Introduction
Idiopathic Pulmonary Fibrosis (IPF) is one of the interstitial lung diseases (ILD) and is a progressive lung disease of unknown cause and pathologically compatible with the usual interstitial pneumonia (UIP) pattern [
1]. Despite being the most prevalent form of interstitial lung diseases, its overall incidence is low. The annual incidence in the United States of America has been found between 1.2 and 76.4 per 100,000 [
2,
3]. The most common population is male smokers over the age of 65 [
4]. IPF is a difficult disease to treat and treatment alternatives are limited. Pirfenidone and nintedanib are two antifibrotic drugs indicated for the treatment of IPF patients. There are studies showing that both drugs reduce the decrease in FVC (Force Vital Capacity), which is a determinant of the severity of restriction in IPF, decrease the exacerbation frequency and hospitalizations, and increase survival rate [
5,
6,
7].
Interstitial lung diseases other than IPF can also progress to fibrosis. However, antifibrotic therapy is indicated when fibrosis is progressive and this is called Progressive Pulmonary Fibrosis (PPF). To diagnose with PPF at least two of the following criteria must be present within the last year: worsening of respiratory symptoms, physiologic evidence of disease progression (absolute decrease in FVC ⩾5% or absolute decrease in DLCO (corrected for Hb⩾10%), radiologic evidence of disease progression (increase in the extent or severity of traction bronchiectasis and bronchiolectasis, or development of new ground-glass opacities or new reticulations with traction bronchiectasis)[
8]. Recent studies show that antifibrotic drugs with proven efficacy in IPF may also be effective in progressive pulmonary fibrosis [
9,
10]. Although the evidence regarding Pirfenidone is not clear in this regard, there are important studies showing the positive effects of nintedanib on pulmonary function tests in this group of patients [
11].
There have been many studies on these two drugs separately in both IPF and non-IPF patients, but there are only a few studies comparing these two drugs between each other. The criteria for which drug is preferred in diagnosed patients are not clear. The aim of our study is to compare the effects of pirfenidone and nintedanib on lung function and radiologic findings in IPF.
Methods
The data of patients who were started Pirfenidone or Nintedanib treatment with the diagnosis of Idiopathic Pulmonary Fibrosis Progressive Pulmonary Fibrosis according to An Official ATS/ERS/JRS/ALAT Clinical Practice guideline and treated for at least one year in our department between January 1, 2010 and December 31, 2022 were retrospectively analyzed. The included patients had not previously used steroids or similar drugs for lung disease. The study was initiated after approval of the local ethics committee.
File information, pulmonary function test parameters and radiologic data, gender, age and comorbidity information of all patients were obtained from the hospital database. Pulmonary function tests were performed using a COSMED Quark 2021 model device. Patients were divided into two groups as nintedanib and pirfenidone group and both groups were compared in terms of progression in pulmonary function tests and radiologic findings within 1 year of diagnosis. For this purpose, the difference of pulmonary function test parameters (FVC (mL-%), FEV1 (mL-%) and 6-minute walk test values at the 3rd, 6th, 9th and 12th months of all patients from the baseline values at the time of diagnosis were analyzed and these differences were compared between the two groups.Thorax CT findings were also compared between the two groups for the presence of progression. Patients whose radiologic and PFT data at initial presentation were not available, patients whose treatment was discontinued before 1 year due to side effects or unresponsiveness or death, and patients who were switched between the two drugs before 1 year had elapsed were excluded from the study.
Statistical Analysis
Kolmogorov-Smirnov test was used to test the normal distribution of the continuous variables. The data characterized by a normal distribution are expressed as mean±standard deviation. Student's t-tests was used for the comparison of the data which had a normal distribution. Mann-Whitney-U test was used for the comparison of the non-normally distributed data. The discrete variables were compared using Chi-squared test. P <0.05 was considered to be statistically significant. The data were analyzed using the SPSS statistical software (version 13.01, serial number 9069728, SPSS Inc., Chicago).
Results
After exclusion of patients who did not meet the inclusion criteria, 109 patients were included in the study. There were no deaths within 1 year among the patients included in the study. The gender distribution of patients was as follows: 87(79.8%) male and 22(20.2%) female. The mean age was 69.90±8.65 years in men and 68.64±10.19 years in women (p=0.55).
The number of patients receiving pirfenidone treatment was 82(75.2%) and the number of patients receiving nintedanib treatment was 27(24.8%). Demographic characteristics of the patients are given in
Table 1.
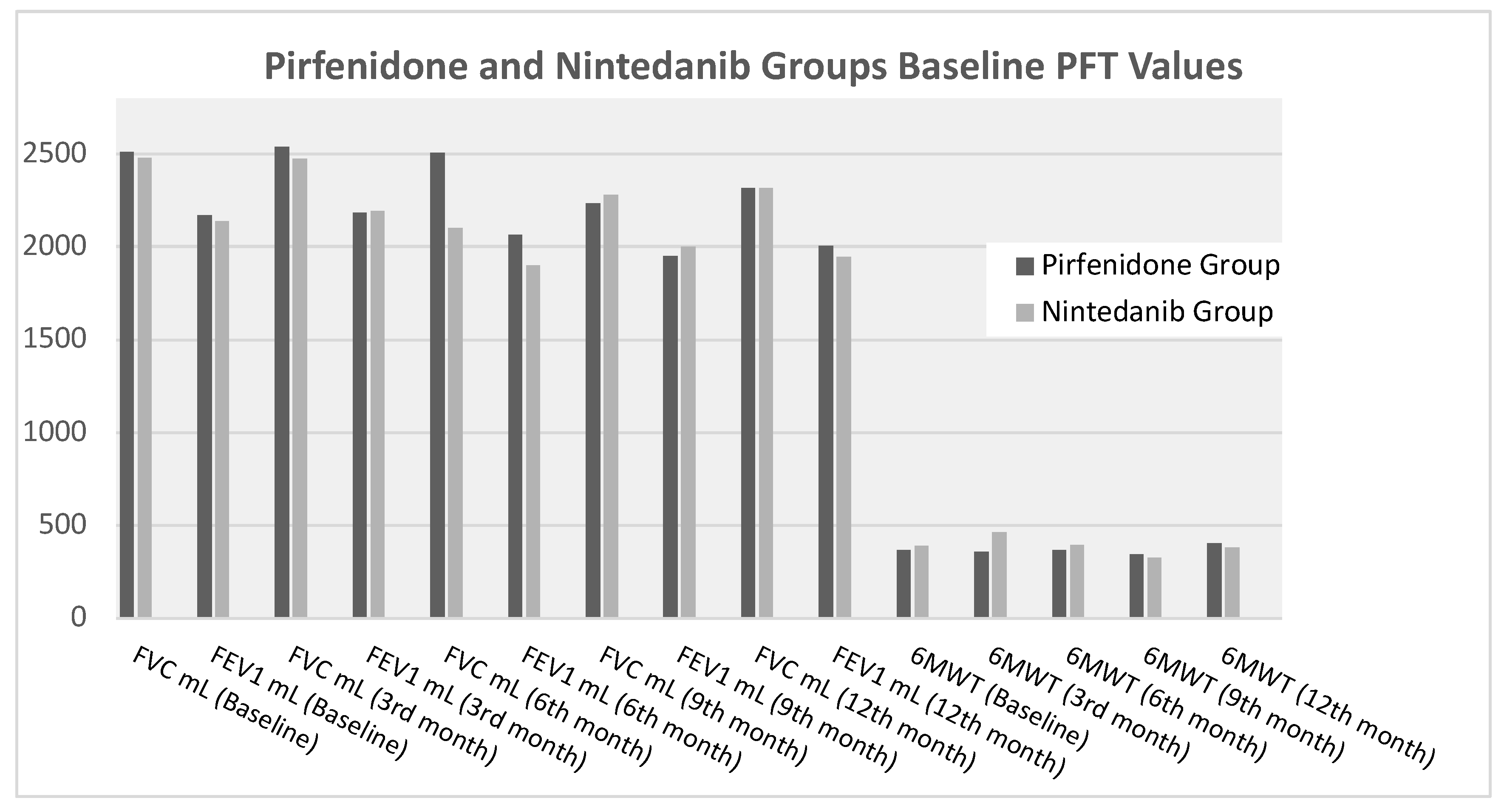
Figure 1 shows the comparison of baseline, 3-month, 6-month, 9-month and 12-month PFT and 6MWT parameters of pirfenidone and nintedanib group. There is no statistically significant difference between the two groups.
When the PFT values at 3rd, 6th, 9th and 12th months compared to baseline in both groups, there was no statistically significant difference in any parameter between the two groups. (Tablo 2). At the 3rd, 6th, 9th and 12th months, there was no statistical difference between the FVC% values and baseline values between the two groups. Figure 2 shows the change in FVC % values in both groups over a 12-month period.
Table 2.
Differences in PFT Values Compared to Baseline in Two Groups.
Table 2.
Differences in PFT Values Compared to Baseline in Two Groups.
| |
Pirfenidone Group
N=82(75,2%) |
Nintedanib Group
N=27(24,8%) |
|
| Changes of PFT Values Compared to Baseline |
Mean ± Std. Deviation
Mean (Min-Max) |
Mean ± Std. Deviation
Mean (Min-Max) |
P |
| FVC mL (3rd month) |
88,15±489,46
50,00 (-920-1060) |
153,75±461,270
180,00 (-500-950) |
,694 |
| FEV1 mL (3rd month) |
147,86±570,11
90,00 (-1260-2230) |
145,00±293,063
125,00 (-130-740) |
,970 |
| FVC mL (6th month) |
45,7143±489,70
60,00 (-920,00-1040,00) |
257,27±246,58025
290,00 (-40,00-610,00) |
,164 |
| FEV1 mL (6th month) |
140,58±713,33
30,00 (-870,00-2891,00) |
177,27±214,20042
170,00 (-80,00-660,00) |
,321 |
| FVC mL (9th month) |
235,92±531,62
130,00 (-450,00-1850,00) |
74,28±371,07309
40,00 (-480,00-630,00) |
,594 |
| FEV1 mL (9th month) |
204,81±442,14
90,00 (-570,00-1090,00) |
60,00±278,50793
40,00 (-440,00-430,00) |
,733 |
| FVC mL (12th month) |
200,00±484,42
125,00 (-570-1420) |
131,67±339,35
70,00 (-370-750) |
,770 |
| FEV1 mL (12th month) |
215,48±489,05
120,00 (-510-2250) |
185,83±278,12
155,00 (-210-620) |
,946 |
| 6MWT (3rd month) |
-24,08±120,96
17,00 (-340-102) |
-21,00±77,782
-21,00 (-76-34) |
,713 |
| 6MWT (6th month) |
7,41±86,15
8,50 (-187,00-136,00) |
-97,50±274,04
-,50 (-476,00-215,00) |
,638 |
| 6MWT (9th month) |
28,87±103,65
30,50 (-187,00-153,00) |
-68,00±212,32
,00 (-306,00-102,00) |
,406 |
| 6MWT (12th month) |
-34,93±115,54
-8,50 (-374-68) |
-1,14±242,53
110,00 (-527-170) |
,126 |
The pirfenidone and nintedanib groups were compared with respect to the presence of ground-glass opacity, increased reticulation, honeycombing, and traction bronchiectasis, which are the most common radiological findings in interstitial lung diseases at the time of diagnosis. There was no statistically significant difference in the incidence of these radiological findings between the two groups (
Table 3).
When both groups were evaluated in terms of the presence of radiological progression after 1 year, progression was observed in 11 (13.4%) patients in pirfenidone group and in 5 (18.5%) patients in nintedanib group and there was no statistically significant difference between the two groups in this respect. (p= 0.538).
Table 4 presents the details of patients who were discontinued or switched due to disease progression or the emergence of adverse effects necessitating a change in treatment. Among patients receiving nintedanib, only two experienced severe diarrhea to the extent that they required treatment discontinuation.
Discussion
Our study is an observational real-life study comparing 1-year treatment outcomes in patients with IPF treated with Pirfenidone and Nintedanib.
Pirfenidone and nintedanib are two antifibrotic drugs that have been approved and proven to be effective in the treatment of idiopathic pulmonary fibrosis and are used worldwide. Many clinical studies have shown that these two drugs slow down the rate of decline in functional residual capacity, decrease the number of exacerbations and improve survival rates in IPF [
5,
12,
13]. Nintedanib has been shown to be effective in interstitial lung diseases with non-IPF fibrosis as well as IPF.Lung involvement is common in systemic sclerosis and is the most important cause of mortality. In patients with ILD associated with systemic sclerosis, the annual rate of decline in FVC was found to be lower in patients treated with nintedanib than placebo [
14]. Studies have also shown that nintedanib significantly reduced the annual rate of decline in FVC compared to placebo in patients with lung disease with progressive pulmonary fibrosis other than scleroderma [
15]. There is insufficient data on the efficacy of pirfenidone in non-IPF ILD [
6]. There is no doubt that the two drugs are effective in IPF, but the number of studies comparing the efficacy of nintedanib and pirfenidone in slowing or preventing the progression of the disease is very limited.
Bargagli et al. conducted a retrospective study investigating the efficacy of these two drugs in IPF patients. In this study, FVC, FEV1, TLC and DLCO values at baseline, 6th and 12th months were compared in 82 IPF patients diagnosed in the last 12 months who were treated with pirfenidone (n=52) and nintedanib (n=30). No significant difference was found in the percentages of FVC, FEV1, TLC and DLCO at baseline between patients treated with the two drugs (p = 0.59, p = 0.37, p = 0.21, p = 0.48, respectively). At 6-month follow-up, there was no significant difference in the % of predicted values of FVC, FEV1, DLCO or TLC between patients treated with pirfenidone and nintedanib (p = 0.54, p = 0.38, p = 0.76, p = 0.31, respectively). Due to the late approval of nintedanib in Italy, only the pirfenidone group was able to complete the 12-month period. When the pirfenidone group was evaluated within itself, no statistical difference was found between the baseline, 6th and 12th month values of the mentioned parameters. The study showed that treatment with both drugs had similar rates of decrease in FVC at 6-month follow-up [
16].
In another study by Cerri et al, 142 IPF patients were divided into three groups: pirfenidone-treated (n=78), nintedanib-treated (n=28) and control group that received no treatment (n=36) and patients were compared at 6th, 12th and 24th months for factors such as pulmonary function test, arterial blood gas, liver function test, side effects and treatment adherence. After adjustment for baseline differences in FVC, a statistically significant reduction in this parameter was observed over the time period in the control group compared with the treated groups (p = 0.0053), while no significant difference was observed between the pirfenidone and nintedanib groups over the 24-month time course. After adjustment for baseline differences in DLCO, a statistically significant decrease in this parameter was observed in untreated patients (p = 0.037), but no statistically significant difference was observed between the pirfenidone and nintedanib groups and DLCO remained stable over the 24-month time course [
17].
In a retrospective study by Cameli et al. analyzing 10 years of data, 263 IPF patients were included in the study. Of these patients, 139 were treated with pirfenidone and 124 with nintedanib. The median survival time of the patients was 1224 days, and there was no difference between pirfenidone and nintedanib groups in terms of survival time and the time until FVC decreased by more than 10% (p=0.8786 and p=0.1677, respectively). At the end of the 1-year period, a smaller decrease in DLCO values was found in the nintedanib group compared to the other group (p=0.016), but this difference closed as the follow-up period increased. The lesser decrease in DLCO in the nintedanib group was attributed to the antiangiogenic property of nintedanib [
18].
In a study comparing 840 patients treated with pirfenidone and 713 patients treated with nintedanib in terms of mortality, hospitalization and care costs, both groups were similar in terms of two-year all-cause mortality (HR: 0.90, 95% CI: 0.76; 1.07), one-year all-cause mortality (HR: 1.09, 95% CI: 0.95; 1.25) and respiratory-related hospitalizations (HR: 0.89, 95% CI: 0.72; 1.08). Also, no significant difference was observed between the two groups in terms of total (€- 807, 95% CI: €- 2977; €1220) and respiratory-related costs (€- 1282, 95% CI: €- 3423; €534). However, this study did not analyze the change in pulmonary function and was very different from our study [
19].
In another study, patients with IPF diagnosed with pirfenidone or nintedanib were followed up and at the end of 12 months, FVC values of patients treated with nintedanib were found to be higher than those of the pirfenidone group (mean difference, 106 mL; 95% CI, 34-178). However, it was observed that this difference narrowed by the end of 24-month follow-up [
20].
In our study, we divided 109 patients with IPF into two groups as pirfenidone and nintedanib groups. As expected, the majority of the patients were male [N=87 (79.8%)], mean age was 69.64±8.94 years and there was no statistical difference between the two groups. No significant difference was found in baseline, 3rd month, 6th month, 9th and 12th month PFT and 6MWT parameter values of patients in pirfenidone and nintedanib groups. When the differences of 3rd, 6th, 9th and 12th month PFT values compared to baseline in both groups were compared, no statistically significant difference was found in any parameter between the two groups. In our study, unlike the studies cited above, we did not compare the absolute PFT values of both groups at the 3rd, 6th, 9th and 12th months in our study. By comparing the changes of the values of both groups at the specified months with the basal values, we aimed to reveal the change for favorable or unfavorable more accurately.As a result, we did not find any statistically significant difference in any parameter between the two groups when the differences of 3rd, 6th, 9th and 12th month PFT values compared to baseline were compared in both groups. Another difference of our study from the cited studies is that we compared both groups in terms of radiological progression. The rates of ground-glass opacity, increased reticulation, traction bronchiectasis and honeycombing, which are the most frequently observed findings in IPF, were statistically similar in the pirfenidone and nintedanib groups at the time of diagnosis. When both groups were evaluated for the presence of radiological progression after 1 year, progression was observed observed in 11 (13.4%) patients in pirfenidone group and in 5 (18.5%) patients in nintedanib group and there was no statistically significant difference between the two groups in this respect. (p= 0.538).
In almost all previous studies comparing the efficacy of pirfenidone and nintedanib in IPF patients, the efficacies of these two drugs were found to be equivalent. In line with the literature, our study showed that the effects of pirfenidone and nintedanib on pulmonary function test parameters, 6MWT and radiologic progression were similar at 1-year follow-up.
The limitations of our study are that it was a retrospective study, the number of patients was small and DLCO (Carbon Monoxide Diffusion Test) values could not be compared.
Author Contributions
Olcay AYCICEK:Concept, design, definition of intellectual content, literature search, clinical studies, data analysis, statistical analysis, manuscript preparation, manuscript editing. Serra KESKIN: Data acquisition, data analysis, Muhammed HACIOSMANOGLU: Statistical analysis, manuscript preparation, Funda OZTUNA:Manuscript preparation, manuscript editing, Yılmaz BULBUL:Statistical analysis, manuscript preparation, Tevfik OZLU: Manuscript review The manuscript has been read and approved by all authors, the authorship requirements outlined earlier in this document are met, and each author believes that the manuscript represents honest work.
Ethics approval
Local ethic committee ethic has been approved.
Consent for publication
All authors declare that this article or any part of it has not been published in any other place, institution or organization. All authors reviewed and approved the article. All authors agreed to submit the manuscript to the Tuberculosis and Thorax.
Availability of data and material
The corresponding author has all responsibility for the data, can be contacted with the corresponding author for data.
Conflicts of interest/Competing interests
Not applicable
Financial And Material Support
None of the authors received financial or material support.
Code availability
Not applicable
Consent to participate
The study was prepared in accordance with the rules of the Helsinki Declaration.
References
- Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. The Lancet. 2017, 389, 13–19. [Google Scholar]
- Aycicek O, Cetinkaya E, Ucsular FD et al. Research Burden of Interstitial Lung Diseases in Turkey – RBILD. Sarcoidosis Vasc Diffuse Lung Dis. 2022, 39, e2022006. [Google Scholar]
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and Prevalence of IdiopathicPulmonary Fibrosis. Am J Respir Crit Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Sese L,Nunes H, Cottin V, Israel-Biet D, Crestani B, Guillot-Dudoret Setal. Gender Differences in Idiopathic Pulmonary Fibrosis: Are Men and Women Equal? Front. Med. 2021, 8, 713698. [Google Scholar] [CrossRef] [PubMed]
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U.,Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Amati F, Stainer A,Polelli V,Mantero M,Gramegna A,Blasi F. Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7849. [Google Scholar] [CrossRef] [PubMed]
- Petnak T, Lertjitbanjong P,Thongprayoon C, Moua T. Impact of Antifibrotic Therapy on Mortality and Acute Exacerbation in Idiopathic Pulmonary Fibrosis A Systematic Review and Meta-Analysis. Chest 2021, 160, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Raghu G,Remy-Jardin M,Richeldi L,Thomson CC, Inoue Y,Johkoh T et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine 2011, 205, 18–47. [Google Scholar]
- Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina M, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020, 8, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Behr J, Prasse A, Kreuter M, Johow J, Rabe KF, Bonella F, et al.;RELIEF Investigators. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 2021;9:476-486.
- Amati F, Stainer A, Polelli V, Mantero M, Gramegna A, Blasi F et al. Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review. Int. J. Mol. Sci. 2023;24:7849.
- Margaritopoulos GA, Trachalaki A, Wells AU, Vasarmidi E, Bibaki E, Papastratigakis G at al. Pirfenidone improves survival in IPF: results from a real-life study. Pulmonary Medicine 2018, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Nathana SD, Costabelb U, Alberac C, Behrd J, Wuytse WA, Kirchgaesslerf KU et al. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respiratory Medicine 2019, 153, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Distler O, Highland K, Gahlemann M, Azuma A, Fischer A, Mayes MD et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. N Engl J Med. 2019;380:2518-2528.
- Flaherty KR, Wells A, Cottin V, Devaraj A, Walsh S.L.F, Inoue Y et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381:1718-1727.
- Bargagli E,Piccioli C,Rosi E, Torricelli E,Turi L,Piccioli E et al.Pirfenidone and Nintedanib in idiopathic pulmonary fibrosis:Real-life experience in an Italian referral centre. Pulmonolgy.2019;25(3):149-153.
- Cerri S, Monari M, Guerrieri A, Donatelli P, Bassi I, Garuti M et al. Real-life comparison of pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis: A 24-month assessment. Respiratory Medicine. 2019;159;105803.
- Cameli P,Refini RM,Bergantini L,d’Alessandro M,Alonzi V,Magnoni C et al. Long-Term Follow-Up of Patients With Idiopathic Pulmonary Fibrosis Treated With Pirfenidone or Nintedanib: A Real-Life Comparison Study Frontiers In Molecular Biosciences. 2020;4:7:581828.
- Marijic P,Schwarzkopf L,Schwettmann L,Ruhnke T,Trudzinski F, Michael M.Pirfenidone vs. nintedanib in patients with idiopathic pulmonary fbrosis: a retrospective cohort study. Respir Res. 2021;22:268.
- Kim JS , Murray S,Yow E, Anstrom KJ, Kim HJ, Flaherty KR et al. Comparison of Pirfenidone and Nintedanib. Post Hoc Analysis of the Clean UP-IPF Study. Chest. 2024;165(5):1163-1173.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).