Submitted:
10 January 2025
Posted:
10 January 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Layer Structure
3.2. Carbonate Anion
3.3. Phosphate Anion
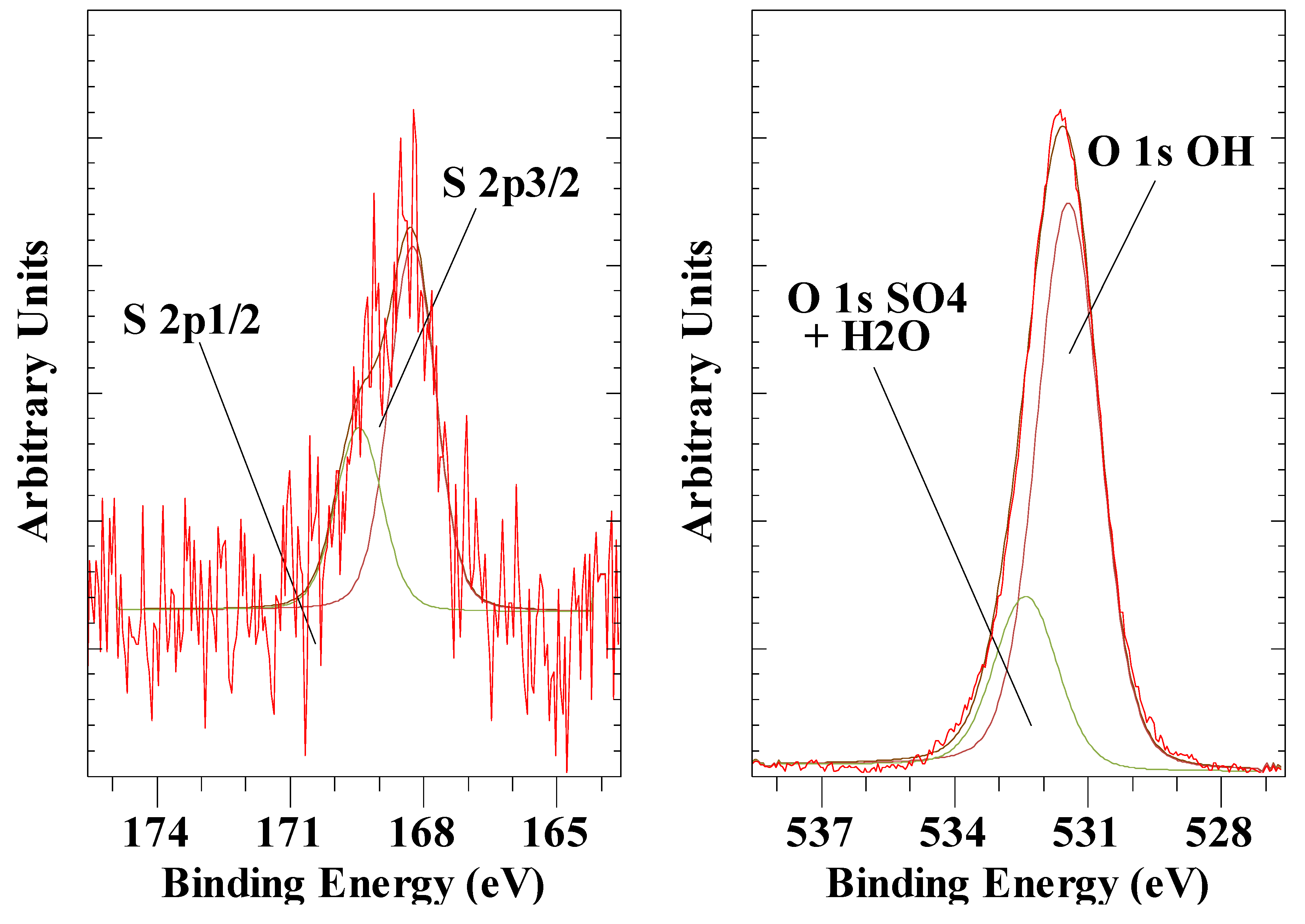
3.4. Sulphate Anion
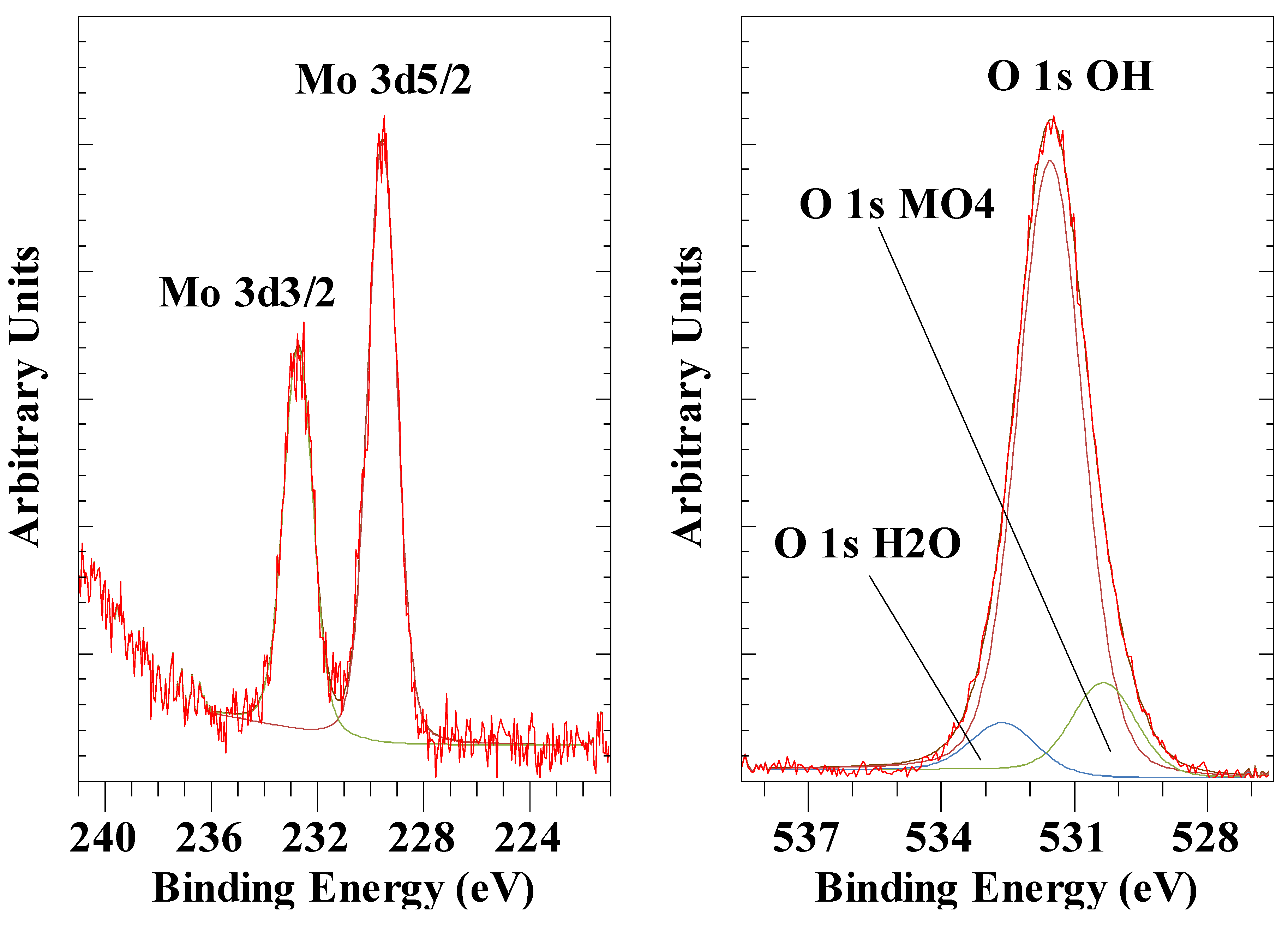
3.5. Molybdate Anion
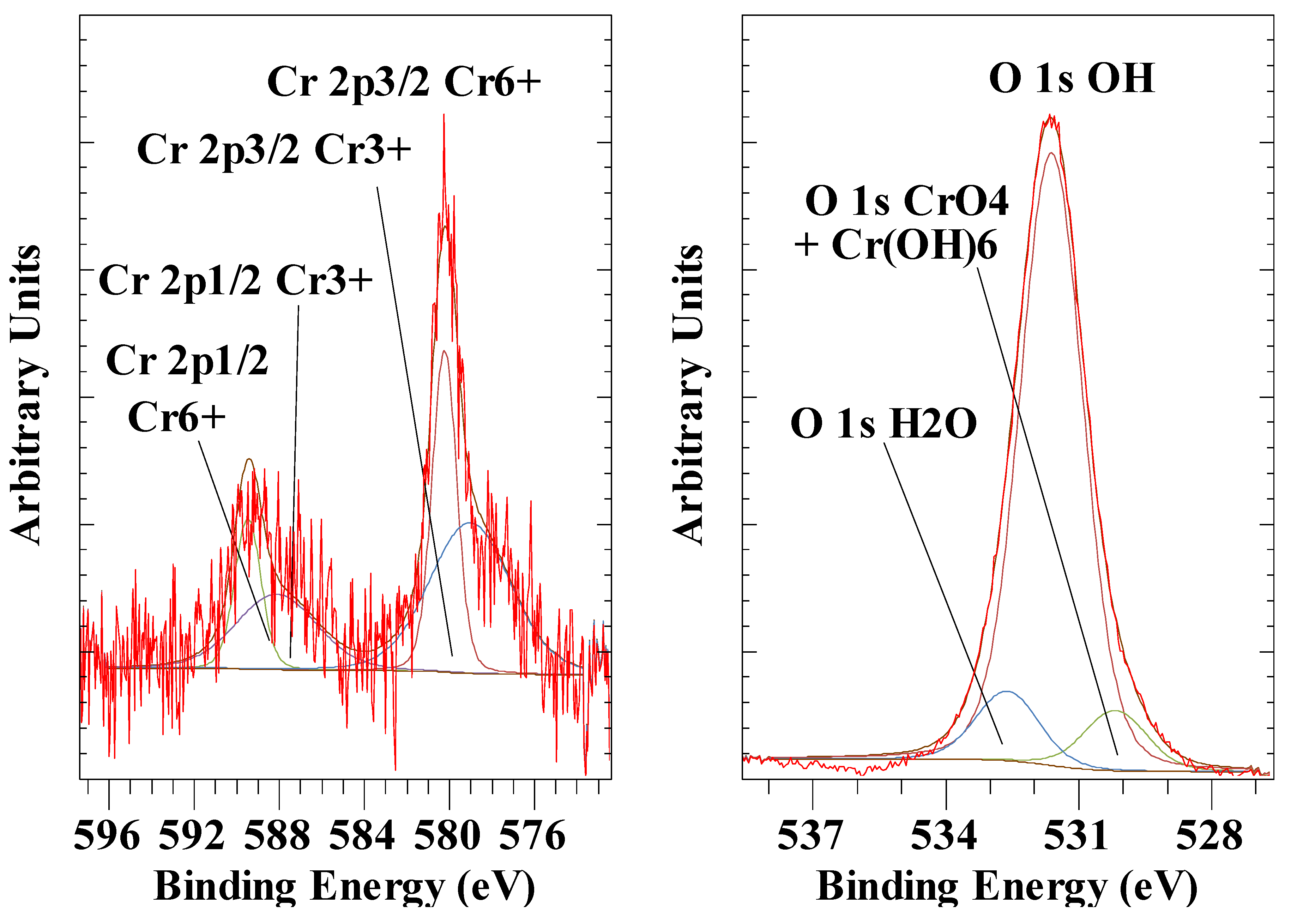
3.6. Chromate Anion
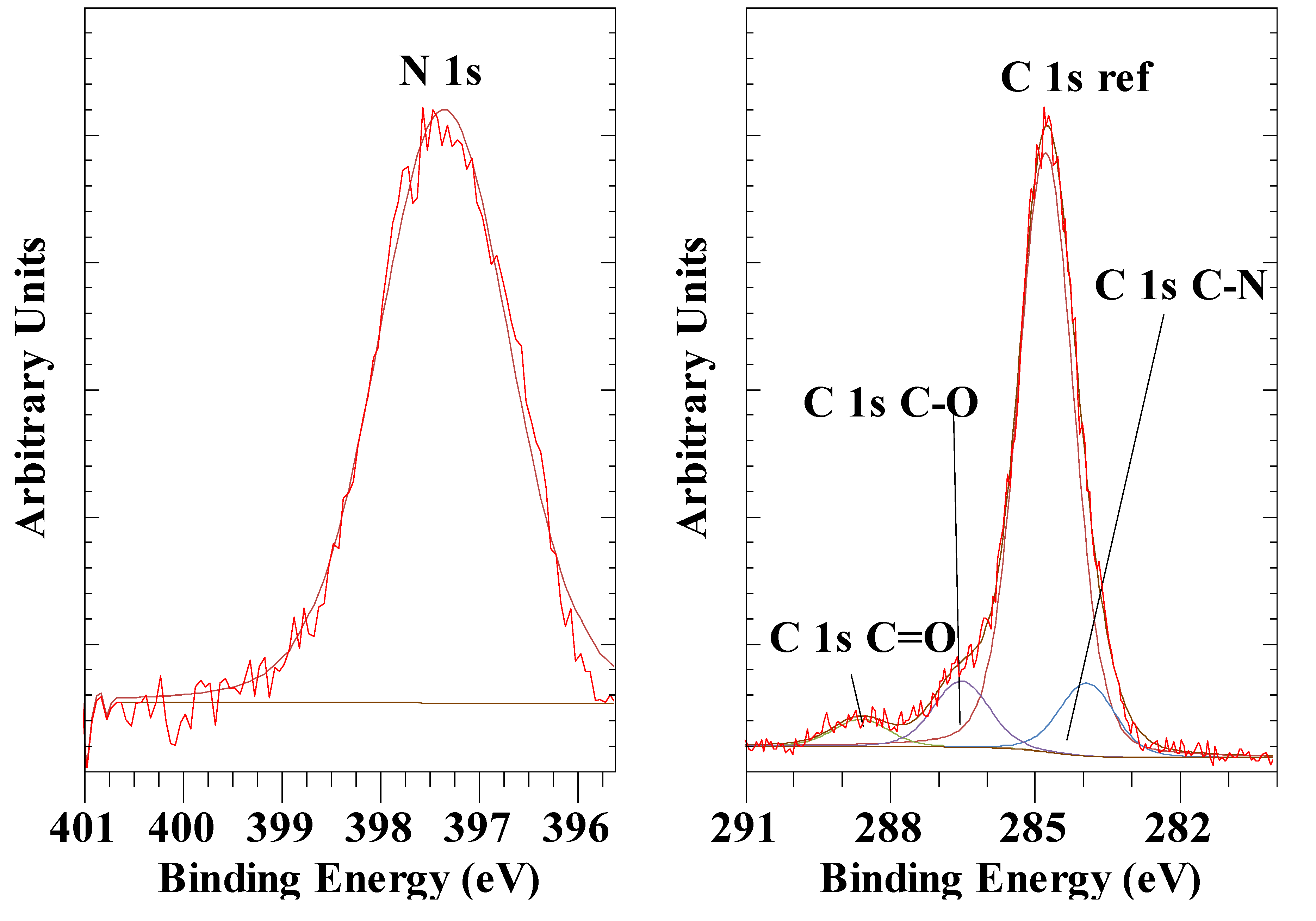
3.7. Hexacyanoferrate Anions
3.8. Influence of Interlayer Anions on the LDH Layer Structure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Applied Clay Science 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides, Duan, X., Evans, D.G., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2006; pp. 1–87. [Google Scholar]
- Wypych, F.; de Freitas, R.A. Chapter 10 - Layered double hydroxides and hydroxide salts: Structure and properties. In Developments in Clay Science, Wypych, F., de Freitas, R.A., Eds.; Elsevier: 2022; Volume 10, pp. 317-350.
- Miyata, S.; Kumura, T. Synthesis of new hydrotalcite-like compounds and their physico-chemical properties Chem. Letters 1973, 843–848. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and Catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpää, M.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Science of The Total Environment 2024, 912, 169160. [Google Scholar] [CrossRef] [PubMed]
- Kameliya, J.; Verma, A.; Dutta, P.; Arora, C.; Vyas, S.; Varma, R.S. Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications. Inorganics 2023, 11, 121. [Google Scholar] [CrossRef]
- Karmakar, A.K.; Hasan, M.S.; Sreemani, A.; Das Jayanta, A.; Hasan, M.M.; Tithe, N.A.; Biswas, P. A review on the current progress of layered double hydroxide application in biomedical sectors. The European Physical Journal Plus 2022, 137, 801. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent advances in the application of layered double hydroxides in analytical chemistry: A review. Analytica Chimica Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Zhou, B.; Meng, F.; Wang, Y.; Wen, G. Recent advance of layered double hydroxides materials: Structure, properties, synthesis, modification and applications of wastewater treatment. Journal of Environmental Chemical Engineering 2023, 11, 111191. [Google Scholar] [CrossRef]
- Nalawade, P.; Aware, B.N.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. Journal of Scientific & Industrial Research 2009, 68, 267–272. [Google Scholar]
- Evana, E.; Marchidan, R.; Mănăila, R. NO3– - CO32– anion exchange during washing of Ni-Al hydroxy- compounds. Bull.Soc. Chim. Belge 1992, 2, 101–107. [Google Scholar] [CrossRef]
- Miyata, S. The synthesis of hydrotalcite-like compounds and their structures and physico-chemical properties - I: the systems Mg2+-Al3+-NO3–, Mg2+-Al3+-Cl–, Mg2+-Al3+-ClO4–, Ni2+-Al3+-Cl– and Zn2+-Al3+-Cl–. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Miyata, S.; Okada, A. Synthesis of hydrotalcite-like compounds and their physico-chemical properties – the system Mg2+ - Al3+ - SO42– and Mg2+ - Al3+ - CrO42–. Clays Clay Miner. 1977, 25, 14–18. [Google Scholar] [CrossRef]
- Reichle, W.T.; Kang, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. J. Catalysis 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Musumeci, A.W.; Kloprogge, J.T.; Frost, R.L.; Martens, W.N. Synthesis and characterization of hydrotalcites containing interlayer sulphate, molybdate and chromate anions. In Proceedings of the 13th International Clay Conference, August 21-27, Tokyo, Japan; 2005; p. 127. [Google Scholar]
- Frost, R.L.; Musumeci, A.W.; Kloprogge, J.T.; Adebajo, M.O.; Martens, W.N. Raman spectroscopy of hydrotalcites with phosphate in the interlayer: implications for the removal of phosphate from water. J. Raman Spectrosc. 2006, 37, 733–741. [Google Scholar] [CrossRef]
- Frost, R.L.; Musumeci, A.W.; Martens, W.N.; Adebajo, M.O.; Bouzaid, J. Raman spectroscopy of hydrotalcites with sulphate, molybdate and chromate in the interlayer. J. Raman Spectrosc. 2005, 36, 925–931. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wharton, D.; Hickley, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32–, NO3–, SO42– and ClO4– in Mg/Al-hydrotalcite. Amer. Miner. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Ookubo, A.; Ooi, K.; Tani, F.; Hayashi, H. Phase Transition of Cl--Intercalated Hydrotalcite-like Compound during Ion Exchange with Phosphates. Langmuir 1994, 10, 407–411. [Google Scholar] [CrossRef]
- Palmer, S.J.; Soisonard, A.; Frost, R.L. Determination of the mechanism(s) for the inclusion of arsenate, vanadate, or molybdate anions into hydrotalcites with variable cationic ratio. J. Colloid Interface Sci. 2009, 329, 404–409. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Y.; Han, T.; Fang, M.; Chen, K.; Tan, X. Phosphate functionalized layered double hydroxides (phos-LDH) for ultrafast and efficient U(VI) uptake from polluted solutions. J. Hazard. Mater. 2020, 399, 123081. [Google Scholar] [CrossRef] [PubMed]
- Pálinkó, I.; Sipos, P.; Berkesi, O.; Varga, G. Distinguishing Anionic Species That Are Intercalated in Layered Double Hydroxides from Those Bound to Their Surface: A Comparative IR Study. The Journal of Physical Chemistry C 2022, 126, 15254–15262. [Google Scholar] [CrossRef]
- Radha, S.; Vishnu Kamath, P. Electronic spectra of anions intercalated in layered double hydroxides. Bulletin of Materials Science 2013, 36, 923–929. [Google Scholar] [CrossRef]
- Mora, M.; Jiménez-Sanchidrián, C.; Rafael Ruiz, J. Raman spectroscopy study of layered-double hydroxides containing magnesium and trivalent metals. Materials Letters 2014, 120, 193–195. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, Y.; Zhang, L.; Zhang, T.; Dong, G.; Li, D.; Cheng, S.; Ma, S.; Song, S.; Quintana, M. Anion regulation strategy of lithium-aluminum layered double hydroxides for strengthening resistance to deactivation in lithium recovery from brines. Chemical Engineering Journal 2023, 472, 145026. [Google Scholar] [CrossRef]
- Ciocan, C.E.; Dumitriu, E.; Cacciaguerra, T.; Fajula, F.; Hulea, V. New approach for synthesis of Mo-containing LDH based catalysts. Catalysis Today 2012, 198, 239–245. [Google Scholar] [CrossRef]
- Schutz, A.; Biloen, P. Interlamellar chemistry of hydrotalcites. I. Polymerzation of silcate anions. J. Solid State Chem. 1987, 68, 360–368. [Google Scholar] [CrossRef]
- Amini, R.; Rahimpour, E.; Jouyban, A. An optical sensing platform based on hexacyanoferrate intercalated layered double hydroxide nanozyme for determination of chromium in water. Anal. Chim. Acta 2020, 1117, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Musumeci, A.W.; Bouzaid, J.; Adebajo, M.O.; Martens, W.N.; Theo Kloprogge, J. Intercalation of hydrotalcites with hexacyanoferrate(II) and (III)—a thermoRaman spectroscopic study. J. Solid State Chem. 2005, 178, 1940–1948. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Koch, C.B. Synthesis and Properties of Hexacyanoferrate Interlayered in Hydrotalcite. I. Hexacyanoferrate(II). Clays Clay Miner. 1994, 42, 170–179. [Google Scholar] [CrossRef]
- Idemura, S.; Suzuki, E.; Ono, Y. Electronic State of Iron Complexes in the Interlayer of Hydrotalcite-Like Materials. Clays Clay Miner. 1989, 37, 553–557. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.; Wang, R.C.; Colon, J.L.; Cearfield, A. Systematic preparation of polyoxometalate pillared layered double hydroxides via direct aqueous reaction. Mater. Res. Soc. Symp. Proc. 1991, 233, 63–80. [Google Scholar] [CrossRef]
- Carpani, I.; Berrettoni, M.; Ballarin, B.; Giorgetti, M.; Scavetta, E.; Tonelli, D. Study on the intercalation of hexacyanoferrate(II) in a Ni, Al based hydrotalcite. Solid State Ionics 2004, 168, 167–175. [Google Scholar] [CrossRef]
- Crespo, I.; Barriga, C.; Rives, V.; Ulibarri, M.A. Intercalation of iron hexacyano complexes in zn,al-hydrotalcite. Solid State Ionics 1997, 101-103, 729–735. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Applied Biological Chemistry 2019, 62, 12. [Google Scholar] [CrossRef]
- Bao, W.; Tang, Y.; Yu, J.; Yan, W.; Wang, C.; Li, Y.; Wang, Z.; Yang, J.; Zhang, L.; Yu, F. Si-doped ZnAl-LDH nanosheets by layer-engineering for efficient photoelectrocatalytic water splitting. Applied Catalysis B: Environment and Energy 2024, 346, 123706. [Google Scholar] [CrossRef]
- Lv, H.; Rao, H.; Liu, Z.; Zhou, Z.; Zhao, Y.; Wei, H.; Chen, Z. NiAl layered double hydroxides with enhanced interlayer spacing via ion-exchange as ultra-high performance supercapacitors electrode materials. Journal of Energy Storage 2022, 52, 104940. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Pan, Q.; Ba, J.; Tang, T.; Luo, W. One-Step Synthesis of NiFe Layered Double Hydroxide Nanosheet Array/N-Doped Graphite Foam Electrodes for Oxygen Evolution Reactions. ChemistryOpen 2019, 8, 1027–1032. [Google Scholar] [CrossRef]
- Mahmoud, R.K.; Taha, M.; Zaher, A.; Amin, R.M. Understanding the physicochemical properties of Zn–Fe LDH nanostructure as sorbent material for removing of anionic and cationic dyes mixture. Scientific Reports 2021, 11, 21365. [Google Scholar] [CrossRef]
- Li, X.; Fortunato, M.; Cardinale, A.M.; Sarapulova, A.; Njel, C.; Dsoke, S. Electrochemical study on nickel aluminum layered double hydroxides as high-performance electrode material for lithium-ion batteries based on sodium alginate binder. Journal of Solid State Electrochemistry 2022, 26, 49–61. [Google Scholar] [CrossRef]
- Shen, W.; Hu, T.; Liu, X.; Zha, J.; Meng, F.; Wu, Z.; Cui, Z.; Yang, Y.; Li, H.; Zhang, Q.; et al. Defect engineering of layered double hydroxide nanosheets as inorganic photosensitizers for NIR-III photodynamic cancer therapy. Nature Communications 2022, 13, 3384. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Fourier Transform Infrared and Raman spectroscopic study of the local structure of Mg, Ni and Co — hydrotalcites. J.Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Frost, R.L.; Musumeci, A.W.; Kloprogge, J.T.; Weier, M.; Adebajo, M.; Martens, W.N. Thermal decomposition of hydrotalcite with hexacyanoferrate(II) and hexacyanoferrate(III) anions in the interlayer. J. Therm. Anal. Cal. 2006, 86, 205–209. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wood, B.J. Handbook of Mineral Spectroscopy Volume 1 X-ray Photoelectron Spectra; Elsevier: Amsterdam, 2020; Volume 1, p. 505. [Google Scholar]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Peng, C.; Yu, J.; Zhao, Z.; Dai, J.; Fu, J.; Zhao, M.; Wang, W. Synthesis and Properties of a Clean and Sustainable Deicing Additive for Asphalt Mixture. PLoS ONE 2015, 10, e0115721. [Google Scholar] [CrossRef]
- Rey, F.; Fornes, V.; Rojo, J.M. Thermal decomposition of hydrotalcites. An infrared and nuclear magnetic resonance spectroscopic study. J. Chem. Soc., Faraday Trans., 1992, 88, 2233–2238. [Google Scholar] [CrossRef]
- Alzamora, L.E.; Ross, J.R.; Kruissink, E.C.; Reijen, L.L.v. Coprecipitated Nickel-Alumina catalysts for methanation at high temperature. J. Chem. Soc., Faraday Trans. I 1981, 77, 665–681. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ponce, C.P.; Ortillo, D.O. X-ray Photoelectron Spectroscopic Study of Some Organic and Inorganic Modified Clay Minerals. Materials 2021, 14, 7115. [Google Scholar] [CrossRef]
- Naumkin, A., V; Kraut-Vass, A.; Gaarenstroom, S.W.; C.J., P. NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 29 November).
- Gupta, N.K.; Saifuddin, M.; Kim, S.; Kim, K.S. Microscopic, spectroscopic, and experimental approach towards understanding the phosphate adsorption onto Zn–Fe layered double hydroxide. J. Mol. Liquids 2020, 297, 111935. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Constantino, V.R.L.; Pinto, F.G.; Vergütz, L.; Tronto, J.; Da Costa, L.M. Layered Double Hydroxides: New Technology in Phosphate Fertilizers Based on Nanostructured Materials. ACS Sustain. Chem. Eng. 2017, 5, 399–409. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Eulálio, D.; Guimarães, L.D.M.; Pinto, F.G.; Costa, L.M.D.; Tronto, J. Layered Double Hydroxides as Hosting Matrices for Storage and Slow Release of Phosphate Analyzed by Stirred-Flow Method. Mater. Res. 2018, 21, e20171004. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.-g.; Yang, Y.-m.; Yu, S.-j.; Shan, R.-r.; Yu, H.-q.; Zhu, B.-c.; Du, B. Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: Kinetics, isotherms and mechanisms. Sep. Pur. Techn. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Ross, S.D. Phosphates and other Oxy-anions of Group V. In The Infrared Spectra of Minerals, Farmer, V.C., Ed.; Mineralogical Society of Great Britain and Ireland: 1974; Volume 4, pp. 383-422.
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Shabanian, M.; Hajibeygi, M.; Raeisi, A. 2 - FTIR characterization of layered double hydroxides and modified layered double hydroxides. In Layered Double Hydroxide Polymer Nanocomposites, Thomas, S., Daniel, S., Eds.; Woodhead Publishing: 2020; pp. 77-101.
- Cheng, X.; Huang, X.; Wang, X.; Sun, D. Influence of calcination on the adsorptive removal of phosphate by Zn–Al layered double hydroxides from excess sludge liquor. J. Hazard. Mater. 2010, 177, 516–523. [Google Scholar] [CrossRef]
- He, H.; Kang, H.; Ma, S.; Bai, Y.; Yang, X. High adsorption selectivity of ZnAl layered double hydroxides and the calcined materials toward phosphate. J. Colloid Interf. Sci. 2010, 343, 225–231. [Google Scholar] [CrossRef]
- Bish, D.L.; Livingstone, A. The crystal chemistry and paragenesis of honessite and hydrohonessite: the sulfate analogues of reevesite. Miner. Mag. 1981, 44, 339–343. [Google Scholar] [CrossRef]
- Nickel, E.H.; Clarke, R.M. Carrboydite, a hydrated sulfate of nickel and aluminum: a new mineral from Western Australia. Amer. Miner. 1976, 61, 366–372. [Google Scholar]
- Nickel, E.H.; Wildman, J.E. Hydrohonessite- a new hydrated Ni-Fe hydroxy-sulphate mineral; its relationship to honessite, carrboydite, and minerals of the pyroaurite group. Miner. Mag. 1981, 44, 333–337. [Google Scholar] [CrossRef]
- Wahlqvist, M.; Shchukarev, A. XPS spectra and electronic structure of Group IA sulfates. J. Electr. Spectros. Rel. Phenom. 2007, 156-158, 310–314. [Google Scholar] [CrossRef]
- Ross, S.D. Inorganic Infrared and Raman Spectra; McGraw-Hill Book Company: London, 1972; p. 140. [Google Scholar]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. Synthesis and spectroscopic characterization of deuterated hydrotalcite. J. Mater. Sci. Letters 2001, 21, 603–605. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W. Mechanosynthesis and characterization of Hydrotalcite like Mg–Al–SO4-LDH. Mater. Letters 2016, 165, 192–195. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z. Intercalation of sulfate anions into a Zn–Al layered double hydroxide: their synthesis and application in Zn–Ni secondary batteries. RSC Advanc. 2016, 6, 68584–68591. [Google Scholar] [CrossRef]
- Frost, R.L.; Theiss, F.L.; López, A.; Scholz, R. Vibrational spectroscopic study of the sulphate mineral glaucocerinite (Zn,Cu)10Al6(SO4)3(OH)32⋅18H2O – A natural layered double hydroxide. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2014, 127, 349–354. [Google Scholar] [CrossRef]
- Dutta, P.K.; Puri, M. Anion exchange in lithium aluminate hydroxides. J. Phys. Chem. 1989, 93, 376–381. [Google Scholar] [CrossRef]
- Bish, D.L. Anion-exchange in takovite: applications to other hydroxide minerals. Bull. Miner. 1980, 103, 170–175. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Adebajo, M.O.; Kloprogge, J.T.; Martens, W.N.; Frost, R.L. X-ray diffraction and Raman spectroscopic studies of Zn-substituted carrboydite-like compounds. Mater. Chem. Phys. 2006, 100, 174–186. [Google Scholar] [CrossRef]
- Thao, N.T.; Trung, N.D.; Van Long, D. Activity of Molybdate-Intercalated Layered Double Hydroxides in the Oxidation of Styrene with Air. Catal. Letters 2016, 146, 918–928. [Google Scholar] [CrossRef]
- Behera, G.C.; Parida, K.M. A comparative study of molybdenum promoted vanadium phosphate catalysts towards epoxidation of cyclohexene. Appl. Catal. A: General 2013, 464-465, 364–373. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Mendoza-Sanchez, B.; Fernandez, V.; Veenstra, R.; Dukstiene, N.; Roberts, A.; Fairley, N. Generalized molybdenum oxide surface chemical state XPS determination via informed amorphous sample model. Appl. Surf. Sci. 2015, 326, 151–161. [Google Scholar] [CrossRef]
- Klemkaitė-Ramanauskė, K.; Žilinskas, A.; Taraškevičius, R.; Khinsky, A.; Kareiva, A. Preparation of Mg/Al layered double hydroxide (LDH) with structurally embedded molybdate ions and application as a catalyst for the synthesis of 2-adamantylidene(phenyl)amine Schiff base. Polyhedron 2014, 68, 340–345. [Google Scholar] [CrossRef]
- Mitchell, P.C.H.; Wass, S.A. Propane dehydrogenation over molybdenum hydrotalcite catalysts. Appl. Catal. A: General 2002, 225, 153–165. [Google Scholar] [CrossRef]
- Nejati, K.; Akbari, A.R.; Davari, S.; Asadpour-Zeynali, K.; Rezvani, Z. Zn–Fe-layered double hydroxide intercalated with vanadate and molybdate anions for electrocatalytic water oxidation. New J. Chem. 2018, 42, 2889–2895. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Zhang, M.; Yang, P.; Yang, L.; Cao, D.; Li, J. One-step synthesis of lamellar molybdate pillared hydrotalcite and its application for AZ31 Mg alloy protection. Solid State Sci. 2009, 11, 376–381. [Google Scholar] [CrossRef]
- Ross, S.D. Sulphates and other Oxy-anions of Group VI. In The Infrared Spectra of Minerals, Farmer, V.C., Ed.; Mineralogical Society of Great Britain and Ireland: London, 1974; Volume 4, pp. 423–444. [Google Scholar]
- Colombo, K.; Maruyama, S.; Yamamoto, C.; Wypych, F. Intercalation of Molybdate Ions into Ni/Zn Layered Double Hydroxide Salts: Synthesis, Characterization, and Preliminary Catalytic Activity in Methyl Transesterification of Soybean Oil. J. Brazil. Chem. Soc. 2016, 28, 1315–1322. [Google Scholar] [CrossRef]
- Alidokht, L.; Oustan, S.; Khataee, A.; Neyshabouri, M.; Reyhanitabar, A. Removal of chromate from aqueous solution by reduction with nanoscale Fe–Al layered double hydroxide. Res. Chem. Intermed. 2018, 44, 2319–2331. [Google Scholar] [CrossRef]
- Treverton, J.A.; Davies, N.C. An XPS study of chromate pretreatment of aluminium. Metals Techn. 1977, 4, 480–489. [Google Scholar] [CrossRef]
- Amonette, J.E.; Rai, D. Identification of Noncrystalline (Fe,Cr)(OH)3 by Infrared Spectroscopy. Clays Clay Miner. 1990, 38, 129–136. [Google Scholar] [CrossRef]
- Gomes, A.S.O.; Yaghini, N.; Martinelli, A.; Ahlberg, E. A micro-Raman spectroscopic study of Cr(OH)3 and Cr2O3 nanoparticles obtained by the hydrothermal method. J. Raman Spectrosc. 2017, 48, 1256–1263. [Google Scholar] [CrossRef]
- Del Arco, M.; Carriazo, D.; Martín, C.; Pérez Grueso, A.M.; Rives, V. Characterization of Chromate-Intercalated Layered Double Hydroxides. Mater. Sci. Forum 2006, 514-516, 1541–1545. [Google Scholar] [CrossRef]
- Prasanna, S.V.; Vishnu Kamath, P. Chromate uptake characteristics of the pristine layered double hydroxides of Mg with Al. Solid State Sci. 2008, 10, 260–266. [Google Scholar] [CrossRef]
- Prasanna, S.V.; Rao, R.A.P.; Kamath, P.V. Layered double hydroxides as potential chromate scavengers. J. Colloid Interf. Sci. 2006, 304, 292–299. [Google Scholar] [CrossRef]
- Ross, S.D. Inorganic infrared and Raman spectra; McGraw-Hill: London, 1972; p. 414. [Google Scholar]
- Holgado, M.J.; Rives, V.; Sanromán, M.S.; Malet, P. Hexacyanoferrate-interlayered hydrotalcite. Solid State Ion. 1996, 92, 273–283. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Kikkawa, S.; Koizumi, M. Ferrocyanide anion bearing Mg, Al hydroxide. Mater. Res. Bull. 1982, 17, 191–198. [Google Scholar] [CrossRef]
- Idemura, S.; Suzuki, E.; Ono, Y. Electronic State of Iron Complexes in the Interlayer of Hydrotalcite-Like Materials. Clays Clay Miner. 1989, 37, 553–557. [Google Scholar] [CrossRef]
- Mao, G.; Tsuji, M.; Tamaura, Y. Synthesis and CO2 Adsorption Features of a Hydrotalcite-Like Compound of the Mg2+-Al3+-Fe(CN)64- System with High Layer-Charge Density. Clays Clay Miner. 1993, 41, 731–737. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. Intercalation of Hexacyanoferrate(III) Ions in Layered Double Hydroxides: A Novel Precursor To Form Ferri-/Antiferromagnetic Exchange Coupled Oxides and Monodisperse Nanograin Spinel Ferrites. J Phys Chem C 2009, 113, 9560–9567. [Google Scholar] [CrossRef]
- Meng, W. , Li, F.; Evans, D.G.; Duan, X. Preparation and thermal decomposition of magnesium/iron(III) layered double hydroxide intercalated by hexacyanoferrate(III) ions. J. Mater. Sci. 2004, 39, 4655–4657. [Google Scholar] [CrossRef]
- Yao, K.; Taniguchi, M.; Nakata, M.; Shimazu, K.; Takahashi, M.; Yamagishi, A. Mass transport on an anionic clay-modified electrode as studied by a quartz crystal microbalance. J. Electroanal. Chem. 1998, 457, 119–128. [Google Scholar] [CrossRef]
- Crespo, I.; Barriga, C.; Rives, V.; Ulibarri, M.A. Intercalation of iron hexacyano complexes in Zn,Al-hydrotalcite. Solid State Ion. 1997, 101-103, 729–735. [Google Scholar] [CrossRef]
- Fernández, J.M.; Ulibarri, M.A.; Labajos, F.M.; Rives, V. The effect of iron on the crystalline phases formed upon thermal decomposition of Mg-Al-Fe hydrotalcites. J. Mater. Chem. 1998, 8, 2507–2514. [Google Scholar] [CrossRef]
- Gordon, B.M.; Williams, L.L.; Sutin, N. The Kinetics of the Oxidation of Iron(II) Ions and of Coördination Complexes1a. J. Amer. Chem. Soc. 1961, 83, 2061–2064. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Mentasti, E.; Baiocchi, C. Kinetics and mechanism of oxidation of quinols by hexachloroiridate(IV) in aqueous acidic perchlorate media. J. Phys. Chem. 1976, 80, 2979–2982. [Google Scholar] [CrossRef]
- Jones, L.H. Nature of Bonding in Metal Cyanide Complexes as Related to Intensity and Frequency of Infrared Absorption Spectra. Inorg. Chem. 1963, 2, 777–780. [Google Scholar] [CrossRef]
- Braterman, P.S.; Tan, C.; Zhao, J. Orientational effects in the infrared spectrum of the double layer material, magnesium aluminum hydroxide ferrocyanide. Mater. Res. Bull. 1994, 29, 1217–1221. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S.; Brister, B.D.; Wang, Z.; Yarberry, F. Physical and Chemical Interactions between Mg:Al Layered Double Hydroxide and Hexacyanoferrate. J. Solid State Chem. 2001, 161, 249–258. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Weier, M.; Crespo, I.; Ulibarri, M.A.; Barriga, C.; Rives, V.; Martens, W.N.; Frost, R.L. Intercalation of iron hexacyano complexes in Zn,Al hydrotalcite. Part 2. A mid-infrared and Raman spectroscopic study. J. Solid State Chem. 2004, 177, 1382–1387. [Google Scholar] [CrossRef]
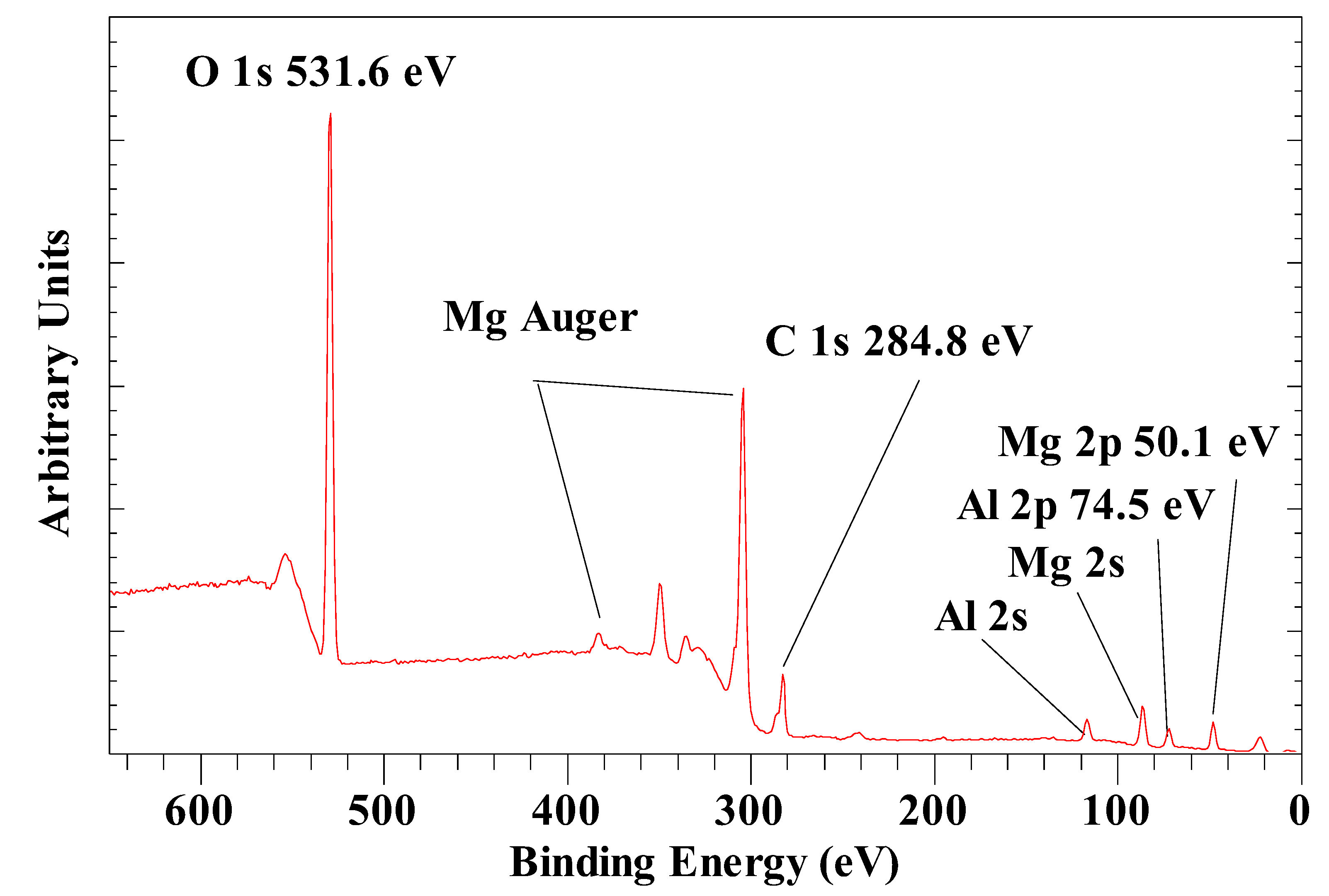
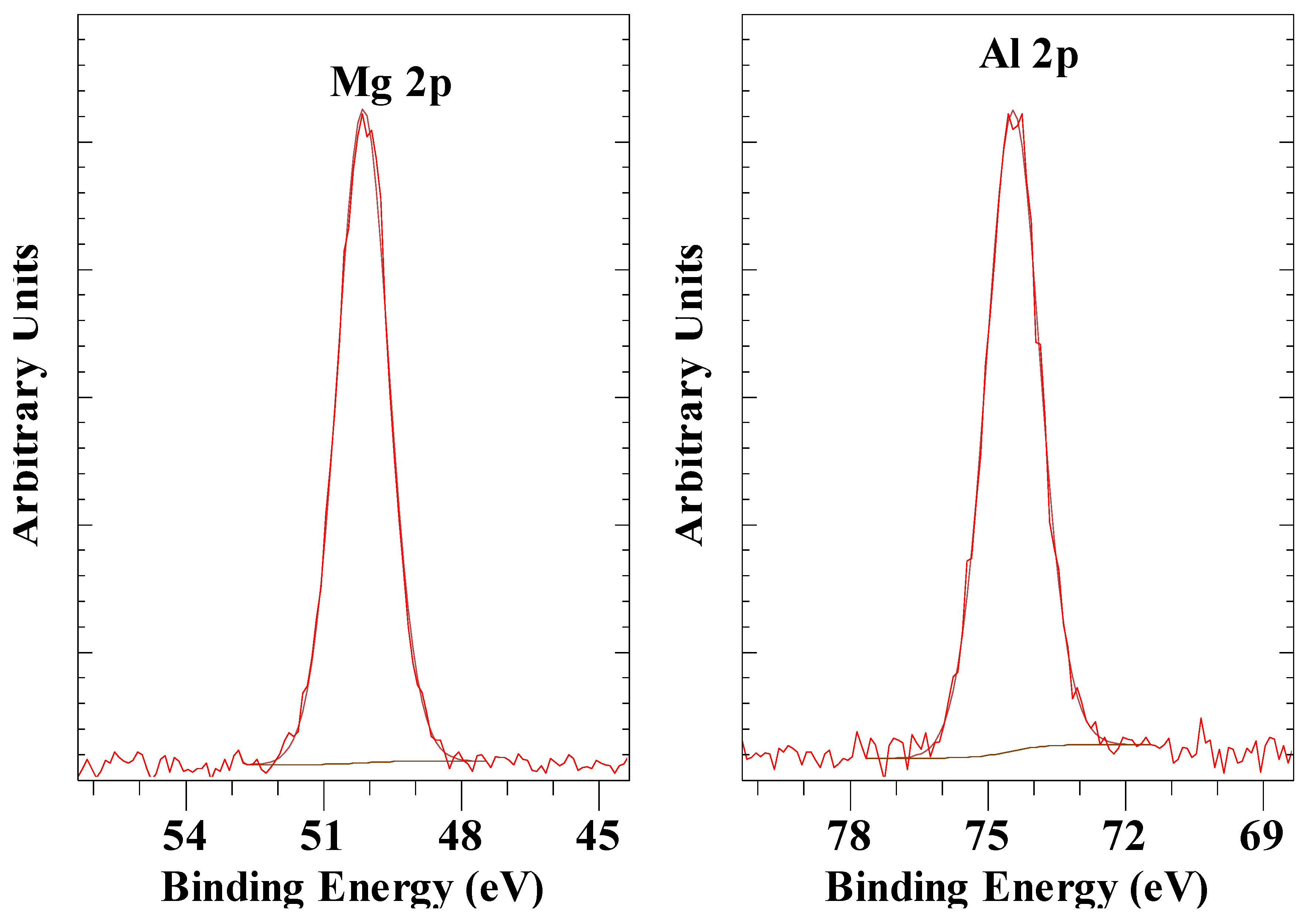
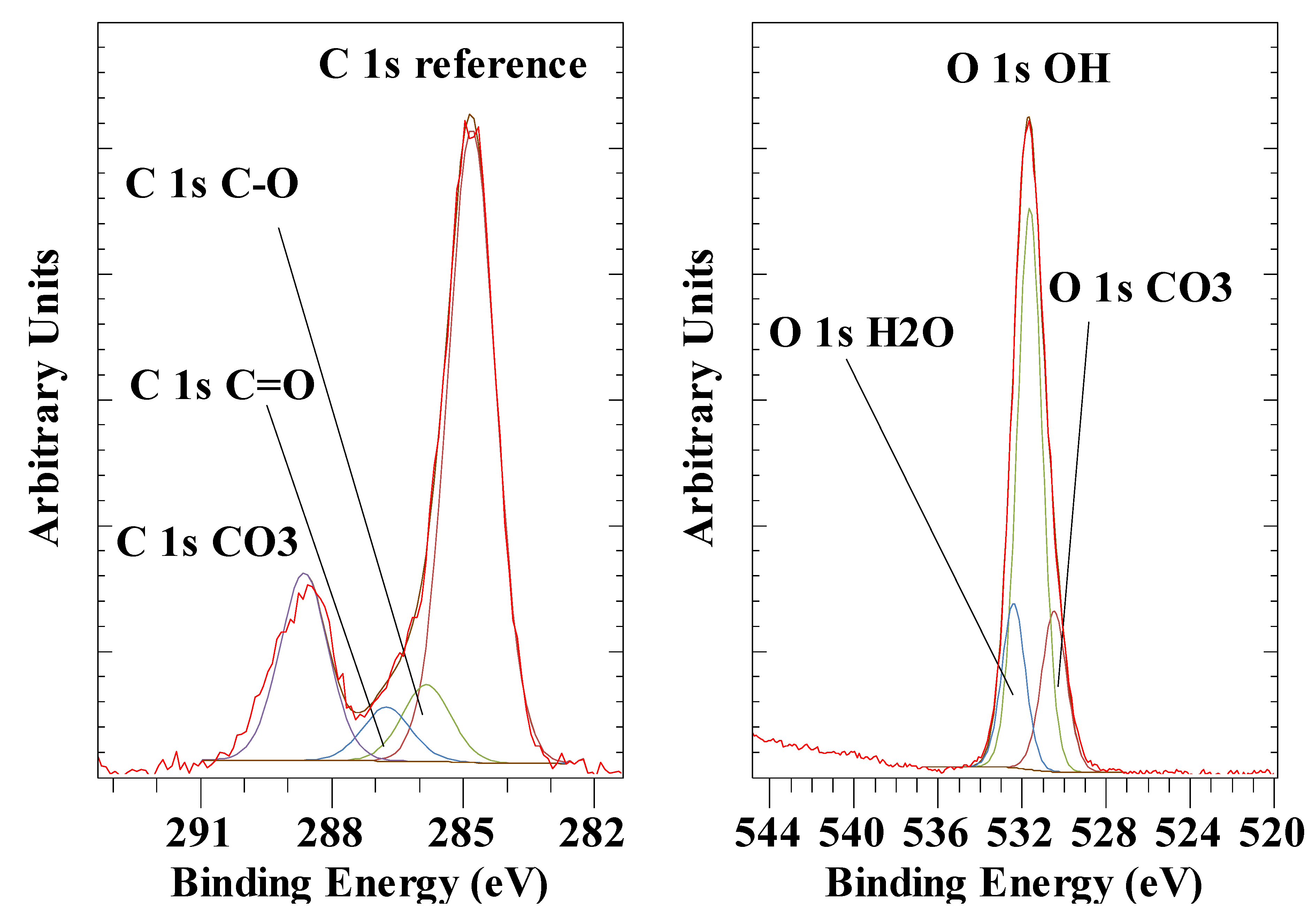
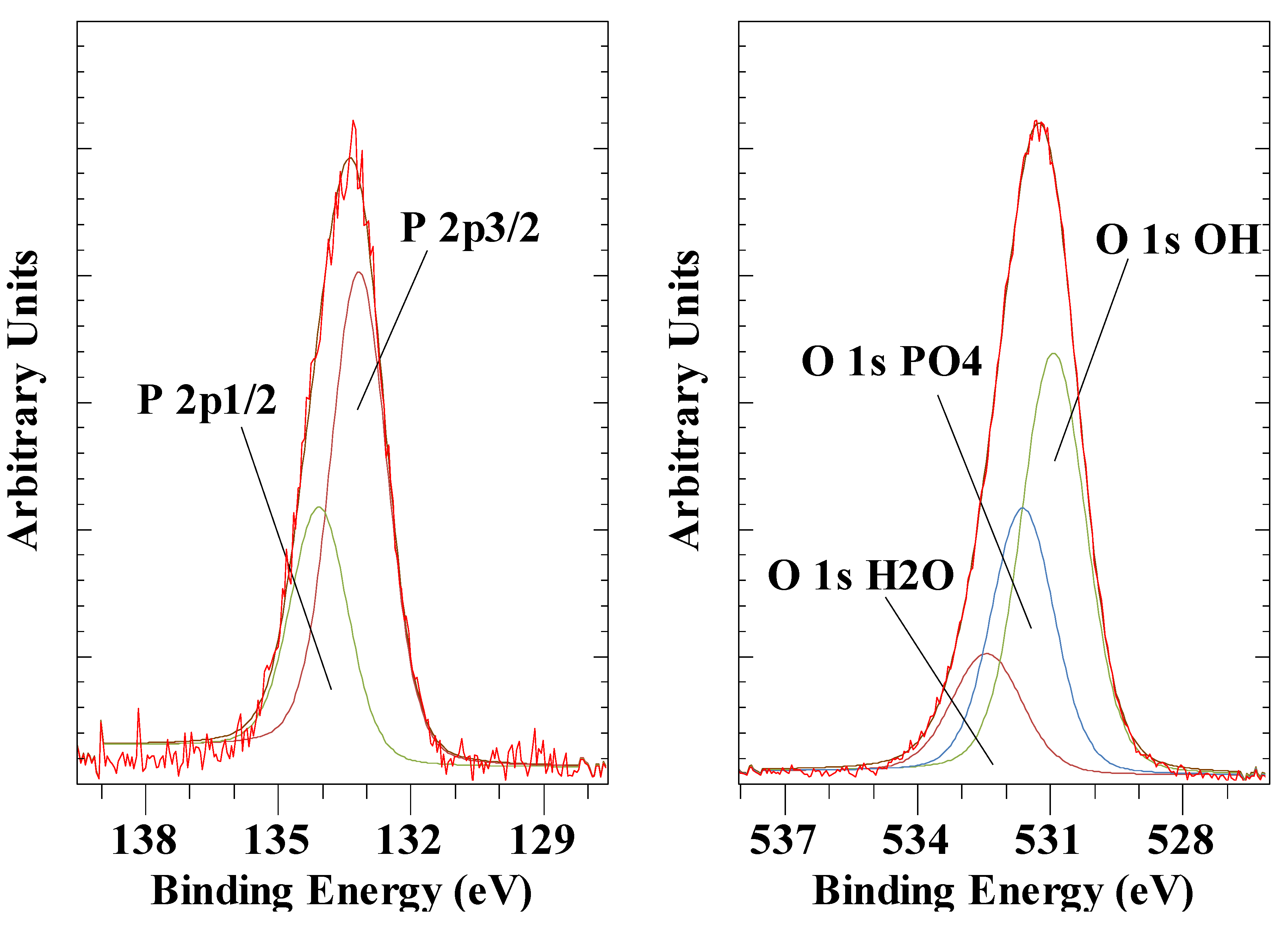
| Anion | Al 2p | Mg 2p | O 1s OH |
| CO32- | 74.5 | 50.1 | 531.6 |
| PO43- pH 11.9 | 74.4 | 50.1 | 531.7 |
| PO43- pH 12.5 | 74.5 | 50.1 | 531.7 |
| SO42- | 74.3 | 49.9 | 531.7 |
| MoO42- | 74.2 | 49.8 | 531.5 |
| CrO42- | 74.3 | 49.9 | 531.6 |
| Fe(CN)64- | 74.4 | 50.0 | 531.7 |
| Fe(CN)63- | 74.4 | 50.0 | 531.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





