1. Introduction
Knowledge of human biological rhythms has contributed to improving several sectors of modern life, including health, education, and sports [
1,
2,
3]. From a biological perspective, adjusting to environmental rhythmicity enables organisms to anticipate periodic events like daily changes in temperature, light or food availability, as well as to efficiently regulate behavioral and metabolic processes [
4]. Many physiological functions show endogenous ~24-h oscillations strongly entrained by the light-dark cycle [
5], but circadian rhythms are also sensitive to nonphotic inputs such as mealtimes and social stimuli [
6]. Exercise influences the human circadian system, with the effects depending on when during the day a person exercises [
7,
8].
Both cognitive and motor performance exhibit circadian variations [
3,
9,
10]. Attentional levels are generally low in the early morning and reach highest levels around mid-afternoon [
11], while most components of exercise performance peaks in the early evening [
12]. Individual differences in the entrainment of circadian clocks to the rhythmic environment are often referred to as ''chronotypes'', with early ''larks'' and late ''owls'' at the extremes of a normal distribution [
13]. Previous studies revealed that peak performance times vary among individuals depending on their chronotype [
11,
14] with late chronotypes demonstrating poorer cognitive and motor performance in the morning, compared to early chronotypes [
15]. On the other hand, morning exercise has been found to help individuals with late chronotypes overcome morning challenges by advancing their circadian phase [
16]. Furthermore, a comprehensive intervention that included targeted light exposure, meals and caffeine, along with exercise in the morning, reported improvements in cognitive and physical performance during ‘suboptimal’ morning hours [
17].
Physical activity enhances cognitive function, with different effects depending on the duration, frequency, intensity and type of exercise [
18]. The effect of exercise also depends on which specific cognitive skill is evaluated and what time of day it is tested [
19,
20,
21]. Precisely, there is strong support for positive effects of moderate-intensity exercise on cognition [
22]. These include improvements in reaction speed induced by acute physical exercise in male trained athletes [
23], young sport students [
24], and improvements in attention in response to moderate-intensity exercise in young female physical education students [
25]. Professional dancers perform at moderate to vigorous intensities, requiring not only physical strength and endurance but also a high level of visual attention for precision, group coordination, and inhibitory control [
26,
27]. While there is abundant research on sports and cognition across various age groups, studies on dance as a potential cognitive enhancer have been largely focused on elderly populations [
28,
29]. Moreover, most research on exercise and cognition has been conducted in controlled laboratory settings, often using bicycle or treadmill ergometry [
30]. However, less is known about the effect of dance on cognition in real-world contexts, particularly from a chronobiological perspective [
31].
Here, we examined the interplay among circadian parameters, physical activity features and attention in young adult subjects in the real life setting of the Uruguayan National Dance School (ENFAS) [
32,
33,
34]. Although most of these dance students had shown late chronotypes [
35], their 4-hour daily training began in the early morning (08:30 h). This situation prompted us to examine how movement and circadian parameters interacted in a challenging schedule for late-morning dancers, and how they are associated with attentional attributes.
We expected to observe differences in cognitive performance before and after training, with faster responses following the 4-hour morning practice compared to the early morning measurement. Additionally, we predicted that the later the individual’s circadian phase, the lower their attentional levels. We also expected to find an association between time spent in moderate-intensity exercise during training and improved performance.
2. Results
2.1. Circadian and Exercise Characterization
We characterized 22 young dancers, mostly female, who regularly trained during the morning (08:30 - 12:30, Monday to Friday) (
Table 1). The majority were late chronotypes (MCTQ, MSFsc above 05:00), with very high social disruption (MCTQ, SJL > 2 hours) and intermediate circadian preference (MEQ, between 41 and 59). Regarding the characterization by objective accelerometry, we first assessed the midpoint of the least active five hours on free days (L5cf) as a proxy of the individual circadian phase, which was above 04:30 in average (
Table 1). In addition, we found that participants exhibited moderate inter-daily stability (IS), suggesting moderate consistency in their daily activity patterns; low intra-daily variability (IV), reflecting low fragmentation of the rest-activity pattern; and a high relative amplitude (RA), indicating a healthy balance between the most and least active times of day.
Comparing self-reported (MCTQ) and objective measures (actimetry), we found a correlation in sleep duration on both workdays (R2=0.48, p=0.003) and free days (R2=0.32, p=0.0016), and a correlation in sleep onset on training days (R2=0.32, p=0.014). Interestingly, self report SD was shorter than actimetric SD during training days and longer during free days (mean difference: 1.1 , p=5.23e-05, d=0.6; mean difference: 0.94, p=0.018, d=1.01). We found a correlation between the circadian phase, as estimated by L5c on free days, with chronotype, as estimated by MSFsc (R2=0.29, p=0.023). However, L5c and the mid-sleep point did not correlate on either training days or free days (p>0.05).
We also used accelerometry to assess exercise features. Participants exhibited high levels of moderate physical activity (MPA) during training hours, with an average of 167 (23) mg and 48 (22) mins per session. Additionally, they engaged in moderate to vigorous physical activity (MVPA) for 168 (69) min per day. Interestingly, we detected a bimodal distribution in the time spent in MPA during the training hours. The most active dancers spent on average 62.4 (7) min in MPA during the 4-hour training, while the less active group spent 20.6 (12.9) min.
2.2. Cognitive Performance
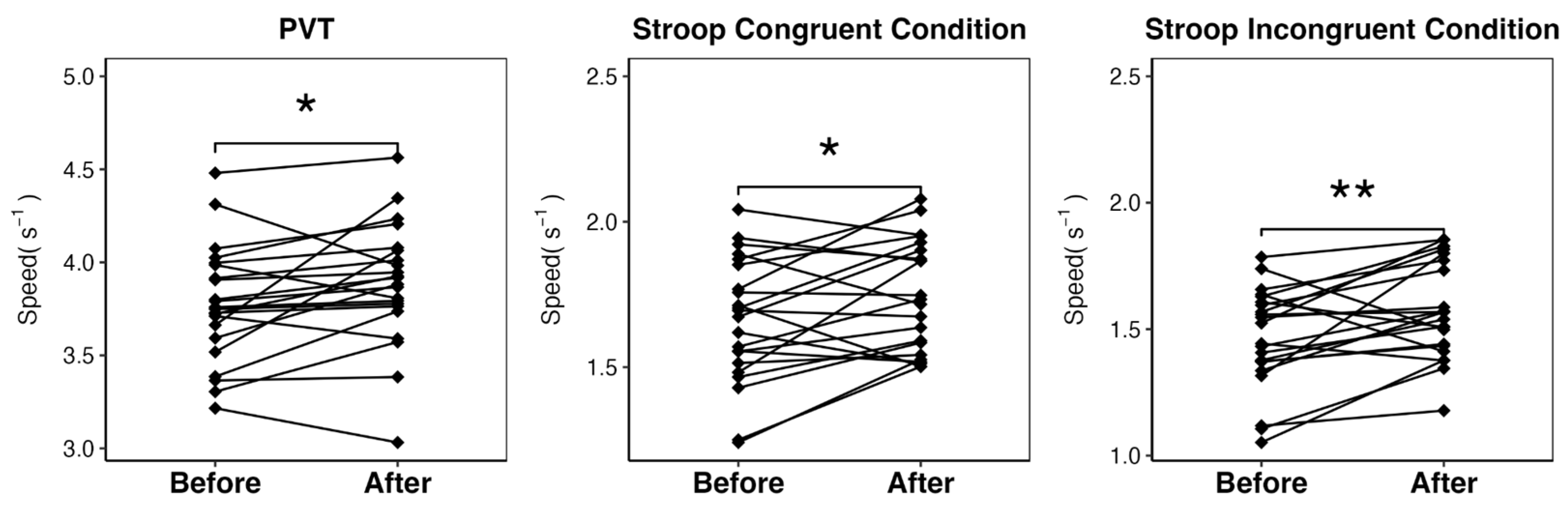
Regarding attentional performance, consistent with circadian variation, we found significant differences on both PVT and Stroop tasks before and after training, with faster reaction at 12:30 (
Figure 1). More pronounced effects were observed in the task requiring greater cognitive effort (Stroop IC) (
Table 3).
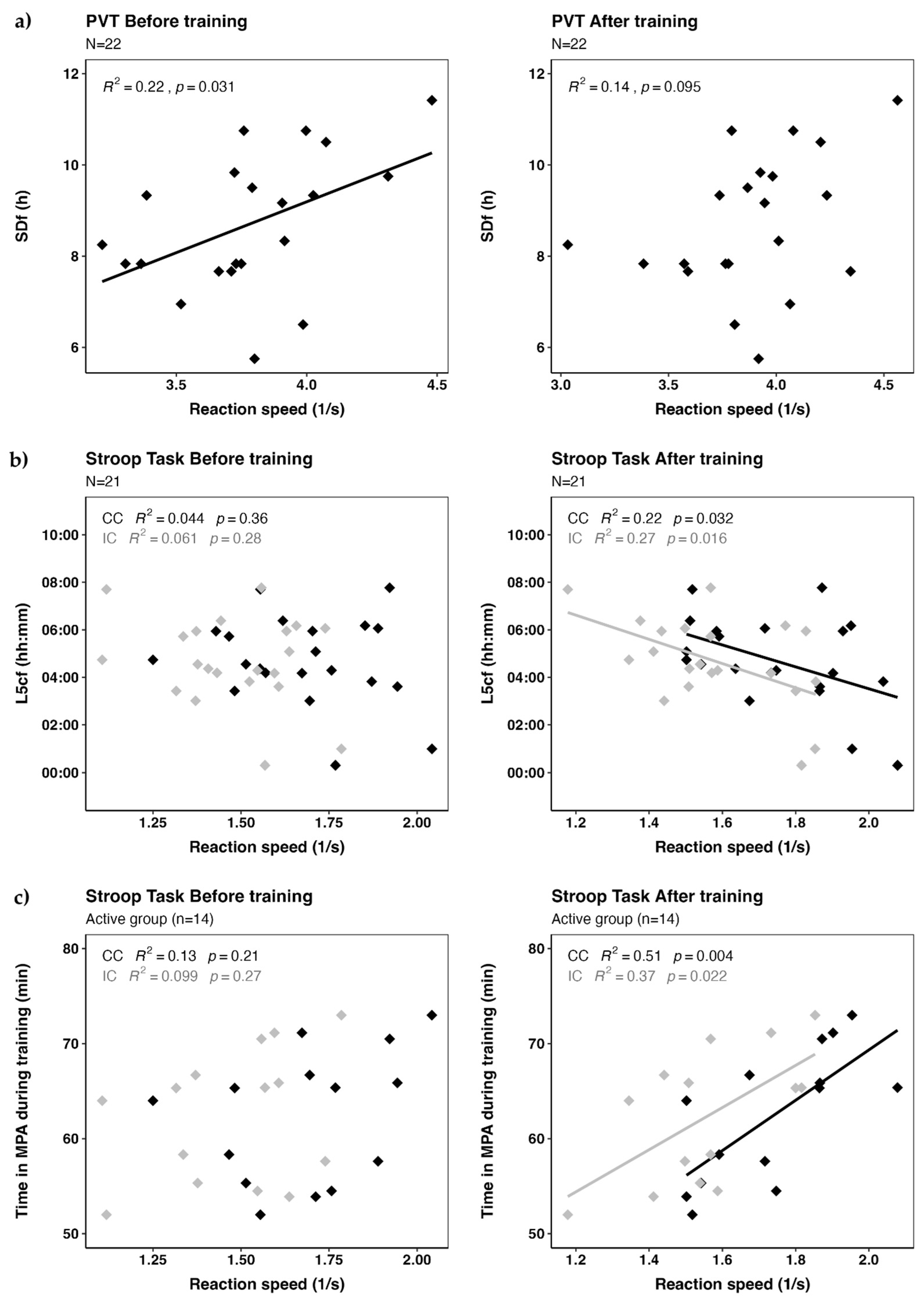
We found a significant correlation between PVT performance before training and sleep duration on free days as estimated by MCTQ, but not after training, nor with actimetry data (
Figure 2a). We found significant correlations between Stroop performance after training for both conditions and L5c on free days, but no correlation was found before training (
Figure 2b). We did not find correlations between speed and time spent in MPA for the whole sample. When we examined the correlation between reaction speed and the exercise variable for the active group (n=14), we found significant correlations between the time spent in MPA and Stroop performance after training for both congruent and incongruent conditions (
Figure 2c).
We then estimated regression models to study the effect of both circadian (L5c on free days) and exercise (time spent in MPA) parameters on reaction speed after training in the active group of dancers (
Table 4). The best-fitting model revealed a significant effect of the circadian parameter for the incongruent condition, whereas the exercise parameter was significant for the congruent condition. In the less active group, the analysis did not yield significant results.
3. Discussion
3.1. Study Findings
Consistent with previous reports of changes in cognitive performance throughout the day [
10], we confirmed that dancers trained in the morning had a better cognitive performance after training (at noon) than before (early morning). Also as expected [
36], we observed more pronounced effects in the task requiring greater cognitive effort (Stroop incongruent condition) compared to simpler tasks such as the PVT and the Stroop congruent condition. As a novel contribution of this study on dancers with late chronotypes in average [
32,
35], we found that: a) the longer the sleep in the free days the better the cognitive performance (PVT) early in the morning; b) the later the circadian phase (estimated by L5c) the worse the Stroop performance after training; and c) the longer the MPA during training the better the Stroop performance after training.
The Uruguayan Dance School model has been extensively studied to compare circadian and sleep traits between the morning (08:30 to 12:30) and the night shift (20:00 to 24.00) [
32,
33,
34,
35]. In this study, we analyzed for the first time dancers' cognitive performance. To do that, we focused on morning-shift dancers, who are challenged to train early in the morning despite having late chronotypes on average. The population of dancers assessed here showed the same characteristics previously reported for dancers trained in the morning shift by self reports and/or actigraphy [
32,
33]: a) late chronotypes; b) high SJL ; c) intermediate circadian preference; d) later sleep timing in free days than in training days; and e) no sleep deficit. Although both subjective (MSFsc) and objective (L5c on free days) proxies of circadian phase correlated as expected, we did not find significant differences in L5c between training and free days as previously reported in morning-shift dancers [
33]. A possible explanation could be the changes observed in chronotype and circadian rhythms during the COVID pandemic [
37,
38]. During the first semester these dancers attended remote classes, which probably influenced their sleep habits. So, the 15-days period of actimetry measures, done one month after resuming in-person training, may not have coincided with individual subjective assessment.
Both circadian rhythms and sleep homeostasis modulate cognitive performance [
9,
10]. While selective visual attention has been reported to be most strongly modulated by sleep homeostasis, inhibitory control appeared to be most strongly modulated by circadian phase [
36]. The positive correlation between PVT performance at 08:30 and weekend sleep duration (
Figure 2a) could be explained by the misalignment of circadian rhythms during the week, which likely led to accumulated fatigue. Those who managed to sleep longer on the weekend may have achieved more restorative rest, improving cognitive performance on vigilance tasks like the PVT. However, our study did not find associations between PVT reaction speed and sleep duration estimated by accelerometry. This discrepancy between both instruments reflects the complexity of assessing circadian parameters in populations with irregular sleep patterns and underscores the importance of using a comprehensive assessment approach. While questionnaires provide insights into sleep habits, actimetry could be thought of as a long-exposure photograph of sleep patterns within a limited time window.
A challenging task demanding higher-order cognitive function, for example, response inhibition, is expected to be more susceptible to circadian modulation [
36]. Regarding the attentional Stroop task, we found an association between circadian phase (estimated by L5c on free days) and reaction speed after training in both congruent and incongruent conditions, with faster reaction times observed in dancers with earlier circadian phases (
Figure 2b). It is interesting to note that despite the inconsistencies observed in the actimetric data to evaluate sleep patterns, L5c on free days still stands as an objective proxy of circadian phase. Our results are in line with previous findings showing that cognitive performance earlier in the day is worse in late chronotypes than in early ones [
15]. Surprisingly, no similar association was found before training; however, the heavily skewed chronotype distribution towards extreme eveningness in our sample may explain this lack of associations early in the morning, together with the difficulty of the Stroop task.
The short-term effects of exercise on cognitive performance have been extensively reported [
23,
39,
40], but the effect of long-term training on attentional processes of young adults has been less explored [
41,
42]. Dancers are a great model to test this since they train regularly and spend even more time in MPA and MVPA than the recommended for their age group [
26]. We did not find the expected correlation between response speed and the time spent in MPA during training when we tested all the participants. However, among the participants who actually displayed a more active profile (
Figure 2c), time spent in MPA was significantly associated with an improvement in attentional performance after training, in both selective attention (congruent condition) and inhibition control (incongruent condition). Interestingly, our model revealed differences when both circadian and exercise parameters were considered. The circadian effect was more pronounced under the most challenging condition, while time spent in MPA had a greater impact under the easier condition. Although the interaction between the circadian phase proxy and time in moderate exercise was not statistically significant in the models we ran, it suggested a potential trend that warrants further exploration. The impairing effect of later circadian phases may have been counteracted by the positive effect of moderate exercise, although a larger sample would be needed to detect this effect with greater confidence. Nevertheless, despite the small sample size, our results align with previous research [
22,
25] and provide evidence that exercise can enhance cognitive performance in young adults with late chronotypes, particularly performing at challenging times of the day. Despite extensive research on the effect of exercise on cognition in athletes, there remains a significant gap in studies on young professional dancers from a chronobiological perspective. The ecological model of the Uruguayan National Dance School, in which dancers train in the morning despite their late chronotypes, helps bridge that gap.
3.2. Limitations
As mentioned before, the study was conducted during COVID pandemic, which limited its design and scope. Tests were completed immediately before and after the training session in the dance school’s own facilities. These time and space constraints, inherent to the ecological nature of the model, led us to present abbreviated versions of the attentional tasks, which may have limited our ability to capture greater effects. Due to the anonymization process of the data, we could not assess the type of dancer (folkloric or contemporary) the participants were. We speculate that dance style contributed to distinct activity profiles. Ideally, we would have examined performance across a range of chronotypes at different times of day. However, in the context of this dance school the participants were predominantly late chronotypes, and the goal was to assess their performance within their training natural environment. Finally, as we recruited a very specific group of trained dancers following a regular schedule, our findings may not be extrapolated to other populations. Future research should specify the type of dance practiced during the training sessions, increase the sample size, include a sedentary control group, and incorporate measures taken in the evening. Those conditions would allow a finer analysis, distinguishing more clearly the complexity of the effects outlined here.
4. Materials and Methods
4.1. Participants
In September-October 2021, twenty-two dancers (18 females, 18-28 years) attending the morning shift of the Uruguayan Public School for Professional Training in Contemporary and Folkloric Dance (Escuela Nacional de Formación Artística Sodre, ENFAS, Ministerio de Educación y Cultura, Uruguay) participated in this study. Participants were screened for prior diagnoses of psychiatric, neurological or sleep disorders and medications known to affect sleep. All participants gave written informed consent. This study was evaluated by the Ethics Committee of the School of Psychology, Universidad de la República, and complied with the principles outlined by the Declaration of Helsinki [
43].
4.2. Circadian and Sleep Habits Characterization by Self-Report
Circadian characterization was assessed using the Spanish version of the Munich Chronotype Questionnaire (MCTQ) [
44]. The mid-sleep point on free days corrected for sleep debt on workdays (MSFsc) was used as a proxy of individual chronotype [
45]. Circadian preference was assessed using the Spanish version of the Morningness-Eveningness Questionnaire (MEQ) [
46].
4.3. Circadian and Physical Activity Objective Measures
Physical activity, circadian and sleep data were acquired using portable accelerometers (GeneActive Original Activinsights) on the non-dominant wrist for 15 d with a sampling frequency of 10 Hz. Data were extracted with the GENEactiv software and analysed with GGIR package [
47]. As a circadian phase proxy, we examined the L5h center (the timing of the least active 5 hrs in a day) on free days. We estimated sleep onset, sleep end and sleep duration for both training and free days. Circadian rhythms parameters included inter-daily stability (IS, ranging from 0 to 1, representing the degree of consistency of activity patterns from day to day), intra-daily variability (IV, ranging from 0 to 2, with higher values indicating a more fragmented rhythms), and Relative Amplitude (RA, ranging from 0 to 1, reflecting the difference between the activity during the 10 most active hours, M10, and the activity during the 5 least active hours, L5), calculated as (M10 −L5)/(M10 + L5) [
48,
49]. Exercise characterization was performed based on accelerometer-derived movement behaviour features from literature [
50]. The PA intensity was categorized into three levels, Light (LPA, PA < 93.2 mg), Moderate (MPA, 93.2 mg <PA < 418.3 mg) and Vigorous PA (VPA, PA ≥ 418.3 mg) [
51]. We assessed time spent in MPA during the training window, from 08:30 to 12:29, Monday through Friday. After detecting a bimodal distribution in the time spent in MPA, we categorized the dancers into two groups -an active group and a less active group- using the mean as the cutoff.
4.4. Performance
We administered a 2 min version of the psychomotor vigilance task (PVT) to assess alertness [
15] and a visual version of the Stroop Task (ST) to assess selective attention and inhibitory control, before and after the morning dance training [
52]. Participants were randomly assigned to have their first session at either 08:30 or 12:30 over three consecutive days (Tuesday, Wednesday, and Thursday), with each session lasting 10 minutes.
For the PVT task, a yellow ms counter was used as a stimulus. Participants were instructed to observe a red circle on the computer screen and to press the spacebar as soon as the counter stimulus appeared on the screen, which stopped the counter and displayed the reaction time in ms for a period of 1 s. The interstimulus interval, defined as the period between the last response and the appearance of the next stimulus, varied randomly between 2 and 10 s [
53]. A 10-s pre-practice test was performed. Reaction time (RT) in ms was recorded. A response was considered valid if the RT was ≥ 100 ms and < 1000 ms. Reaction speed was taken as the dependent variable, obtained from the reciprocal transformation of RT (1/RT). To calculate the individual mean reaction rate, the transformed values were averaged [
53,
54].
For the Stroop task, words describing four colors (red, yellow, green, and blue) were presented in the same color that they described (CC, congruent condition) or in a non-matching color (IC, incongruent condition). Participants were asked to press the key representing the color of the text, but not the meaning of the word. The interval between stimuli was fixed (1100 ms). The target stimulus remained on the screen for 2000 ms or until a response was registered. Four blocks of stimuli were presented with 32 trials each, 50% congruent, 50% incongruent (128 trials in total), and reaction times in ms (RT) were recorded. A response was regarded as valid if RT was ≥ 200 ms. Response speed was the dependent variable. To calculate the individual mean response speed, each RT (in seconds) was reciprocally transformed (1/RT) and the transformed values were then averaged [
53,
54]. Incorrect answers were discarded.
4.5. Data Analysis
We conducted all statistical analyses using R statistical software [
55] in the RStudio environment [
56]. We used paired t-tests to compare sleep parameters on free versus training days, and performance before and after the training session. We measured effect size using Cohen’s d. We conducted regression models to estimate the association between cognitive performance, circadian phase proxies and exercise variables. Values of p ≤ 0.05 were considered statistically significant.
5. Conclusions
Overall, our study expands the current understanding of the interplay between circadian rhythms, exercise, and their impact on cognition. Attentional performance is expected to improve after noon due to the circadian rhythmicity, but our results also suggest different associations between sleep, circadian phase and moderate exercise on tasks demanding varying degrees of difficulty. The less demanding task at the more challenging time was associated with sleep duration on free days. The more challenging task, which required inhibitory control, was associated with circadian phase at the most favorable time, whereas the task demanding selective attention was associated with time spent in moderate exercise during training, although only on more active dancers. These results reflect the delicate complexity of attentional processes and their modulation by both behaviour and endogenous biological rhythms. Within the framework of this model, regular morning dance training could enhance cognitive performance, potentially counteracting the impairment typically associated with later chronotypes at a challenging time of day. Our findings could be used to inform the design of healthier training schedules for both dancers and athletes, taking into account time of day, sleep habits and individual circadian phases.
Author Contributions
Conceptualization, M.M., A.C., B.T. and A.S.; methodology, M.M., A.C., B.T. and A.S.; formal analysis, M.M.; investigation, M.M., B.T. and A.S.; data curation, M.M.; writing—original draft preparation, M.M., B.T. and A.S.; writing—review and editing, M.M., A.C., B.T. and A.S.; visualization, M.M.; supervision, B.T. and A.S.; project administration, B.T. and A.S..; funding acquisition, B.T. and A.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CSIC, Universidad de la República, Uruguay, Programa de Grupos I+D – 2018 # 92.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the School of Psychology, Universidad de la República (2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, A.S., upon reasonable request.
Acknowledgments
We would like to thank all the dancers who kindly volunteered as participants of this study as well as the directors, teachers and coordinators of the ENFAS for their support and logistic help. We also thank Natalia Coirolo and Janaína Muñoz for their collaboration in collecting data and Alfonso Pérez for his collaboration in programming attentional tasks. We are very grateful to Graciela Muniz for statistical advice and Kent Dunlap for his collaboration in previous versions of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ENFAS |
Escuela Nacional de Formación Artística del SODRE |
| IS |
Interdaily stability |
| IV |
Intraday variability |
| MCTQ |
Munich Chronotype Questionnaire |
| MEQ |
Morningness-Eveningness Questionnaire |
| MPA |
Moderate Physical Activity |
| MVPA |
Moderate to Vigorous Physical Activity |
| MSFsc |
Mid-sleep point on free days corrected for sleep debt on workdays |
| PVT |
Psychomotor vigilance task |
| RA |
Relative Amplitude |
References
- Klerman, E.B.; Brager, A.; Carskadon, M.A.; Depner, C.M.; Foster, R.; Goel, N.; Harrington, M.; Holloway, P.M.; Knauert, M.P.; LeBourgeois, M.K.; Lipton, J.; Merrow, M.; Montagnese, S.; Ning, M.; Ray, D.; Scheer, F.A.J.L.; Shea, S.A.; Skene, D.J.; Spies, C.; Staels, B.; St-Onge, M.; Tiedt, S.; Zee, P.C.; Burgess, H.J. Keeping an Eye on Circadian Time in Clinical Research and Medicine. Clinical & Translational Med 2022, 12. [Google Scholar] [CrossRef]
- Dunster, G.P.; de la Iglesia, L.; Ben-Hamo, M.; Nave, C.; Fleischer, J.G.; Panda, S.; de la Iglesia, H.O. Sleepmore in Seattle: Later School Start Times Are Associated with More Sleep and Better Performance in High School Students. Sci. Adv. 2018, 4, eaau6200. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Waterhouse, J. Sports Performance: Is There Evidence That the Body Clock Plays a Role? Eur J Appl Physiol 2009, 106, 321–332. [Google Scholar] [CrossRef]
- Paranjpe, D.A.; Sharma, V.K. Evolution of Temporal Order in Living Organisms. J Circadian Rhythms 2005, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Holzberg, D.; Albrecht, U. The Circadian Clock: A Manager of Biochemical Processes Within the Organism: The Circadian Clock. Journal of Neuroendocrinology 2003, 15, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.E.; Skene, D.J. Nonphotic Entrainment in Humans? J Biol Rhythms 2005, 20, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human Circadian Phase–Response Curves for Exercise. J Physiol 2019, 597, 2253–2268. [Google Scholar] [CrossRef]
- Edwards, B.J.; Reilly, T.; Waterhouse, J. Zeitgeber -Effects of Exercise on Human Circadian Rhythms: What Are Alternative Approaches to Investigating the Existence of a Phase-Response Curve to Exercise? Biological Rhythm Research 2009, 40, 53–69. [Google Scholar] [CrossRef]
- Blatter, K.; Cajochen, C. Circadian Rhythms in Cognitive Performance: Methodological Constraints, Protocols, Theoretical Underpinnings. Physiology & Behavior 2007, 90, 196–208. [Google Scholar] [CrossRef]
- Valdez, P. Homeostatic and Circadian Regulation of Cognitive Performance. Biological Rhythm Research 2019, 50, 85–93. [Google Scholar] [CrossRef]
- Valdez, P. Circadian Rhythms in Attention. Yale J Biol Med 2019, 92, 81–92. [Google Scholar]
- Atkinson, G.; Reilly, T. Circadian Variation in Sports Performance: Sports Medicine 1996, 21, 292–312. [CrossRef]
- Roenneberg; Pilz; Zerbini; Winnebeck. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology 2019, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Facer-Childs, E.; Brandstaetter, R. The Impact of Circadian Phenotype and Time since Awakening on Diurnal Performance in Athletes. Current Biology 2015, 25, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Facer-Childs, E.R.; Boiling, S.; Balanos, G.M. The Effects of Time of Day and Chronotype on Cognitive and Physical Performance in Healthy Volunteers. Sports Med - Open 2018, 4, 47. [Google Scholar] [CrossRef]
- Lang, C.; Richardson, C.; Short, M.A.; Gradisar, M. Low-Intensity Scheduled Morning Exercise for Adolescents with a Late Chronotype: A Novel Treatment to Advance Circadian Phase? SLEEP Advances 2022, 3, zpac021. [Google Scholar] [CrossRef]
- Facer-Childs, E.R.; Middleton, B.; Skene, D.J.; Bagshaw, A.P. Resetting the Late Timing of ‘Night Owls’ Has a Positive Impact on Mental Health and Performance. Sleep Medicine 2019, 60, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Medicine & Science in Sports & Exercise 2019, 51, 1242–1251. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The Effects of Acute Exercise on Cognitive Performance: A Meta-Analysis. Brain Research 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Müller, P.; Gronwald, T.; Müller, N.G. Dose–Response Matters! – A Perspective on the Exercise Prescription in Exercise–Cognition Research. Front. Psychol. 2019, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T. Exercise-Cognition Interaction: Neuroscience Perspectives; Elsevier, Academic Press: London, UK, 2016. [Google Scholar]
- McMorris, T. The Acute Exercise-Cognition Interaction: From the Catecholamines Hypothesis to an Interoception Model. International Journal of Psychophysiology 2021, 170, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, E.; Riedel, W.; Jeukendrup, A.; Jolles, J. Cognitive Performance after Strenuous Physical Exercise. Percept Mot Skills 1996, 83, 479–488. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Alderman, B.L.; Chu, C.-H.; Wang, C.-C.; Song, T.-F.; Chen, F.-T. Acute Exercise Has a General Facilitative Effect on Cognitive Function: A Combined ERP Temporal Dynamics and BDNF Study: Acute Exercise, BDNF, ERPs, and Cognition. Psychophysiol 2017, 54, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Znazen, H.; Slimani, M.; Hadadi, A.; Alzahrani, T.; Tod, D.; Bragazzi, N.L.; Souissi, N. Acute Effects of Moderate versus High-Intensity Strength Exercise on Attention and Mood States in Female Physical Education Students. Life 2021, 11, 931. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.D.; Willis, E.A.; Ainsworth, B.E.; Barreira, T.V.; Hastert, M.; Kracht, C.L.; Schuna, J.M.; Cai, Z.; Quan, M.; Tudor-Locke, C.; Whitt-Glover, M.C.; Jacobs, D.R. 2024 Adult Compendium of Physical Activities: A Third Update of the Energy Costs of Human Activities. Journal of Sport and Health Science 2024, 13, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Isoglu-Alkac, U.; Ermutlu, M.N.; Eskikurt, G.; Yücesir, İ.; Demirel Temel, S.; Temel, T. Dancers and Fastball Sports Athletes Have Different Spatial Visual Attention Styles. Cogn Neurodyn 2018, 12, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kattenstroth, J.-C.; Kalisch, T.; Holt, S.; Tegenthoff, M.; Dinse, H.R. Six Months of Dance Intervention Enhances Postural, Sensorimotor, and Cognitive Performance in Elderly without Affecting Cardio-Respiratory Functions. Front. Aging Neurosci. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, K.; Lüders, A.; Hökelmann, A.; Lessmann, V.; Kaufmann, J.; Brigadski, T.; Müller, P.; Müller, N.G. Dance Training Is Superior to Repetitive Physical Exercise in Inducing Brain Plasticity in the Elderly. PLoS ONE 2018, 13, e0196636. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. BPL 2017, 2, 127–152. [Google Scholar] [CrossRef]
- Fietze, I.; Strauch, J.; Holzhausen, M.; Glos, M.; Theobald, C.; Lehnkering, H.; Penzel, T. SLEEP QUALITY IN PROFESSIONAL BALLET DANCERS. Chronobiology International 2009, 26, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Coirolo, N.; Silva, A.; Tassino, B. The Impact of Training Shifts in Dancers’ Chronotype and Sleep Patterns. Sleep Sci. 2020. [Google Scholar] [CrossRef]
- Coirolo, N.; Casaravilla, C.; Tassino, B.; Silva, A. Evaluation of Environmental, Social, and Behavioral Modulations of the Circadian Phase of Dancers Trained in Shifts. iScience 2022, 25, 104676. [Google Scholar] [CrossRef]
- Estevan, I.; Coirolo, N.; Tassino, B.; Silva, A. The Influence of Light and Physical Activity on the Timing and Duration of Sleep: Insights from a Natural Model of Dance Training in Shifts. Clocks & Sleep 2023, 5, 47–61. [Google Scholar] [CrossRef]
- Marchesano, M.; Coirolo, N.; Tassino, B.; Silva, A. Impact of Training-Shift Change on Chronotype and Social Jetlag: A Longitudinal Study on Dancers. Biological Rhythm Research 2023, 1–8. [Google Scholar] [CrossRef]
- Burke, T.M.; Scheer, F.A.J.L.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P. Sleep Inertia, Sleep Homeostatic and Circadian Influences on Higher-Order Cognitive Functions. J Sleep Res 2015, 24, 364–371. [Google Scholar] [CrossRef]
- Leone, M.J.; Sigman, M.; Golombek, D.A. Effects of Lockdown on Human Sleep and Chronotype during the COVID-19 Pandemic. Current Biology 2020, 30, R930–R931. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Bowen, A.E.; Cooper, E.; Brinton, J.T.; Higgins, J.; Nadeau, K.J.; Wright, K.P.; Simon, S.L. A Naturalistic Actigraphic Assessment of Changes in Adolescent Sleep, Light Exposure, and Activity Before and During COVID-19. J Biol Rhythms 2022, 37, 690–699. [Google Scholar] [CrossRef]
- Hsieh, S.-S.; Huang, C.-J.; Wu, C.-T.; Chang, Y.-K.; Hung, T.-M. Acute Exercise Facilitates the N450 Inhibition Marker and P3 Attention Marker during Stroop Test in Young and Older Adults. JCM 2018, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Alderman, B.; Wu, C.-H.; Chi, L.; Chen, S.-R.; Chu, I.-H.; Chang, Y.-K. Effects of Acute Aerobic and Resistance Exercise on Cognitive Function and Salivary Cortisol Responses. Journal of Sport and Exercise Psychology 2019, 41, 73–81. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, F.T.; González-Víllora, S.; Baena-Morales, S.; Pastor-Vicedo, J.C.; Clemente, F.M.; Badicu, G.; Murawska-Ciałowicz, E. Effect of Physical Exercise Program Based on Active Breaks on Physical Fitness and Vigilance Performance. Biology 2021, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M. de Sousa, A.; Medeiros, A.R.; Del Rosso, S.; Stults-Kolehmainen, M.; Boullosa, D.A. The Influence of Exercise and Physical Fitness Status on Attention: A Systematic Review. International Review of Sport and Exercise Psychology 2019, 12, 202–234. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Jama 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. J Biol Rhythms 2003, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A Marker for the End of Adolescence. Current Biology 2004, 14, R1038–R1039. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A Self-Assessment Questionnaire to Determine Morningness-Eveningness in Human Circadian Rhythms. Int J Chronobiol 1976, 4, 97–110. [Google Scholar]
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A Research Community–Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes From Multi-Day Raw Accelerometer Data. Journal for the Measurement of Physical Behaviour 2019, 2, 188–196. [Google Scholar] [CrossRef]
- Rock, P.; Goodwin, G.; Harmer, C.; Wulff, K. Daily Rest-Activity Patterns in the Bipolar Phenotype: A Controlled Actigraphy Study. Chronobiology International 2014, 31, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.V.; Van Hees, V.T.; Dawkins, N.P.; Maylor, B.D.; Plekhanova, T.; Henson, J.; Edwardson, C.L.; Brady, E.M.; Hall, A.P.; Davies, M.J.; Yates, T. Accelerometer-Assessed Physical Activity in People with Type 2 Diabetes: Accounting for Sleep When Determining Associations with Markers of Health. Sensors 2023, 23, 5382. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.V.; Edwardson, C.L.; Davies, M.J.; Khunti, K.; Harrington, D.M.; Yates, T. Beyond Cut Points: Accelerometer Metrics That Capture the Physical Activity Profile. Medicine & Science in Sports & Exercise 2018, 50, 1323–1332. [Google Scholar] [CrossRef]
- Hildebrand, M.; Van Hees, V.T.; Hansen, B.H.; Ekelund, U. Age Group Comparability of Raw Accelerometer Output from Wrist- and Hip-Worn Monitors. Medicine & Science in Sports & Exercise 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. Journal of Experimental Psychology 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Basner, M.; Mollicone, D.; Dinges, D.F. Validity and Sensitivity of a Brief Psychomotor Vigilance Test (PVT-B) to Total and Partial Sleep Deprivation. Acta Astronautica 2011, 69, 949–959. [Google Scholar] [CrossRef]
- Dinges, D.F.; Orne, M.T.; Whitehouse, W.G.; Orne, E.C. Temporal Placement of a Nap for Alertness: Contributions of Circadian Phase and Prior Wakefulness. Sleep 1987, 10, 313–329. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing., 2022. https://www.R-project.org/.
- Posit team. RStudio: Integrated Development Environment for R, 2023. http://www.posit.co/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).