Submitted:
10 January 2025
Posted:
13 January 2025
You are already at the latest version
Abstract
Liposomal drug delivery systems have become the revolutionary technology in the field of modern therapeutics, providing improved efficacy and fewer side effects. Herein, we review the most recent developments in targeting strategies and innovations in liposomal formulations. We discuss the passive targeting mechanisms, such as the enhanced permeability and retention effect, through which liposomes can accumulate in the tumor tissue. We also consider active targeting techniques that use particular ligands for enhancing the specificity of drug delivery to target cells. The review discusses different kinds of liposomes, such as PEGylated and pH-sensitive formulations, for enhancing drug stability and controlled release in response to specific environmental conditions. We have also discussed factors that affect the stability and circulation time of the liposomal formulations, with major emphasis on the lipid composition and formulation strategies. Discussion on challenges in the mass production of liposomes, together with potential solutions to achieve uniformity and reproducibility. In general, the incorporation of advanced targeting strategies and innovative formulations underlines the potential of liposomal drug delivery systems to considerably improve therapeutic results, especially in oncology and personalized medicine. This comprehensive review aims to highlight the current status of liposomal research and the future directions of this area to pave the way for more effective and targeted therapeutic interventions.
Keywords:
1. Targeting Strategies for Liposomal Formulations
1.1. Passive Targeting
1.1.1. Mechanisms
1.1.2. Advantages:
1.2. Active Targeting
1.2.1. Methods
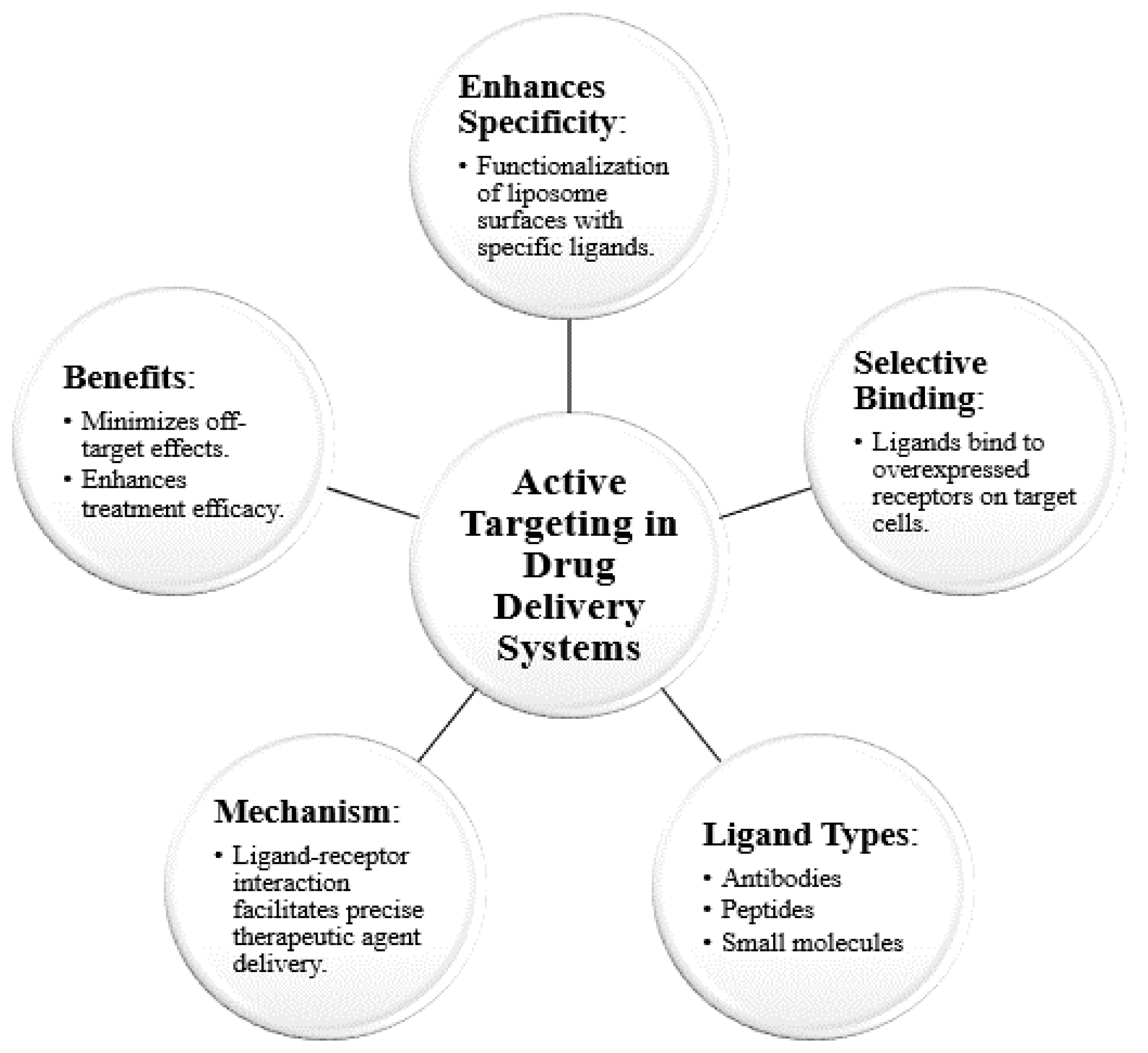

1.2.2. Applications:
| Aspect | Details | Examples/Applications | Citations |
|---|---|---|---|
| Ligands Used | Antibodies, peptides, small molecules | Trastuzumab for HER2-positive tumors, integrin-targeting peptides for metastatic cancers | [5,6,7] |
| Conjugation Strategies | Functionalization of liposomes through antibody, peptide, or small molecule conjugations | HER2-specific monoclonal antibodies, folic acid for ovarian cancer cells | [5,6] |
| Target Types | Receptors overexpressed on tumor cells or diseased tissues | EGFR in lung cancer, integrin αvβ3 in metastatic melanoma | [7,8] |
| Therapeutic Benefits | Increased drug accumulation at target site, reduced systemic toxicity, enhanced therapeutic efficacy | Enhanced drug delivery to tumor sites, reduced side effects in cancer therapies | [7,8] |
| Example Liposome Types | Functionalized liposomes (e.g., trastuzumab-liposomes, integrin-targeting liposomes) | Liposomes with HER2 antibodies, integrin αvβ3-binding peptides | [7,8] |
1.3. Role of Ligands
1.3.1. Specific Ligands
1.3.2. Significance
| Aspect | Details | Examples/Applications | Citations |
|---|---|---|---|
| Ligand Types | Folate, monoclonal antibodies (e.g., trastuzumab) | Folate for ovarian cancer; HER2 antibodies for breast cancer | [9,10] |
| Target Receptors | Folate receptors, HER2, EGFR | Folate receptors in ovarian cancer; HER2 in breast cancer | [9,10] |
| Function | Enhance binding to overexpressed receptors, improve internalization into target cells | Increased drug accumulation in tumors | [9,10] |
| Therapeutic Benefits | Reduced systemic toxicity, improved therapeutic index, disease-specific drug delivery | HER2-targeting liposomes for breast cancer; folate-targeted liposomes | [9,10] |
| Versatility | Ligands can be customized based on disease-specific biomarkers | Antibody-targeting for cancer; small molecule ligands for folate receptors | [9,10] |
2. Innovations in Liposomal Formulations
2.1. Types of Liposomes
2.1.1. Overview
2.1.2. Specific Properties
2.2. Stability and Circulation Time
2.2.1. Factors Affecting Stability
2.2.2. Formulation Strategies
| Factor/Strategy | Details | Impact on Stability and Circulation |
|---|---|---|
| Lipid Composition | Type of lipids affects bilayer rigidity, size, and integrity | Enhanced membrane stability; reduced leakage and degradation |
| Surface Charge | Positive or neutral charges influence interaction with biological membranes | Improved uptake (positive); better circulation stability (neutral/zwitterionic) |
| Cholesterol Incorporation | Increases bilayer rigidity | Reduces permeability and improves resistance to external stresses |
| PEGylation | Coating with polyethylene glycol | Creates steric hindrance; reduces immune clearance and prolongs circulation |
| Polymer Stabilizers | Use of hydrophilic polymers to prevent aggregation | Enhances storage and systemic stability |
2.3. Manufacturing Challenges
2.3.1. Scale-Up Issues
2.3.2. Overcoming Challenges
| Challenge | Details | Solution/Strategy |
|---|---|---|
| Batch-to-Batch Variability | Differences in liposome size, drug encapsulation efficiency, and release profiles | Standardized protocols and automated systems |
| Process Reproducibility | Inconsistencies in liposome characteristics during large-scale manufacturing | Microfluidics for precise control; use of advanced mixing technologies |
| Complexity in Composition Control | Difficulty in achieving uniform lipid composition and drug distribution | Application of QbD principles to identify and control critical process parameters |
| Manual Errors in Production | Human intervention leading to variability | Automation and robotics to minimize errors and ensure reproducibility |
3. Conclusion
References
- Sengar, A. Targeting methods: A short review including rationale, goal, causes, strategies for targeting. International Journal of Research Publication and Reviews 2023, 4, 1379–1384. [Google Scholar]
- Jagrati, K.M. , & Sengar, A. Liposomal vesicular delivery system: An innovative nano carrier. World Journal of Pharmaceutical Research 2024, 13, 1155–1169. [Google Scholar] [CrossRef]
- Prajapati, R.N. , Jagrati, K., Sengar, A., & Prajapati, S.K. Nanoparticles: Pioneering the future of drug delivery and beyond. World Journal of Pharmaceutical Research 2024, 13, 1243–1262. [Google Scholar]
- Sengar, A. , Jagrati, K., & Khatri, S. Enhancing therapeutics: A comprehensive review on naso-pulmonary drug delivery systems for respiratory health management. World Journal of Pharmaceutical Research 2024, 13, 1112–1140. [Google Scholar]
- Sengar, A. , Vashisth, H., Chatekar, V.K., Gupta, B., Thange, A.R., & Jillella, M.S. R. S. N. From concept to consumption: A comprehensive review of chewable tablets. World Journal of Pharmaceutical Research 2024, 13, 176–189. [Google Scholar]
- Allen, T.M. Liposomes: Opportunities in drug delivery. Drugs of the Future 2002, 27, 1–10. [Google Scholar] [CrossRef]
- Klibanov, A.L. , & Maruyama, K. Liposomes for drug delivery: A review. Advanced Drug Delivery Reviews 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Lasic, D.D. Liposomes: From physics to applications. In Liposomes: A Practical Approach (pp. 1-20). Oxford University Press.
- Torchilin, V.P. (2007). Micelles from lipid-core micelles to liposomes: A new approach to drug delivery. Drug Delivery 1998, 14, 1–10. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A. , Yadav, S., & Niranjan, S.K. Formulation and evaluation of mouth-dissolving films of Propranolol Hydrochloride. World Journal of Pharmaceutical Research 2024, 13, 850–861. [Google Scholar]
- Sengar, A. , Saha, S., Sharma, L., Hemlata, Saindane, P.S., & Sagar, S.D. Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research 2024, 13, 1063–1071. [Google Scholar]
- Gabizon, A. , & Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin:From bench to bedside. Clinical Pharmacokinetics 2000, 39, 85–95. [Google Scholar] [CrossRef]
- Allen, T.M. , & Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. , & Kato, Y. Liposomal drug delivery systems: A review of their applications in cancer therapy. Journal of Drug Delivery Science and Technology 2010, 20, 1–10. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—the first FDA-approved nano-drug: Lessons learned. Nature Reviews Drug Discovery 2012, 9, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H. , & Reddy, S.M. Liposomes: A review of their applications in drug delivery. Journal of Controlled Release 2011, 153, 1–15. [Google Scholar] [CrossRef]
- Sengar, A. , Tile, S.A., Sen, A., Malunjkar, S.P., Bhagat, D.T., & Thete, A.K. (2024, ). Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research, 13, 1424–1435. 21 July.
- Sengar, A. Precision in practice: Nanotechnology and targeted therapies for personalized care. International Journal of Advanced Nano Computing and Analytics 2024, 3, 56–67. [Google Scholar] [CrossRef]
- Sengar, A. (2024, ). Liposomes and beyond: Pioneering vesicular systems for drug delivery. Preprint. https://www.preprints.org/manuscript/202412. 26 December 2230. [Google Scholar]
- Sengar, A. (2025). "Liposomal Drug Delivery Systems: An Intro as a Primer for Advanced Therapeutics." Preprints. [CrossRef]
- Sengar, A. (2025). Innovations and Mechanisms in Liposomal Drug Delivery: A Comprehensive Introduction. Preprints. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




