from the Sponge Agelas dispar. Can. J. Chem. 1975, 53, 1690–1691. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Mangoni, A.; Taglialatela-Scafati, O. Clathramides, Unique Bromopyrrole Alkaloids from the Caribbean Sponge Agelas clathrodes. Tetrahedron 1996, 52, 13713–13720. [Google Scholar] [CrossRef]
- Assmann, M.; Lichte, E.; van Soest, R.W. M.; Köck, M. New Bromopyrrole Alkaloid from the Marine Sponge Agelas wiedenmayeri. Org. Lett. 1999, 1, 455–457. [Google Scholar] [CrossRef]
- Kusama, T.; Tanaka, N.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Bromopyrrole Alkaloids from a Marine Sponge Agelas sp. Chem. Pharm. Bull. 2014, 62, 499–503. [Google Scholar] [CrossRef]
- Kubota, T.; Iwai, T.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J. Agelasines O–U, New Diterpene Alkaloids with a 9-N-Methyladenine Unit from a Marine Sponge Agelas sp. Tetrahedron 2012, 68, 9738–9744. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tang, Y.; Zhang, Q.; Bourne, G.T.; Christoph, C.A.; Leet, J.E.; Knight, J.C.; Pettit, R.K.; Chapuis, J.-C.; Doubek, D.L.; Ward, F.J.; Weber, C.; Hooper, J.N. A. Isolation and Structures of Axistatins 1–3 from the Republic of Palau Marine Sponge Agelas axifera Hentschel. J. Nat. Prod. 2013, 76, 420–424. [Google Scholar] [CrossRef]
- Uemoto, H.; Tsuda, M.; Kobayashi, J. Mukanadins A–C, New Bromopyrrole Alkaloids from Marine Sponge Agelas nakamurai. J. Nat. Prod. 1999, 62, 1581–1583. [Google Scholar] [CrossRef]
- Schroif-Gregoire, C.; Appenzeller, J.; Debitus, C.; Zaparucha, A.; Al-Mourabit, A. Debromokeramadine from the Marine Sponge Agelas cf. mauritiana: Isolation and Short Regioselective and Flexible Synthesis. Tetrahedron 2015, 71, 3609–3613. [Google Scholar]
- Zhu, Y.; Wang, Y.; Gu, B.-B.; Yang, F.; Jiao, W.-H.; Hu, G.-H.; Yu, H.-B.; Han, B.-N.; Zhang, W.; Shen, Y.; Lin, H.-W. Antifungal Bromopyrrole Alkaloids from the South China Sea Sponge Agelas sp. Tetrahedron 2016, 72, 2964–2971. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, Novel Bicycle Diterpenoids with a 9-Methylladeninium Unit Possessing Inhibitory Effects on Na-K-ATPase from the Okinawan Sea Sponge Agelas sp. Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Capon, R.J.; Faulkner, D.J. Antimicrobial Metabolites from a Pacific Sponge, Agelas sp. J. Am. Chem. Soc. 1984, 106, 1819–1822. [Google Scholar] [CrossRef]
- Li, T.; Wang, B.; de Voogd, N.J.; Tang, X.-L.; Wang, Q.; Chu, M.-J.; Li, P.-L.; Li, G.-Q. Two New Diterpene Alkaloids from the South China Sea Sponge Agelas aff. nemoechinata. Chin. Chem. Lett. 2016, 27, 1048–1051. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Kobayashi, J.; Kobayashi, M.; Ohizumi, Y.; Hirata, Y. Agelasidines, Novel Hypotaurocyamine Derivatives from the Okinawan Sea Sponge Agelas nakamurai Hoshinot. J. Org. Chem. 1985, 50, 2494–2497. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J.; Tanner, R.S.; Kelly-Borges, M. Agelasines H and I, 9-Methyladenine-Containing Diterpenoids from an Agelas Sponge. J. Nat. Prod. 1998, 61, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Hedner, E.; Charnock, C.; Samuelsen, Ø.; Larsson, R.; Gundersen, L.-L.; Bohlin, L. (+)-Agelasine D: Improved Synthesis and Evaluation of Antibacterial and Cytotoxic Activities. J. Nat. Prod. 2006, 69, 381–386. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialatela-Scafati, O. Two Novel Pyrrole-Imidazole Alkaloids from the Mediterranean Sponge Agelas oroides. Tetrahedron Lett. 2000, 41, 9917–9922. [Google Scholar] [CrossRef]
- Appenzeller, J.; Mihci, G.; Martin, M.-T.; Gallard, J.-F.; Menou, J.-L.; Boury-Esnault, N.; Hooper, J.; Petek, S.; Chevalley, S.; Valentin, A.; Zaparucha, A.; Al-Mourabit, A.; Debitus, C. Agelasines J, K, and L from the Solomon Islands Marine Sponge Agelas cf. mauritiana. J. Nat. Prod. 2008, 71, 1451–1454. [Google Scholar] [CrossRef]
- Ishida, K.; Ishibashi, M.; Shigemori, H.; Sasaki, T.; Kobayashi, J. Agelasine G, A New Antileukemic Alkaloid from the Okinawan Marine Sponge Agelas sp. Chem. Pharm. Bull. 1992, 40, 766–767. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W. M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E. G.; Proksch, P. From Anti-Fouling to Biofilm Inhibition: New Cytotoxic Secondary Metabolites from Two Indonesian Agelas Sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Adachi, K.; Shizuri, Y. New Agelasine Compound from the Marine Sponge Agelas mauritiana as an Antifouling Substance against Macroalgae. J. Nat. Prod. 1997, 60, 411–413. [Google Scholar] [CrossRef]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Seiki, M.; Itoh, Y.; Yamashita, J.; van Soest, R.W. M.; Fusetani, N. Ageladine A: An Antiangiogenic Matrixmetalloproteinase Inhibitor from the Marine Sponge Agelas nakamurai. J. Am. Chem. Soc. 2003, 125, 15700–15701. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Hemimycalins C–E; Cytotoxic and Antimicrobial Alkaloids with Hydantoin and 2-Iminoimidazolidine-4-one Backbones from the Red Sea Marine Sponge Hemimycale sp. Mar. Drugs 2021, 19, 691. [Google Scholar] [CrossRef]
- Shaala, L.A. , Youssef, D. T.A. Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica. Mar. Drugs 2020, 18, 525. [Google Scholar]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlyn, C. Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica. Mar. Drugs 2020, 18, 525. [Google Scholar] [CrossRef]
- Acar, J.F. The Disc Susceptibility Test. In Antibiotics in Laboratory Medicine; Lorian, V., Ed.; Williams & Wilkins: Philadelphia, PA, USA, 1980; pp. 24–54. [Google Scholar]
- Youssef, D.T.A.; Asfour, H.Z.; Genta-Jouve, G.; Shaala, L.A. Magnificines A and B, Antimicrobial Marine Alkaloids Featuring a tetrahydrooxazolo[3,2-a]azepine-2,5(3H, 6H)-dione Backbone from the Red Sea Sponge Negombata magnifica. Mar. Drugs 2021, 19, 214. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).