Submitted:
28 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Initial Characterization of the Samples
2.1.1. TEM Studies
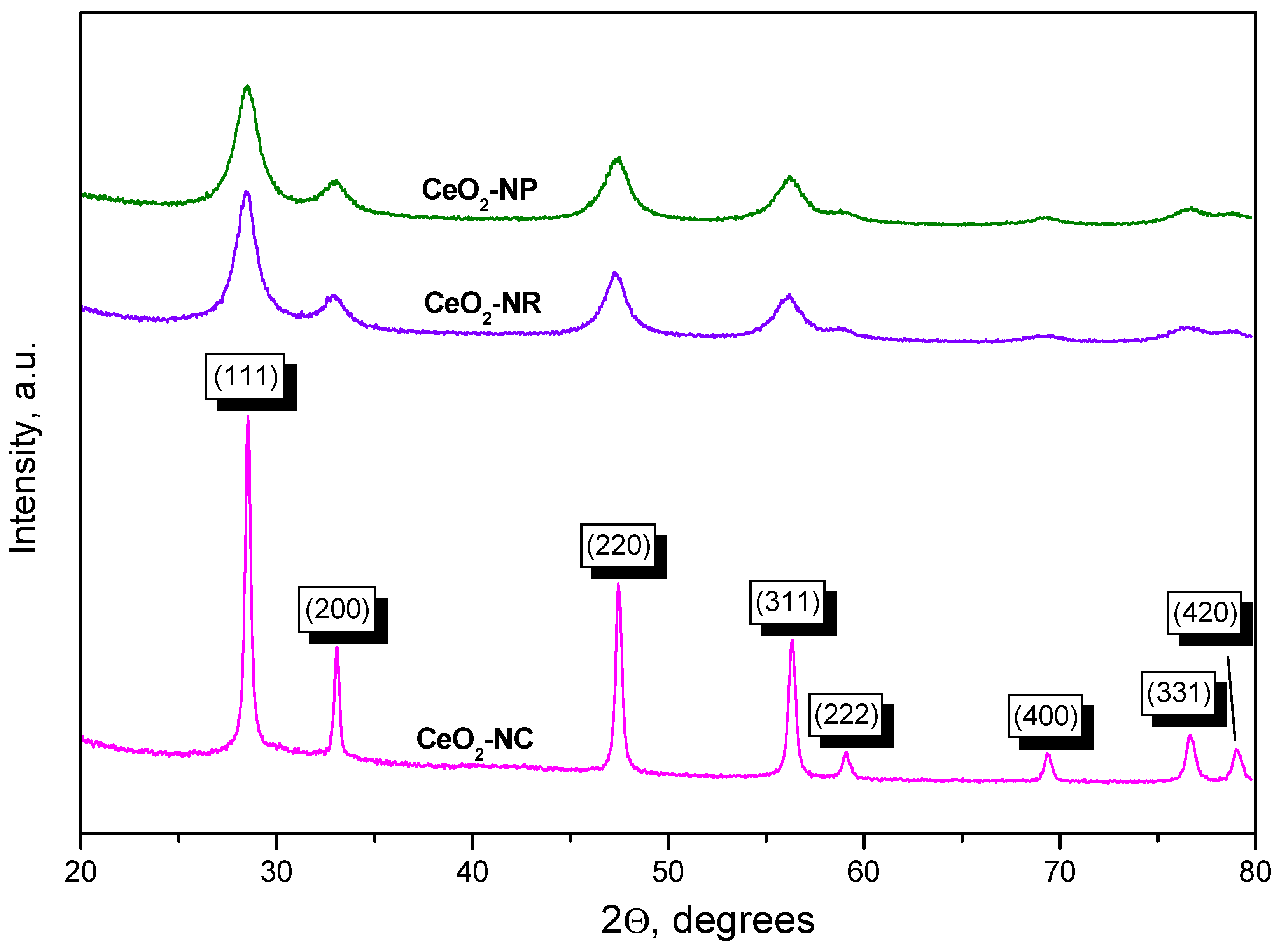
2.1.2. XRD Studies
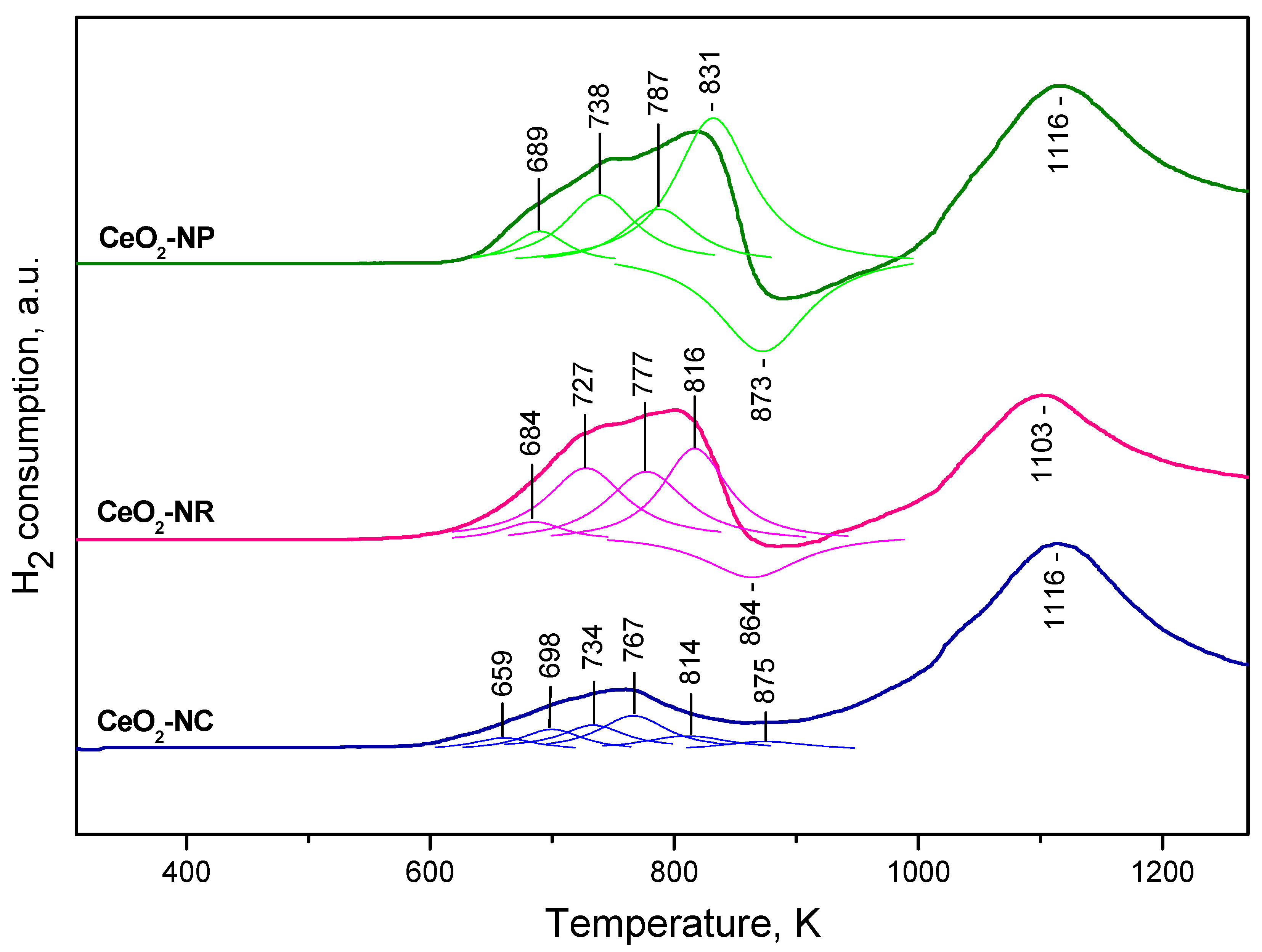
2.1.3. TPR Studies
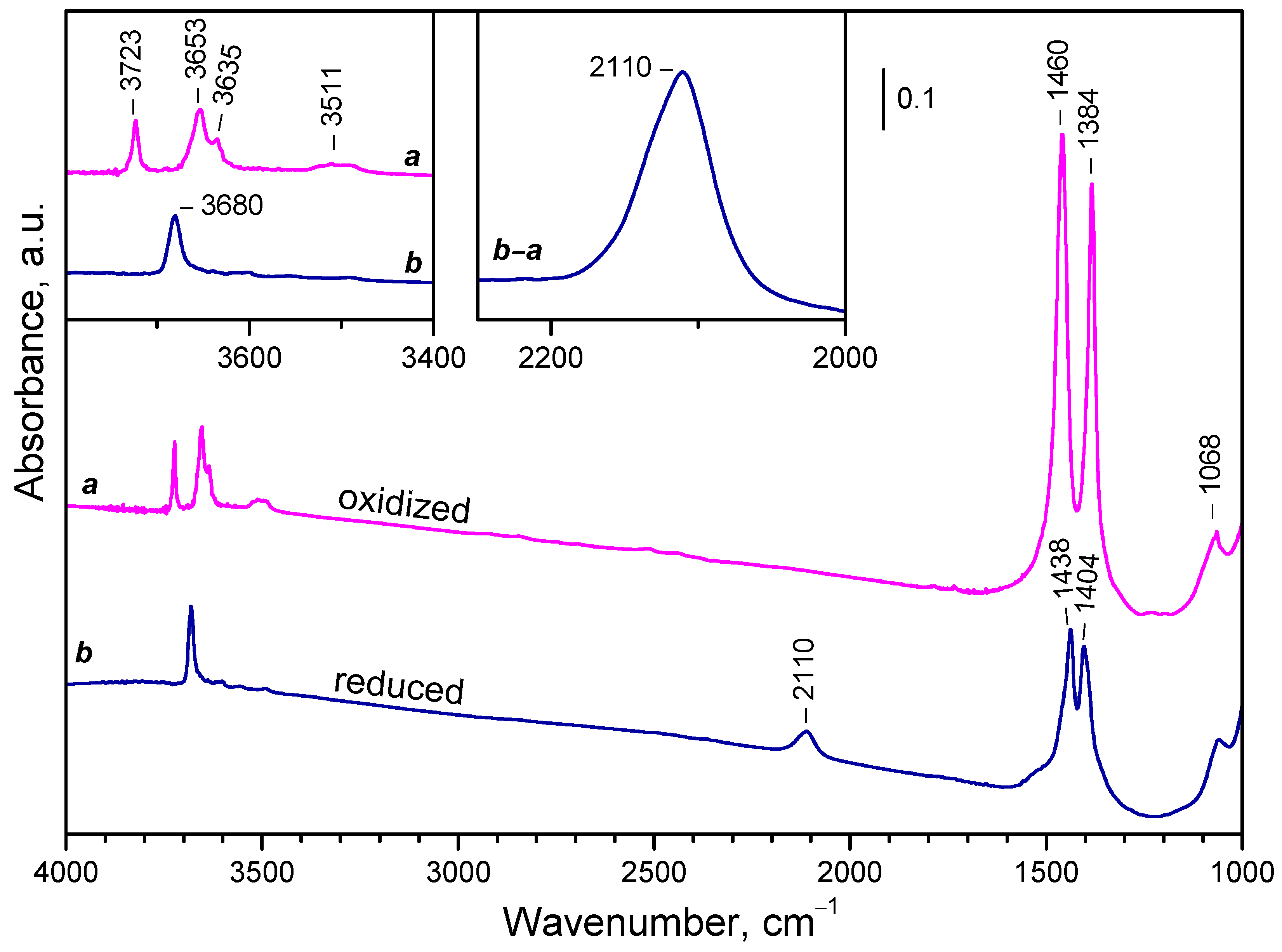
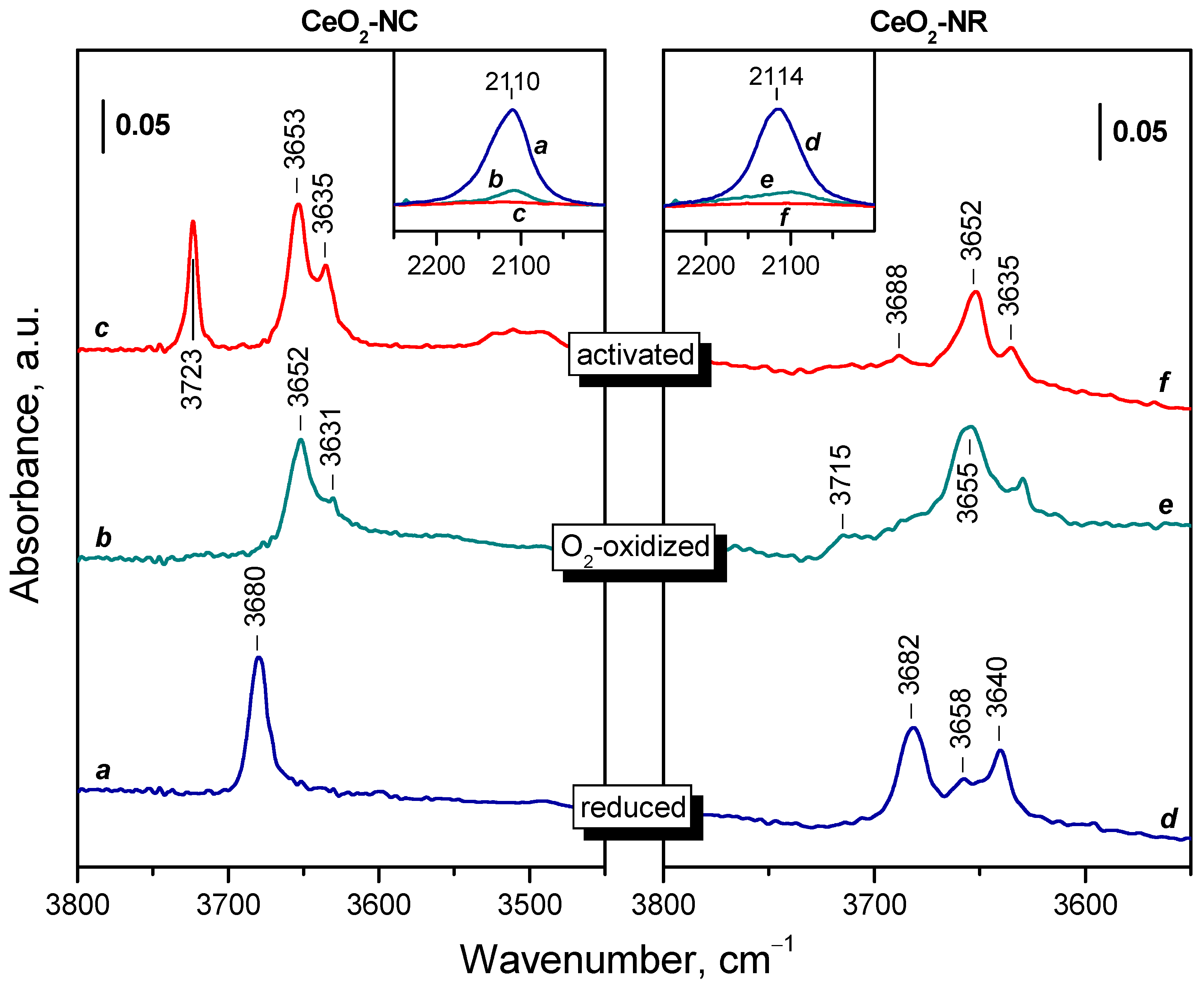
2.2. Background IR Spectra of Oxidized and Reduced Samples
2.2.1. CeO2-NC
2.2.2. CeO2-NR and CeO2-NP
2.2.3. Temperature Changes of the IR Spectra
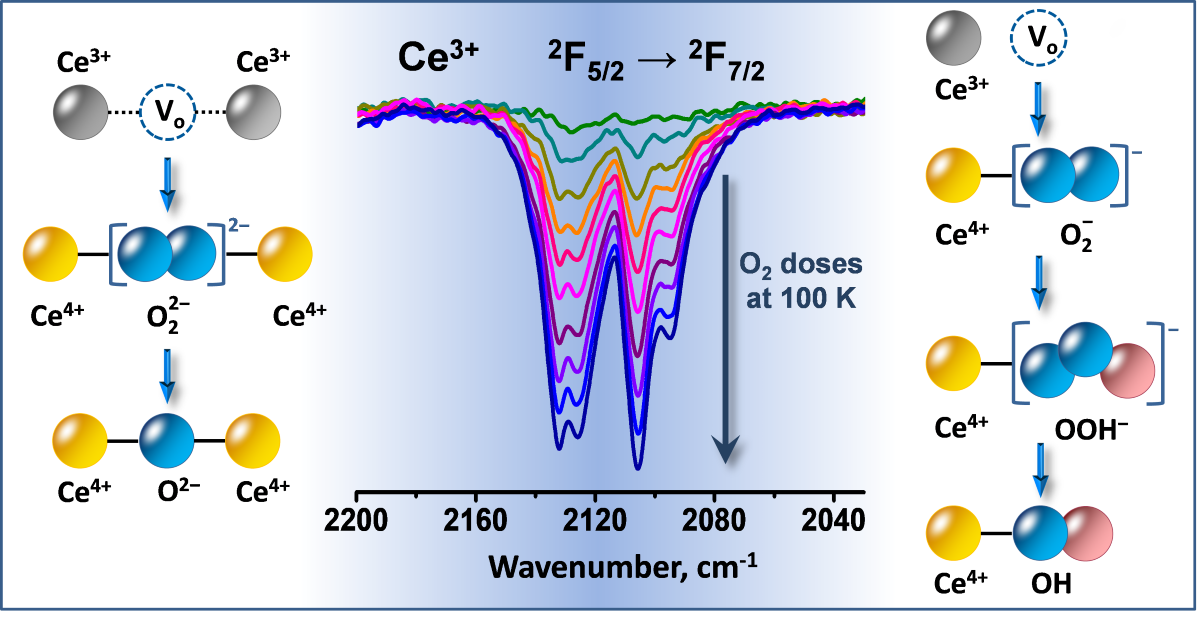
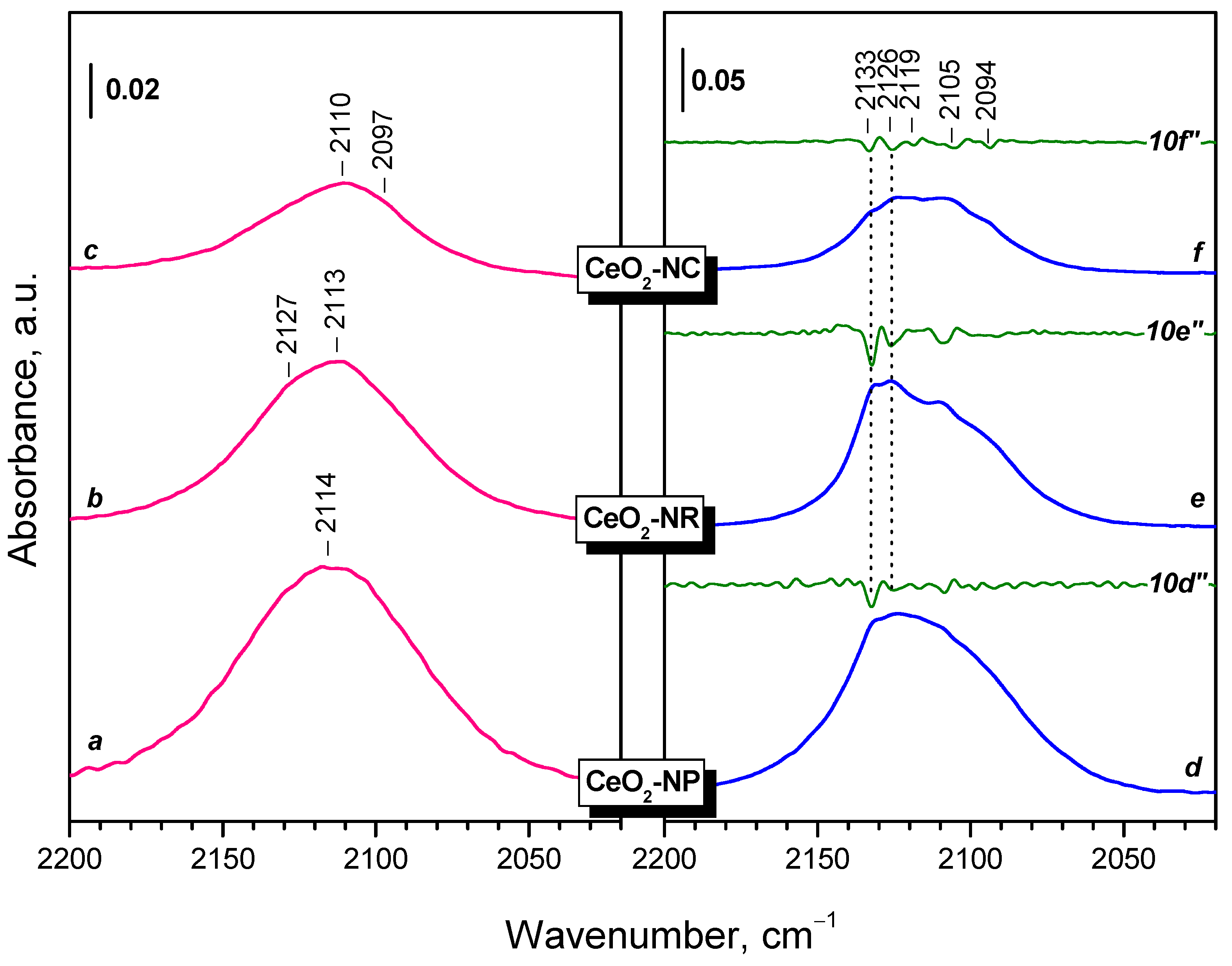
2.3. Interaction of Activated Samples with O2 at Variable Temperatures
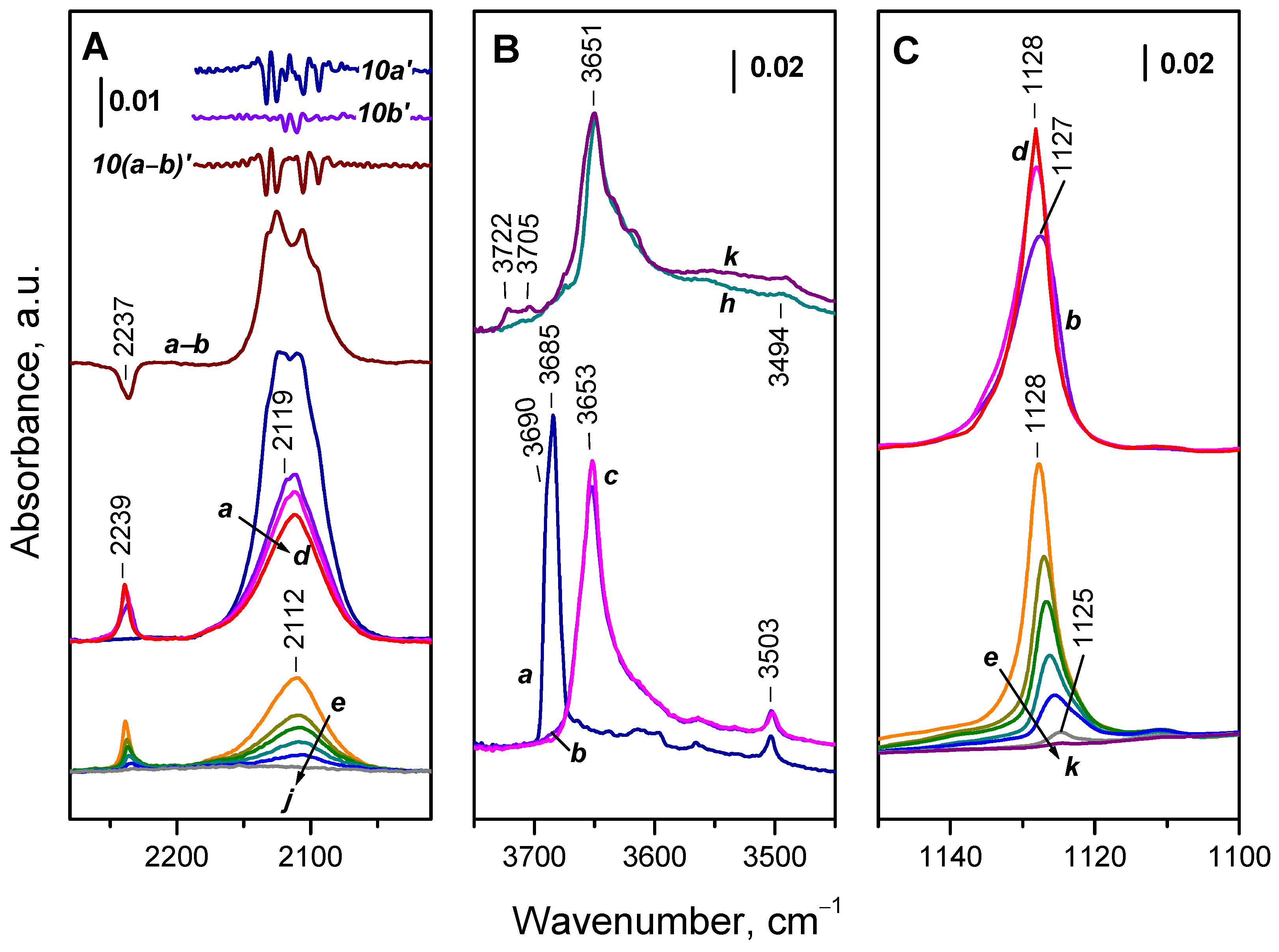
2.4. Interaction of Reduced CeO2-NC with O2 at Variable Temperature
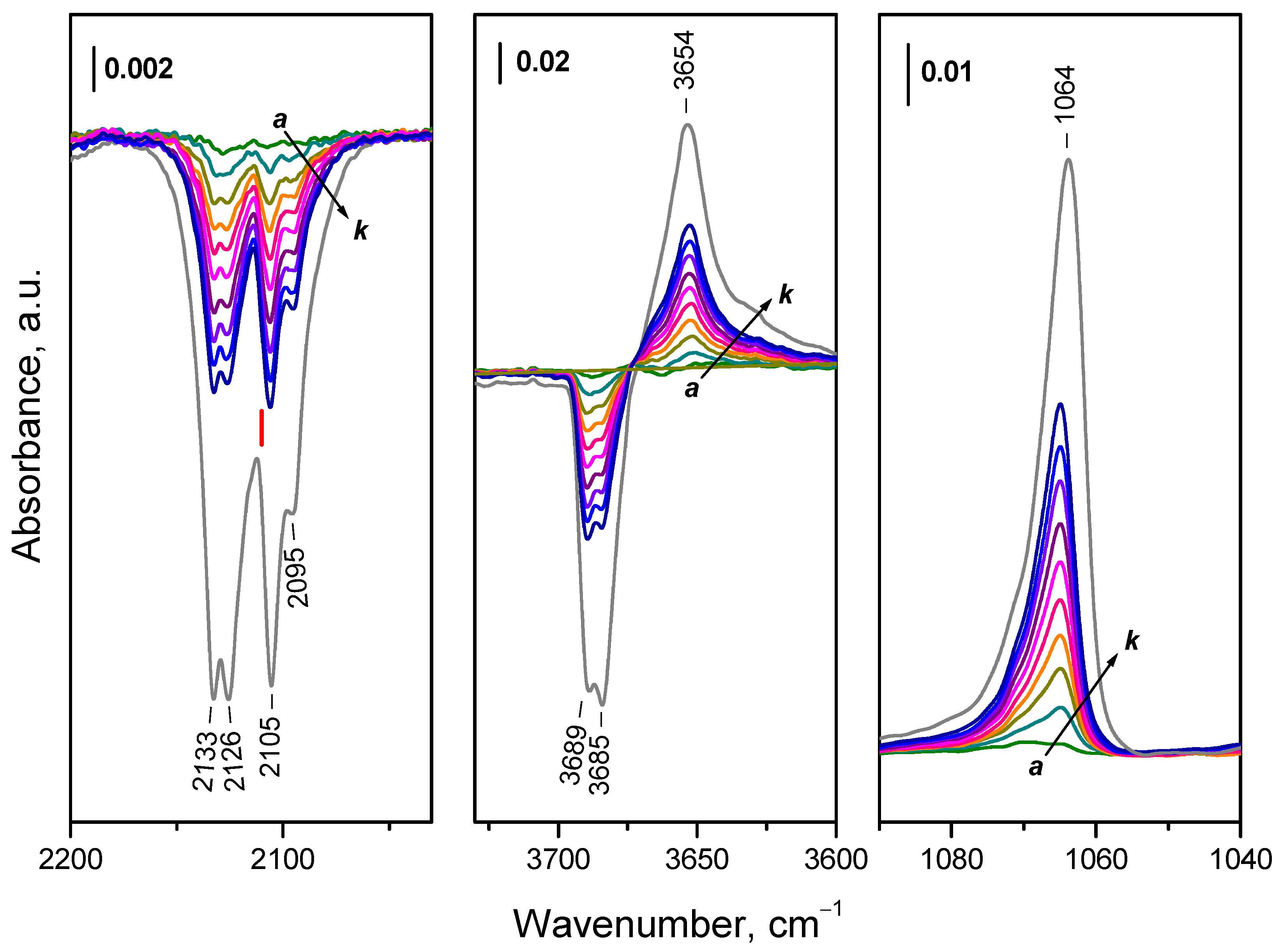
2.4.1. Interaction with 16O2
2.4.2. Interaction with 18O2
2.4.3. Interaction of Hydroxylated CeO2-NC with 16O2
2.4.4. Interaction of CeO2-NC Reduced at Lower Temperatures with 16O2
2.5. Interaction of Reduced CeO2-NP with O2 at Variable Temperature
2.5.1. Interaction with 16O2
2.5.2. Interaction with 18O2
2.6. Interaction of the Samples with Oxygen at Ambient Temperature
2.6.1. CeO2-NC
2.6.2. CeO2-NR
3. Discussion
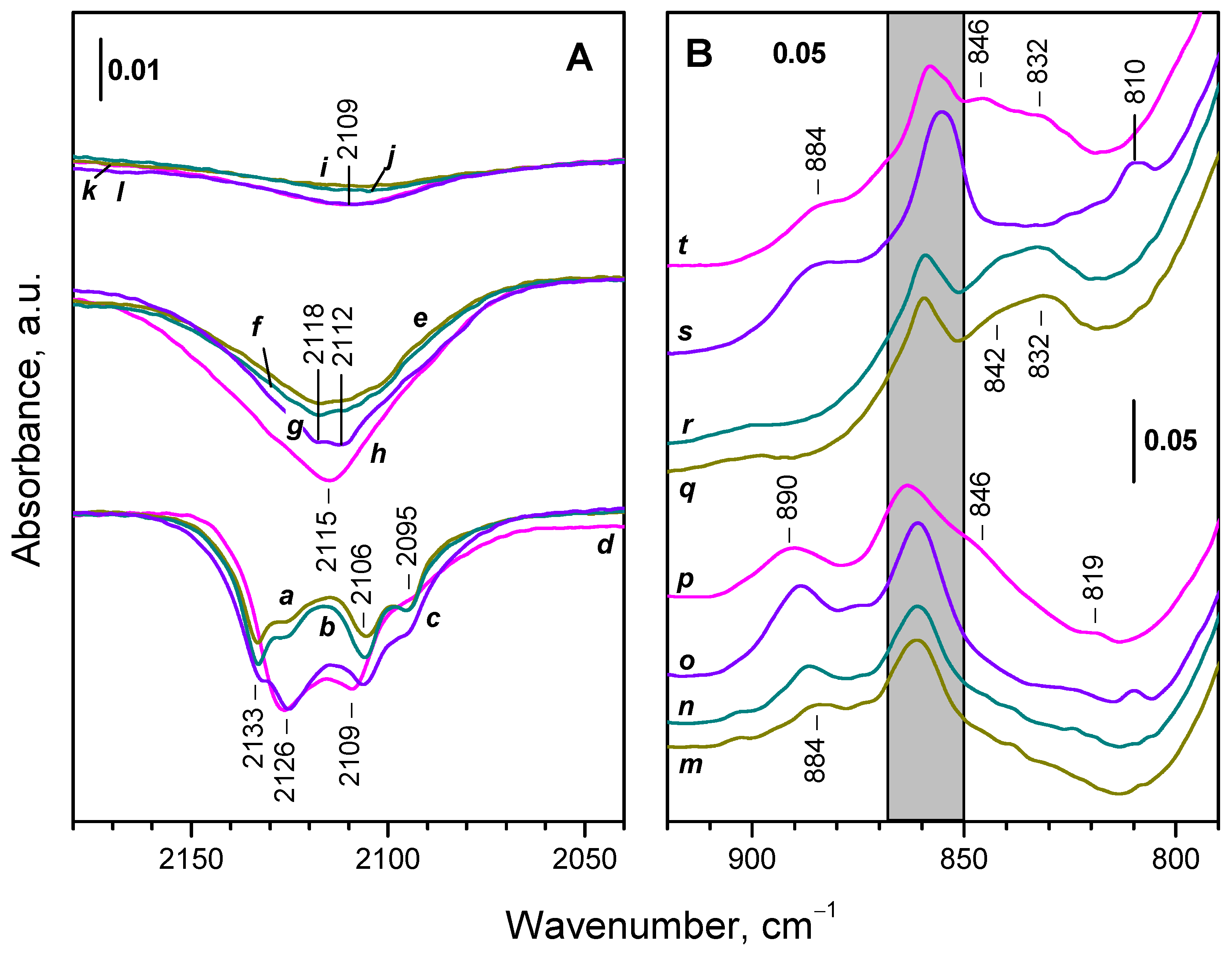
3.1. Species Formed During Adsorption of O2 on Oxidized and Reduced Ceria
3.2. Ce3+ Sites Involved in Fast Oxidation
- Ce3+ cations that are fast oxidized at 100 K;
- Ce3+ cations fast oxidized between 100 and 293 K, and
- Ce3+ cations resisting oxidation at 293 K but oxidized at slightly higher temperature, up to 393 K.
3.3. Fine Structure of Ce3+ Band
3.4. Oxidation of Sorbed H2
4. Materials and Methods
4.1. Synthesis of the Samples
4.2. Gases
4.3. Methods
4.3.1. FTIR Spectroscopy
4.3.2. Temperature-Programmed Reduction
4.3.3. Transmission Electron Microscopy
4.3.4. X-ray Diffraction
4.3.5. BET Surface Area
5. Conclusions
- No autoreduction of Ce4+ to Ce3+ occurs during evacuation of pure ceria nanoparticles at 573-773 K.
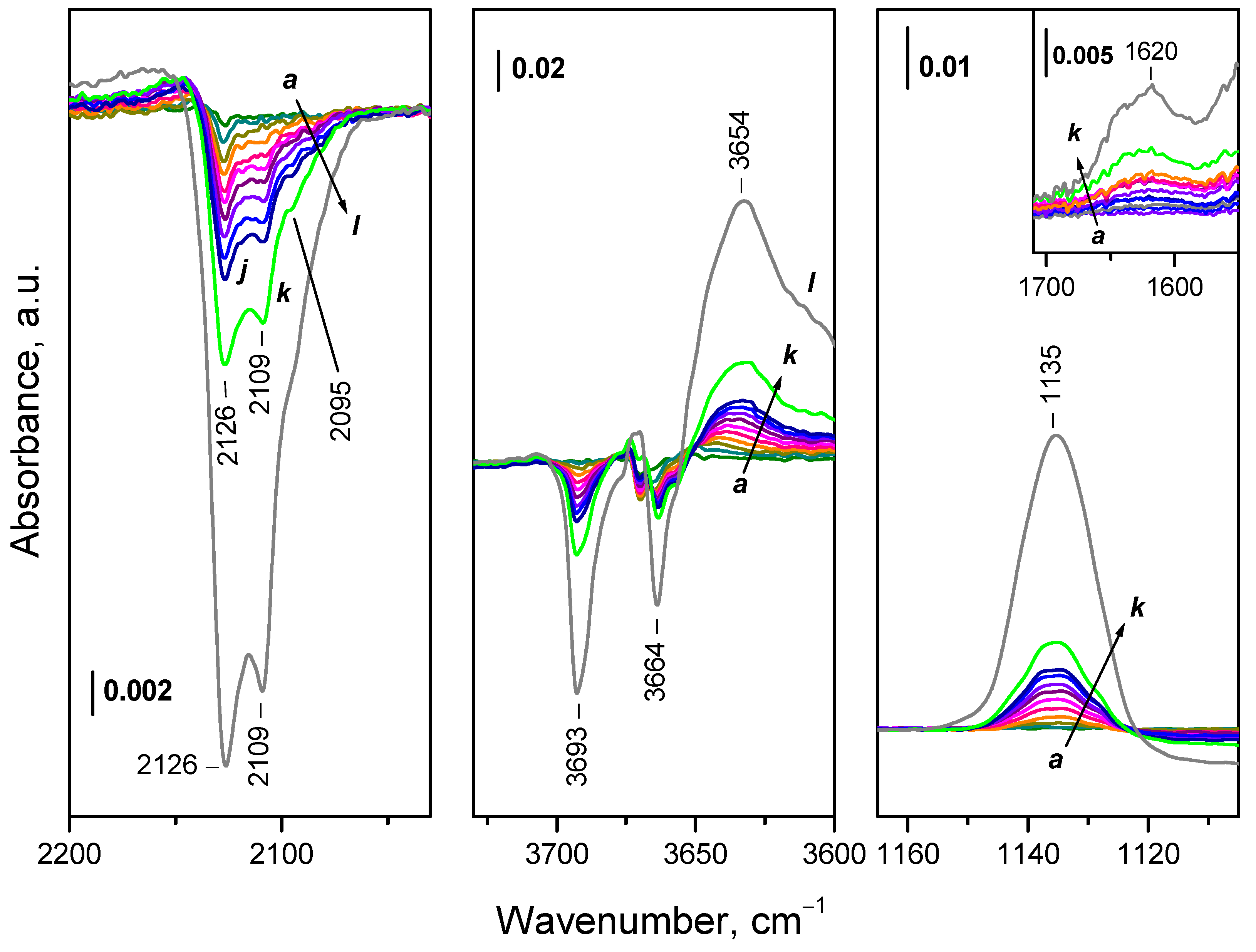
- Reduction of ceria with H2 at 773 K leads to formation of Ce3+ cations, which are monitored in the IR spectra by a band at 2133-2094 cm-1. This band possess a fine structure, well resolved at 100 K. The positions of the individual components depend on the environment of Ce3+, including the presence of nearby OH groups and likely of residual carbonates.
- Even at 100 K part of Ce3+ sites on reduced ceria are fast oxidized by O2. These sites are situated on the surface and include all Ce3+ cations bound to OH groups and carbonates.
- Depending on the location of the Ce3+ sites, O2− or O22− are produced during the fast oxidation of reduced ceria at 100 K.
- Some Ce3+ sites resist oxidation at 100 K but are oxidized at higher temperatures, in the temperature interval 100 – 400 K. These sites are also assumed to be surface situated, but a location in subsurface layers is not excluded.
- Peroxide (O22−) species decompose to give lattice oxygen, while superoxides first convert to hydroperoxides (OOH−) and then to terminal OH groups
- H2 dissolves in reduced ceria and is not completely removed upon evacuation at temperatures < 773 K. Part of this hydrogen is also fast oxidized at 100 K.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trovarelli, A.; de Leitenburg, C.; Boaro, M.; Dolcetti, G. The utilization of ceria in industrial catalysis, Catal. Today 1999, 50, 353-367. [CrossRef]
- Chung, C.-H.; Tu, F.-Y.; Chiu, T.-A.; Wu, T.-T.; Yu, W.-Y. Critical roles of surface oxygen vacancy in heterogeneous catalysis over ceria-based materials: a selected review, Chem. Lett. 2021, 50, 856-865. [CrossRef]
- Chuen, W.C.; Haile, S.M. A thermochemical study of ceria: exploiting an old material for new modes of energy conversion and CO2 mitigation. Phil. Trans. R. Soc. A 2010, 368, 3269-3294. [CrossRef]
- Ganduglia-Pirovano, M. V.; Hofmann, A.; Sauer, J., Oxygen vacancies in transition metal and rare earth oxides: Current state of understanding and remaining challenges. Surf. Sci. Rep. 2007, 62, 219-270. [CrossRef]
- Li, H.; Xia, P.; Pan, S.; Qi, Z.; Fu, C.; Yu, Z.; Kong, W.; Chang, Y.; Wang, K.; Wu, D.; Yang, X. The advances of ceria nanoparticles for biomedical applications in orthopaedics. Int. J. Nanomed. 2020, 15, 7199-7214. [CrossRef] [PubMed]
- Rozhin, P.; Melchionna, M.; Fornasiero, P.; Marchesan, S. Nanostructured ceria: biomolecular templates and (bio)applications. Nanomaterials 2021, 11, 2259. [CrossRef] [PubMed]
- Song, G.; Cheng, N.; Zhang, J.; Huang, H.; Yuan, Y.; He, X.; Luo, Y.; Huang, K. Nanoscale cerium oxide: synthesis, biocatalytic mechanism, and applications. Catalysts 2021, 11, 1123. [CrossRef]
- Putna, E.S.; Vohs, J.M.; Gorte, R.J. Evidence for weakly bound oxygen on ceria films. J. Phys. Chem. 1996, 100, 17862-17865. [CrossRef]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts. I. Oxygen storage. J. Catal. 1984, 86, 254-265. [CrossRef]
- Laachir, A.; Perrichon, V.; Badri, A.; Lamotte, J.; Catherine, E.; Lavalley, J. C.; El Fallah, J.; Hilaire, L.; Le Normand, F.; Quéméré, E.; Sauvion, G. N.; Touret, O. Reduction of CeO2 by hydrogen. Magnetic susceptibility and Fourier-transform infrared, ultraviolet and X-ray photoelectron spectroscopy measurements. J. Chem. Soc., Faraday Trans. 1991, 87, 1601-1609. [CrossRef]
- Perrichon, V.; Laachir, A.; Bergeret, G.; Frety, R.; Tournayan, L. Reduction of cerias with different textures by hydrogen and their reoxidation by oxygen. J. Chem. Soc., Faraday Trans. 1994, 90, 773-781. [CrossRef]
- Holgado, J. P.; Munuera, G. XPS/TPR study of the reducibility of M/CeO2 catalysts (M=Pt, Rh): Does junction effect theory apply? In Stud. Surf. Sci. Catal., Frennet, A.; Bastin, J. M., Eds. Elsevier: 1995; Vol. 96, pp 109-122.
- Terribile, D.; Trovarelli, A.; de Leitenburg, C.; Dolcetti, G. Unusual oxygen storage/redox behavior of high-surface-area ceria prepared by a surfactant-assisted route, Chem. Mater. 1997, 9, 2676-2678. [CrossRef]
- Rao, G. R. Influence of metal particles on the reduction properties of ceria-based materials studied by TPR. Bull. Mater. Sci. 1999, 22, 89-94. [CrossRef]
- Daturi, M.; Finocchio, E.; Binet, C.; Lavalley, J.-C.; Fally, F.; Perrichon, V.; Vidal, K.; Hickey, N.; Kaspar, J. Reduction of high surface area CeO2-ZrO2 mixed oxides. J. Phys. Chem. B 2000, 104, 9186-9194. [CrossRef]
- Giordano, F.; Trovarelli, A.; Leitenburg, C.; Giona, M. A model for the temperature-programmed reduction of low and high surface area ceria. J. Catal. 2000, 193, 273-282. [CrossRef]
- Fally, F.; Perrichon, V.; Vidal, H.; Kaspar, J.; Blanco, G.; Pintado, J. M.; Bernal, S.; Colon, G.; Daturi, M.; Lavalley, J.C. Modification of the oxygen storage capacity of CeO2–ZrO2 mixed oxides after redox cycling aging. Catal. Today 2000, 59, 373–386. [CrossRef]
- Ayastuy, J. L.; Gil-Rodríguez, A.; González-Marcos, M. P.; Gutiérrez-Ortiz, M. A. Effect of process variables on Pt/CeO2 catalyst behaviour for the PROX reaction. Int. J. Hydrogen Energy 2006, 31, 2231-2242. [CrossRef]
- Gao, Y.; Wang, W.; Chang, S.; Huang, W. Morphology effect of CeO2 support in the preparation, metal–support interaction, and catalytic performance of Pt/CeO2 catalysts. ChemCatChem 2013, 5, 3610-3620. [CrossRef]
- Zabilskiy, M.; Djinović, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 materials: effect of the exposed ceria surfaces on catalytic activity in N2O decomposition reaction, ACS Catal. 2015, 5, 5357−5365. [CrossRef]
- Chen, D.; He, D.; Lu, J.; Zhong, L.; Liu, F.; Liu, J.; Yu, J.; Wan, G.; He, S.; Luo, Y. Investigation of the role of surface lattice oxygen and bulk latticeoxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl. Catal. B 2017, 218, 249–259. [CrossRef]
- Sohn, H.; Celik, G.; Gunduz, S.; Dogu, D.; Zhang, S.; Shan, J.; Tao, F. F.; Ozkan, U. S. Oxygen mobility in pre-reduced nano- and macro-ceria with Co loading: an AP-XPS, in-situ DRIFTS and TPR study. Catal. Lett. 2017, 147, 2863-2876. [CrossRef]
- Wang, H.; Luo, S.; Zhang, M.; Liu, W.; Wu, X.; Liu, S. Roles of oxygen vacancy and Ox− in oxidation reactions over CeO2 and Ag/CeO2 nanorod model catalysts. J. Catal. 2018, 368, 365–378. [CrossRef]
- Gonzalez-A, E.; Rangel, R.; Solís-Garcia, A.; Venezia, A. M.; Zepeda, T. A. FTIR investigation under reaction conditions during CO oxidation over Ru(x)-CeO2 catalysts. Mol. Catal. 2020, 493, 111086. [CrossRef]
- Cao, T.; You, R.; Li, Z.; Zhang, X.; Li, D.; Chen, S.; Zhang, Z.; Huang, W. Morphology-dependent CeO2 catalysis in acetylene semihydrogenation reaction, Appl. Surf. Sci. 2020, 501, 144120. [CrossRef]
- Schweke, D.; Shelly, L.; Ben David, R.; Danon, A.; Kostirya, N.; Hayun, S. Comprehensive study of the ceria–H2 system: Effect of the reaction conditions on the reduction extent and intermediates. J. Phys. Chem. C 2020, 124, 6180-6187. [CrossRef]
- Bogeat, A. B.; Blanco, G.; Pintado, J. M.; Goma, D.; Gamez, J. J. C. Tailoring CO2 adsorption and activation properties of ceria nanocubes by coating with nanometre-thick yttria layers. Surf. Interfaces 2021, 26, 101353. [CrossRef]
- Mi, R.; Li, D.; Hu, Z.; Yang, R. T. Morphology effects of CeO2 nanomaterials on the catalytic combustion of toluene: a combined kinetics and diffuse reflectance infrared fourier transform spectroscopy study, ACS Catal. 2021, 11, 7876−7889. [CrossRef]
- Wang, Y.; Liu, Z.; Confer, M. P.; Li, J.; Wang, R. In-situ DRIFTS study of chemically etched CeO2 nanorods supported transition metal oxide catalysts. Mol. Catal. 2021, 509, 111629. [CrossRef]
- Zhang, B.; Deng, L.; Liebau, M.; Ren, Y.; Luo, C.; Liu, B.; Zhang, S.; Gläser, R. Promotion effect of niobium on ceria catalyst for selective catalytic reduction of NO with NH3, J. Rare Earths 2022, 40, 1535-3545. [CrossRef]
- Lee, J.; Ryou, Y. S.; Chan, X.; Kim, T. J.; Kim, D. H. How Pt interacts with CeO2 under the reducing and oxidizing environments at elevated temperature? The origin of improved thermal stability of Pt/CeO2 compared to CeO2. J. Phys. Chem C 2016, 120, 25870–25879. [CrossRef]
- Agarwal, S.; Lefferts, L.; Mojet, B. L. Ceria nanocatalysts: shape dependent reactivity and formation of OH. ChemCatChem 2013, 5, 479-489. [CrossRef]
- Kovacevic, M.; Agarwal, S.; Mojet, B. L.; van Ommen, J. G.; Lefferts, L. The effects of morphology of cerium oxide catalysts for dehydrogenation of ethylbenzene to styrene. Appl. Catal. A 2015, 505, 354–364. [CrossRef]
- Agarwal, S.; Mojet, L. L.; Lefferts, L.; Datye, A. K. Ceria nanoshapes - structural and catalytic properties. In Catalysis by materials with well-defined structures, Elsevier, Amsterdam, 2015, pp. 31-70.
- Dang, F.; Kato, K.; Imai, H.; Wada, S.; Haneda, H.; Kuwabara, M. Characteristics of CeO2 nanocubes and related polyhedra prepared by using a liquid-liquid interface, Cryst. Growth Des. 2010, 10, 4537-4541. [CrossRef]
- Huttunen, P. K.; Labadini, D.; Hafiz, S. S.; Gokalp, S.; Wolff, E. P.; Martell, S. M.; Foster, M. DRIFTS investigation of methanol oxidation on CeO2 nanoparticles. Appl. Surf. Sci. 2021, 554, 149518. [CrossRef]
- Kuan, W.-F.; Yu, W.-Y.; Tu, F.-Y.; Chung, C.-H.; Chang, Y.-C.; Lin, M. M.; Yu, T.-H.; Chen, L.-J. Facile reflux preparation of defective mesoporous ceria nanorod with superior catalytic activity for direct carbon dioxide conversion into dimethyl carbonate. Chem. Eng. J. 2022, 430, 132941. [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes, J. Phys. Chem. B 2005, 109, 24380-24385. [CrossRef] [PubMed]
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-controlled ceria-based nanostructures for catalysis applications. ChemSusChem 2013, 6, 1821-1833. [CrossRef] [PubMed]
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F.; Stadler, B. Exploring the properties and applications of nanoceria: is there still plenty of room at the bottom? Environ. Sci. Nano 2014, 1, 390-405. [CrossRef]
- Schilling, C.; Ganduglia-Pirovano, M. V.; Hess, C. Experimental and theoretical study on the nature of adsorbed oxygen species on shaped ceria nanoparticles. J. Phys. Chem. Lett. 2018, 9, 6593-6598. [CrossRef] [PubMed]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475-8505. [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H. M.; Overbury, S. H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595-16606. [CrossRef] [PubMed]
- Wu, W.; Savereide, L. M.; Notestein, J.; Weitz, E. In-situ IR spectroscopy as a probe of oxidation/reduction of Ce in nanostructured CeO2. Appl. Surf. Sci. 2018, 445, 548-554. [CrossRef]
- Wu, Z.; Li, M.; Mullins, D. R.; Overbury, S. H. Probing the surface sites of CeO2 nanocrystals with well-defined surface planes via methanol adsorption and desorption, ACS Catal. 2012, 2, 2224-2234. [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G. The catalysts of three-dimensionally ordered macroporous Ce1-xZrxO2-supported gold nanoparticles for soot combustion: the metal–support interaction, J. Catal. 2012, 287, 13–29. [CrossRef]
- Guillén-Hurtado, N.; Atribak, I., Bueno-López, A.; García-García, A. Influence of the cerium precursor on the physico-chemical features and NO to NO2 oxidation activity of ceria and ceria–zirconia catalysts. J. Mol. Catal. A 2010, 323, 52–58. [CrossRef]
- Liu, L.; Cao, Y.; Sun, W.; Yao, Z.; Liu, B.; Gao, F.; Dong, L. Morphology and nanosize effects of ceria from different precursors on the activity for NO reduction. Catal. Today 2011, 175, 48-54. [CrossRef]
- Zotin, F.M.Z.; Tournayan, L.; Varloud, J.; Perrichon, V.; Frety, R. Temperature-programmed reduction: limitation of the technique for determining the extent of reduction of either pure ceria or ceria modified by additives. Appl. Catal. A 1993, 98, 99-114. [CrossRef]
- Afrin, S.; Bollini, P. On the utility of Ce3+ spin-orbit transitions in the interpretation of rate data in ceria catalysis: theory, validation, and application. J. Phys. Chem. C 2023, 127, 234-247. [CrossRef]
- Binet, C.; Badri, A.; Lavalley, J.-C. A spectroscopic characterization of the reduction of ceria from electronic transitions of intrinsic point defects. J. Phys. Chem. 1994, 98, 6392-6398. [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207-225. [CrossRef]
- Mihaylov, M. Y.; Ivanova, E. Z.; Aleksandrov, H. A.; Petkov, P. St.; Vayssilov, G.N.; Hadjiivanov, K.I. FTIR and density functional study of NO interaction with reduced ceria: identification of N3− and NO2− as new intermediates in NO conversion. Appl. Catal. B 2015, 176-177, 107-119. [CrossRef]
- Mihaylov, M. Y.; Ivanova, E. Z.; Aleksandrov, H. A.; Petkov, P. St.; Vayssilov, G.N.; Hadjiivanov, K.I. Formation of N3− during interaction of NO with reduced ceria. Chem. Commun. 2015, 51, 5668-5671. [CrossRef]
- Mihaylov, M. Y.; Ivanova, E. Z.; Vayssilov, G.N.; Hadjiivanov, K.I. Revisiting ceria-NOx interaction: FTIR studies. Catal. Today 2020, 357, 613-620. [CrossRef]
- Li, H.; Zhang, P.; Li, G.; Lu, J.; Wu, Q.; Gu, Y. Stress measurement for nonstoichiometric ceria films based on Raman spectroscopy. J. Alloys Compounds 2016, 682, 132-137. [CrossRef]
- Mihaylov, M. Y.; Ivanova, E. Z.; Aleksandrov, H. A.; Petkov, P. St.; Vayssilov, G.N.; Hadjiivanov, K.I. Species formed during NO adsorption and NO + O2 co-adsorption on ceria: a combined FTIR and DFT study. Mol. Catal. 2018, 451, 114-124. [CrossRef]
- Chen, L.; Fleming, P.; Morris, V.; Holmes, J. D.; Morris, M. A. Size-related lattice parameter changes and surface defects in ceria nanocrystals. J. Phys. Chem. C 2010, 114, 12909-12919. [CrossRef]
- Descorme, C.; Madier, Y.; Duprez, D. Infrared study of oxygen adsorption and activation on cerium–zirconium mixed oxides. J. Catal. 2000, 196, 167-173. [CrossRef]
- Li, C.; Domen, K.; Maruya, K.; Onishi, T. Dioxygen adsorption on well-outgassed and partially reduced cerium oxide studied by FT-IR. J. Am. Chem. Soc. 1989, 111, 7683-7687. [CrossRef]
- Li, C.; Domen, K.; Maruya, K.-I.; Onishi, T. Oxygen exchange reactions over cerium oxide: An FT-IR study. J. Catal. 1990, 123, 436-442. [CrossRef]
- Pushkarev, V. V.; Kovalchuk, V. I.; d'Itri, J. L. Probing defect sites on the CeO2 surface with dioxygen. J. Phys. Chem. B 2004, 108, 5341-5348. [CrossRef]
- Baranchikov, A.E.; Polezhaeva, O.S.; Ivanov, V.K.; Tretyakov, Y.D. Lattice expansion and oxygen non-stoichiometry of nanocrystalline ceria, CrystEngComm 2010, 12, 3531–3533. [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seala, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [CrossRef]
- Kim, K.; Yi, D. K.; Paik, U. Increase in Ce3+ concentration of ceria nanoparticles for high removal rate of SiO2 in chemical mechanical planarization. ECS J. Solid State Sci. Technol. 2017, 6, 681-685. [CrossRef]
- Cardenas, L.; Molinet-Chinaglia, C.; Loridant, S. Unraveling Ce3+ detection at the surface of ceria nanopowders by UPS analysis. Phys. Chem. Chem. Phys. 2022, 24, 22815-22822. [CrossRef]
- Morgan, D.J. Photoelectron spectroscopy of ceria: reduction, quantification and the myth of the vacancy peak in XPS analysis. Surf. Interface Anal. 2023, 55, 845-850. [CrossRef]
- Xu, Y.; Wang, F.; Liu, X.; Liu, Y.; Luo, M.; Teng, B.; Fan, M.; Liu, X. Resolving a decade-long question of oxygen defects in Raman spectra of ceria-based catalysts at atomic level. J. Phys. Chem. C 2019, 123, 18889-18894. [CrossRef]
- Filtschew, A.; Hofmann, K.; Hess, C. Ceria and its defect structure: new insights from a combined spectroscopic approach. J. Phys. Chem. C 2016, 120, 6694-6703. [CrossRef]
- Schmitt, R.; Nenning, A.; Kraynis, O.; Korobko, R.; Frenkel, A. I.; Lubomirsky, I.; Haile, S. M.; Rupp, J. L. M. A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 2020, 49, 554-592. [CrossRef] [PubMed]
- Wang, X.; Li, M.; Wu, Z., In situ spectroscopic insights into the redox and acid-base properties of ceria catalysts. Chin. J. Catal. 2021, 42, 2122–2140. [CrossRef]
- Loridant, S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts. Catal. Today 2021, 373, 98-111. [CrossRef]
- Gao, Y.; Li, R.; Chen, S.; Luo, L.; Cao, T.; Huang, W. Morphology-dependent interplay of reduction behaviors, oxygen vacancies and hydroxyl reactivity of CeO2 nanocrystals. Phys. Chem. Chem. Phys. 2015, 17, 31862-31871. [CrossRef] [PubMed]
- Lakshmanan, P.; Averseng, F.; Bion, N.; Delannoy, L.; Tatibouët, J.-M.; Louis, C. Understanding of the oxygen activation on ceria- and ceria/alumina-supported gold catalysts: a study combining 18O/16O isotopic exchange and EPR spectroscopy. Gold Bull. 2013, 46, 233-242. [CrossRef]
- Lee, K.-M.; Brito, M.; DeCoster, J.; Linskens, K.; Mehdi, K.; Lee, W.-I.; Kim, E.; Kim, H.; Kwon, G.; Nam, C.-Y., Kim, J. Influence of oxidizing and reducing pretreatment on the catalytic performance of CeO2 for CO oxidation, Mol. Catal. 2022, 538, 112465. [CrossRef]
- Ortega, P.P.; Hangai, B.; Moreno, H.; Rocha, L.S.R.; Ramírez, M.A.; Ponce, M.A.; Longo, E.; Simões, A.Z. Tuning structural, optical, and gas sensing properties of ceria-based materials by rare-earth doping, J. Alloys Compounds 2021, 888, 161517. [CrossRef]
- Pan, J.; Wang, S.; Chen, A.; Chen, Y.; Wang, M.; Chen, Y. Visible-light-active mesoporous ceria (CeO2) nanospheres for improved photocatalytic performance, J. Alloys. Compounds 2022, 898, 162895. [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A. K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying defects in ceria-based nanocrystals by UV resonance Raman spectroscopy. J. Phys. Chem. C 2009, 113, 19789-19793. [CrossRef]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Zhou, Y.; Shao, Y.; Zhang, L.; Li, T.; Shi, J., Mild generation of surface oxygen vacancy on CeO2 for improved CO2 photoreduction activity, Nanoscale 2020, 12, 12374-12382. [CrossRef]
- Bruce, L.A.; Hoang, M.; Hughes, A.E.; Turney, T.W., Surface area control during the synthesis and reduction of high area ceria catalyst supports. Appl. Catal. A 1996, 134, 351-362. 050. [CrossRef]
- Badri, A.; Binet, C.; Lavalley, J.-C., An FTIR study of surface ceria hydroxy groups during a redox process with H2. J. Chem. Soc., Faraday Trans. 1996, 92, 4669-4673. [CrossRef]
- Tsyganenko, A.A.; Filimonov, V.N. Infrared spectra of surface hydroxyl groups and crystalline structure of oxides. J. Mol. Struct. 1973, 19, 579-589. [CrossRef]
- Vayssilov, G. N.; Mihaylov, M.; Petkov, P. S.; Hadjiivanov, K. I.; Neyman, K. M. Reassignment of the vibrational spectra of carbonates, formates, and related surface species on ceria: a combined density functional and infrared spectroscopy investigation. J. Phys. Chem. C 2011, 115, 23435-23454. [CrossRef]
- Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy, Adv. Catal. 2014, 47, 99-318. [CrossRef]
- Zecchina, A.; Otero Areán, C. Diatomic molecular probes for mid-IR studies of zeolites, Chem. Soc. Rev. 1996, 25, 187-197. [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, part B (6th Ed.), John Wiley & Sons, Hoboken, N.J. (2009).
- Preda, G.; Migani, A.; Neyman, K. M.; Bromley, S. T.; Illas, F.; Pacchioni, G. Formation of superoxide anions on ceria nanoparticles by interaction of molecular oxygen with Ce3+ sites. J. Phys. Chem. C 2011, 115, 5817-5822. [CrossRef]
- Bashir, S. M.; Idriss, H. The reaction of propylene to propylene-oxide on CeO2: An FTIR spectroscopy and temperature programmed desorption study. J. Chem. Phys. 2020, 152, 044712. [CrossRef] [PubMed]
- Lin, W.Y.; Frei, H. Photochemical and FT-IR probing of the active site of hydrogen peroxide in Ti silicalite sieve, J. Am. Chem. Soc. 2002, 124, 9292-9298. [CrossRef]
- Anpo, M.; Costentin, G.; Giamello, E.; Lauron-Pernot, H.; Sojka, J. Characterisation and reactivity of oxygen species at the surface of metal oxides, J. Catal. 2021, 393, 259-280. [CrossRef]
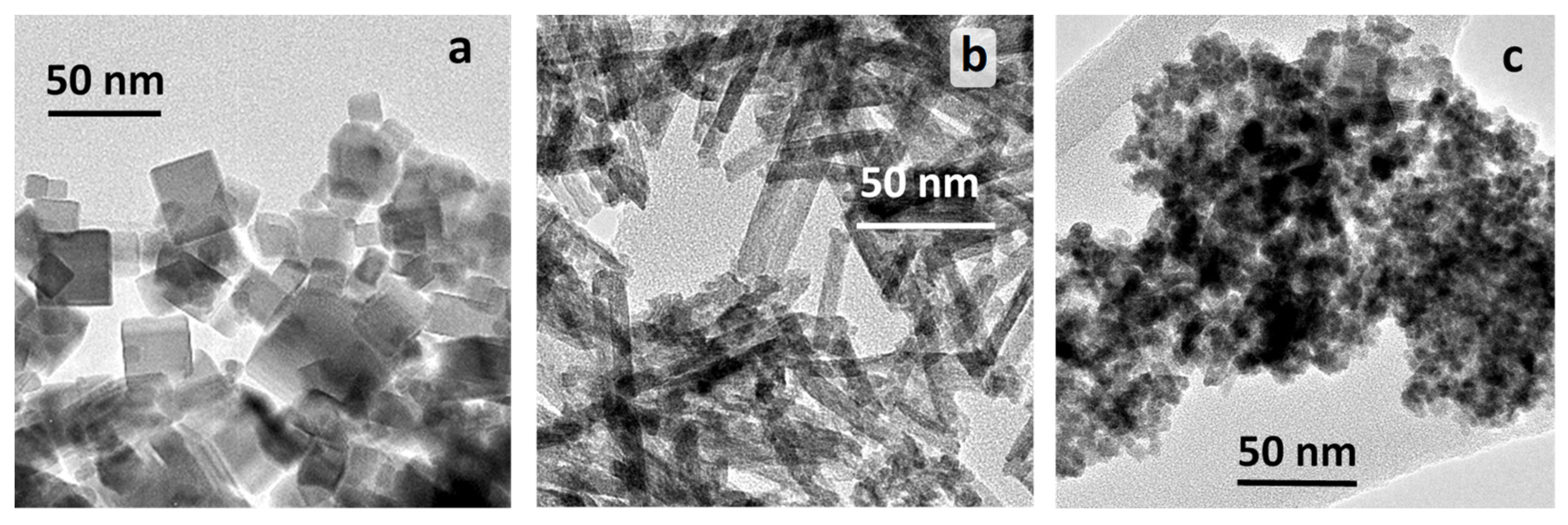

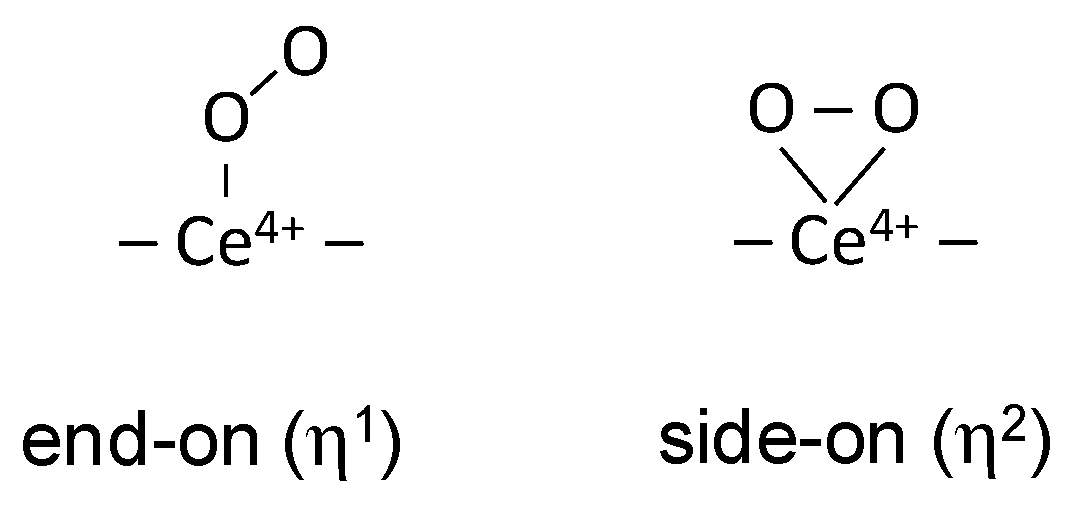
| Sample | Particle shape | SBET, m2 g-1 | Mean crystallyte size, nm1 |
|---|---|---|---|
| CeO2-NC | cubes | 27 | 30.1 |
| CeO2-NR | rods | 110 | 6.7 |
| CeO2-NP | polyhedra | 140 | 7.2 |
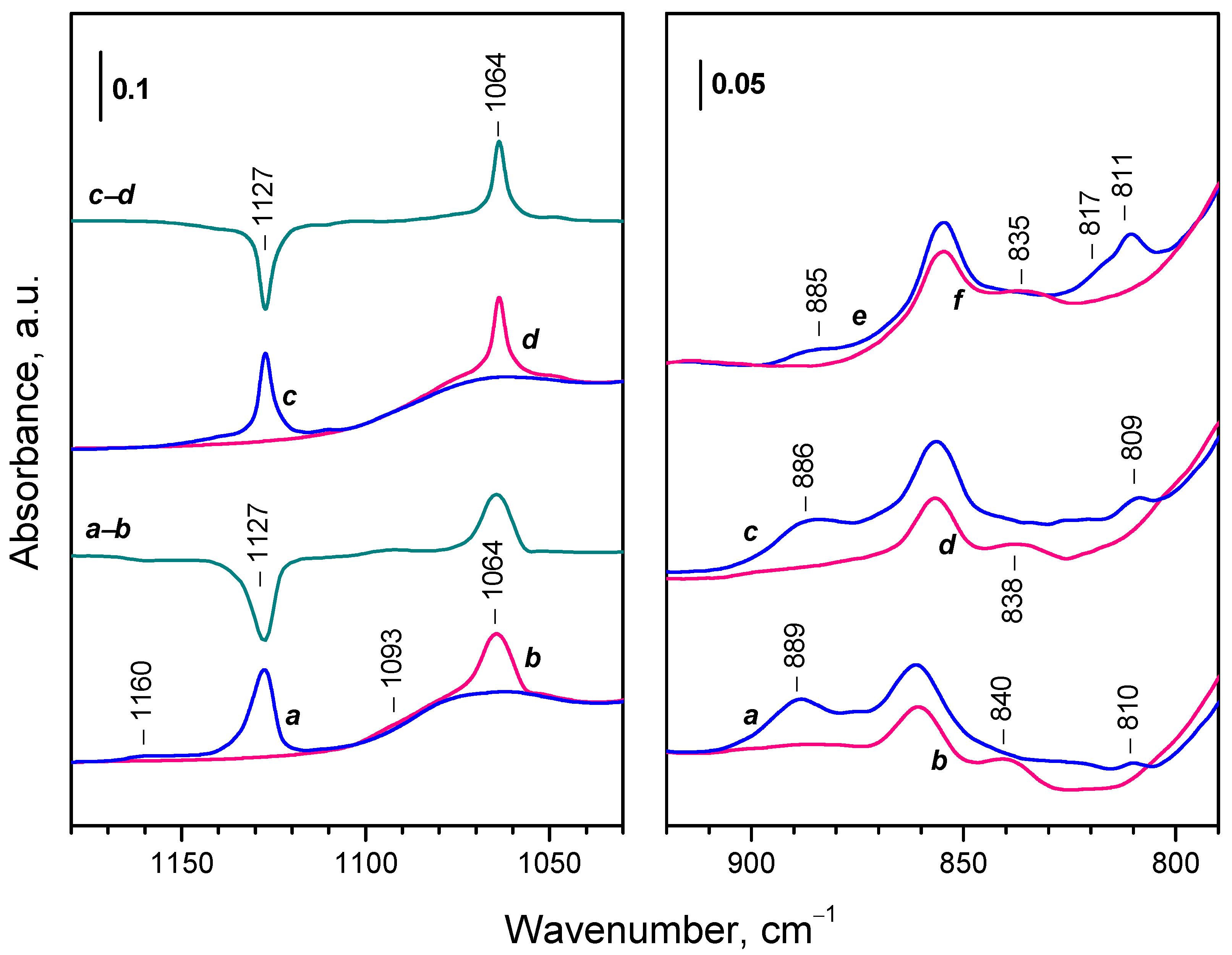
| Sample | Assignment | ν(16O−16O), cm-1 | ν(18O−18O), cm-1 | i1 |
|---|---|---|---|---|
| CeO2-NC | superoxide | 1128 | 1064 | 1,060 |
| CeO2-NC | superoxide | 1160 | 1093 | 1,061 |
| CeO2-NP | superoxide | 1128 | 1065 | 1,059 |
| CeO2-NP | superoxide | 1137 | 1072 | 1,061 |
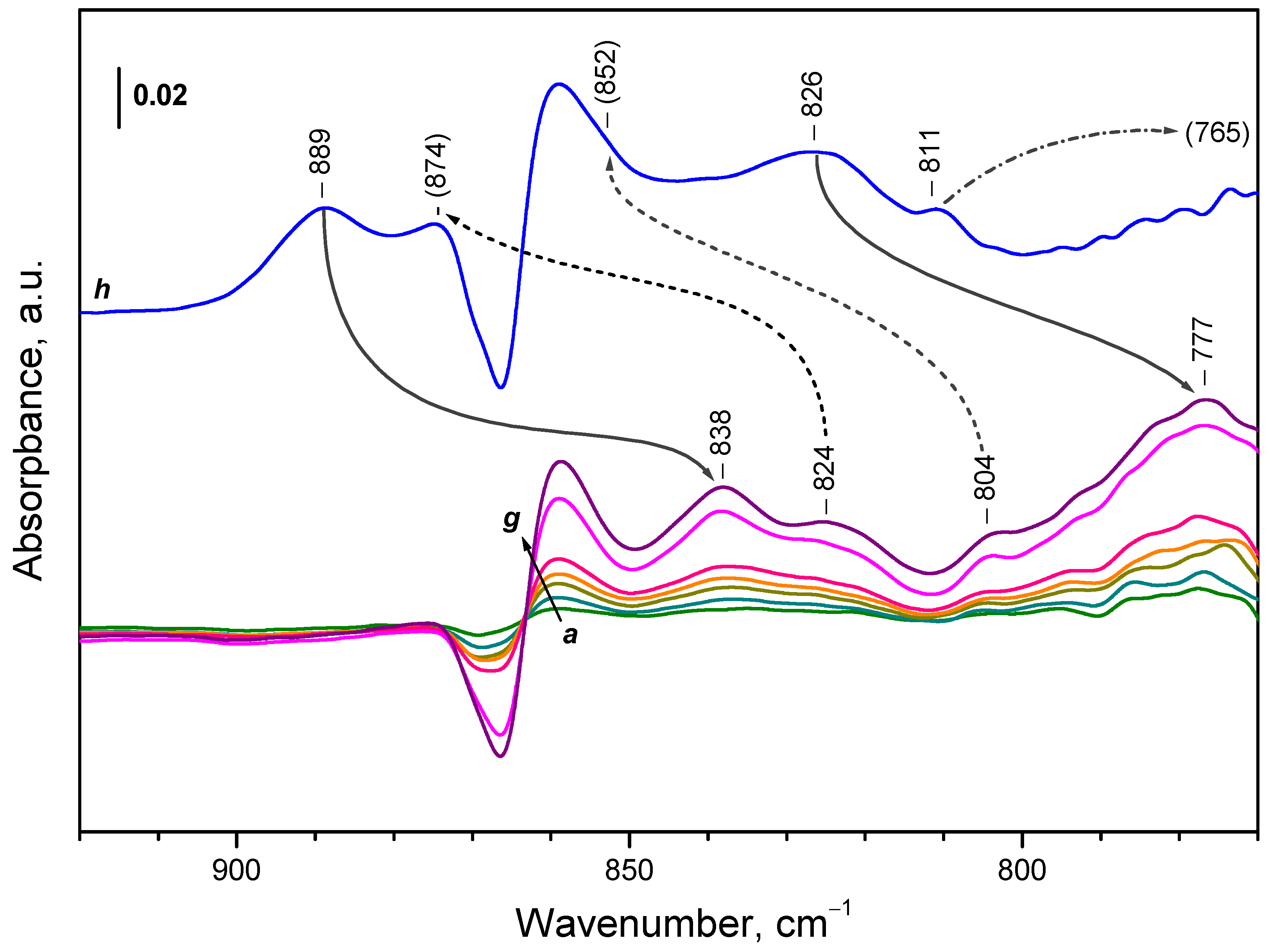
| CeO2-NC | peroxide | 889 | 838 | 1,061 |
| CeO2-NC | peroxide | 874 | 824 | 1,061 |
| CeO2-NC | peroxide | 852 | 804 | 1,060 |
| CeO2-NC | peroxide | 826 | 777 | 1,063 |
| CeO2-NC | peroxide | 811 | 765 2 | - |
| CeO2-NP | peroxide | 889 | 840 | 1,058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





