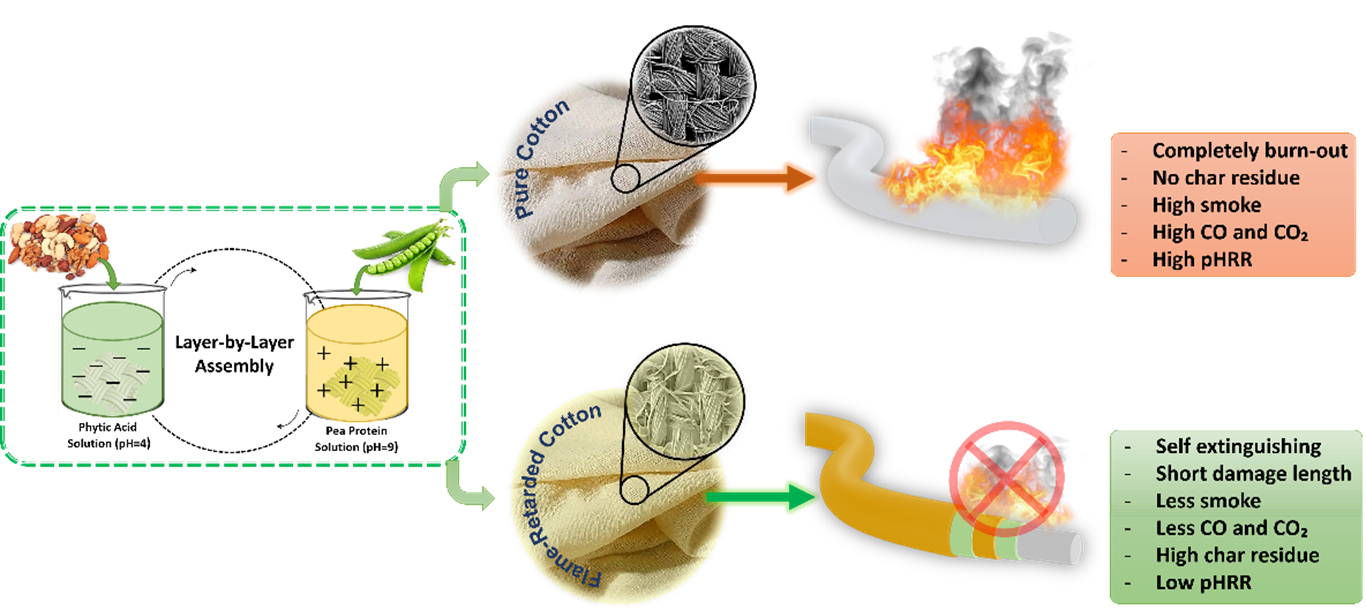
1. Introduction
Among various natural textiles, cotton fabric, made from biodegradable cellulose, offers numerous benefits including superior hygroscopicity, softness, comfort, and breathability. Due to its distinctive characteristics, cotton fabric is extensively utilized in various domains such as apparel, furniture, military uniforms, home decor, and industrial applications [
1,
2,
3]. Despite its many advantages, cotton fabric’s primary shortcoming is its flammability. This limitation restricts its suitability for high-performance applications that necessitate flame resistance. Cotton is composed of carbohydrate chain segments, making it highly flammable. Upon ignition, it undergoes significant degradation, producing highly combustible volatiles that lead to rapid fire spread and substantial smoke release, thereby heightening the risk of fatalities and severe societal damage [
4]. Consequently, there is an urgent need to enhance the flame retardancy of cotton fabric to comply with mandatory flammability standards. Thus, modifying cotton fabric to impart superior flame retardant properties is essential.
Halogenated flame retardants are acknowledged as the most efficacious agents for enhancing the fire resistance of cotton fabrics [
5,
6]. Nonetheless, their combustion is accompanied by the release of toxic fumes, including carcinogenic dioxins, which pose severe threats to human health and contribute substantially to environmental pollution. Consequently, numerous countries have determined to prohibit the use of halogenated flame retardants [
7,
8]. Instead, flame retardants containing phosphorus, nitrogen, boron, and silicon elements are extensively employed for this purpose [
9]. Among these, phosphorus-based flame retardants are particularly noted for their efficacy and low toxicity when applied to cotton fabrics, as conducted by Nguyen et al.’s investigation [
10]. During the combustion, phosphorus-containing flame retardants (FRs) generate nonvolatile phosphorus-based acids. These acids can esterify and dehydrate decomposed cellulose, leading to the formation of a coherent char residue. This char acts as a barrier, impeding the transfer of oxygen and heat between the gaseous and condensed phases, thereby facilitating the cessation of combustion [
11]. Commercially available flame retardants, such as Pyrovatex CP® and PROBAN®, incorporate reactive phosphorus or N-CH2OH groups synthesized with formaldehyde. Consequently, treated cotton fabrics tend to emit formaldehyde during their use [
12]. From an environmental protection and ecological outlook, there is a growing emphasis on substituting toxic, harmful, and halogen-containing flame retardants with environmentally friendly alternatives [
13]. To this end, various methods have been employed to develop eco-friendly fire-retardant coatings for cotton fabrics. On the other hand, there are several methods of applying the flame retardant materials to the cotton fabrics including sol–gel processes [
14], layer-by-layer (LbL) assembly [
15,
16], plasma treatment [
17], and polyelectrolyte deposition techniques [
18].
Due to its ease of processing and the versatility of its components, the layer-by-layer (LbL) assembly process has been widely utilized with various materials [
19], such as chitosan (CS)/ammonium polyphosphate (APP) [
20,
21], CS/montmorillonite [
22], CS/phytic acid (PA) [
23,
24], and protein/PA [
25,
26]. Utilizing the LbL assembly method, Liu et al. [
27] integrated 3-aminopropyl triethoxysilane, chitosan, and sodium phytate to create a nanocoating that capable cotton fabrics with self-extinguishing properties at a coating load of 32.2 wt%. Ammonium polyphosphate (APP)-derived intumescent flame retardants have garnered significant attention due to their low toxicity and high efficiency. They are frequently utilized in the preparation of layer-by-layer (LbL) assembly coatings. In a particular study, Fang et al. [
28] treated cotton fabric with APP and chitosan using the LbL assembly technique. The results showed that increasing the number of bilayers to 20 or more, significantly reduced the heat release rate to approximately one-fifth of that of untreated fabric. Despite the main advantages of the LbL assembly technique, such as simplicity and high customizability, it involves a multistep adsorption process that requires specialized equipment and long-term operations, thus hindering large-scale production. Therefore, LbL assembly technique operation steps needs to be minimized [
29]. As mentioned before, natural fiber fabrics are highly flammable. Thus, the development of high-performance flame retardants or advanced flame-retardant technologies is crucial to ensure the safety and reliability of natural polymer-based composites. Layer-by-layer (LbL) assembly offers a promising alternative to traditional additive flame retardants due to its high flame-retardant efficiency, environmental acceptability, and minimal impact on the intrinsic properties of polymers [
30]. LbL assembly is versatile, cost-effective, and applicable to various materials, including polyelectrolytes, nanoparticles, and biomolecules. It has been utilized for applications such as gas barriers, antimicrobial coatings, biosensing, charge storage, antireflection, and drug delivery [
31]. Recently, it has been applied to design flame retardant coating, as well. The LbL method provides several advantages over traditional flame retardant techniques. It constructs flame-retardant multilayer films on the substrate’s surface, directly interfering with combustion and avoiding the challenges of incorporating flame retardants into the substrate, which can adversely affect its mechanical properties [
32,
33]. Additionally, LbL assembly allows for the fabrication of multilayer films with controllable thickness, composition, and function using simple, versatile, and mild experimental conditions, such as room temperature, atmospheric pressure, and low concentrations of assembly materials (below 1 wt%), making it a cost-effective route for fabricating coatings [
34,
35]. Consequently, In Qiu et al.’s investigation [
31], flame retardant coatings fabricated through the straightforward and eco-friendly layer-by-layer assembly method are particularly significant, as they enhance the flame retardant properties of polymers without altering their intrinsic characteristics.
It is worthy to note that, the layer-by-layer (LbL) assembly technique has been effectively utilized to construct thermally insulating and fire-shielding coatings composed of inorganic nanoparticles or hybrid organic-inorganic systems [
36,
37]. Since its initial application, significant advancements have been made, resulting in enhanced coating efficiency and, at times, unmatched properties. For instance, in the context of cotton, initial systems struggled to preserve fabric structure post-flammability tests, whereas current systems can now achieve self-extinguishing capabilities while maintaining the integrity of most of the fabric [
38]. The range of reagents and substrates has expanded to include various nanoparticles and eco-friendly polyelectrolytes, which have been applied to fabrics, foams, and thin films [
39,
40,
41]. Moreover, in-depth studies on deposition parameters have provided a better understanding of the relationship between coating morphology and final properties [
32,
42].
This work represents an advanced strategy focused on the novel employment of a bio-based and eco-friendly layer-by-layer coating to fabricate a facile and efficient structure capable of enhancing the fire safety of cotton fabrics for the indoor use. In the present project, phytic and pea protein was used as positive and negative charge coating with the aim of enhancing the anti-flammability of the cotton fabric in air (characterized by combustion and flammability tests, thermogravimetric analysis and spectroscopy, respectively). More specifically, the pea protein exhibits an intumescent-like and char forming agent system with excellent synergy, in which phytic acid is capable of generating phosphoric acid at high temperatures, thus promoting the char formation. Moreover, pea protein undergoes dehydration in the presence of phosphoric acid, generating water vapor, and its synergism with phytic acid favors the production of efficient residual char, which significantly enhances the resistance of the cotton fabric toward combustion significantly. Finally, FTIR, SEM, and Raman spectroscopy were conducted to characterize the morphological structure of the flame retardant coating before and after the combustion, and the performance of the flame retarded cotton fabrics were discussed. For this purpose, thermal gravimetric analysis and cone calorimetry analysis were tested to analyze the thermal decomposition and fire-retardant properties of the treated cotton fibers. Finally, the mechanical properties were characterized by tensile tests.
2. Experimental
2.1. Materials
Pure cotton fabrics (100%, 220 g/m2) was supplied by the Shaoxing Manheng Textiles Company, China, and used as the substrate. Phytic acid (PA, 50 wt% aqueous solution) were purchased from Shanghai Macklin Biochemical Co., Ltd., China. Pea protein were purchased from Shanghai Haiwanyile Biotechnology Co., Ltd. All reagents were used to prepare 6 wt% phytic acid solution and 1.5 wt% pea protein solution for layer-by-layer deposition using deionized water.
2.2. Preparation of Flame-Retardant Solution
Pea protein was dissolved in 60 °C deionized (DI) water and adjusted the pH value to 9 using the 1 M HCl solution which was stirred for 1 h to prepare 1.5 wt% pea protein solution. 6 wt% PA solution was prepared by diluting the concentrated PA solution in DI water and its pH was adjusted to 4 using the 1 M NaOH solution.
2.3. Treatment of Native Cotton Fabrics with LBL Flame Retardant
Before the LBL deposition, the cotton fabric was washed with DI water and dried to remove the impurities. The process of preparing cotton fabric flame retardant composite is prepared by alternatively adsorbing the positive and negative polyelectrolyte according to LBL technology, as shown in
Figure 1. Cotton fibers usually containing negative charge due to the presence of carboxyl and hydroxyl groups. Therefore, cotton fabric is firstly immersed in the positive pea protein solution for 5 minutes, then washed with deionized water and dried at 80 °C for 30 min. After that, the cotton fabric was subsequently immersed in the negative PA solution for 5 min, then washed with DI water and dried at 80 °C in the oven for 30 min to complete the 1 bi-layer (BL). The above process was repeated to obtain 3, 6, 9 and 12 BL pea protein/PA flame retardant coating on cotton fabrics which were named by 3BL, 6BL, 9BL, 12BL, as shown in
Table 1.
2.4. Characterizations
The surface morphology and elemental distribution on cotton fabrics and residual chars were analyzed using a scanning electron microscope (SEM) (JSM 7800F Prime, OXFROD X-Max 80, Japan) at an accelerating voltage of 5 kV, equipped with an energy-dispersive X-ray spectrometer (EDX). To improve electrical conductivity, samples were sputter-coated with chromium under vacuum before microscopy.
Fourier-transform infrared (FTIR) spectra were recorded with a Spectrum 100 T FTIR spectrometer (Thermo Fisher Nicolet iS50, USA) across a wavenumber range of 4000 to 500 cm⁻¹, averaging 16 scans per spectrum at a resolution of 4 cm⁻¹. The FTIR spectrometer utilized an attenuated total reflectance technique to analyze raw chemicals and cotton fabrics before and after coating.
The thermal stability of the cotton fabric was evaluated using a TGA (Netzsch TG 209 F1, Germany) instrument with a heating rate of 10 °C/min under nitrogen atmosphere. To ensure accuracy, each specimen was characterized twice. The theoretical values were calculated using a linear combination of the values from pristine cotton, PA, and Pea protein, based on the following equation:
The experimental TG values of pristine cotton, PA and Pea protein are denoted as , and , respectively. The weight percentages of pristine cotton, PA and Pea protein are represented by v, x, and y, respectively. For the above calculation, the weight gain of each bilayer was approximately 16.5 g/m2 and used to calculate the weight of bilayers for each individual specimen. Thermogravimetric analysis was carried out at a heating rate of 10 °C/min from 35 °C to 800 °C under a nitrogen atmosphere. Approximately 10 mg of the sample was used for the analysis.
Limiting oxygen index (LOI) was determined using a limiting oxygen indexer instrument (HC-2C, Nanjing Shangyuan Analytical Instrument Co., Ltd.) according to the GB/T 5454-1997 standard, with specimen dimensions of 150 mm × 58 mm. The vertical burning test was performed using a vertical burning instrument with specimen dimensions of 300 mm × 78 mm, following the GB/T 5455-2014 standard.
A Laser Raman spectrometer (Thermo Scientific DXR, USA) was employed to record spectra in the range of 500-2000 cm⁻¹ with an excitation wavelength of 532.17 nm.
Flammability tests were performed using a Cone calorimeter (FTT, UK) according to the ISO 5660-1 standard, with a heat flux of 25 kW/m² and specimen dimensions of 100 mm × 100 mm × 1 mm.
The mechanical properties of the cotton fabrics were evaluated using an electronic universal testing machine (Instron, Model 6025/5800R) following ASTM D-5035-11, with specimen dimensions of 100 mm × 25 mm. A 1 kN crosshead and a tensile speed of 300 mm/min were used to determine the tensile properties.
The uptake weight of the flame retardant coating on the cotton was calculated using the following formula:
where W
1 is the weight of the initial cotton, and W
2 is the weight of the treated cotton.
3. Results and Discussion
3.1. Structural and Morphological Characterizations
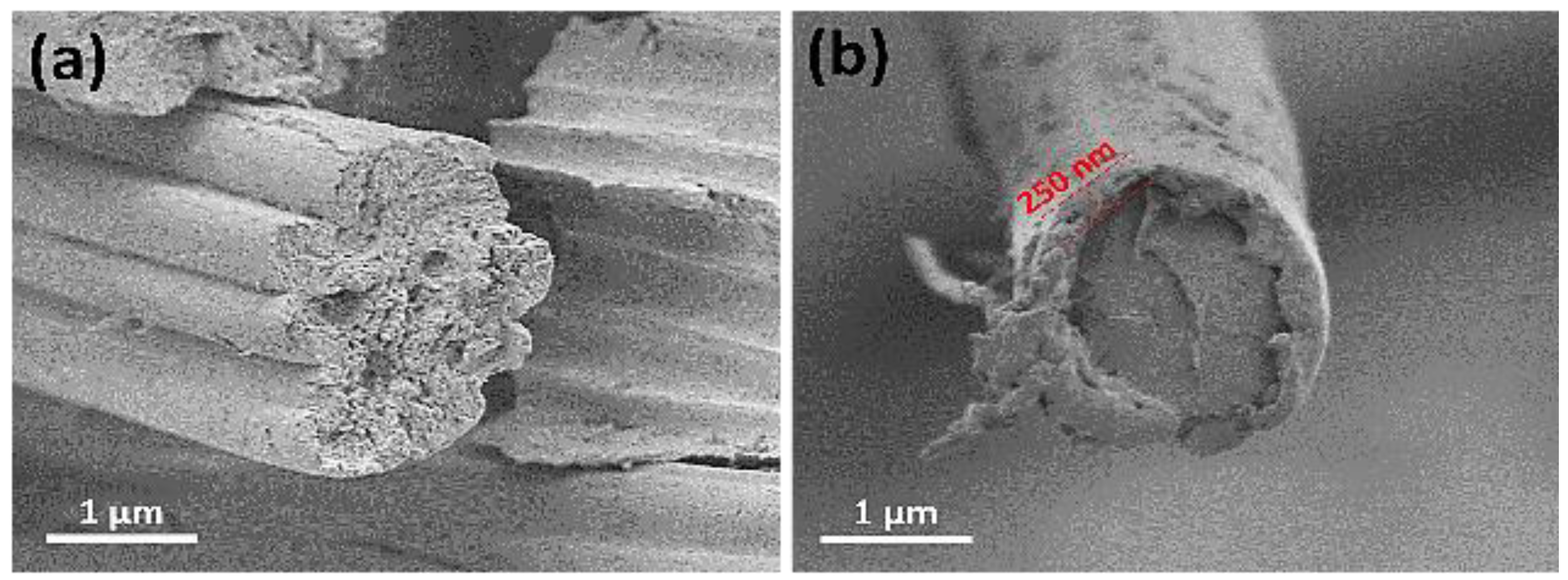
The formation of the flame-retardant (FR) coating on cotton fabrics was examined using scanning electron microscopy (SEM). SEM images revealed that the applied FR one bilayer coating forms a uniform thin film, approximately 250 nm thick (as shown in
Figure 1b), surrounding the cotton fibers. The transparency of the coating was assessed through both physical and psychophysical methods.
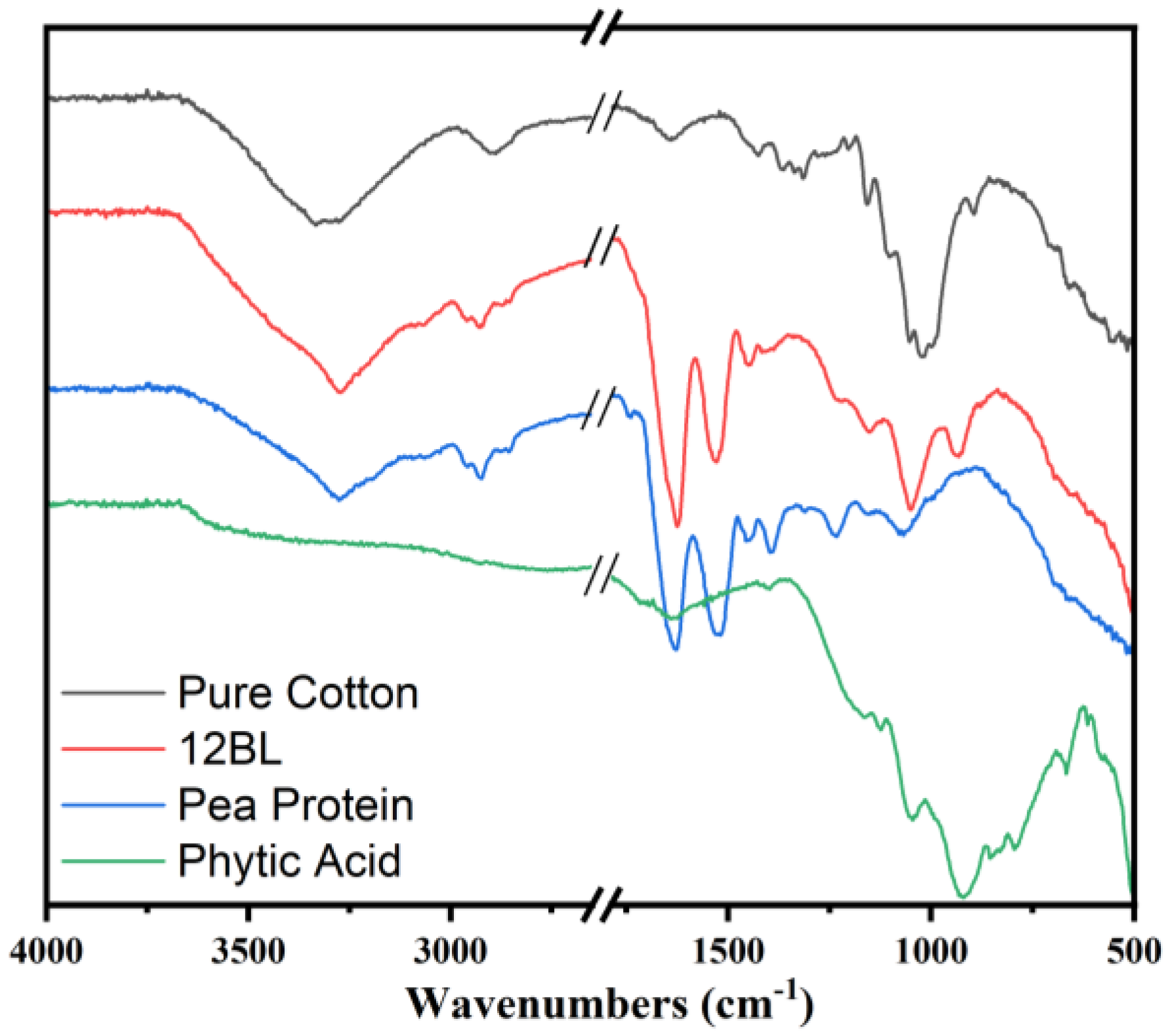
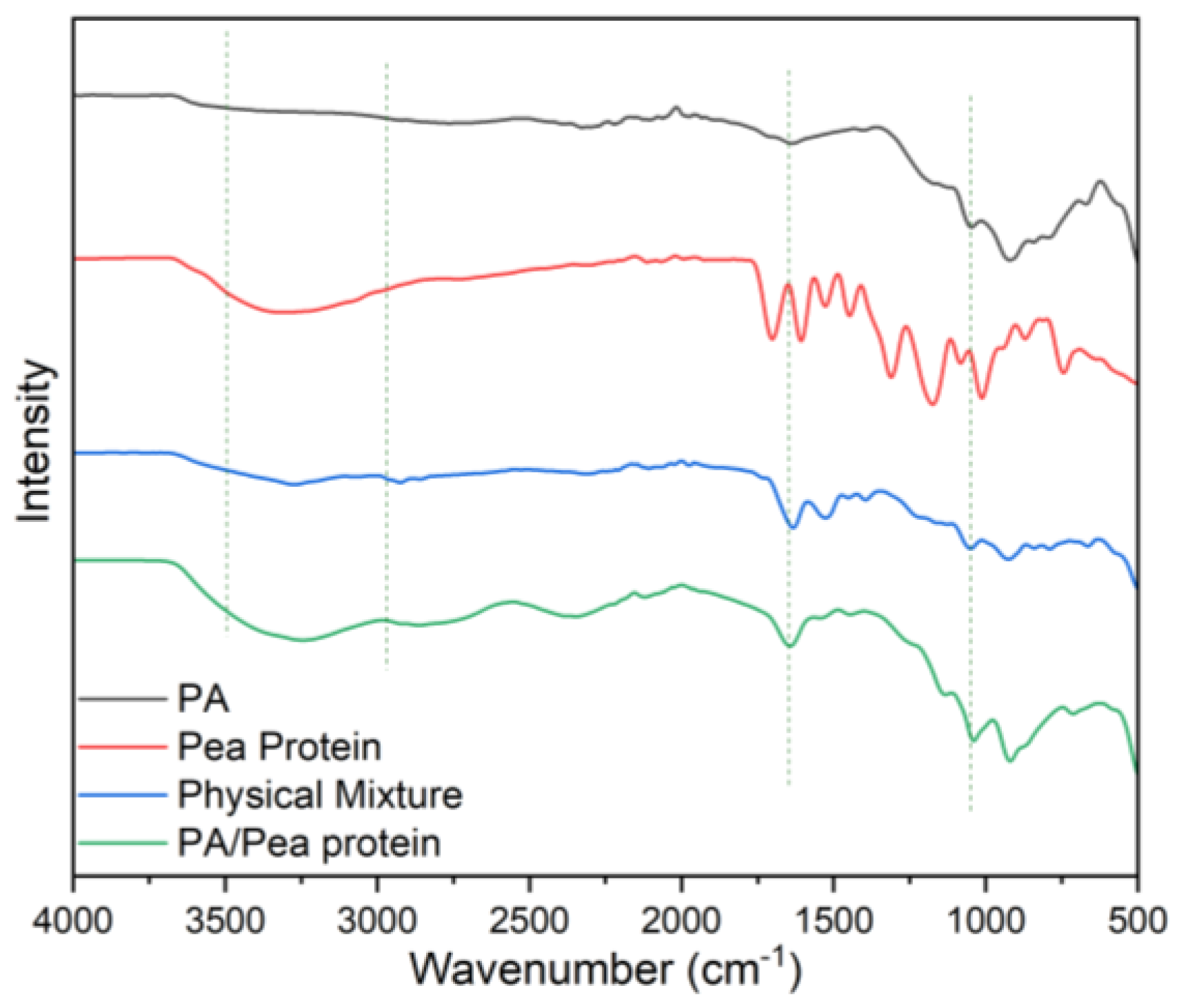
The Fourier Transform Infrared (FTIR) spectra presented in the image compare the characteristic absorption bands of pure cotton, pea protein, phytic acid, and the flame-retardant-coated cotton with 12 bilayers (12BL). The pure cotton spectrum (black line) shows typical cellulose peaks, including a broad absorption band around 3340 cm⁻¹, which corresponds to O-H stretching vibrations, and a peak near 2900 cm⁻¹ attributed to C-H stretching. Additionally, the peaks at 1420 cm⁻¹ and 1370 cm⁻¹ are associated with C-H bending in cellulose, while the band near 1060 cm⁻¹ corresponds to the C-O stretching in cellulose. In the spectrum of phytic acid (green line), the characteristic P=O stretching band appears around 1250 cm⁻¹, and the peak at 900 cm⁻¹ is indicative of P-O stretching, confirming the presence of phosphorus-based flame retardants. The pea protein spectrum (blue line) shows prominent peaks around 1650 cm⁻¹ and 1540 cm⁻¹, which correspond to the amide I and amide II bands, respectively, indicating protein structures.
The 12BL spectrum (red line) reflects the integration of the pea protein and phytic acid with the cotton. The peaks from both phytic acid and pea protein can be seen, with the characteristic peaks of cellulose still present, but slightly shifted or reduced in intensity due to the coating. For instance, the P=O stretching at 1250 cm⁻¹ and the amide I and II bands from the protein indicate the successful deposition of the flame-retardant bilayers on the cotton fabric. This shift and reduction in intensity signify effective interaction between the cotton fabric and the flame-retardant layers.
3.2. Thermal Stability
The dissemination and formation of char from decomposed products are critical factors influencing the flame-retardant properties of polymeric materials. Therefore, it is crucial to investigate the thermal stability of polymer composites. The thermal gravimetric analysis (TGA) data for pristine cotton and its air-free flame-retardant (FR) coatings are summarized in
Table 1. The parameter T
5% denotes the temperature at which 5% of the mass is lost and serves as an indicator of thermal stability.
Pristine cotton exhibits a primary degradation step occurring between 240 °C and 450 °C, with a T5% value of 80 °C. After the TGA analysis, there was minimal ash residue left for pristine cotton. In contrast, the 3-bilayer composite, which includes the flame-retardant coating, shows a lower T5% compared to pristine cotton. This is attributed to the lower decomposition temperature of the coating compositions used. However, increasing the incorporation of phytic acid and pea protein in the 6BL, 9BL and 12BL resulted in higher T5% values compared to the 3BL composite. Despite these increases, the T5% values of the bilayer compounds remain lower than those of pristine cotton. Moreover, the amount of T5% values in 9BL demonstrates that it is in optimum point and to prove this result the 12BL demonstrated decreases.
This reduced thermal stability is attributed to the lower thermal stability of Pea protein and phytic acid, which accelerates the decomposition process within the FR coatings. The addition of the number of bilayers significantly enhanced the residual char yield of the composites. At 800 °C, the residual char yields for 3BL, 6BL, 9BL and 12BL were 22.4%, 25.3%, 34.7% and 28.8%, respectively, which are substantially higher than the 2.1% residual yield of pristine cotton. To validate the effectiveness of the FR coating, theoretical residual yields were calculated (
Table 1). In all FRC composites, the experimental residual yields exceeded the calculated values, indicating synergistic effects between cotton fabric, Phytic acid and pea protein, and number of layered-by-layered coating that previously mentioned.
3.3. Flammability
UL-94 and LOI tests are widely used methods to evaluate the effectiveness of flame retardant coatings on treated cotton fabrics [
38,
39]. As indicated in
Table 2, the Limiting Oxygen Index (LOI) of the untreated cotton fabric was 19%, indicating a high fire hazard. In contrast, the LOI value of 3BL reached 23%, representing a 26% increment compared to the untreated fabric. This indicates that the coating transformed the fabric from highly flammable to highly flame-retardant, only at 3 bilayers.
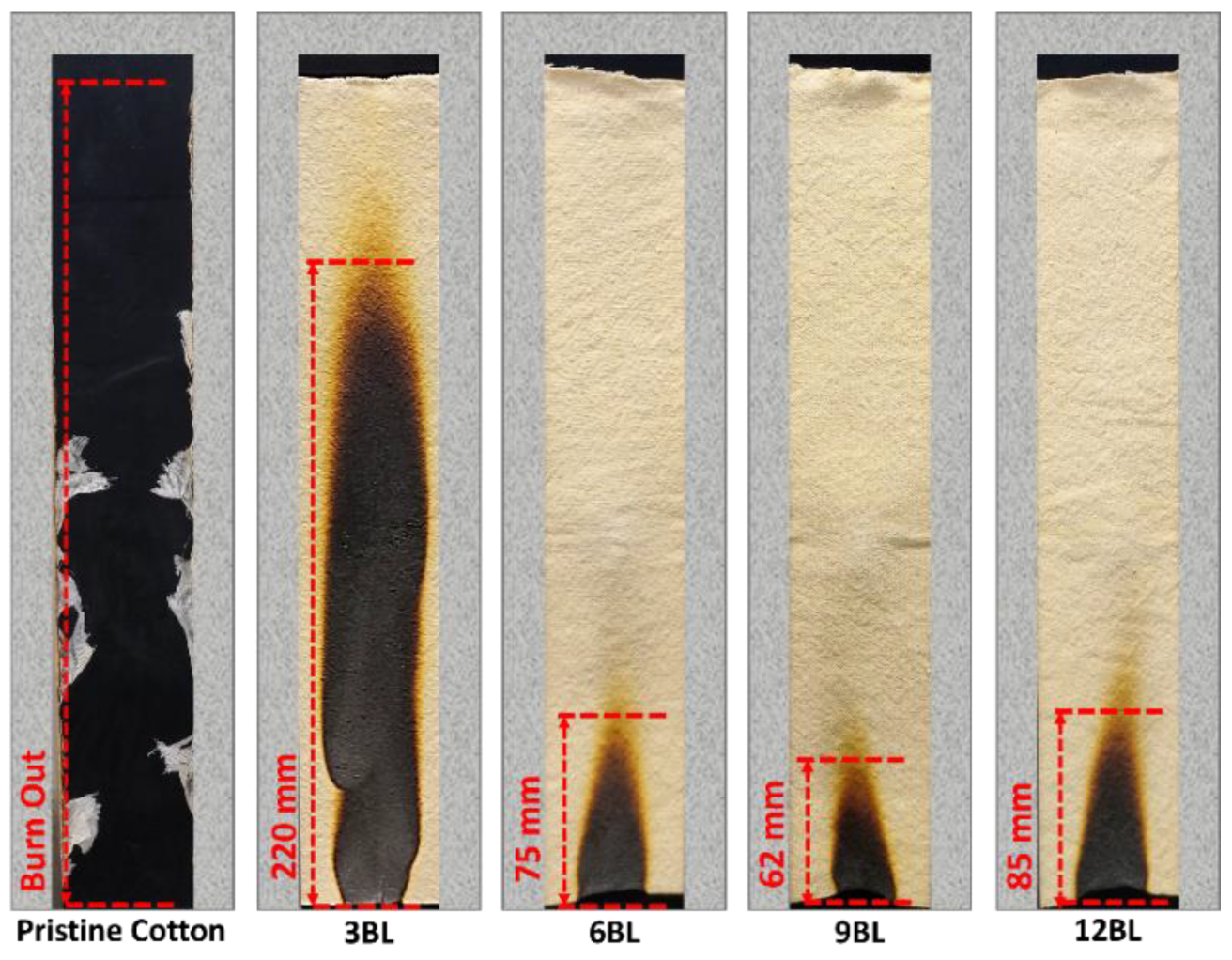
Moreover, as illustrated in the digital images in
Figure 7, the pristine cotton fabric immediately ignited upon exposure to fire, with flames rapidly spreading to the top of the sample, resulting in complete destruction. Remarkably, when pristine cotton fabrics were treated with 3 bilayers, they exhibited an improved ability to form char, resulting in a stable but crumbly char residue (
Figure 3), however, it did not pass the UL-94 vertical burning test.
Increasing the number of bilayers progressively enhanced the fabric’s resistance to ignition, increased the LOI value, and resulted in an intact char residue. Among these, 6BL showed significant difficulty in catching fire, easily passed the vertical test, and achieved an LOI value than 3BL. Notably, this pattern is consistent between 9BL and 6BL, with 9BL exhibiting superior performance. However, as previously mentioned, 9BL represents the optimal configuration.
Figure 7 further demonstrates that increasing the bilayer to 12BL results in a decline in performance.
From
Figure 7, it is evident that less cotton fabric was damaged after ignition, and the structural morphology of the residual char remained intact. This indicates that the combination of Phytic acid (PA) and pea protein (PE) effectively hindered flame propagation even at low weight percentages [
31,
32].
3.4. Cone Calorimetry Test
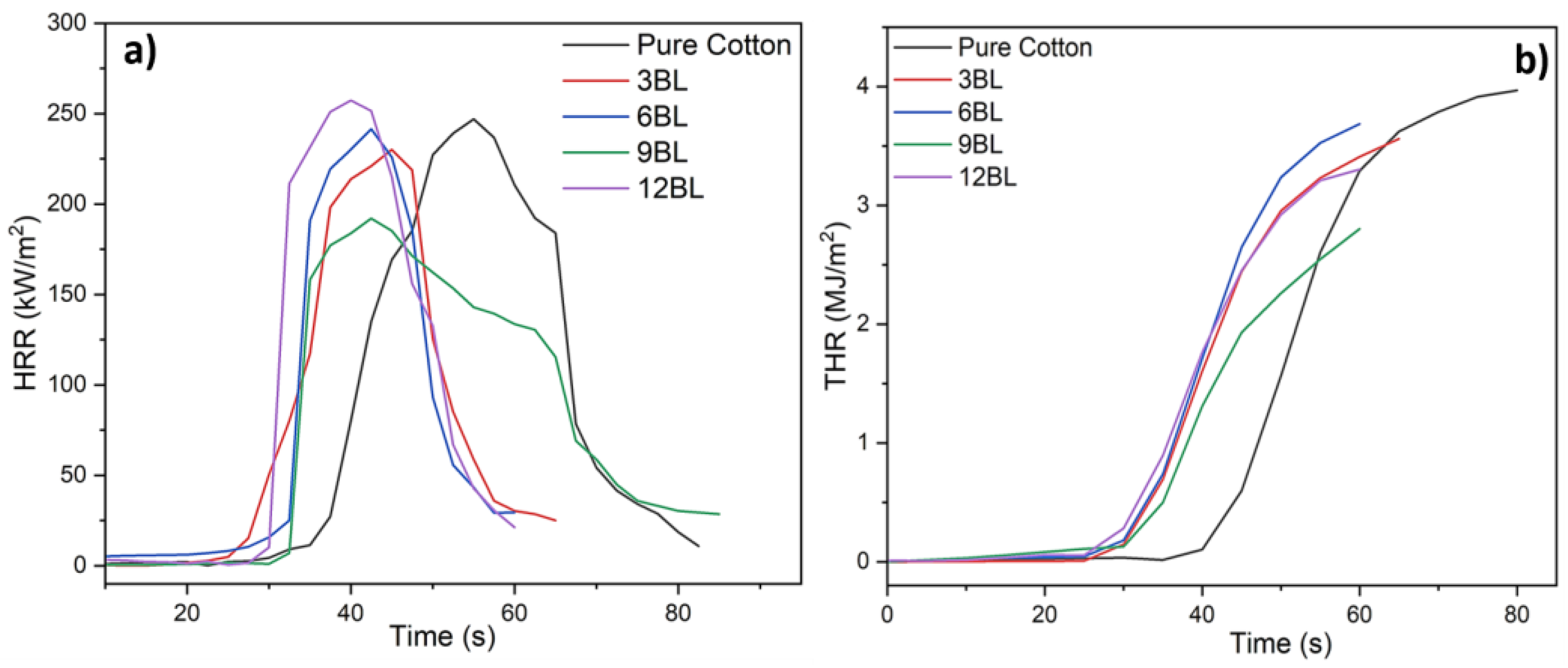
The fire resistance properties of untreated cotton and cotton fabrics treated with different Bilayers (3BL, 6BL, 9BL and 12BL) of a specific solution were further examined using cone calorimetry. The results, depicted in
Figure 4 and summarized in
Table 3, include the heat release rate (HRR), total heat release rate (THR), and mass loss data of both untreated cotton and flame-retardant-coated cotton fabric samples. It is noteworthy that all pHRR values of the flame-retardant cotton samples were significantly lower compared to the untreated cotton control (250 kW/m
2). The pHRR values exhibit a gradual decrease as the loading of the flame-retardant coating increases. Notably, 9BL demonstrates the lowest pHRR value of 203 kW/m
2, which is 30% lower than that of untreated cotton. The THRs of the flame-retardant-coated cotton fabrics are significantly lower than those of untreated cotton, reduced from 3.89 to 3.55, 3.64, and 1.82 MJ/m
2, respectively, indicating an effective suppression of total heat release. The decreased THR suggests that a greater amount of carbonaceous compounds remained in the condensed phase, potentially attributed to the strong synergistic effect of the bilayers’ number. This effect leads to a reduced conversion of organic volatiles into fuel. The inclusion of low percentage of phytic acid and pea protein as the flame-retardant system resulted in a substantial increment in char formation, less smoke production, and more fire safety. The formation of char residues and THR were in agreement with the findings from thermogravimetric analysis (TGA).
Figure 4 indicates that the coated cotton fabrics ignited slightly faster than untreated cotton due to the rapid decomposition of the coating materials. The char residues of the flame-retardant-coated cotton fabrics are significantly higher compared to untreated cotton (
Table 3). The smoke emission parameters, including the smoke production rate (TSR), total smoke production (TSP), CO production and CO/CO
2 proportion are also provided in
Table 3. The TSR and TSP values of the 9BL cotton fabrics showed significant reduction compared to those of untreated and treated cotton fabric.
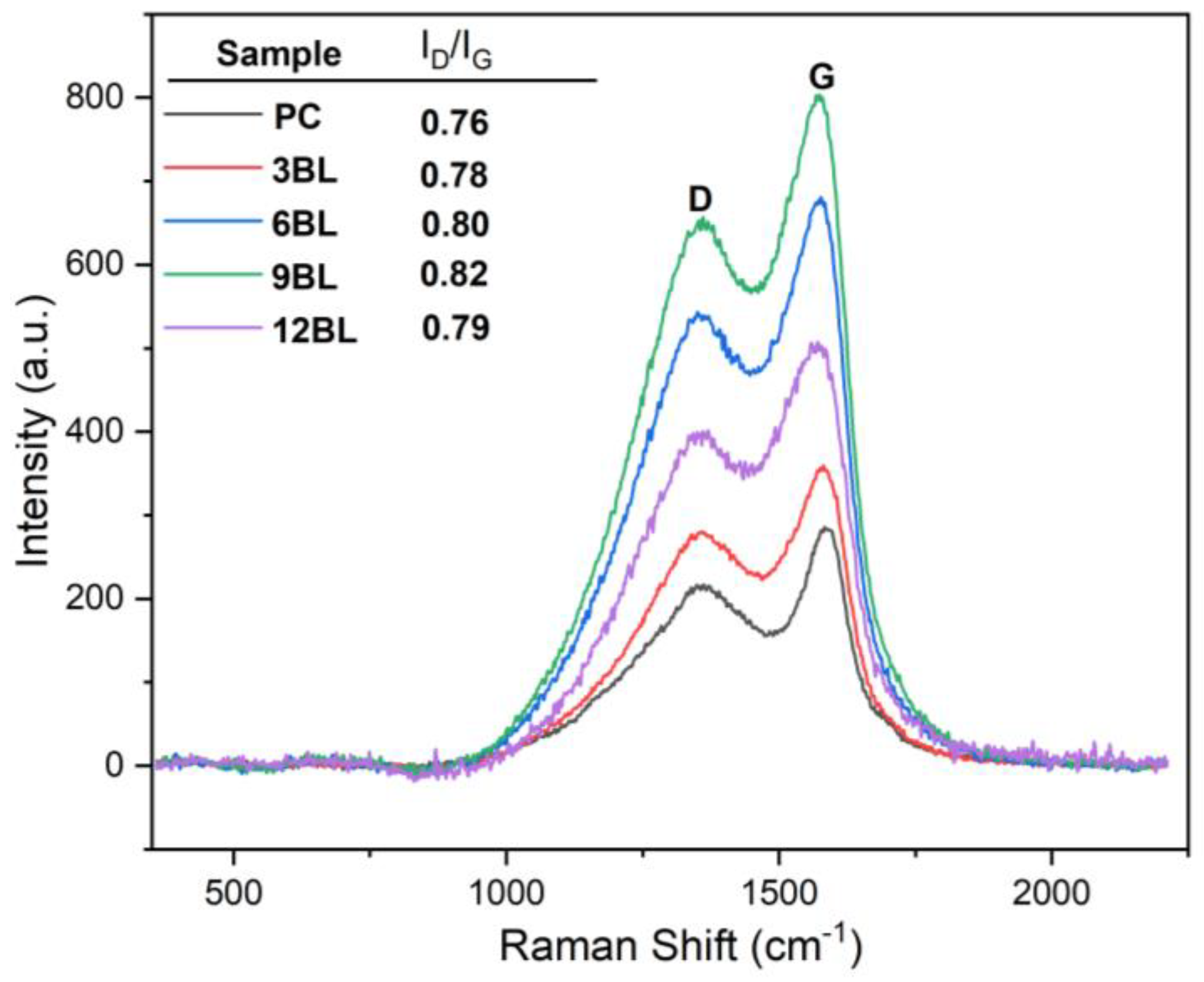
Raman spectroscopy is a widely used and effective technique for evaluating the degree of graphitization in residual carbon char, which is closely related to the flame-retardant properties. In this study, Raman spectroscopy was employed to analyze the char residues of both untreated cotton fabric and the flame-retardant-coated samples. The residual char obtained after the cone calorimetry test was utilized for this characterization. The Raman spectra of the residual chars exhibited two absorption peaks at 1360 cm⁻¹ and 1568 cm⁻¹, known as the D and G bands, respectively. The ratio of the integrated intensity of these two bands (I
D/I
G) was used to determine the graphitization degree of the residual chars [
40]. The D peak (disorder band) indicates the presence of disorder in the carbon planar structure due to defects or functional groups. The G peak (graphite band) arises from the E
2g mode of graphite and involves vibrations of sp²-bonded carbon atoms in a 2D-hexagonal lattice.
The base sample has the lowest ID/IG ratio, showing less disorder compared to the layer-by-layer flame retardant treated samples. As the number of layers increases, the ID/IG ratios rise from 0.78 to 0.82 for 3BL to 9BL, indicating more disorder or defect density. For the 12BL sample, a slight decrease in the ID/IG ratio to 0.79 suggests stabilization or fewer defects at this processing stage. In general, highly graphitized carbon materials have better thermal stability, meaning they retain their structure at high temperatures.
In flame retardant applications, the formation of a stable char layer is crucial, as it shields the material from further combustion. Such materials can form more stable, less flammable char layers. The 9BL sample, with the highest I
D/I
G ratio (0.82), shows the most disorder but still maintains good flame retardant properties. The higher D peak in 9BL may aid in better char formation during combustion. For instance, in some studies, alongside increase in the I
D/I
G ratio, a significant increase in LOI values observed, suggesting improved performance due to its structural defects. These defects could promote cross-linking reactions, forming a protective char layer that insulates the material, reduces heat release, and lowers flammability. The strong char formed in 9BL could result from both structural features and flame retardants, creating a more cohesive heat barrier [
43,
44,
45].
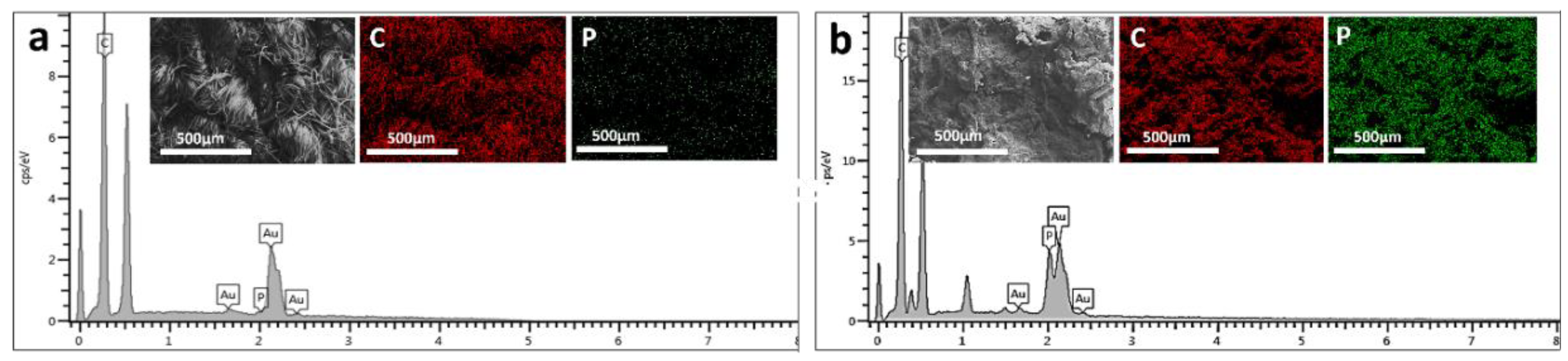
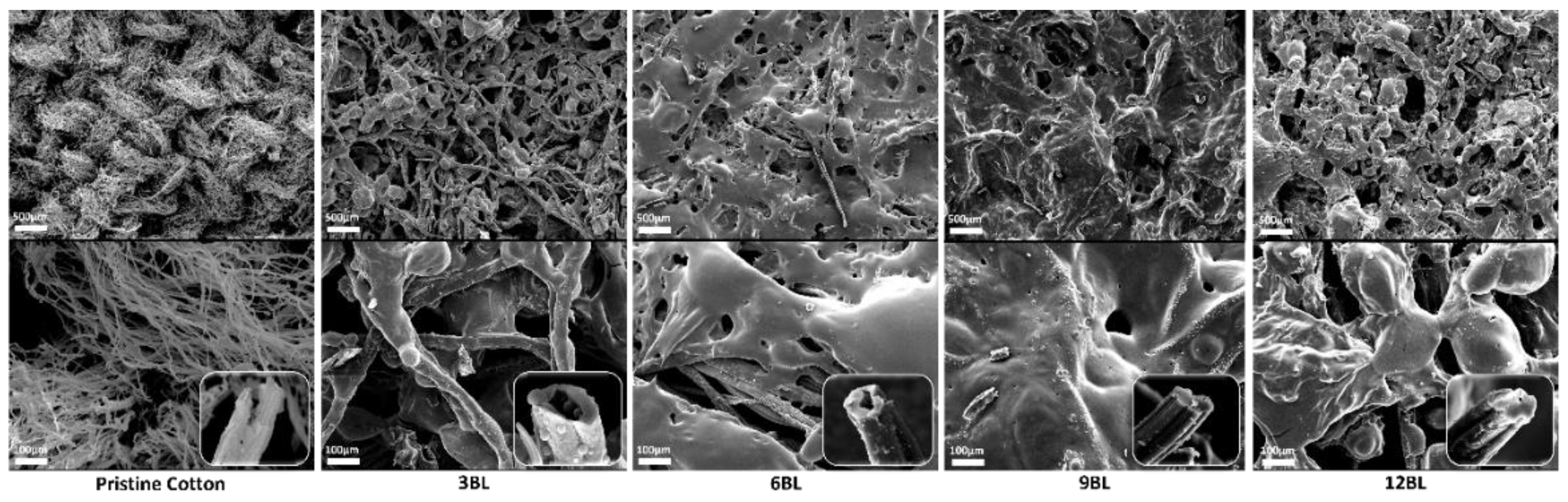
To gain a comprehensive understanding of the flame-retardant mechanism, we conducted SEM and EDX spectroscopy characterization of the char residues on the FR-coated cotton fabric.
Figure 6 presents micrographs of both untreated cotton fabric and relevant FR-coated samples after the cone calorimetry tests at two different magnifications. The observations revealed that in the case of 3BL, a continuous and dense protective layer uniformly covered the surface of the cotton fibers. In contrast, the char residue of the untreated cotton fabric appeared fluffy, fragile, thin, and unstable.
Additionally, the weft-warp structure of the FR-coated samples remained intact, similar to the sample before the cone test. The EDX spectroscopy results showed a uniform dispersion of phosphorus (P) element within the char layer (
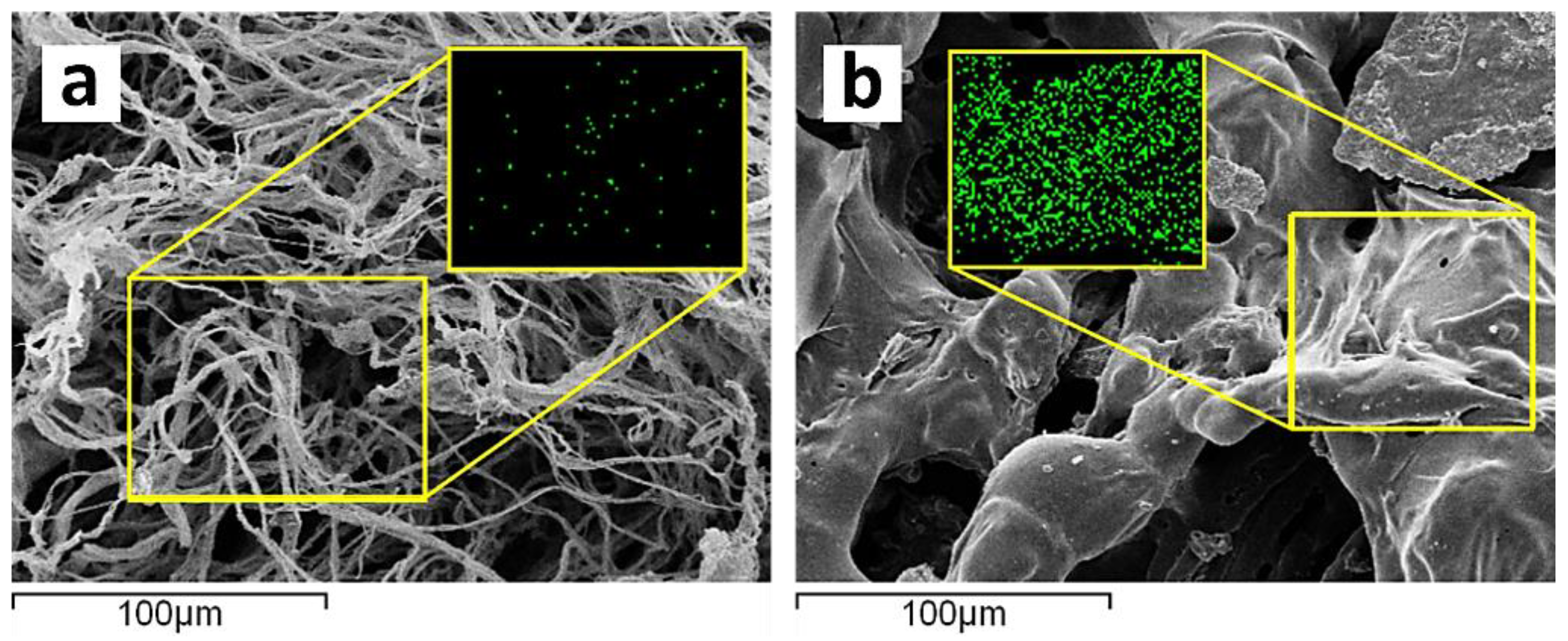
Figure 8), indicating the effective action of the flame retardant coating in promoting condensation. It is hypothesized that polyphosphoric acids derived from ammonium polyphosphate could catalyze the cotton fabric, bilayers. The combination of these observations suggests that the char layer acts as an efficient insulation layer, preventing the transfer of oxygen and heat to the substrate.
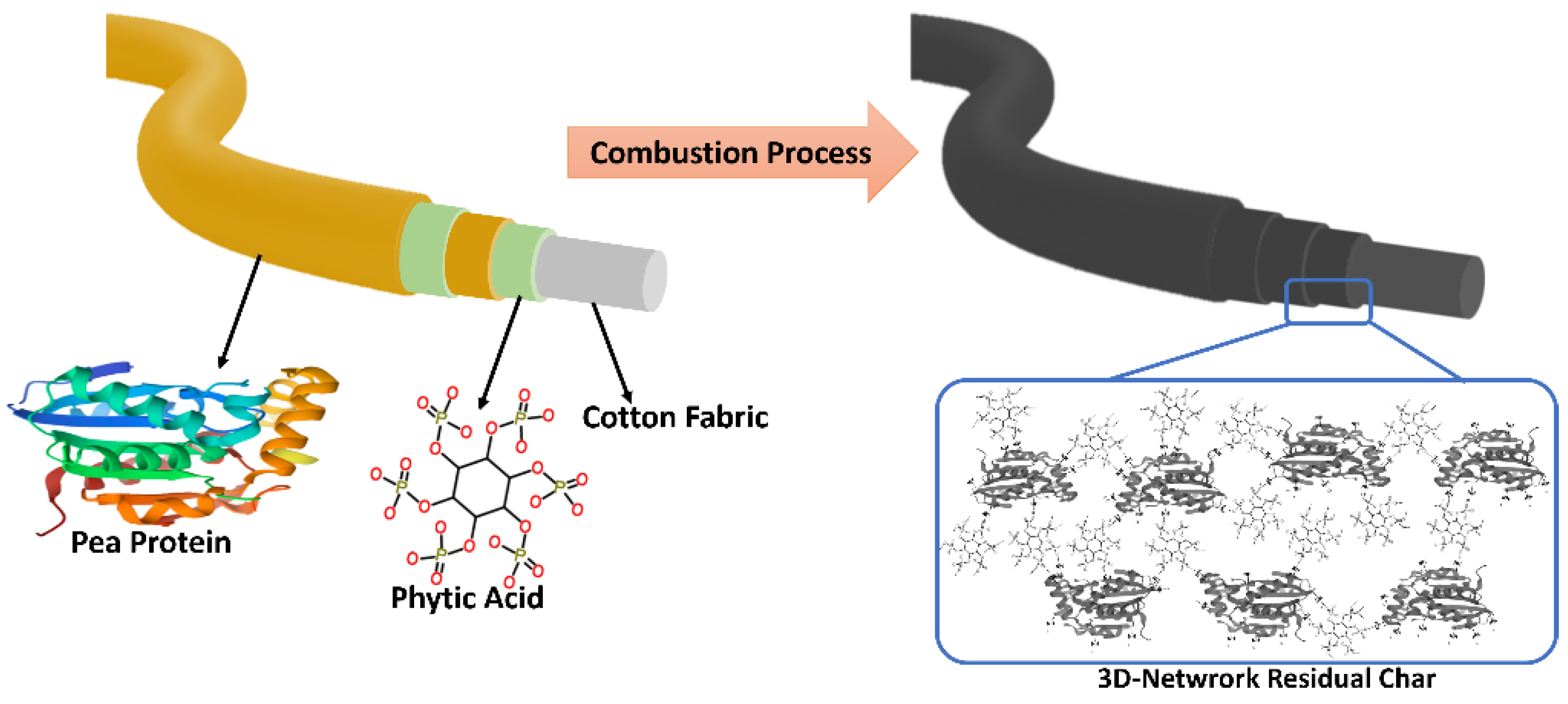
The reaction of phytic acid and pea protein during combustion was considered in order to propose the flame-retardant mechanism of the coatings. The reactions among the pea protein and the phytic acid, particularly, a crosslinking network between amino groups in pea protein and phosphate groups from phytic acid as a dominant reaction (
Figure 9). The reaction occurs by phosphorylation of the amino groups (–NH₂) of the pea protein, and this esterification can result in crosslinking between phosphate groups (–PO₄²⁻) of phytic acid and the amine groups in the protein.
To explain the flame-retardant mechanism of the coatings, the combustion interactions between phytic acid and pea protein were considered. A key reaction between the pea protein and phytic acid involves a crosslinking network formed between the amino groups in the pea protein and the phosphate groups from the phytic acid (
Figure 9). This reaction occurs through the phosphorylation of the amino groups (–NH₂) in the pea protein, and this esterification leads to crosslinking between the phosphate groups (–PO₄²⁻) from phytic acid and the amine groups in the protein. This type of crosslinking enhances the thermal stability of the char, improving fire retardancy performance [
46]. According to the FTIR results shown in
Figure 9, the amide I band at 1650 cm⁻¹, which represents the physical mixture, disappears due to the interaction with the protein’s carbonyl group. Additionally, some peaks below 1000 cm⁻¹, which correspond to the individual fingerprints of phytic acid and pea protein, merged or changed due to new chemical interactions.
On the other hand, peaks around 1000-1200 cm⁻¹, associated with the P–O and P=O vibrations, shifted or intensified, indicating an interaction between the phosphate groups and the protein side-chains. Additionally, sharper or more distinct peaks in the 3000-3500 cm⁻¹ region, related to hydrogen bonding, revealed the formation of stronger hydrogen bonds in the chemical mixture. As a result, the chemical interactions between phytic acid and pea protein (as seen in the FTIR spectra) lead to a uniform chemical blend with strong hydrogen bonds, covalent or ionic phosphate-protein connections, and amide involvement. It can be concluded that these hydrogen bonds help stabilize the structure at lower temperatures.
The phosphate groups catalyze the formation of char and promote crosslinking, while the protein’s carbon and nitrogen components contribute to carbonization, forming a nitrogen-doped carbonaceous network. After combustion, the resulting char forms a 3D-network composed of phosphorus-rich and nitrogen-doped carbon structures (
Figure 10). This network is thermally stable, mechanically strong, and chemically homogeneous, reflecting the synergistic interactions seen in the FTIR spectra of the chemical mixture [
47,
48].
3.5. Mechanical Properties
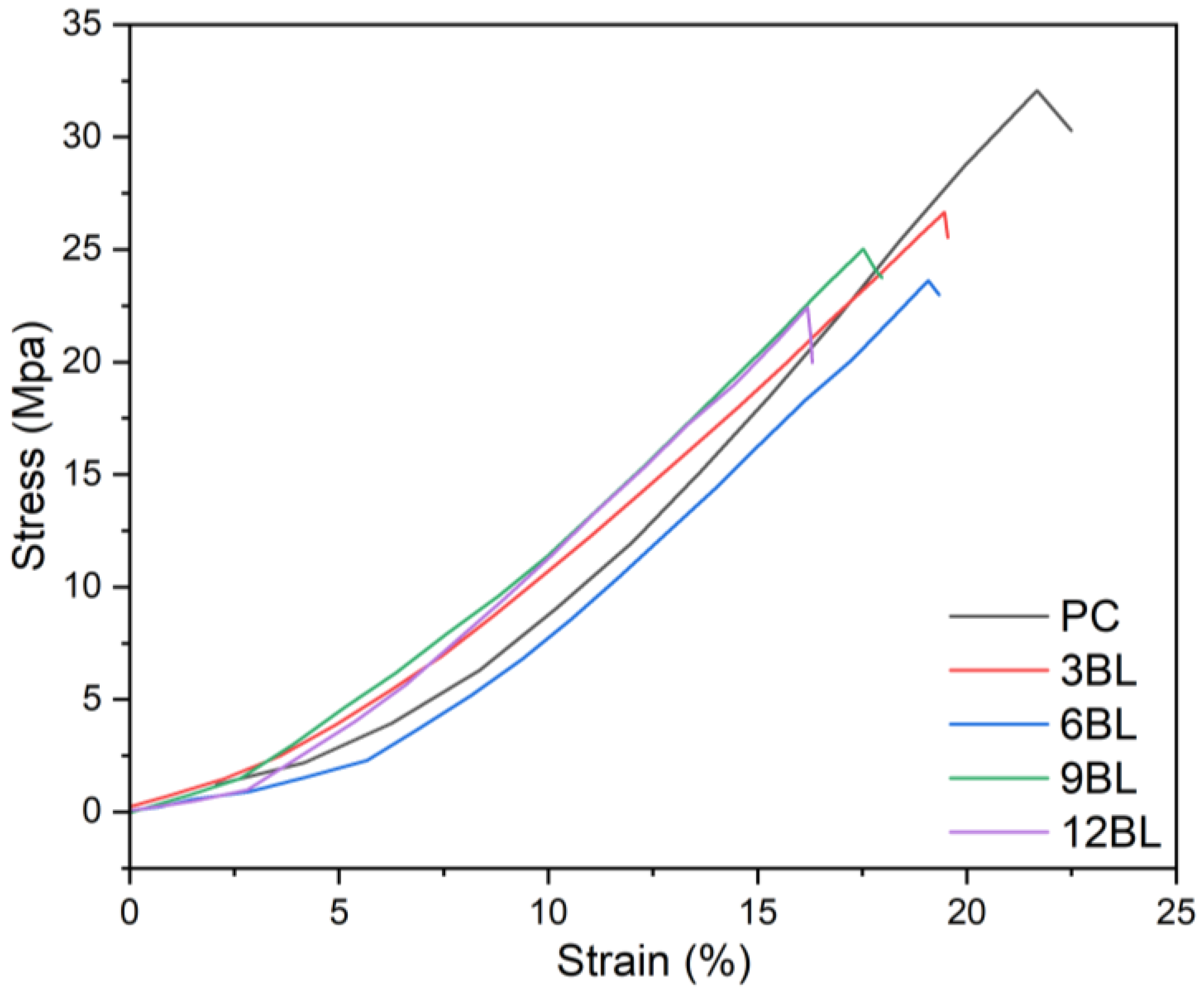
The tensile test results shown in the
Figure 11 compared the mechanical behavior of PC fabric with flame-retardant-coated cotton fabrics with 3, 6, 9 and 12 bilayers of flame retardant coatings. As illustrated, the stress-strain curves indicate that pure cotton fabric (PC) reaches an optimal tensile strength of around 35 MPa with a strain of approximately 20%, reflecting better mechanical integrity. In contrast, the flame-retardant-coated fabrics show a decrement in stress and strain as the bilayer count increases. For example, the 3BL fabric shows a similar trend to pure cotton but with reduced peak strength and stretch. As the number of bilayers increases, especially with 6BL, 9BL, and 12BL fabrics, the stress at failure demonstrates even more drops, with the 12BL fabric displaying the weakest mechanical performance. This suggests that, adding more flame retardant bilayers compromises mechanical integrity, possibly due to increased brittleness or stiffness from the coating materials. However, the 12BL fabric still achieves stress levels above 20 MPa, retaining a substantial portion of its strength.
Figure 1.
The cotton fibers’ cross section SEM before (a) and after (b) coating deposition.
Figure 1.
The cotton fibers’ cross section SEM before (a) and after (b) coating deposition.
Figure 2.
The upper part of the image shows scanning electron microscopy (SEM) micrographs of both untreated and treated cotton fabrics and elemental distribution mapping. The lower part depicts elemental distribution mapping of carbon (C) and phosphorus (P), with panel (a) showing the untreated cotton fabric and panel (b) showing the treated cotton fabric.
Figure 2.
The upper part of the image shows scanning electron microscopy (SEM) micrographs of both untreated and treated cotton fabrics and elemental distribution mapping. The lower part depicts elemental distribution mapping of carbon (C) and phosphorus (P), with panel (a) showing the untreated cotton fabric and panel (b) showing the treated cotton fabric.
Figure 3.
Representative FTIR spectra of pure cotton fabric, and flame retardant coated (12BL) in the presence of pea protein and phytic acid.
Figure 3.
Representative FTIR spectra of pure cotton fabric, and flame retardant coated (12BL) in the presence of pea protein and phytic acid.
Figure 3.
Digital images depicting untreated and treated cotton fabrics following the vertical burning test.
Figure 3.
Digital images depicting untreated and treated cotton fabrics following the vertical burning test.
Figure 4.
(a) HRR and (b) THR curves of the pristine and treated cotton fabrics obtained from the cone calorimetry test.
Figure 4.
(a) HRR and (b) THR curves of the pristine and treated cotton fabrics obtained from the cone calorimetry test.
Figure 5.
Raman spectra analysis of the residual char of pristine cotton.
Figure 5.
Raman spectra analysis of the residual char of pristine cotton.
Figure 6.
SEM micrographs of the char residues for pristine cotton, 3BL, 6BL, 9BL and 12BL after the cone calorimetry test.
Figure 6.
SEM micrographs of the char residues for pristine cotton, 3BL, 6BL, 9BL and 12BL after the cone calorimetry test.
Figure 7.
Digital image of the residual chars of the pure and flame retarded cotton fabrics after the cone calorimeter tests.
Figure 7.
Digital image of the residual chars of the pure and flame retarded cotton fabrics after the cone calorimeter tests.
Figure 8.
Elemental mapping (EDAX) of the residual chars of the pure cotton (PC) (a), and 9BL sample (b).
Figure 8.
Elemental mapping (EDAX) of the residual chars of the pure cotton (PC) (a), and 9BL sample (b).
Figure 9.
Representative FTIR spectra of the pure FR materials used in this work, and their physical and chemical mixture.
Figure 9.
Representative FTIR spectra of the pure FR materials used in this work, and their physical and chemical mixture.
Figure 10.
Schematic flame-retardant mechanism of the FR coating, left; the chemical structure of the flame retardant coating, and right; illustration of the network generated during the combustion between the phytic acid and pea protein.
Figure 10.
Schematic flame-retardant mechanism of the FR coating, left; the chemical structure of the flame retardant coating, and right; illustration of the network generated during the combustion between the phytic acid and pea protein.
Figure 11.
Stress–strain curves of pristine cotton (PC) and flame-retardant treated cotton fabrics.
Figure 11.
Stress–strain curves of pristine cotton (PC) and flame-retardant treated cotton fabrics.
Table 1.
TGA results.
| Sample |
T5% (°C) |
Residual yield @ 800 °C (under N2), wt% |
Residual yield @ 800 °C (under air), wt% |
| Calculated |
Experimental |
|
| Phytic acid (PA) |
155 |
-- |
61.7 |
5.0 |
| Pea Protein (PE) |
79 |
-- |
10.3 |
5.0 |
| Pure Cotton (PC) |
80 |
-- |
2.1 |
3.78 |
| 3BL |
72 |
10.1 |
22.4 |
18.3 |
| 6BL |
88 |
13.9 |
25.3 |
20.7 |
| 9BL |
111 |
16.9 |
34.7 |
28.4 |
| 12BL |
95 |
19.5 |
28.8 |
23.6 |
Table 2.
Flammability assessment outcomes for both untreated and treated textiles.
Table 2.
Flammability assessment outcomes for both untreated and treated textiles.
| Sample |
LOI (%) |
Damage Length (mm) |
After Fame Time (s) |
| Pristine Cotton |
17 |
Burn out/damaged char |
38 |
| 3BL |
23 |
220 |
15 |
| 6BL |
26 |
75 |
3 |
| 9BL |
29 |
62 |
1 |
| 12BL |
26 |
85 |
3 |
Table 3.
The results for both untreated and treated cotton fabrics from cone colometry test.
Table 3.
The results for both untreated and treated cotton fabrics from cone colometry test.
| Sample |
pHRR (kW/m2) |
THR (MJ/m2) |
FIGRA (kW/m2⋅s) |
Residual Mass (%) |
TSR (m2/m2) |
TSP (m2) |
MARHE (kW/m2) |
CO (kg/kg) |
CO/CO2
|
| Pure џCotton |
250 |
3.89 |
6.25 |
2.6 |
42.5 |
0.60 |
55.5 |
0.200 |
0.0128 |
| 3BL |
230 |
3.55 |
5.85 |
35.2 |
68.8 |
0.58 |
60.2 |
0.044 |
0.0354 |
| 6BL |
241 |
3.64 |
6.42 |
38.6 |
67.1 |
0.62 |
66.2 |
0.033 |
0.0304 |
| 9BL |
192 |
1.82 |
5.02 |
41.8 |
13.0 |
0.17 |
50.9 |
0.034 |
0.0270 |
| 12BL |
255 |
3.20 |
6.80 |
37.4 |
44.3 |
0.49 |
59.5 |
0.045 |
0.0326 |