Submitted:
06 January 2025
Posted:
07 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- a)
-
Multifocal candidiasis: Simultaneous isolation of Candida spp. in 2 or more of the following foci [20]:
- Respiratory focus: Isolation of tracheal aspirate samples
- Digestive focus: Simultaneous isolation of gastric aspirate and pharyngeal swab
- Urinary focus: Positive urine cultures
- Drains: Positive culture of samples obtained from different drains
- b)
- Disseminated candidiasis: Presence of endophthalmitis due to Candida spp. or isolation from biopsies, usually sterile organic fluids or blood culture with a negative catheter tip
- c)
- Invasive candidiasis: Patients who present both multifocal and disseminated candidiasis are included in this group
- d)
- Colonization by Candida spp.: When the isolation has occurred in a single focus not suggestive of dissemination or at the skin level, feces or vaginal smear
- Heart, liver, spleen and brain: Presence of yeast in histology associated with polymorphonuclear inflammatory foci in different areas of the parenchyma studied.
- Blood and spleen: Cultures obtained by puncture of cardiac cavities or splenic parenchyma with a sterile needle and prior cauterization of the puncture point
- Intestine: Presence in histology of pseudohyphae or spores that extend to the muscle and/or serosa, together with the presence of an important polymorphonuclear infiltrate. The culture is assessed from samples obtained from the peritoneal cavity or bile fluid
- Lung: Histology is evaluated if pseudohyphae and spores are found in the parenchyma, alveoli and vessels, along with polymorphonuclear infiltrates. The culture has been obtained from areas of the parenchyma distant from the main bronchi and trachea
- Kidneys: Presence of yeast with a polymorphonuclear inflammatory component in the kidney parenchyma. The culture is obtained from areas of the parenchyma and not from the urinary tract
- Negative histology and postmortem cultures in significant organs positive for yeast without identification of other microorganisms: These cultures have been considered significant in relating yeast to the cause of death
- Negative histology and postmortem cultures in significant organs positive for yeast with identification of other microorganisms: Yeast has not been considered as a cause of death
- Negative cultures and positive histology in a patient who has received correct antifungal treatment: Death is attributed to yeast
- Negative cultures and positive histology in more than one organ, in a patient who has received incorrect antifungal treatment: Death is attributed to yeast
- Negative cultures and positive histology in a single organ, in a patient who has received incorrect antifungal treatment: Yeasts have not been considered as the cause of death
- Cultures and histology positive for yeast: These have been considered the cause of death
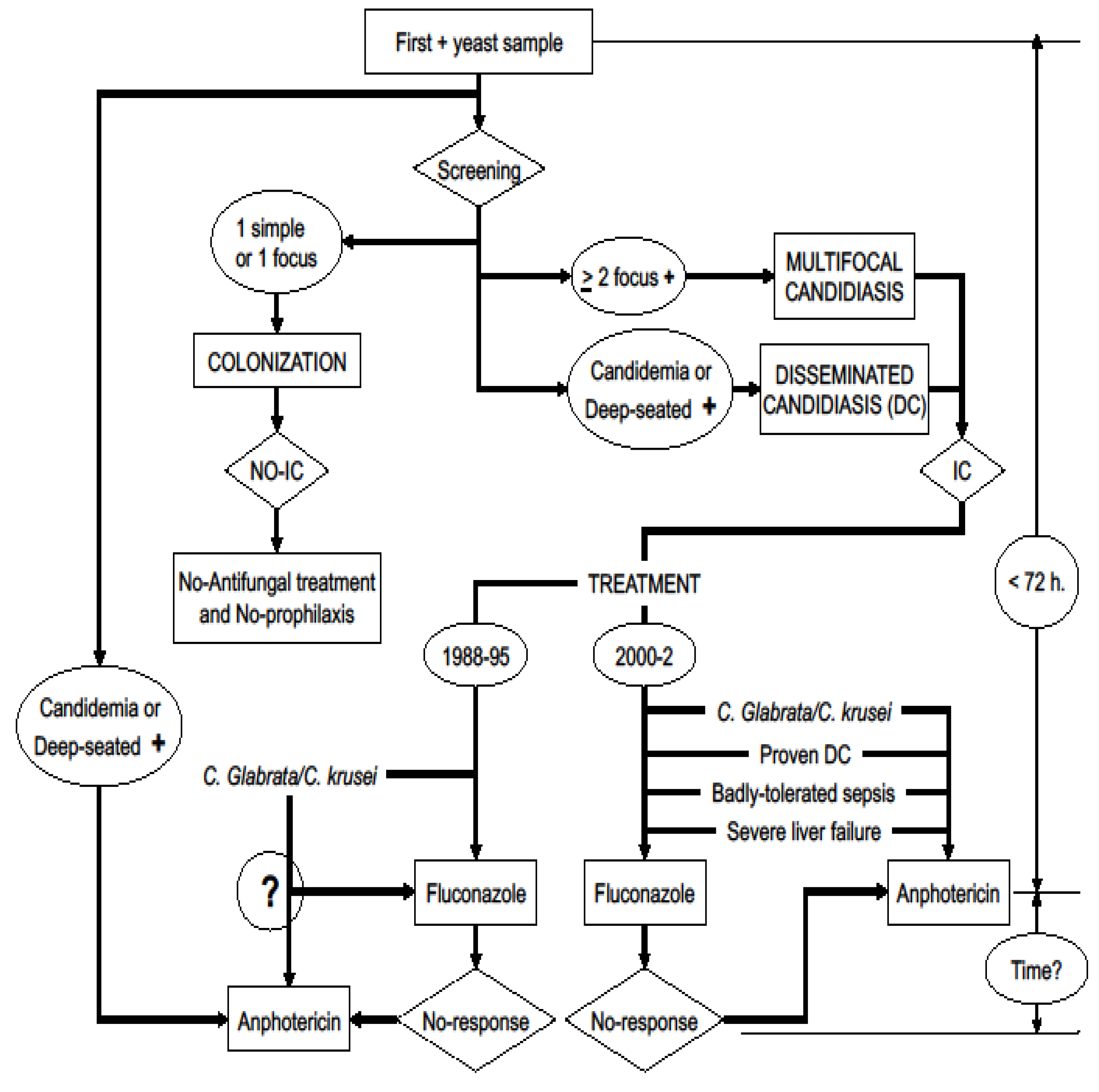
- Definition of attributable mortality based on the postmortem study: It refers to patients who die because of a certain factor that is being analyzed. In a certain way, the “gold standard” for the diagnosis of invasive candidiasis will be biopsy [31] or the postmortem study [26]. In this study, the factor to be analyzed was candidiasis acquired in the ICU in non-neutropenic patients, as the main cause or factor that could influence the patient’s death. Following this work methodology, a classification algorithm for high probability of death attributable to yeast is defined (Figure 1).
- Definition of attributable mortality based on the clinical study: The cases in which the postmortem study was not performed have been used as a reference group. The group of patients with a moderate probability of death attributable to yeast is defined based on the clinical cause of death, the existence of a multifocality of yeast isolations and the performance of incorrect antifungal therapy before death.
- Definition of global attributable mortality: Global mortality attributable to yeast has been considered the sum of cases that have been defined as attributable mortality based on the postmortem study and those defined as attributable mortality based on the clinical study, in relation to the total of the deceased population
3. Results
3.1. Demographics
3.1.1. Characteristics of the Population Studied
3.1.2. Characteristics of Fungal Infection
- Positive samples: The samples that were most frequently positive, both in the first positive culture and in screening, were the bronchial secretion cultures (66% and 86% respectively) (Table 2).
- Blood cultures: The percentage of patients with candidemia, in relation to the number of patients treated during said period, has been 0.5% (18/3389). In total there were 24 positive blood cultures in 18 patients. In 4 of them the isolation was in the first sample, in 14 in the screening and in 6 in the follow-up blood cultures. 27 catheters with colonized tips were detected. Four in the first sample, 16 in the screening and 7 in the follow-up cultures. In 8 patients the association of a positive catheter with a positive blood culture was verified (44% of patients with a positive blood culture).
- Identified yeast species: The most frequently isolated yeast has been Candida albicans, both in the first positive culture and in the screening (80% and 87% respectively), followed by Candida glabrata, also in both cases (11% and 18% respectively) (Table 3).
-
Classification according to location of the yeasts: The patients were grouped into multifocal candidiasis in 89 cases (61%), disseminated candidiasis 31 cases (22%) and colonization by Candida sp in 25 cases (17%). In total, 120 invasive candidiasis have been detected, representing 3.5% of the population treated during the period studied (120/3389):
- a)
- Multifocal candidiasis: The respiratory focus was identified in 96% (85), the digestive focus in 93% (83), the urinary focus in 42% (37) and the drainage in 2% (2). In 67% (60) 2 simultaneous foci were demonstrated, in 33% (29) 3 foci and there were no cases with 4 foci.
- b)
- Disseminated candidiasis: They were classified as such because they demonstrated the presence of endophthalmitis in 3 cases (one of them with a positive blood culture). In 7 cases the evidence was positive blood cultures and negative catheter tip. There were 12 cases in which the yeast was identified in samples obtained from puncture of abscesses, in 2 cases from cerebrospinal fluid and in another 2 from tissue biopsies. In 5 cases evidence of dissemination was demonstrated in the postmortem study.
- c)
- Colonization: Yeasts were identified in bronchial secretions 15 cases, pharynx 15 cases, stool culture 10 cases, gastric aspirate 8 cases, urine culture 5 cases, vagina 2 cases, wounds 2 cases, catheters 2 cases, catheter together with positive blood culture 1 case, skin 1 case and nasal 1 case
3.1.3. Antifungal Treatment
3.1.4. Mortality
3.1.5. Postmortem Study
3.1.6. Attributable Mortality
- Attributable mortaity according to statistical analysis, if the ICU mortality of the studied population was 35% and that of the population treated in the ICU during the study carried out was 9.6%, the attributable mortality according to statistical analysis is 25%.
- Attributable mortality according to postmortem study, of the 16 patients in whom yeast was identified in the postmortem study, there were 6 who died from respiratory failure, 5 from septic shock or MODS, 2 from non-septic shock, 2 from brain death and 1 case from hepatocellular insufficiency. According to the criteria based on the postmortem study, death was attributed to yeast in 10 cases (28%) (Table 5).
- Attributable death based on the clinical study, of the 31 cases in which no postmortem study was performed, 7 cases (23%) were considered to meet the criteria for death attributable to yeast (Table 6).
- Overall attributable mortality, assessing the data on attributable death according to the postmortem study together with that based on the clinical study, it can be considered that there were 17 cases out of 67 (25%) in which death could be attributed to yeast. This number of patients represents a mortality related to Candida spp. of 12% in the 145 patients studied.
3.2. Figures and Tables
| Risk factors for candidiasis | Number of cases (%) |
|---|---|
| Antibiotics | 144 (99) |
| Central venous catheter | 143 (99) |
| Urinary catheter | 140 (97) |
| Antacid therapy | 141 (97) |
| Naso-orogastric catheter | 131 (90) |
| Arterial catheter | 125 (86) |
| Oro-nasotracheal tube and / or tracheostomy | 124 (85) |
| Surgical procedures | 85 (59) |
| Vasoactive drugs | 72 (50) |
| Total parenteral nutrition | 68 (47) |
| Drainages | 65 (45) |
| Blood derivatives | 62 (43) |
| Corticoids | 56 (39) |
| Hemodyalisis | 8 (5) |
| Splenectomy | 5 (3) |
| CAUSES OF DEATH | Number of cases (%) |
|---|---|
| Septic shock – Multiorganic dysfunction syndrome | 18 (50) |
| Respiratory failure (hypoxemia) | 8 (22) |
| Brain death | 4 (11) |
| Non-septic shock | 3 (8) |
| Hepatic failure | 3 (8) |
| CAUSES OF ADMISSION | Number of cases (%) |
|---|---|
| Respiratory failure | 17 (12) |
| Cardiovascular | 23 (16) |
| Sepsis | 9 (6) |
| Respiratory infection | 19 (13) |
| Multiple trauma | 52 (36) |
| Neurologic (non-cranioencephalic trauma) | 9 (6) |
| Digestive and others | 16 (11) |
| Location | Positive cultures only | Positive histology only | Positive cultures and histology | All positive cultures (n=24) (%) | All positive histology (n=36) (%) |
|---|---|---|---|---|---|
| Lung | 5 | 3 | 3 | 8 (33) | 6 (17) |
| Trachea | 4 | 4 (17) | |||
| Bowel | 2 | 1 | 1 | 3 (12) | 2 (6) |
| Heart | 1 | 2 | 1 (4) | 2 (6) | |
| Kidney | 1 | 2 | 1 (4) | 2 (6) | |
| Urinary tract | 3 | 3 (12) | |||
| Liver | 2 | 2 (8) | |||
| Spleen | 2 | 2 (8) | |||
| CNS | 1 | 1 (3) | |||
| Positive samples | 20 | 9 | 4 | 24 | 13 |
| Patients | Cause of death | CD Risk | Histology | Cultures | Antifungal treatment |
| JGB | Non-septic shock | Multifocality | > 1 S.O. | Negative | Not given* |
| ACG | Septic shock | Multifocality | S.O. | S.S. | Given |
| JLS | Respiratory failure | Multifocality | Non-S.O. | S.S. | Given |
| MTN | Respiratory failure | Multifocality | S.O. | S.S. | Not given |
| APS | Septic shock | Multifocality | S.O. | S.S. | Not given |
| JFC | Respiratory failure | Multifocality | S.O. | S.S. | Given |
| VLG | Septic shock | Multifocality | > 1 S.O. | Negative | Not given * |
| AMG | Respiratory failure | Multifocality | S.O. | Negative | Given * |
| BCP | Septic shock | Multifocality | S.O. | Negative | Given * |
| AMM | Septic shock | Multifocality | S.O. | S.S. | Not given |
| Patients | Cause of death | CD Risk | Candida spp. | Antifungal treatment | FUC |
|---|---|---|---|---|---|
| JMS | Septic shock | Multifocality | C. glabrata | Incorrect | Not made |
| ECA | Septic shock | Multifocality |
C. glabrata C. albicans |
Incorrect | Positive |
| FVP | Respiratory failure | Multifocality |
C. glabrata C. albicans C. tropicalis |
Incorrect | Positive |
| BGB | MODS | Multifocality | C. albicans | Correct | Positive |
| TLC | Septic shock | Multifocality |
C. glabrata C. albicans C. krusei |
Incorrect | Not made |
| GMM | MODS | Multifocality | C. albicans | Incorrect | Positive |
| JG | MODS | Multifocality |
C. glabrata C. albicans C. tropicalis |
Incorrect | Not made |
| VARIABLES | NAM | AM | P |
|---|---|---|---|
| Age | 51 (22) | 69 (14) | 0.001* |
| Previous diseases | 2 (0-4) | 3 (1-4) | 0.009† |
| Apache III | 72 (24-136) | 90 (58-183) | 0.040† |
| Abdominal surgery on admission | 24/128 (19) | 9/17 (53) | 0.001‡ |
| More than one foci (risk classification) | 100/128 (78) | 17/17 (100) | 0.043§ |
| Candida glabrata at screening | 20/128 (16) | 6/15 (40) | 0.020‡ |
| Candida tropicalis at screening | 6/128 (5) | 3/15 (20) | 0.036§ |
| Urine cultures at FUC | 20/128 (16) | 13/75 (75) | <0.001§ |
| Variables | attributable Mortality | ||
|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | P | |
| Abdominal surgery | 9.17 | 1.77-47.39 | 0.008 |
| Antifungal treatment | <0.01 | <0.01-0.10 | <0.001 |
| Candida glabrata at screening | 7.38 | 1.24-43.98 | 0.028 |
| Previous diseases | 1.82 | 0.98-3.37 | 0.055 |
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carpenter, H.; Wilkins, R.M. Autopsy bacteriology: Review of 2033 cases. Arch Pathol 1964, 77, 73–81. [Google Scholar] [PubMed]
- Dolan, C.T.; Brown, A.L.; Ritts, R.E. Microbiological examination of postmortem tissues. Arch Pathol 1971, 92, 206–211. [Google Scholar] [PubMed]
- DuMoulin, G.C.; Paterson, D.G. Clinical relevance of postmortem microbiologic examination: A review. Hum Pathol 1985, 16, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Martín Álvarez, R.; Pérez Sáenz, J.L. Microbiología postmortem. Med Clin (Barc) 1983, 81, 667–669. [Google Scholar]
- Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in Intensive Care Units in 2017. JAMA 2020, 323(15): 1478-1487. [CrossRef]
- Fraser, V.J.; Jones, M.; Dunkel, J.; Storfer, S.; Medoff, G.; Dunagan, W.C. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis 1992, 15, 414–421. [Google Scholar] [CrossRef]
- Harvey, R.L.; Myers, J.P. Nosocomial fungemia in a large community teaching hospital. Arch Intern Med 1987, 147, 2117–2120. [Google Scholar] [CrossRef]
- Klein, J.J.; Watanakunakorn, C. Hospital-Acquired fungemia. Its natural course and clinical significance. Am J Med 1979, 67, 51–58. [Google Scholar] [CrossRef]
- Komshian, S.V.; Uwaydah, A.K.; Sobel, J.D.; Crane, L.R. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev Infect Dis 1989, 11, 379–390. [Google Scholar] [CrossRef]
- Meunier-Carpentier, F.; Kiehn, T.E.; Armstrong, D. Fungemia in immunocompromissed host. Changing patterns, antigenemia, high mortality. Am J Med 1981, 71, 363–370. [Google Scholar] [CrossRef]
- Pittet, D.; Tarara, D.; Wenzel, R.P. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1994, 271, 1598–1601. [Google Scholar] [CrossRef]
- Wenzel, R.P. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis 1995, 20, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Fagon, J.Y.; Chastre, J.; Hance, A.J.; Montravers, P.; Novara, A.; Gibert, C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med 1993, 94, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Nolla M, Chanovas M, Torres JM, Nolla J, Garcés J. Cellular Immunity alterations and presence of Candida in patients admitted to the intensive care unit. In: Aochi O, Amaha K, Takeshita H (eds). Intensive and Critical Care medicine. Excerpta Medica. International Congress Series 885, Amsterdam 1990, pg 1135.
- Brooks, R.G. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Arch Intern Med 1989, 149, 2226–2228. [Google Scholar] [CrossRef] [PubMed]
- Donahue SP, Greven CM, Zuravleff JJ, Eller AW, Nguyen MH, Peacock JE Jr, Wagener MM, Yu VL. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophtalmology 1994, 101, 1302–1309. [Google Scholar] [CrossRef]
- Ibàñez-Nolla J, Torres-Rodríguez JM, Nolla M, et al. The utility of serology in diagnosing candidosis in non-neutropenic critically ill patients. Mycoses 2001, 44, 47–53. [Google Scholar] [CrossRef]
- Ibáñez-Nolla J, Nolla-Salas M, León MA, et al. Early diagnosis of candidiasis in non-neutropenic critically ill patients, J Infect 2004, 48, 181–192. [CrossRef]
- Nolla-Salas M, Monmany J, Gich I, Ibàñez-Nolla J. Early Treatment of Candidiasis in Non-Neutropenic Critically Ill Patients. World Conference on Magic Bullets. Celebrating Paul Ehrlich’s 150th Birthday. Nürnberg, Germany, 2004, 347.
- Ibáñez-Nolla, J.; Nolla-Salas, M. Multifocal Candidiasis can be considered a form of Invasive Candidiasis in critically non neutropenic patients. IJID. 2024, 147. [Google Scholar] [CrossRef]
- Lefemine, A.; Acuff, R.; Vo, N.; Waycaster, M. Delayed hypersentivity on a surgical service. J Am Coll Nutr 1988, 7:355-359.
- Maxwell, A.P.; McCluskey, D.R. Assessment of cell-mediated immunity in a British population using multiple skin test antigens. Clin Allerg 1986, 16, 365–369. [Google Scholar] [CrossRef]
- Balows A, Harsler WJ. Manual of Clinical Microbiology. 5th ed., section III. American Society for Microbiology, Washington, 1991. DC. 209-553.
- Ludwig, J. Current methods of autopsy practice. Sunders Company WB, Philadelphia 1979.
- Bodey, G.; Bueltmann, B.; Duguid, W.; Gibbs, D.; Hanak, H.; Hotchi, M.; Mall, G.; Martino, P.; Meunier, F.; Milliken, S.; Naoe, S.; Okudaira, M.; Scevola, D.; van’t Wout, J. Fungal infections in cancer patients. An International Autopsy Survey. Eur J Clin Microbiol Infect Dis 1992, 11, 99–109. [Google Scholar] [CrossRef]
- Hughes, W.T. Systemic candidiasis. A study of 109 fatal cases. Pediatr Infect Dis 1982, 1: 11-18.
- Jandrlic, M.; Kalenic, S.; Labar, B.; Nemet, D.; Jakic-Razumovic, J.; Mrsic, M.; Plecko, V.; Bogdanic, V. An autopsy study of systemic fungal infections in patients with hematologic malignancies. Eu J Clin Microbiol Infect Dis 1995, 14, 768–774. [Google Scholar] [CrossRef]
- DeGregorio, M.W.; Lee, W.M.F.; Linker, C.A.; Jacobs, R.A.; Ries, C.A. Fungal infections in patients with acute leukemia. Am J Med 1982, 73, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Myerowitz, R.L.; Pazin, G.J.; Allen, C.M. Disseminated candidiasis. Changes in incidence, underlying diseases and pathology. Am J Clin Path 1977, 68, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Falagas ME, Snydman DR, George MJ, Werner B, Ruthazer R, Griffith J, Rohrer RH, Freeman R, Boston Center for Liver Transplantation CMVIG Study Group. Incidence and predictors of Cytomegalovirus pneumonia in orthotopic liver transplant recipients. Transplantation 1996, 61, 1716–1720. [Google Scholar] [CrossRef]
- Franklin, C.; Metry, M. Life-threatening Candida infections in the intensive care unit. J Intensive Care Med 1992, 7: 127-137.
- Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994, 220, 751–758.
- Reagan, D.R.; Pfaller, M.A.; Hollis, R.J.; Wenzel, R.P. Evidence of nosocomial spread of Candida albicans causing bloodstream infection in a neonatal intensive care unit. Diagn Microbiol Infect Dis 1995, 21, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Hollis, R.J.; Pfaller, M.A.; Wenzel, R.P.; Doebbeling, B.N. Investigation of the sequence of colonization ans candidemia in nonneutropenic patients. J Clin Microbiol 1994, 32, 975–980. [Google Scholar] [CrossRef]
- Horn, R.; Wong, B.; Kiehn, T.E.; Armstrong, D. Fungemia in a cancer hospital: Changing frequency, earlier onset, and results of therapy. Rev Infect Dis 1985, 7: 646-655.
- Meunier, F.; Aoun, M.; Bitar, N. Candidemia in immunocompromised patients. Clin Infect Dis 1992, 14 (Suppl. 1); S120-S125.
- Nolla-Salas, J.; Sitges-Serra, A.; León-Gil, C.; Martínez-González, J.; León-Regidor, M.A.; Ibáñez-Lucía, P.; Torres-Rodríguez, J.M. Candidemia in nonneutropenic critically ill patients: Analisys of prognostic factors and assessment of systemic antifungal therapy. Intensive Care Med 1997, 23, 23–30. [Google Scholar] [CrossRef]
- Anaissie E, Solomkin JS. Fungal infection. In: Wilmore DW, Cheung LY, Harken AH - American College of Surgeons (eds). FALL, Scientific American Surgery. Scientific American Inc., New York 1994, pg 1-19.
- Fung JC, Donta ST, Tilton RC. Candida detection system (Cand-Tec) to differentiate between Candida albicans colonization and disease. J Clin Microbiol 1986, 24, 542–547.
- Kujath, P.; Lerch, K.; Kochendörfer, P.; Boos, C. Comparative study of the efficacy of fluconazole versus amphotericin B/flucytosine in surgical patients with systemic mycoses. Infection 1993, 21, 376–382. [Google Scholar] [CrossRef]
- Pellinen, T.J.; Valtonen, V.V.; Luomanmaki, K.; Sivonen, A.; Virtanen, K.S. The microbial colonization due to medical devices in intensive care patients with special emphasis on Candida albicans. Ann Clin Res 1983, 15, 62–65. [Google Scholar]
- Trilla, A. Epidemiology of nosocomial infections in adult intensive care units. Intensive Care Med 1994, 20 (Suppl. 3): S1-S4.
- Dlouhy P, Svejda J, Veselska A, Kalhousova V. Candida sepsis: Risk factors, pathogenesis and the clinical picture. Cas Lek Ces 1993, 132, 393–396.
- Burchard, K.W.; Minor, L.B.; Slotman, G.J.; Gann, D.S. Fungal sepsis in surgical patients. Arch Surg 1983, 118, 217–221. [Google Scholar] [CrossRef] [PubMed]
- McQuillen, D.P.; Zingman, B.S.; Meunier, F.; Levitz, S.M. Invasive infections due to Candida krusei: Report of ten cases of fungemia that include three cases of endophthalmitis. Clin Infect Dis 1992, 14, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Rex JH, Bennet JE, Sugar AM, Pappas PG, Van-der-Horst CM, Edwards JE, Washburn RG, Scheld WM, Karchmer AW, Dine AP, Levenstein MJ, Webb CD, Candidemia Study Group and the National Institute of Allergy and Infections Diseases Mycoses Study Group. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med 1994, 331, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Caroll, K.; Jeppson, K.; Reading, J.; Reimer, L. Factors influencing outcome in hospitalized patients with candidemia. Infect Dis Clin Pract 1993, 2: 268-271.
- Develoux, M.; Gajdos, P.; Goulon, M. Les candidoses disséminées. Vingt-trois cas observés dans un service de réanimation. Ann Méd Interne 1982, 133, 406–409. [Google Scholar]
- Dhahri, M.A.; Jebali, A.; Lamine, K.; Labbene, I.; Ferjani, M. Alerte aux septicémies à levures en réanimation polyvalente. Cahiers Anesthésiologie 1995, 43, 13–19. [Google Scholar]
- Eubanks PJ, de Virgilio C, Klein S, Bongard F. Candida sepsis in surgical patients. Am J Surg 1993, 166, 617–620.
- Neumann, P.R.; Rakower, S.R. The risk of positive cultures for Candida in the critically ill patient. Crit Care Med 1978, 6: 73-76.
- Sánchez-Rodríguez, C.; León-Regidor, M.A.; Capell-Font, S.; Pérez-Campos, A.; Planes-Reig, A.; León-Gil, C. Funguemia 1973-1983, Análisis de 67 pacientes. Med Clin (Barc) 1985, 84, 549–553. [Google Scholar]
- Slotman, G.J.; Burchard, K.W. Ketoconazole prevents Candida sepsis in critically ill surgical patients. Arch Surg 1987, 122, 147–151. [Google Scholar] [CrossRef]
- Soutter, D.I.; Todd, T.R. Systemic candidiasis in surgical intensive care unit. Can J Surg 1986, 29, 197–199. [Google Scholar]
- Bassetti M, Azoulay E, Kullberg BJ, et al. Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin Infect Dis 2021, 72, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Bassetti M, Scudeller L, Giacobbe DR, et al. Developing definitions for invasive fungal diseases in critically ill adult patients in intensive care units. Protocol of the Fungal Infections Definitions in ICU patients (FUNDICU) project. Mycoses 2019, 62, 310–319. [Google Scholar] [CrossRef]
- Bassetti M, Giacobbe DR, Agvald-Ohman C, Akove M, Alastruey-Izquierdo A, Arikan-Akdagli S, et al. Invasive Fungal Diseases in Adult Patients in Intensive care Unit (FUNDICU): 2024 consensus definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC and ISHAM. Intensive care Med 2024. https//doi.org/10.1007/s00134-024-07341-7.
- Guiot, H.F.L.; Fibbe, W.E.; Van’t Wout, J.W. Risk factors for fungal infections in patients with malignant hematologic disorders: Implications for empirical therapy and prophylaxis. Clin Infect Dis 1994, 18, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Merz, W.G.; Karp, J.E.; Schron, D.; Saral, R. Increased incidence of fungemia caused by Candida krusei. J Clin Microbiol 1986, 24, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Bille, J.; Schneider, R.; Mosimann, F.; Francioli, P. Clinical significance of Candida isolated from peritoneum in surgical patients. Lancet 1989, 11, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Marsh PK, Tally FP, Kellum J, Callow A, Gorbach SL. Candida infections in surgical patients. Ann Surg 1983, 198, 42–47.
- Aisner J, Schimpff SC, Sutherland JC, Young VM, Wiernik PH. Torulopsis glabrata infections in patients with cancer: Increasing incidence and relationship to colonization. Am J Med 1976, 61, 23–28.
- Bryce, E.A.; Roberts, F.J.; Sekhon, A.S.; Coldman, A.J. Yeast in blood cultures. Evaluation of factors influencing outcome. Diagn Microbiol Infect Dis 1992, 15, 233–237. [Google Scholar] [CrossRef]
- Wey, S.B.; Mori, M.; Pfaller, M.A.; Woolson, R.F.; Wenzel, R.P. Hospital-acquired candidemia. The atributable mortality and excess length of stay. Arch Intern Med 1988, 148, 2642–2645. [Google Scholar] [CrossRef]
- Bross, J.; Talbot, G.H.; Maislin, G.; Hurwitz, S.; Strom, B.L. Risk factors for nosocomial candidemia. A case-control study in adults without leukemia. Am J Med 1989, 87, 614–620. [Google Scholar] [CrossRef]
- Graninger, W.; Presteril, E.; Schneeweiss, B.; Teleky, B.; Georgopoulos, A. Treatment of Candida albicans fungaemia with fluconazole. J Infect Dis 1993, 26, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Meunier, F. Candidiasis. Eur J Clin Microbiol Infect Dis 1989, 8: 438-447.
- Sobel JD. Candida infections in the ICU. Crit Care Clin 1988, 4: 325-344.
- Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risck prediction of hospital mortality for critically ill hospital adults. Chest 1992, 100, 1619–1636. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





