3. Analysis
The comparative analysis of treatment pathways between the STARR and
All of Us datasets reveal variations in the frequencies of sequences leading to amputation or non-amputation outcomes. In both datasets, the administration of anti-platelet and lipid-lowering medications is a prominent feature of the most prevalent treatment pathways, particularly those leading to non-amputation outcomes.
Table 6 illustrates that the anti-platelet therapy followed by lipid lowering has been echoed similarly in both the datasets. This consistency across datasets underscores the effectiveness of these initial treatments in preventing severe outcomes.
However, when examining pathways leading to amputation, the patterns diverge more noticeably between the two datasets. In the STARR data, simpler treatment sequences, such as those involving only anti-platelet or lipid-lowering therapies, are relatively common. These findings suggest that in the STARR cohort, there may be a higher incidence of cases where initial medical management fails to prevent amputation, potentially due to the complexity or progression of the underlying conditions.
In contrast, the
All of Us data shows a broader and more complex range of treatment sequences leading to amputation, particularly those involving revascularization procedures as stated in
Table 7. Notably, the pathway involving endovascular revascularization alone accounts for 13.64% of amputations, highlighting its significant role in this cohort. Additionally, more complex sequences, such as those combining anti-platelet, lipid-lowering, and endovascular revascularization, are more common in the
All of Us data, suggesting a more aggressive or multifaceted approach to treatment. The presence of these complex pathways indicates that patients in the
All of Us dataset may present with more advanced disease or that clinicians are more likely to pursue multiple interventions before resorting to amputation.
All of Us data includes sequences where revascularization, both endovascular and surgical, is more frequently associated with amputation outcomes. For instance, the sequence endovascular revascularization and revascularization surgery appears in 6.25% of amputation cases, which is relatively high compared to the absence of this treatment sequence in the STARR data as mentioned in
Table 8. However, in the STARR dataset, the sequences with revascularization procedures can be found with other treatment procedures like anti platelet, lipid lowering. This difference could reflect a variation in the timing or criteria for surgical interventions between the two cohorts. The STARR dataset exhibits fewer sequential interventions, particularly in cases leading to non-amputation, where simpler combinations of anti-platelet and lipid-lowering therapies are more common. In contrast, the All of Us data demonstrates a higher incidence of pathways involving multiple, complex treatments, particularly in the sequences leading to amputation, where revascularization procedures play a prominent role.
In summary as given in
Table 9, while both datasets highlight the importance of anti-platelet and lipid-lowering therapies in managing patients at risk of amputation, the All of Us data shows a higher incidence of revascularization procedures leading to amputation. These differences may reflect variations in patient populations, disease severity, or treatment practices. Understanding these variations may inform tailored treatment strategies aimed at improving patient outcomes and reducing the incidence of amputation.
3.1. Odds Ratio Analysis of Treatment Pathways
To further explore the relationship between treatment pathways and outcomes, an odds ratio analysis was performed. The odds ratio provides a measure of the association between a particular treatment pathway and the likelihood of amputation or non-amputation outcomes. An odds ratio greater than 1 indicates a higher likelihood of the amputation outcome, while an odds ratio less than 1 indicates a lower likelihood of amputation outcome. The odds ratio was calculated by comparing the frequency counts of amputation and non-amputation outcomes in the STARR and
All of Us datasets, utilizing a 2x2 contingency table, focusing on the pathways that lower the risk of amputation.
Table 10 shows a few treatment pathways from the odds ratio analysis upon the STARR data, that likely lower the risk of amputations.
The pathway consisting of lipid-lowering therapy, anti-platelet therapy, and surgical revascularization has an odds ratio of 0.83, indicating a lower likelihood of amputation. This suggests that this treatment sequence is effective in reducing severe outcomes in patients with peripheral artery disease (PAD). Similarly, the combination of anti-platelet therapy, lipid-lowering medication, and surgical revascularization has an odds ratio of 0.81, highlighting its effectiveness in lowering the risk of amputation.
The use of lipid-lowering therapy combined with anti-platelet therapy alone results in an odds ratio of 0.70, further supporting the benefits of combining these treatments in reducing the risk of amputation. In another case, anti-platelet therapy followed by lipid-lowering medication has an odds ratio of 0.62, emphasizing the positive impact of these treatments on non-amputation outcomes. The sequence involving lipid-lowering therapy, anti-platelet therapy, and endovascular revascularization shows an odds ratio of 0.60, reinforcing the idea that these pathways play a critical role in lowering the risk of amputation in patients with PAD.
The odds ratio analysis of the “
All of Us” data reveals some notable contrasts and similarities when compared with the STARR data. The “
All of Us” data presents an odds ratio of 0.35 for anti-platelet therapy, i.e., a probability of 0.26, indicating a lower likelihood of amputation. The top few rows of the analysis upon
All of Us data are as follows in
Table 11.
The combination of anti-platelet therapy and lipid-lowering medication is highlighted in both datasets as an effective treatment sequence. In the STARR data, this combination yielded an odds ratio of 0.62, i.e., probability of 0.38, indicating a lower likelihood of amputation. The “All of Us” data reinforces this finding with an odds ratio of 0.53, i.e., probability of 0.35, indicating lower likelihood of amputations, further underscoring the effectiveness of this treatment combination in mitigating the risk of severe outcomes.
The pathway consisting of Lipid-lowering treatment alone has an odds ratio of 0.34, i.e., a probability of 0.25, highlighting the effectiveness of the treatment in reducing the risk of amputation.
In the STARR data, the combination of anti-platelet therapy, lipid-lowering medication, and endovascular revascularization showed an odds ratio of 3.21, i.e., probability of 0.76, indicating a higher likelihood of amputation. This suggested that this sequence was associated with more severe cases, where multiple interventions were necessary. The “All of Us” data similarly shows a significant association with an odds ratio of 3.55, i.e., probability of 0.78, reflecting the use of this combination in complex cases that require aggressive treatment strategies.
Another noteworthy comparison is the pathway involving revascularization, both endovascular and surgical. In the STARR data, the pathway starting with anti-platelet therapy followed by surgical revascularization showed a significant odds ratio of 6.41, i.e., probability of 0.87, indicating a higher likelihood of amputation. This result highlighted the critical need for effective blood flow restoration in severe cases. The “All of Us” data presents a similar trend, with an even higher odds ratio of 6.69, i.e., probability of 0.87, for the combination of anti-platelet therapy and endovascular revascularization. This suggests that the association between these treatment sequences and the likelihood of amputation is consistent across different populations.
The analysis also reveals that in both datasets, more complex pathways involving multiple interventions tend to be associated with a higher likelihood of amputation. For instance, the combination of surgical revascularization and lipid-lowering medication in the STARR data showed an odds ratio of 10.18, i.e., probability of 0.91, further increased when anti-platelet therapy and endovascular revascularization were added. The “All of Us” data similarly shows high odds ratios for these complex pathways, reflecting the necessity for a robust, multi-pronged approach in treating patients with severe cerebrovascular disease.
These findings from the “All of Us” data complement and extend the results from the STARR analysis. Both datasets underscore the importance of certain treatment sequences in managing cardiovascular conditions and highlight their significant associations with either preventing or leading to amputation. The odds ratio analysis provides a deeper understanding of the relative risks associated with different pathways, aiding clinicians in making informed decisions about the most effective treatment strategies for their patients.
3.2. Confounder Analysis of Treatment Pathways in PAD
To further enhance our insights from the visualizations in PAD, we identified confounders such as age (> 50, <= 50, > 65, <= 65), gender(male, female), race(white, black, Asian), smoking habit, and risk factors like diabetes, hypertension, heart failure, cerebrovascular disease, Hyperlipidemia and coronary artery disease, within the PAD cohort. We filtered the PAD patients based on these confounders and visualized the treatment pathways for each risk factor-specific cohort, in a manner like the visualizations of the entire PAD cohort presented earlier.
Table 12 and
Table 13 describe the patient counts from the risk factor cohorts we have built.
3.2.1. Hypertension Analysis of Treatment Pathways in PAD
Figure 3 is a Sankey diagram of treatment sequences in PAD patients with Hypertension cohort in our STARR dataset.
Table 14 and
Table 15 describe the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, PAD patients with hypertension show amputation rates comparable to or slightly better than the general PAD population when combination therapies are used. For example, Antiplatelet → Revascularization Surgery → Lipid Lowering and Antiplatelet → Lipid Lowering → Endovascular Revascularization both result in an amputation rate of 7.14%, which aligns closely with the general PAD rate of 7.79%. The most effective pathway, Revascularization Surgery → Lipid Lowering → Antiplatelet → Endovascular Revascularization, achieves the lowest amputation rate of 1.79%. These results suggest that multi-modal approaches can effectively manage PAD in hypertensive patients, potentially yielding better outcomes than for the general population.
Non-amputation outcomes for hypertensive PAD patients in the STARR dataset are strong, particularly for combination therapies. Antiplatelet → Lipid Lowering achieves the highest non-amputation rate of 33%, slightly exceeding the general PAD rate of 32%. Similarly, Lipid Lowering → Antiplatelet produces a non-amputation rate of 27%, outperforming the general PAD rate of 24.1%. Standalone treatments, such as Lipid Lowering alone (15%) and Antiplatelet alone (7.28%), yield outcomes comparable to general PAD data, reinforcing the efficacy of combination therapies in improving non-amputation rates.
Figure 4 is a Sankey diagram of treatment sequences in PAD with Hypertension cohort in our
All of Us dataset.
Table 16 and
Table 17 illustrate the dominant pathways that lead to amputation and non-amputation that can be observed from the figure.
In the All of Us dataset, hypertensive PAD patients experience slightly higher amputation rates for standalone treatments compared to the general PAD population. Endovascular Revascularization alone results in an amputation rate of 13.29%, slightly better than the general PAD rate of 13.6%. However, multi-modal strategies appear to significantly reduce amputation risks. For instance, Endovascular Revascularization → Revascularization Surgery achieves an amputation rate of 6.36%, closely aligning with the general PAD rate of 6.25%. The most effective pathway, Lipid Lowering → Anti platelet → Revascularization Surgery, produces the lowest amputation rate of 2.31%.
Non-amputation outcomes for hypertensive PAD patients in the All of Us dataset are competitive with general PAD outcomes. Lipid Lowering → Antiplatelet achieves the highest non-amputation rate of 28%, slightly above the general PAD rate of 27.29%. Simpler sequences, such as Antiplatelet alone (22%) and Antiplatelet → Lipid Lowering (22%), align closely with the general PAD rates of 23% and 22%. Standalone Lipid Lowering results in a non-amputation rate of 14%, consistent with the general PAD population.
3.2.2. Diabetes Analysis of Treatment Pathways in PAD
Figure 5 is a Sankey diagram of treatment sequences in PAD patients with Diabetes cohort in our STARR dataset.
Table 18 and
Table 19 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, diabetic PAD patients show slightly elevated amputation rates compared to the general PAD population, particularly for combination therapies. For instance, Antiplatelet → Lipid Lowering → Endovascular Revascularization results in an amputation rate of 9.26%, higher than the general PAD rate of 7.79% for the same sequence. Antiplatelet → Revascularization Surgery → Lipid Lowering achieves a better amputation rate of 5.56%, closely aligning with the general PAD rate of 5.19%. The most effective pathways, Lipid Lowering → Antiplatelet → Endovascular Revascularization and Revascularization Surgery → Lipid Lowering → Antiplatelet, yield the lowest amputation rate of 3.7%, which is worse than the general PAD rate of 2.6% but indicates the benefits of multi-modal approaches for diabetic patients.
Non-amputation outcomes in the STARR dataset for diabetic PAD patients are strong, especially with combination therapies. Antiplatelet → Lipid Lowering produces the highest non-amputation rate of 33%, outperforming the general PAD rate of 32%. Similarly, Lipid Lowering → Antiplatelet achieves 31%, better than the general PAD rate of 24.1%. However, standalone treatments, such as Lipid Lowering alone (15%) and Antiplatelet alone (4.7%), are less effective, aligning poorly with the general PAD rates of 16.8% and 10.2%. These findings underscore the importance of combination therapies in improving non-amputation outcomes for diabetic PAD patients.
Figure 6 is a Sankey diagram of treatment sequences in PAD with Diabetes cohort in our
All of Us dataset.
Table 20 and
Table 21 illustrate the dominant pathways that lead to amputation and non-amputation that can be observed from the figure.
In the All of Us dataset, diabetic PAD patients experience higher amputation rates for standalone treatments compared to the general PAD population. Endovascular Revascularization alone results in an amputation rate of 13.75%, higher than the general PAD rate of 13.6%. However, combination therapies significantly improve outcomes. Endovascular Revascularization → Revascularization Surgery lowers the rate to 5.62%, closely aligning with the general PAD rate of 6.25%. The best outcome is achieved with Lipid Lowering → Antiplatelet → Endovascular Revascularization and Lipid Lowering → Antiplatelet, which achieve amputation rates of 3.7% and 1.8%, respectively, demonstrating the efficacy of comprehensive pharmacological and surgical interventions in diabetic patients.
Non-amputation outcomes in the All of Us dataset for diabetic patients are competitive but slightly lower than the general PAD population for some pathways. Lipid Lowering → Antiplatelet achieves the highest non-amputation rate of 29%, slightly above the general PAD rate of 27.29%. Other pathways, such as Antiplatelet → Lipid Lowering (23%) and Antiplatelet alone (21%), align closely with the general PAD rates of 22% and 23%. Standalone Lipid Lowering produces a non-amputation rate of 13%, falling below the general PAD rate of 14%, indicating that combination therapies remain crucial for diabetic PAD patients.
3.2.3. Heart Failure Analysis of Treatment Pathways in PAD
Figure 7 is a Sankey diagram of treatment sequences in PAD patients with heart failure cohort in our STARR dataset.
Table 22 and
Table 23 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, PAD patients with heart failure experience significantly elevated amputation rates compared to the general PAD population. Antiplatelet → Lipid Lowering → Endovascular Revascularization results in an amputation rate of 19.23%, which is much higher than the general PAD rate of 7.79% for a similar sequence. Standalone Lipid Lowering achieves an amputation rate of 11.54%. The most effective pathway, Lipid Lowering → Antiplatelet → Endovascular Revascularization, reduces the rate to 7.69%. These findings highlight the greater challenges associated with managing PAD in patients with heart failure, particularly the increased risk of amputations.
Non-amputation outcomes for heart failure patients in the STARR dataset are relatively strong, particularly for combination therapies. Antiplatelet → Lipid Lowering achieves the highest non-amputation rate of 39%, significantly outperforming the general PAD rate of 32%. Lipid Lowering → Antiplatelet produces a non-amputation rate of 28%, better than the general PAD rate of 24.1%. However, standalone treatments such as Antiplatelet alone (7.43%) yield far poorer results than the general PAD rate of 10.2%, underscoring the necessity of combination therapies to achieve optimal outcomes.
Figure 8 is a Sankey diagram of treatment sequences in PAD with Heart Failure cohort in our
All of Us dataset.
Table 24 and
Table 25 illustrate the dominant pathways that lead to amputation and non-amputation that can be observed from the figure.
In the All of Us dataset, PAD patients with heart failure also experience elevated amputation rates for standalone treatments. Endovascular Revascularization alone results in an amputation rate of 14.15%, worse than the general PAD rate of 13.6%. However, combination therapies yield significantly better outcomes. Endovascular Revascularization → Revascularization Surgery reduces the rate to 7.55%, aligning closely with the general PAD rate of 6.25%. The most effective pathway, Antiplatelet → Lipid Lowering → Endovascular Revascularization, achieves an amputation rate of 2.83%, demonstrating the importance of multi-modal strategies for heart failure patients.
Non-amputation outcomes in the All of Us dataset for heart failure patients are comparable to or slightly worse than the general PAD population. Lipid Lowering → Antiplatelet achieves the highest non-amputation rate of 30%, slightly better than the general PAD rate of 27.29%. Simpler pathways, such as Antiplatelet → Lipid Lowering (25%) and Antiplatelet alone (20%), are relative to the general PAD rates of 22% and 23%, respectively. These results suggest that while pharmacological strategies remain effective, standalone treatments are less effective for heart failure patients.
3.2.4. Cerebrovascular Disease Analysis of Treatment Pathways in PAD
Figure 9 is a Sankey diagram of treatment sequences in PAD patients with Cerebrovascular disease cohort in our STARR dataset.
Table 26 and
Table 27 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
CVD significantly elevates amputation rates compared to general PAD data, especially with less comprehensive treatments. For example, Lipid Lowering followed by Antiplatelet results in the highest amputation rate of 30.77%. However, integrating Endovascular Revascularization alongside Antiplatelet and Lipid Lowering reduces the amputation rate to 11.54%, which is still notably worse than the general PAD rate of 7.79% for similar sequences. The most effective pathways involve Revascularization Surgery combined with Lipid Lowering and Antiplatelet, achieving an amputation rate of 7.69%. This comparison underscores the critical importance of surgical interventions in improving outcomes for CVD patients, though the rates remain higher than in the general population.
Non-amputation rates for CVD patients in the STARR dataset are also significantly impacted. The highest rate of 35%, achieved with Antiplatelet followed by Lipid Lowering, is comparable to the general PAD rate of 32% for the same combination, indicating that pharmacological strategies remain effective for some CVD patients. However, outcomes decline sharply with less comprehensive approaches; for instance, Lipid Lowering alone yields a non-amputation rate of just 8.5%, far lower than the 16.8% seen in general PAD patients. This stark contrast emphasizes the diminished effectiveness of standalone treatments for CVD patients, highlighting the need for combination therapies to improve outcomes in this group.
Figure 10 is a Sankey diagram of treatment sequences in PAD with Cerebrovascular disease cohort in our
All of Us dataset.
Table 28 and
Table 29 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, Endovascular Revascularization used alone leads to an amputation rate of 14.53%, slightly worse than the 13.6% observed in the general PAD population, showing that CVD adds complexity to PAD management even in cases of surgical intervention. Adding Revascularization Surgery reduces the amputation rate to 6.84%, a marked improvement but still higher than the 6.25% for similar pathways in general PAD patients. The best outcomes are achieved with a comprehensive sequence involving Lipid Lowering, Antiplatelet, and Endovascular Revascularization, yielding an amputation rate of 5.13%, which is competitive with the general PAD outcome of 3.98% for similar sequences. This suggests that while aggressive multi-modal approaches are effective for CVD patients, they may not entirely offset the increased risks associated with the condition.
For non-amputation outcomes, Lipid Lowering followed by Antiplatelet produces the highest rate at 27%, close to the 27.29% seen in general PAD data. Other sequences, such as Antiplatelet alone or Antiplatelet followed by Lipid Lowering, result in non-amputation rates of 21% and 23%, respectively, which are slightly lower than the 23% and 22% observed in general PAD outcomes. Interestingly, standalone Lipid Lowering achieves a non-amputation rate of 12%, which is below the 14% for general PAD patients, again reinforcing the importance of combination therapies in improving outcomes for CVD patients.
3.2.5. Coronary Artery Disease Analysis of Treatment Pathways in PAD
Figure 11 is a Sankey diagram of treatment sequences in PAD patients with coronary artery disease cohort in our STARR dataset.
Table 30 and
Table 31 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, CAD significantly impacts the effectiveness of PAD treatment pathways, particularly with less comprehensive approaches. For example, Antiplatelet followed by Lipid Lowering and Endovascular Revascularization results in an amputation rate of 13.95%, notably higher than the general PAD rate of 7.79% for similar sequences. However, more aggressive combinations such as Revascularization Surgery, Lipid Lowering, and Antiplatelet reduce the amputation rate significantly to 4.65%, which is closer to the lower end of general PAD outcomes (e.g., 2.6%). This demonstrates that multi-modal strategies involving surgical intervention are crucial in managing PAD in CAD patients, though they still do not perform as well as in the general population.
Non-amputation rates for CAD patients in the STARR dataset are strikingly high when combination therapies are used. For instance, Antiplatelet followed by Lipid Lowering achieves a non-amputation rate of 40%, outperforming the general PAD rate of 32% for the same sequence. However, less aggressive pathways, such as Lipid Lowering followed by Antiplatelet, achieve only 30%, which is comparable to the upper range of general PAD outcomes. These results indicate that CAD patients benefit significantly from carefully sequenced combination therapies, whereas standalone approaches may lead to suboptimal outcomes.
Figure 12 is a Sankey diagram of treatment sequences in PAD with Coronary artery disease cohort in our
All of Us dataset.
Table 32 and
Table 33 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, Endovascular Revascularization alone results in the highest amputation rate of 15.38%, which is higher than the general PAD rate of 13.6% for the same treatment. However, adding Revascularization Surgery reduces the rate to 6.36%, and the lowest amputation rate of 2.1% is achieved with Antiplatelet followed by Endovascular Revascularization. This combination suggests that surgical and pharmacological interventions, when properly sequenced, can be particularly effective for CAD patients.
For non-amputation outcomes, the best results are seen with Lipid Lowering followed by Antiplatelet, achieving a non-amputation rate of 29%, which is slightly better than the 27.29% observed for general PAD patients. Single-agent therapies, such as Antiplatelet alone, achieve 20%, while Lipid Lowering alone yields only 11%, which is notably worse than the general PAD rate of 14% for similar standalone treatments. These findings reinforce the importance of combination therapies in managing PAD in CAD patients.
3.2.6. Hyperlipidemia Analysis of Treatment Pathways in PAD
Figure 13 is a Sankey diagram of treatment sequences in PAD patients with Hyperlipidemia cohort in our STARR dataset.
Table 34 and
Table 35 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, hyperlipidemia (HLD) patients show varied amputation outcomes based on the treatment pathway. The combination of Antiplatelet → Lipid Lowering → Endovascular Revascularization results in an amputation rate of 7.89%, which aligns closely with the general PAD rate of 7.79% for the same sequence. However, introducing Revascularization Surgery reduces the rate to 5.26%, and the lowest amputation rate of 2.63% is achieved with the pathway Revascularization Surgery → Lipid Lowering → Antiplatelet. This shows that surgical interventions, when combined with pharmacological therapies, provide superior outcomes for HLD patients, closely matching the lower end of general PAD outcomes (e.g., 1.3%).
Non-amputation outcomes are notably strong in HLD patients. The highest rate of 35.80% is achieved with Antiplatelet followed by Lipid Lowering, outperforming the general PAD rate of 32% for the same sequence. However, reversing the sequence to Lipid Lowering followed by Antiplatelet achieves a lower rate of 26.72%, which is still above the general PAD equivalent of 24.1%. These findings suggest that HLD patients benefit greatly from pharmacological management, with slightly improved outcomes compared to the general PAD population.
Figure 14 is a Sankey diagram of treatment sequences in PAD with Hyperlipidemia cohort in our
All of Us dataset.
Table 36 and
Table 37 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, HLD patients experience higher amputation rates when treatments are less comprehensive. For instance, Endovascular Revascularization alone results in a rate of 14.12%, which is slightly higher than the general PAD rate of 13.6% for the same pathway. However, integrating Revascularization Surgery reduces the rate significantly to 5.88%, and the most effective pathway, Antiplatelet → Lipid Lowering → Endovascular Revascularization, achieves a rate of 2.94%. This is slightly worse than the general PAD rate of 2.84% for a similar sequence but demonstrates the effectiveness of comprehensive treatment in HLD patients.
Non-amputation outcomes for HLD patients in the All of Us dataset are competitive with general PAD data. The highest rate of 29% is observed for Lipid Lowering followed by Antiplatelet, which closely matches the general PAD outcome of 27.29% for the same sequence. Less effective pathways, such as Antiplatelet alone and Lipid Lowering alone, yield non-amputation rates of 20% and 15%, respectively, which are slightly worse than the general PAD rates of 23% and 14%. This highlights the importance of combination therapies for improving non-amputation outcomes in HLD patients.
3.2.7. Analysis of Treatment Pathways in PAD Patients with Age > 50
Figure 15 is a Sankey diagram of treatment sequences in PAD filtered for patients who are aged over 50 cohort in our STARR dataset.
Table 38 and
Table 39 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, age over 50 significantly impacts PAD treatment outcomes, particularly in amputation pathways. For instance, Lipid Lowering alone results in the highest amputation rate of 20.25%, which is substantially worse than the general PAD rate of 7.79% for the worst-performing sequence. However, introducing combination therapies leads to better outcomes. For example, Antiplatelet → Lipid Lowering → Endovascular Revascularization reduces the amputation rate to 7.59%, which is comparable to general PAD outcomes for multi-modal treatments. The most effective sequence, Revascularization Surgery → Lipid Lowering → Antiplatelet, achieves the lowest amputation rate of 2.53%. This highlights the importance of integrating surgical interventions and comprehensive pharmacological management to mitigate the increased risks associated with age.
For non-amputation outcomes, the highest rate of 32.43% is achieved with Antiplatelet followed by Lipid Lowering, closely aligning with the general PAD rate of 32% for the same sequence. However, reversing the sequence to Lipid Lowering followed by Antiplatelet results in a non-amputation rate of 24.31%, which is slightly better than the general PAD rate of 24.1%. This suggests that while combination therapies remain effective for older patients, age-related complications might necessitate prioritizing certain sequences for optimal outcomes.
Figure 16 is a Sankey diagram of treatment sequences in PAD with age > 50 cohort in our
All of Us dataset.
Table 40 and
Table 41 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, Endovascular Revascularization alone leads to a high amputation rate of 17.14%, higher than the general PAD rate of 13.6%, indicating that age over 50 increases risks with standalone surgical treatments. However, combining therapies significantly improves outcomes. For instance, Lipid Lowering → Antiplatelet → Endovascular Revascularization reduces the rate to 4.29%, and Antiplatelet → Lipid Lowering → Endovascular Revascularization further lowers it to 3.57%. The best results are achieved with Revascularization Surgery alone, yielding an amputation rate of 2.86%.
For non-amputation outcomes, the highest rate of 28.41% is observed with Lipid Lowering → Antiplatelet, slightly exceeding the general PAD rate of 27.29%. Other sequences, such as Antiplatelet → Lipid Lowering, achieve 21.05%, which is comparable to the general PAD rate of 22%. These findings suggest that age over 50 does not drastically alter non-amputation outcomes when combination therapies are applied but does highlight the need for sequencing optimization to address age-related challenges.
3.2.8. Analysis of Treatment Pathways in PAD Patients with Age <= 50
Figure 17 is a Sankey diagram of treatment sequences in PAD filtered for patients with age <= 50 cohort in our STARR dataset.
Table 42 and
Table 43 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
For patients aged 50 and younger in the STARR dataset, PAD treatment pathways show markedly higher amputation rates compared to the general PAD population. Antiplatelet followed by Lipid Lowering results in an exceptionally high amputation rate of 66.67%. This highlights a poor response to pharmacological therapies alone in younger patients, potentially indicating more aggressive disease progression or a lack of timely intervention. A simpler sequence, Antiplatelet alone, fares slightly better with an amputation rate of 33.33%.
Non-amputation outcomes in this age group are also less favorable compared to the general PAD population. The highest non-amputation rate, 18.49%, is achieved with Lipid Lowering alone, which is higher than the general PAD rate of 16.8% for this sequence. Adding Antiplatelet to Lipid Lowering slightly reduces the non-amputation rate to 17.36% and introducing Endovascular Revascularization after Antiplatelet yields only 5.28%.
Figure 18 is a Sankey diagram of treatment sequences in PAD with age <= 50 cohort in our
All of Us dataset.
Table 44 and
Table 45 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, younger patients show similarly elevated amputation rates, though outcomes are slightly better than in the STARR dataset. Lipid Lowering followed by Antiplatelet achieves an amputation rate of 27.78%, which, while high, is significantly better than the 66.67% observed in the STARR dataset. The reverse sequence, Antiplatelet followed by Lipid Lowering, further reduces the rate to 25%. Non-amputation outcomes are somewhat better in the All of Us dataset than in STARR. Antiplatelet alone achieves the highest non-amputation rate of 29%, comparable to the general PAD rate of 23%. Meanwhile, Lipid Lowering alone achieves 21.51%, which is significantly better than the 18.49% seen in STARR for the same sequence and slightly higher than the general PAD rate of 14%. These results suggest that younger patients in the All of Us dataset respond better to standalone therapies than their counterparts in the STARR dataset but still lag behind general PAD outcomes.
3.2.9. Analysis of Treatment Pathways in PAD Patients with Age > 65
Figure 19 is a Sankey diagram of treatment sequences in PAD filtered for patients with age > 65 cohort in our STARR dataset.
Table 46 and
Table 47 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, older PAD patients aged over 65 show significantly higher amputation rates for certain treatment pathways compared to the general PAD population. For example, Lipid Lowering followed by Antiplatelet results in an amputation rate of 31.37%. Integrating surgical interventions proves more effective, as seen with Antiplatelet → Lipid Lowering → Endovascular Revascularization, which reduces the amputation rate to 5.88%. While this is better than the higher rates seen in the general PAD rate of 7.79% for similar combinations, suggesting that age-related factors increase risks even with optimal treatment.
Non-amputation outcomes in older patients show better alignment with general PAD data. The highest rate of 31.64% is achieved with Antiplatelet followed by Lipid Lowering, which is comparable to the general PAD rate of 32% for this sequence. However, standalone treatments, such as Lipid Lowering alone, achieve only 18.21%, which is slightly better than the general PAD rate of 16.8% for the same approach. These results suggest that while combination therapies remain effective for older patients, age-related factors may reduce the efficacy of standalone strategies.
Figure 20 is a Sankey diagram of treatment sequences in PAD with age > 65 cohort in our
All of Us dataset.
Table 48 and
Table 49 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, older patients face elevated amputation risks, particularly with standalone treatments. Endovascular Revascularization alone leads to an amputation rate of 24.07%, significantly higher than the general PAD rate of 13.6%, highlighting the added risks of surgical intervention in this age group without supporting therapies. However, the combination of Endovascular Revascularization → Revascularization Surgery reduces the rate to 9.26%, and the best outcome is achieved with Revascularization Surgery alone, yielding an amputation rate of 5.56%. While this rate is close to the general PAD minimum of 2.84%, it still underscores the heightened risks associated with age.
Non-amputation outcomes in the All of Us dataset show that combination therapies are crucial for older patients. Lipid Lowering followed by Antiplatelet achieves the highest non-amputation rate of 29.89%, which is slightly better than the general PAD rate of 27.29% for the same sequence. However, simpler strategies, such as Lipid Lowering alone, yield a non-amputation rate of 18.27%, which is only marginally better than the general PAD rate of 14%. These results emphasize that while older patients can achieve comparable outcomes with well-designed treatment pathways, standalone treatments are less effective and riskier.
3.2.10. Analysis of Treatment Pathways in PAD Patients with Age <= 65
Figure 21 is a Sankey diagram of treatment sequences in PAD filtered for patients with age <= 65 cohort in our STARR dataset.
Table 50 and
Table 51 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, PAD patients aged 65 and younger show elevated amputation rates for standalone treatments compared to the general PAD population. For instance, Lipid Lowering alone results in the highest amputation rate of 24.14%. multi-modal treatments prove far more effective. Both Antiplatelet → Lipid Lowering → Endovascular Revascularization and Antiplatelet → Revascularization Surgery → Lipid Lowering achieve an amputation rate of 6.9%, which is only slightly higher than the general PAD rate of 5.19% for similar sequences. These findings suggest that while younger patients may face aggressive disease progression, combination therapies help to mitigate these risks.
Non-amputation outcomes for younger patients in the STARR dataset are promising when combination therapies are used. The highest non-amputation rate, 31.86%, is achieved with Antiplatelet followed by Lipid Lowering, closely matching the general PAD rate of 32% for the same sequence. However, less comprehensive strategies such as Lipid Lowering followed by Antiplatelet result in a non-amputation rate of 18.87%, which is slightly lower than the general PAD rate of 24.1%. This indicates that while younger patients can achieve favorable outcomes with pharmacological strategies, sequencing and the inclusion of surgical interventions play a crucial role in optimizing results.
Figure 22 is a Sankey diagram of treatment sequences in PAD with age <= 65 cohort in our
All of Us dataset.
Table 52 and
Table 53 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, Endovascular Revascularization alone results in an amputation rate of 10.92%, which is better than the 24.14% observed in STARR for Lipid Lowering alone but still worse than the general PAD rate of 13.6%. The combination of Endovascular Revascularization followed by Revascularization Surgery significantly reduces the rate to 5.04%, while the best-performing sequence, Lipid Lowering → Antiplatelet → Endovascular Revascularization, achieves an amputation rate of 4.2%, closely aligning with the general PAD rate of 3.98%.
Non-amputation outcomes in the All of Us dataset highlight the effectiveness of combination therapies. Lipid Lowering followed by Antiplatelet produces the highest non-amputation rate of 24.92%, which is slightly lower than the general PAD rate of 27.29% for this sequence. Meanwhile, Antiplatelet alone achieves a non-amputation rate of 24.48%, comparable to the general PAD rate of 23%. However, less comprehensive strategies, such as Lipid Lowering alone, yield a rate of 18.51%, which is higher than the general PAD rate of 14%. These results suggest that younger patients in the All of Us dataset respond better to treatments than their counterparts in STARR but still lag behind the general PAD population.
3.2.11. Analysis of Treatment Pathways in PAD Patients Who Reported Their Race as ‘White’
Figure 23 is a Sankey diagram of treatment sequences in PAD filtered for patients who reported their race as ‘white’ cohort in our STARR dataset.
Table 54 and
Table 55 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, White patients with PAD exhibit amputation rates that are similar to the general PAD population when comprehensive treatments are applied. For example, Antiplatelet → Lipid Lowering → Endovascular Revascularization and Antiplatelet → Revascularization Surgery → Lipid Lowering both result in an amputation rate of 7.69%, closely aligning with the general PAD rate of 7.79% for similar sequences. The most effective treatment pathway, Revascularization Surgery → Lipid Lowering → Antiplatelet, achieves the lowest amputation rate of 5.13%, which is comparable to the general PAD rate of 2.6%. These findings suggest that multi-modal strategies are equally effective for White patients as they are for the general PAD population, with surgical interventions playing a crucial role in reducing amputation risks.
For non-amputation pathways, the highest rate of 31.84% is observed for Antiplatelet followed by Lipid Lowering, comparable to the general PAD rate of 32% for the same sequence. However, less comprehensive approaches, such as Lipid Lowering followed by Antiplatelet, yield a lower non-amputation rate of 23.15%, which is comparable to the general PAD rate of 24.1%. Standalone treatments like Lipid Lowering alone produce a non-amputation rate of 15.38%, better than the general PAD rate of 16.8%. These results indicate that White patients respond well to combination therapies, achieving outcomes on par with or slightly better than the general PAD population.
Figure 24 is a Sankey diagram of treatment sequences in PAD filtered for patients who reported their race as ‘white’ cohort in our
All of Us dataset.
Table 56 and
Table 57 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, amputation rates for White patients are slightly worse than the general PAD population for standalone treatments but improve significantly with combination therapies. For instance, Endovascular Revascularization alone results in an amputation rate of 15.38%, higher than the general PAD rate of 13.6%. Adding Revascularization Surgery reduces the rate to 8.97%, and the most effective sequence, Lipid Lowering → Antiplatelet → Endovascular Revascularization, achieves an amputation rate of 3.85%, closely aligning with the general PAD rate of 3.98%.
Non-amputation outcomes in the All of Us dataset is competitive with general PAD results. The highest rate of 29.59% is achieved with Lipid Lowering followed by Antiplatelet, slightly better than the general PAD rate of 27.29% for the same sequence. Other combinations, such as Antiplatelet alone or Antiplatelet followed by Lipid Lowering, yield non-amputation rates of 22.61% and 20.72%, respectively, which are slightly lower than the general PAD rates of 23% and 22%. Standalone Lipid Lowering achieves a rate of 15.80%, outperforming the general PAD rate of 14%, suggesting that White patients respond well to pharmacological interventions.
3.2.12. Analysis of Treatment Pathways in PAD Patients Who Reported Their Race as ‘Black’
Figure 25 is a Sankey diagram of treatment sequences in PAD patients who reported their race as ‘black’ cohort in our STARR dataset.
Table 58 and
Table 59 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, Black patients with PAD experience significantly higher amputation rates compared to the general PAD population, particularly with standalone treatments. For instance, Lipid Lowering alone results in an amputation rate of 21.43%. Combination therapies such as Antiplatelet → Lipid Lowering → Endovascular Revascularization reduce the amputation rate to 14.29%, which is still notably higher than the general PAD rate of 7.79% for similar sequences. These findings highlight the elevated risks faced by Black patients with PAD and the need for more aggressive treatment strategies.
Non-amputation rates for Black patients show relatively strong outcomes when combination therapies are used. For example, Lipid Lowering → Antiplatelet achieves the highest non-amputation rate of 31.65%, which is higher than the general PAD rate of 24.1%. However, reversing the sequence to Antiplatelet → Lipid Lowering produces a slightly lower non-amputation rate of 29.29%, which is still competitive with the general PAD rate of 32%. Simpler strategies, such as Antiplatelet alone, yield a non-amputation rate of 10.77%, higher than the general PAD rate of 10.2%.
Figure 26 is a Sankey diagram of treatment sequences in PAD patients who reported their race as ‘black’ cohort in our
All of Us dataset.
Table 60 and
Table 61 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, amputation rates for Black patients are slightly better than in STARR but still worse than the general PAD population. Endovascular Revascularization alone produces an amputation rate of 7.46%, which is worse than the general PAD rate of 13.6%. More comprehensive combinations, such as Lipid Lowering → Antiplatelet → Endovascular Revascularization, achieve an amputation rate of 4.48%, aligning closely with the general PAD rate of 3.98%. Similar outcome is achieved with the addition of Revascularization Surgery to the sequence, maintaining an amputation rate of 4.48%.
Non-amputation outcomes in the All of Us dataset is competitive with the general PAD population. Antiplatelet alone achieves the highest non-amputation rate of 27.65%, slightly outperforming the general PAD rate of 23%. Other combinations, such as Antiplatelet → Lipid Lowering, yield a non-amputation rate of 24.18%, which is slightly higher than the general PAD rate of 22%. Standalone Lipid Lowering produces a non-amputation rate of 11.69%, comparable to the general PAD rate of 14%. These results suggest that while Black patients benefit from combination therapies, they face greater challenges with standalone or less comprehensive treatments.
3.2.13. Analysis of Treatment Pathways in PAD Patients Who Reported Their Race as ‘Asian’
Figure 27 is a Sankey diagram of treatment sequences in PAD patients who reported their race as ‘Asian’ cohort in our STARR dataset.
Table 62 and
Table 63 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, PAD outcomes for Asian patients, given their least patient count, demonstrate high amputation rates, significantly worse than the general PAD population. Both treatment sequences—Lipid Lowering → Antiplatelet and Antiplatelet → Lipid Lowering—result in amputation rates of 50%. This is drastically higher compared to the general PAD rates. Non-amputation outcomes in the STARR dataset are also notably poor for Asian patients. Lipid Lowering alone achieves the highest non-amputation rate of 16.76%, which is far lower than the general PAD rate of 16.8% for the same treatment. Antiplatelet alone produces a dismal non-amputation rate of 7.67%, worse than the general PAD rate of 10.2%. These findings indicate that not only are standalone treatments ineffective in preventing amputations in Asian patients, but even non-amputation outcomes fall well short of general PAD benchmarks.
Figure 28 is a Sankey diagram of treatment sequences in PAD patients who reported their race as ‘Asian’ cohort in our
All of Us dataset.
Table 64 and
Table 65 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
The All of Us dataset paints an even grimmer picture for Asian patients, with Endovascular Revascularization alone resulting in amputation rate of 100%. The highest non-amputation rate of 33.33% is achieved with both Lipid Lowering → Antiplatelet and Antiplatelet → Lipid Lowering, exceeding the general PAD rate of 27.29% for similar sequences. Standalone Lipid Lowering produces a non-amputation rate of 23.08%, which is also slightly better than the general PAD rate of 14%. These results suggest that while surgical interventions fail to deliver positive outcomes, pharmacological strategies remain effective in improving non-amputation outcomes for Asian patients.
3.2.14. Analysis of Treatment Pathways in PAD Patients Who Reported Their Gender as ‘Male’
Figure 29 is a Sankey diagram of treatment sequences in PAD patients who reported their gender as ‘male’ cohort in our STARR dataset.
Table 66 and
Table 67 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, male PAD patients show slightly elevated amputation rates compared to the general PAD population for similar treatment pathways. For instance, Antiplatelet → Lipid Lowering → Endovascular Revascularization results in an amputation rate of 8.93%, which is marginally higher than the general PAD rate of 7.79% for the same sequence. Similarly, Antiplatelet → Revascularization Surgery → Lipid Lowering achieves an amputation rate of 7.14%, slightly better than the general PAD rate of 5.19%. These findings suggest that while combination therapies remain effective for male patients, their outcomes are slightly worse than those seen in the general population, likely due to increased disease severity or other risk factors.
Non-amputation outcomes for male patients in the STARR dataset are slightly better than in the general PAD population for some treatment sequences. For example, Antiplatelet → Lipid Lowering achieves the highest non-amputation rate of 33.45%, comparable to the general PAD rate of 32%. However, simpler sequences such as Lipid Lowering → Antiplatelet yield a lower non-amputation rate of 25.32%, which is still comparable to the general PAD rate of 24.1%. Standalone Lipid Lowering achieves 16.40%, aligning closely with the general PAD rate of 16.8%. These results indicate that male patients respond well to combination therapies, with outcomes closely mirroring those of the general population.
Figure 30 is a Sankey diagram of treatment sequences in PAD patients who reported their gender as ‘male’ cohort in our
All of Us dataset.
Table 68 and
Table 69 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, amputation rates for male patients are slightly elevated for standalone treatments compared to the general PAD population. For instance, Endovascular Revascularization alone produces an amputation rate of 14.29%, higher than the general PAD rate of 13.6%. Adding Revascularization Surgery reduces the rate to 7.62%, which aligns closely with the general PAD rate of 6.25%. These results highlight the importance of combination therapies in reducing amputation risks for male patients, though outcomes remain slightly worse than in the general PAD population.
Non-amputation outcomes in the All of Us dataset for male patients are competitive with the general PAD population. The highest rate of 29.89% is achieved with Lipid Lowering → Antiplatelet, which is slightly higher than the general PAD rate of 27.29%. Other sequences, such as Antiplatelet → Lipid Lowering and Antiplatelet alone, yield non-amputation rates of 21.33% and 21.44%, closely matching the general PAD rates of 22% and 23%, respectively. These findings suggest that while standalone treatments are less effective, pharmacological strategies remain robust for male patients.
3.2.15. Analysis of Treatment Pathways in PAD Patients Who Reported Their Gender as ‘Female’ Cohort
Figure 31 is a Sankey diagram of treatment sequences in PAD patients who reported their gender as ‘Female’ cohort in our STARR dataset.
Table 70 and
Table 71 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, female PAD patients experience significantly higher amputation rates for standalone or less comprehensive treatments compared to the general PAD population. For instance, Lipid Lowering → Antiplatelet results in an alarmingly high amputation rate of 47.62%. However, combination therapies such as Antiplatelet → Exercise Therapy → Lipid Lowering and Endovascular Revascularization → Lipid Lowering achieve substantially lower amputation rates of 4.76%. This underscores the critical importance of multi-modal approaches, particularly for female patients, to mitigate amputation risks effectively.
Non-amputation outcomes for female patients in the STARR dataset show encouraging results with combination therapies. For example, Antiplatelet → Lipid Lowering achieves a non-amputation rate of 30.86%, which is slightly below the general PAD rate of 32% for the same sequence. Simpler pathways, such as Lipid Lowering alone or Antiplatelet alone, result in lower non-amputation rates of 17.77% and 12.71%, respectively, compared to the general PAD rates of 16.8% and 10.2%. These results suggest that while combination therapies remain effective for female patients, standalone treatments are considerably less effective in preventing disease progression.
Figure 32 is a Sankey diagram of treatment sequences in PAD patients who reported their gender as ‘Female’ cohort in our
All of Us dataset.
Table 72 and
Table 73 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, female patients also show elevated amputation rates for standalone treatments compared to the general PAD population. For example, Lipid Lowering → Antiplatelet results in an amputation rate of 27.27%. However, Endovascular Revascularization alone achieves a reduced amputation rate of 13.68%, which is close to the general PAD rate of 13.6%, suggesting that surgical interventions may have similar efficacy for female patients as for the general population.
Non-amputation outcomes in the All of Us dataset for female patients show slightly better results than the general PAD population. Antiplatelet alone achieves a non-amputation rate of 25.06%, which is slightly better than the general PAD rate of 23%. Combination therapies such as Antiplatelet → Lipid Lowering achieve 22.78%, higher than the general PAD rate of 22%. Standalone Lipid Lowering produces a non-amputation rate of 16.48%, slightly better than the general PAD rate of 14%. These findings highlight the importance of combination therapies for female patients while suggesting that even standalone pharmacological strategies can provide moderate benefits.
3.2.16. Analysis of Treatment Pathways in PAD Patients Who Reportedly Smoke
Figure 33 is a Sankey diagram of treatment sequences in PAD patients who reportedly smoke cohort in our STARR dataset.
Table 74 and
Table 75 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the STARR dataset, PAD patients who smoke experience amputation rates that closely align with the general PAD population when combination therapies are applied. For instance, Antiplatelet → Lipid Lowering → Endovascular Revascularization results in an amputation rate of 8.33%, slightly higher than the general PAD rate of 7.79% for the same sequence. Similarly, Antiplatelet → Revascularization Surgery → Lipid Lowering achieves an amputation rate of 5.56%, comparable to the general PAD rate of 5.19%. These results indicate that multi-modal approaches remain effective in managing PAD in smokers, though their outcomes are marginally worse compared to non-smokers, likely due to the compounding effects of smoking on vascular health.
Non-amputation outcomes for smokers in the STARR dataset are also comparable to the general PAD population. Antiplatelet → Lipid Lowering achieves the highest non-amputation rate of 32.14%, close to the general PAD rate of 32% for the same sequence. However, simpler pathways, such as Lipid Lowering → Antiplatelet and Lipid Lowering alone, yield non-amputation rates of 24.62% and 16.45%, which align closely with the general PAD rates of 24.1% and 16.8%, respectively. These findings suggest that smokers benefit from combination therapies, achieving outcomes similar to the general PAD population when multi-modal strategies are used.
Figure 34 is a Sankey diagram of treatment sequences in PAD patients who reportedly smoke cohort in our
All of Us dataset.
Table 76 and
Table 77 illustrate the dominant pathways that lead to amputation and non-amputation which can be observed from the figure.
In the All of Us dataset, amputation rates for smokers are slightly worse than the general PAD population for standalone treatments but improve significantly with combination therapies. Endovascular Revascularization alone produces an amputation rate of 11.54%, which is marginally worse than the general PAD rate of 13.6%. Adding Revascularization Surgery reduces the rate to 6.41%, and the best outcome is achieved with Antiplatelet → Lipid Lowering → Endovascular Revascularization, which results in an amputation rate of 3.85%, closely aligning with the general PAD rate of 3.98%.
Non-amputation outcomes in the All of Us dataset for smokers show slightly lower results compared to the general PAD population. The highest non-amputation rate of 24.90% is achieved with Lipid Lowering → Antiplatelet, which is below the general PAD rate of 27.29% for the same sequence. Other pathways, such as Antiplatelet alone or Antiplatelet → Lipid Lowering, yield non-amputation rates of 22.78% and 21%, respectively, which are lower than the general PAD rates of 23% and 22%. These results indicate that while combination therapies remain effective, smoking may reduce the overall efficacy of certain treatment pathways.
3.3. Summary from Confounder Analysis
In the STARR dataset, out of 5,581 PAD patients, 77 underwent amputation. Among the risk factors assessed, hyperlipidemia was the most prevalent in the amputation group, with 76 patients affected. This represents a substantial proportion compared to other comorbidities, such as diabetes (54 amputations) and hypertension (56 amputations). Notably, heart failure and cerebrovascular disease were associated with fewer amputations, with 26 cases each. The high incidence of hyperlipidemia in the amputation subset underscores its critical role in the progression of PAD leading to limb loss. Similarly, in the All of Us dataset comprising 4,261 PAD patients, 176 experienced amputations. Hyperlipidemia was again the leading risk factor, with 170 amputations reported. There are other significant comorbidities, including hypertension (173 amputations) and diabetes (160 amputations). Heart failure and cerebrovascular disease were associated with fewer amputations, with 106 and 117 cases, respectively. The consistency of hyperlipidemia as a leading risk factor across both cohorts highlights its pervasive impact on PAD outcomes. Certain PAD confounder groups, such as patients identifying as Asian, exhibit negligible amputation counts, likely due to their low representation in both the datasets. Endovascular revascularization treatment is notably present in the heart failure cohort of the STARR dataset, accounting for 20% of the total amputation pathways, while its occurrence has not been found in the dominant non-amputation pathways. This treatment also appears prominently in the cerebrovascular disease and coronary artery disease cohorts, with at least 15% occurrence in both risk factors within the All of Us dataset, while being absent in the dominant non-amputation pathways. Revascularization procedures have also been found to be more prominent in the full PAD cohorts of both STARR and All of Us data, confirming the findings from the confounder analysis. Antiplatelet and lipid-lowering treatments are prominent across all the cohorts, often routing to the non-amputation just like the treatments of smoking cessation and exercise therapy as seen in the sankey visualizations, the prevalence of these treatment sequences vary by slight percentages across different risk factors.
Amputation outcomes in PAD patients across the STARR and
All of Us datasets reveal significant variations based on confounders, underscoring the need for tailored interventions. Hypertension confounders exhibit promising results with multi-modal approaches, such as Revascularization Surgery → Lipid Lowering → Antiplatelet → Endovascular Revascularization, achieving an amputation rate of 1.79% in the STARR dataset. Similarly, diabetic patients show favorable outcomes with sequences like Lipid Lowering → Antiplatelet → Endovascular Revascularization, yielding a low 3.7% amputation rate. On the other hand, patients with heart failure face higher risks, with Antiplatelet → Lipid Lowering → Endovascular Revascularization resulting in a significantly elevated rate of 19.23% in STARR. Racial disparities are evident, with Black patients experiencing elevated risks; for instance, Lipid Lowering alone results in a 21.43% amputation rate (STARR). Similarly, Asian patients appear to demonstrated a very significant amputation rate for standalone Endovascular Revascularization in the
All of Us dataset, but it is to be noted that their patient count is negligible too as shown in
Table 12 and
Table 13. Smoking, while slightly elevating risks, does not drastically affect outcomes when combination therapies like Antiplatelet → Lipid Lowering → Endovascular Revascularization are employed, achieving rates closely aligned with the general PAD population. Age-related confounders also show stark differences, with older patients (>65 years) responding better to aggressive therapies, such as Endovascular Revascularization → Revascularization Surgery (9.26% in
All of Us), while younger patients (<50 years) face disproportionately high risks, such as a 66.67% rate for Antiplatelet → Lipid Lowering in STARR, highlighting the need for more aggressive and early interventions in younger cohorts, however the younger patients count is very low as represented in
Table 12 and
Table 13.
Non-amputation outcomes across these risk factors emphasize the effectiveness of pharmacological and combination therapies in mitigating disease progression, particularly in patients with hypertension, diabetes, and smoking history. For hypertensive patients, Antiplatelet → Lipid Lowering yields a robust 33% non-amputation rate in STARR, slightly exceeding the general PAD rate of 32%. Similarly, diabetic patients benefit significantly from this sequence, achieving 31% in STARR. Smokers and hyperlipidemia patients also show strong results with Lipid Lowering → Antiplatelet combinations, achieving non-amputation rates of 31.65% (STARR) and 29.59% (All of Us), respectively. However, standalone treatments are markedly less effective, with rates often dropping below 15% for Lipid Lowering alone across most confounders, particularly in heart failure (7.43% in STARR) and older patients (>65 years; 18.21% in STARR). Racial disparities persist in the All of Us dataset as well, though pharmacological strategies demonstrate some promise for Black and Asian patients, with sequences like Lipid Lowering → Antiplatelet achieving rates of 27.65% and 33.33%, respectively. These findings underscore the importance of prioritizing combination therapies tailored to individual confounders to improve outcomes, as even minor variations in sequencing can drastically affect both amputation and non-amputation rates, highlighting the need for precision medicine in PAD management.
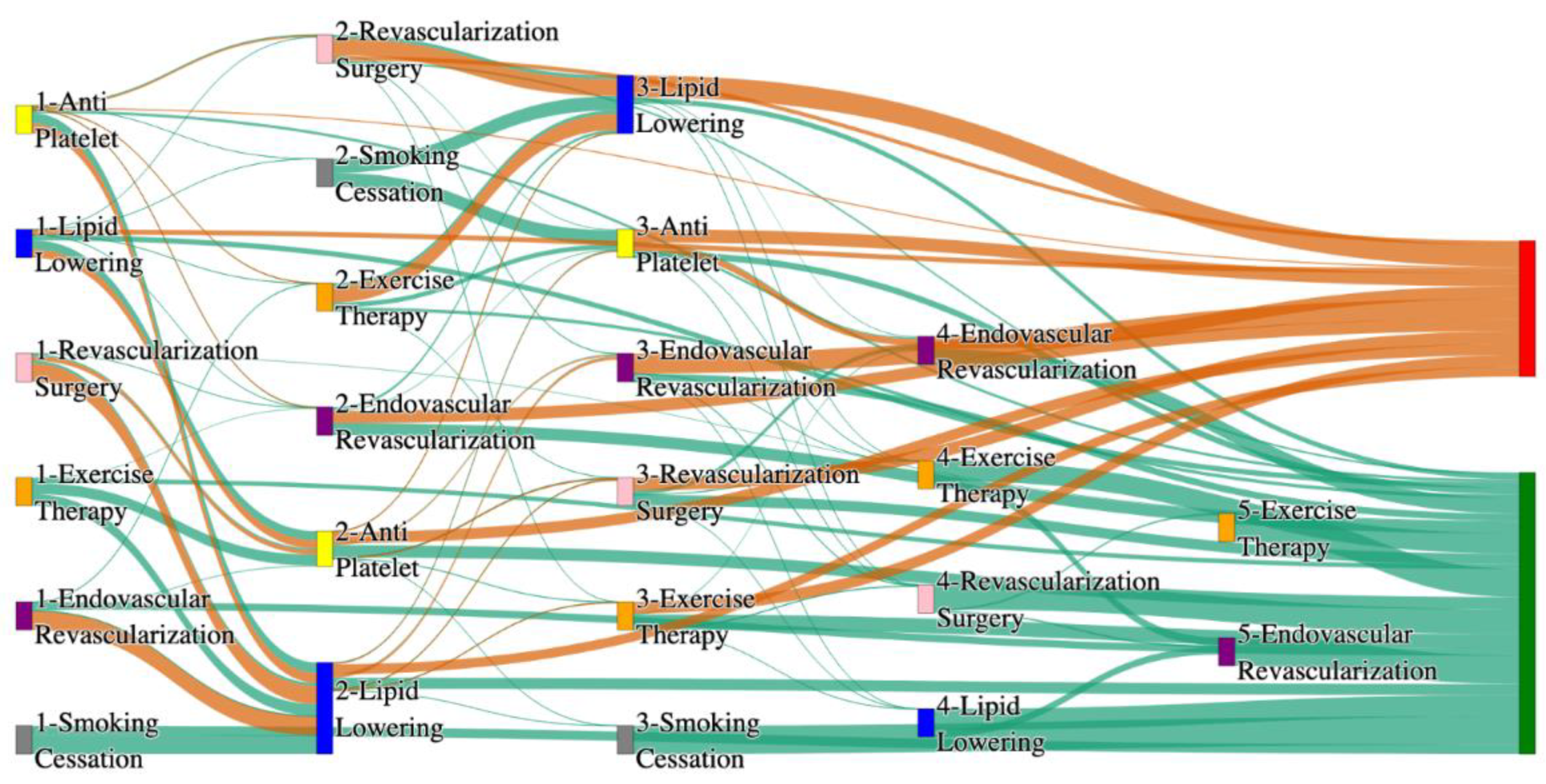
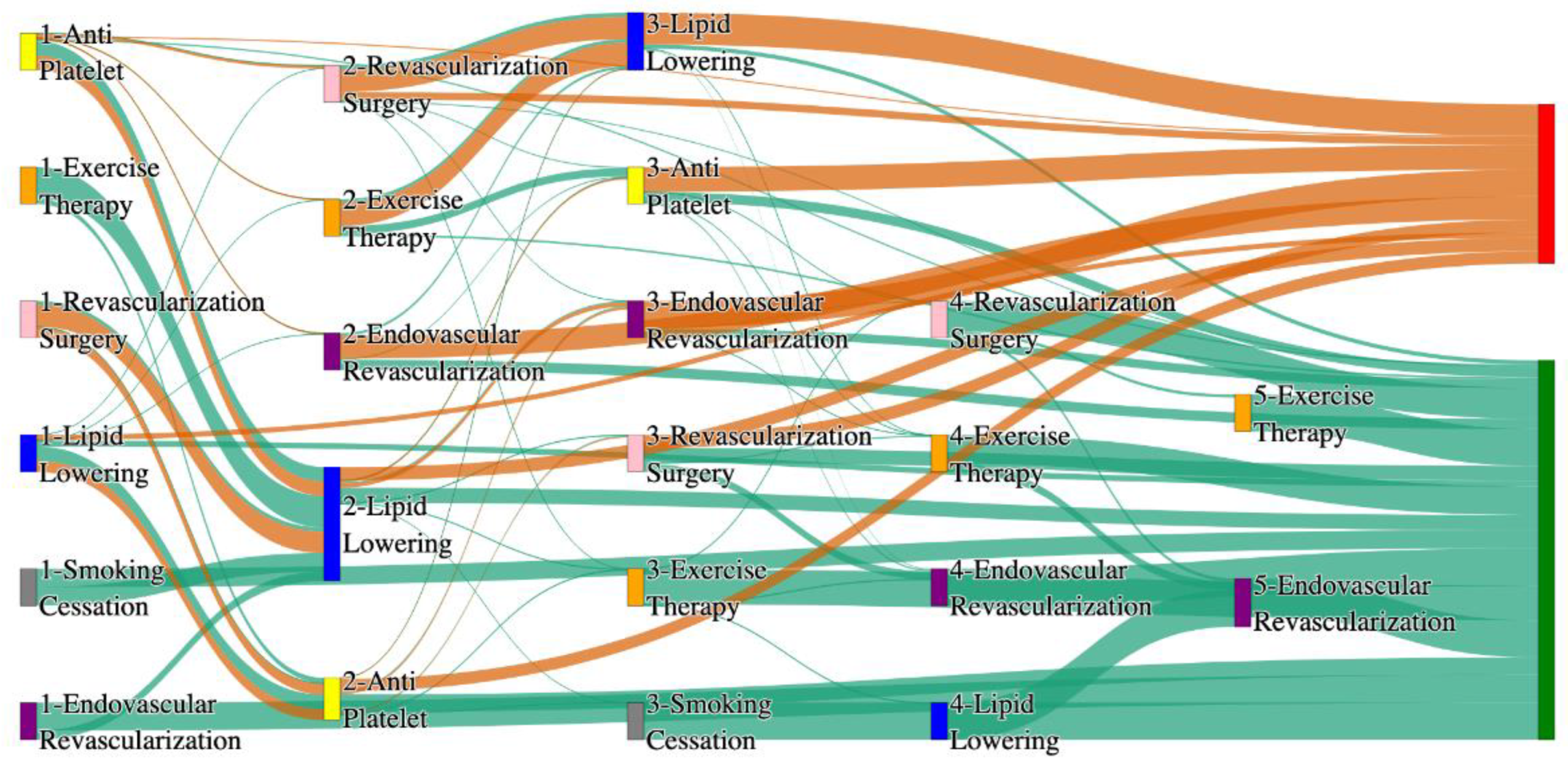
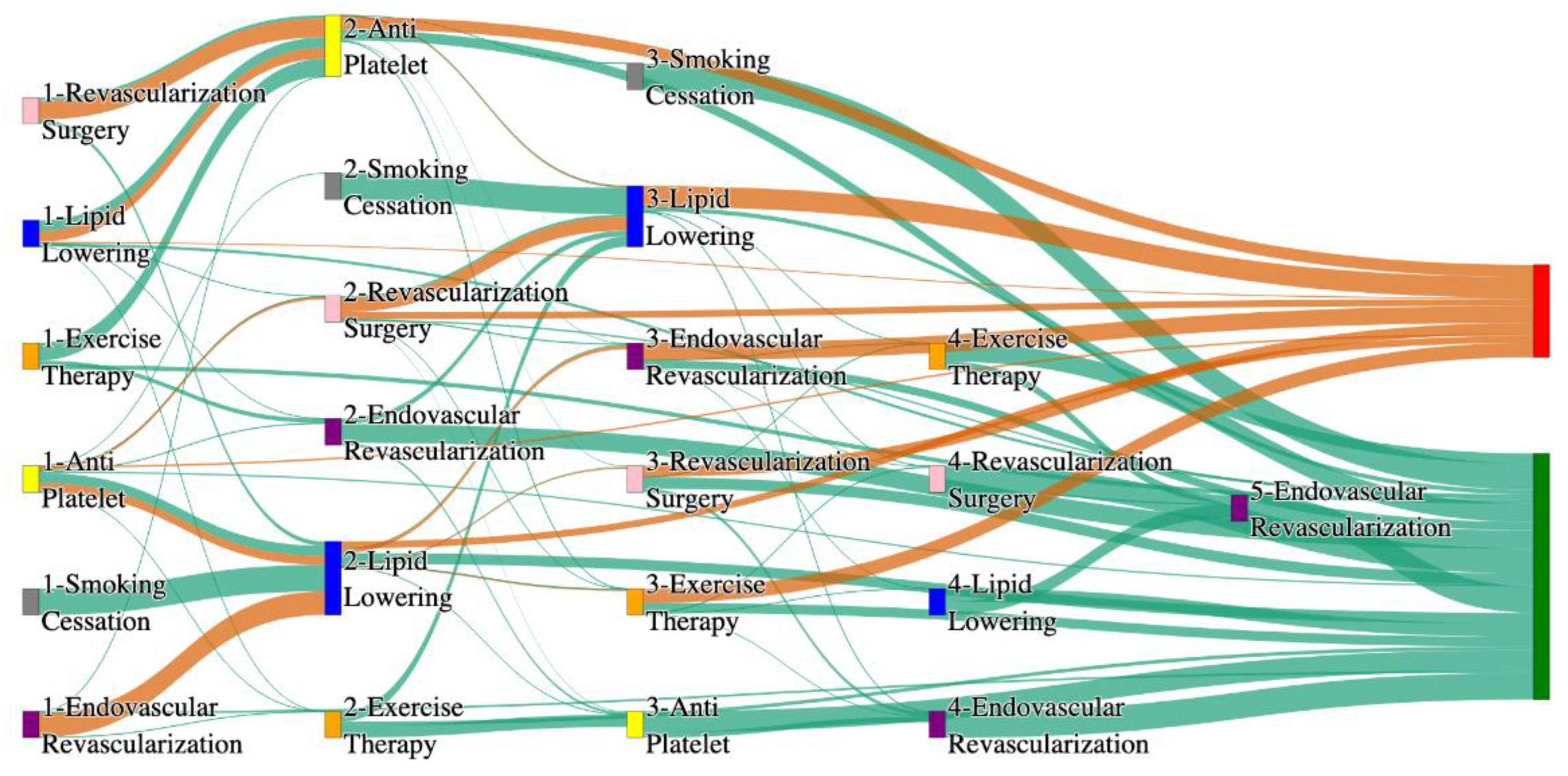
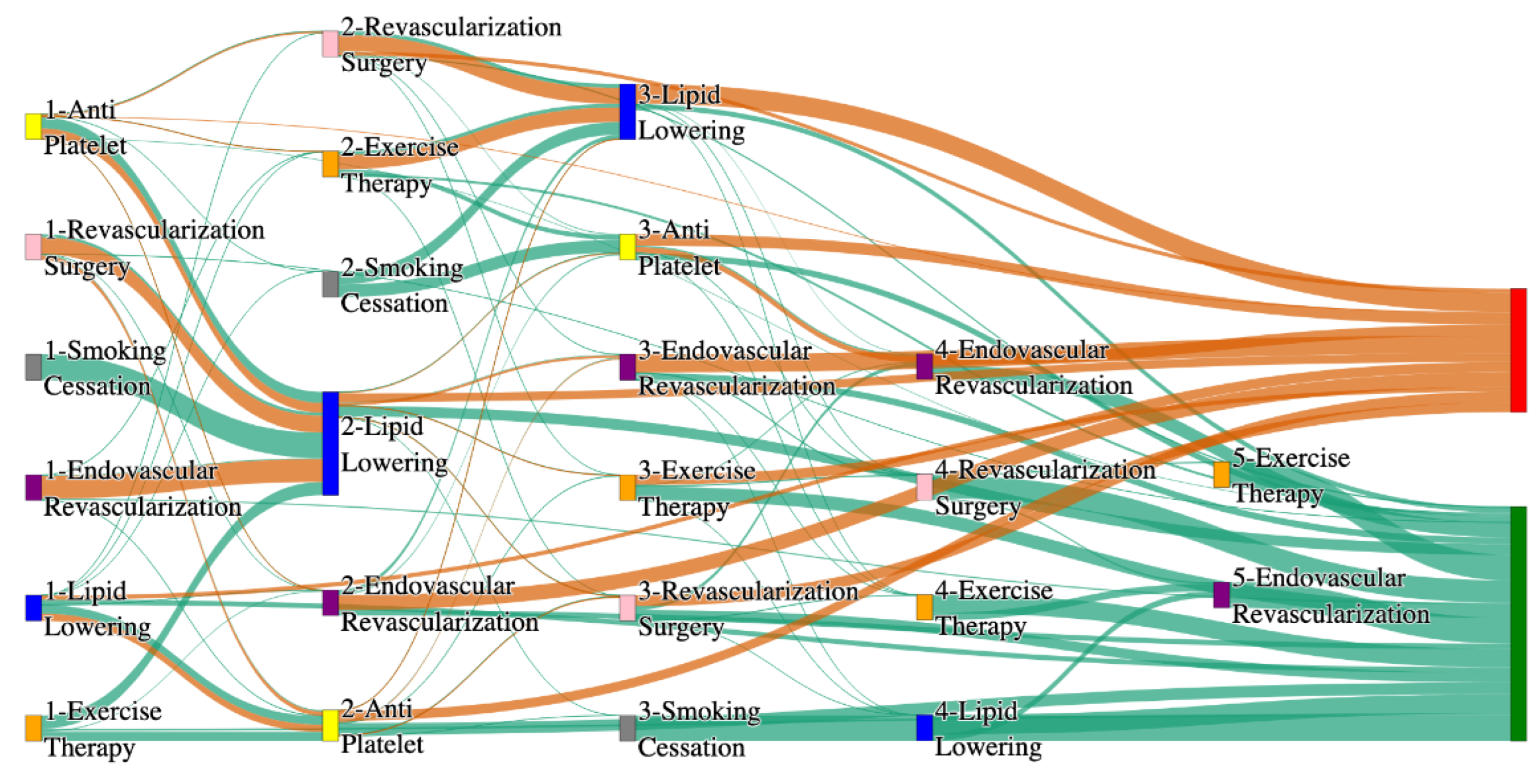
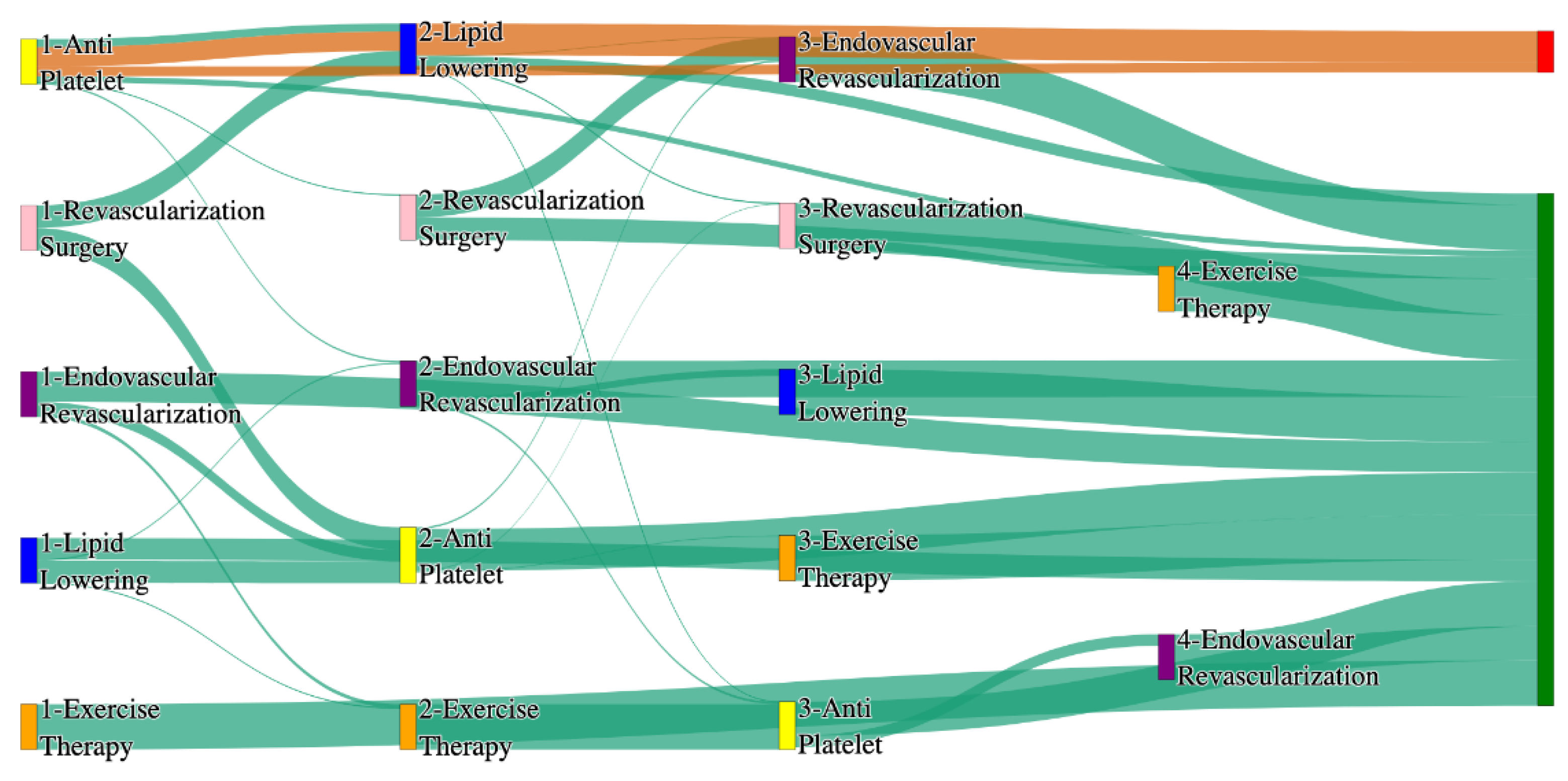
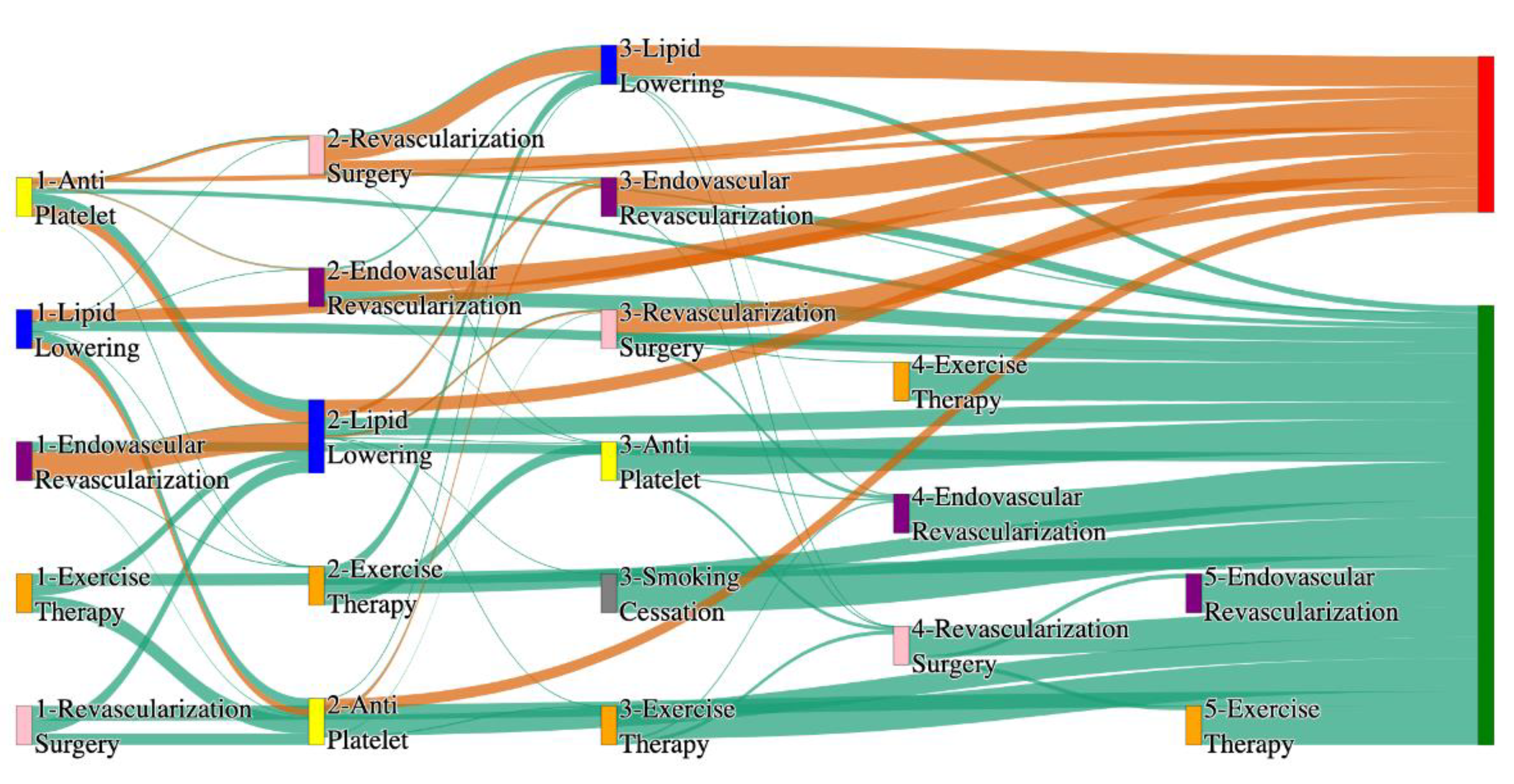
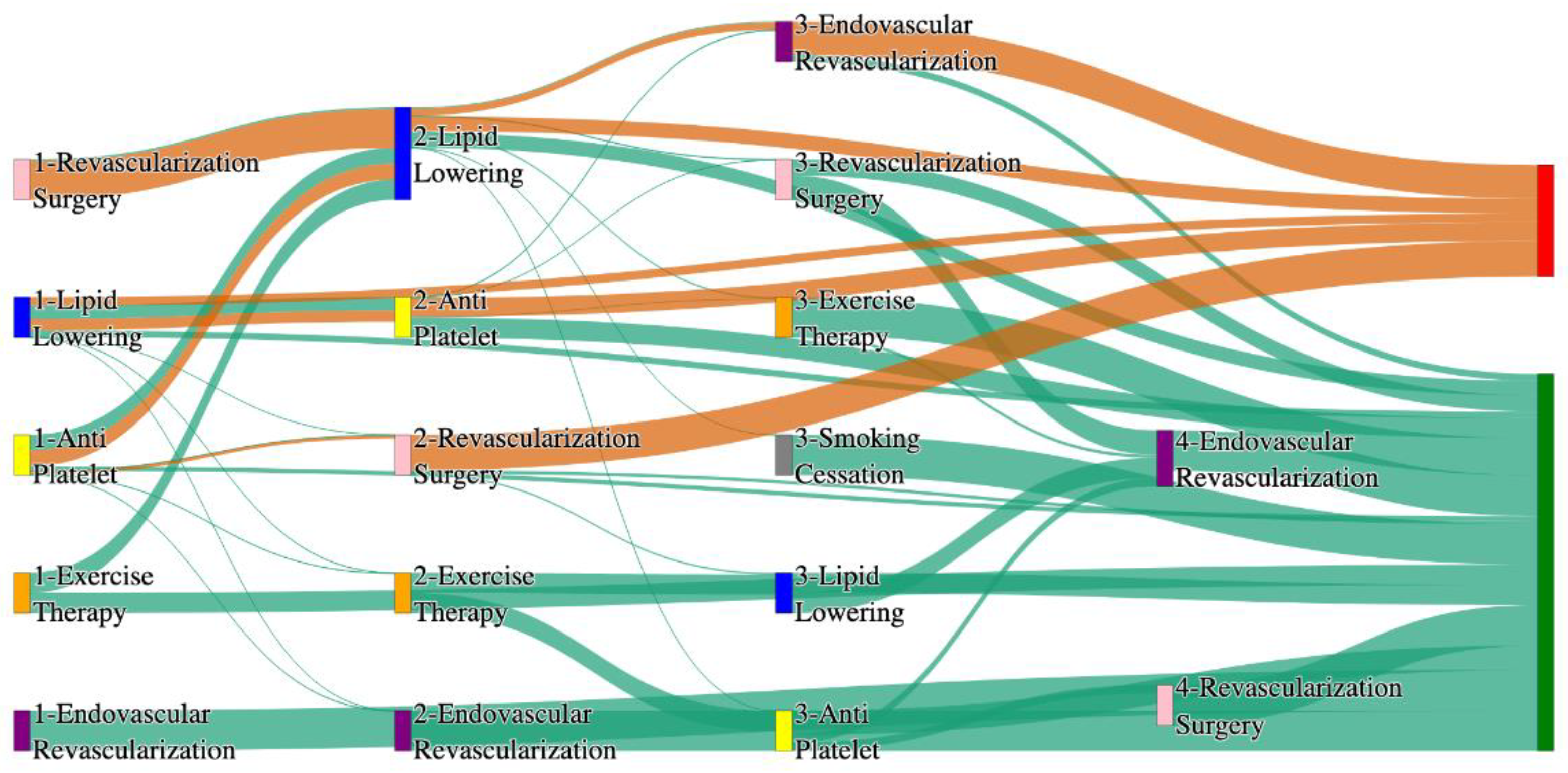
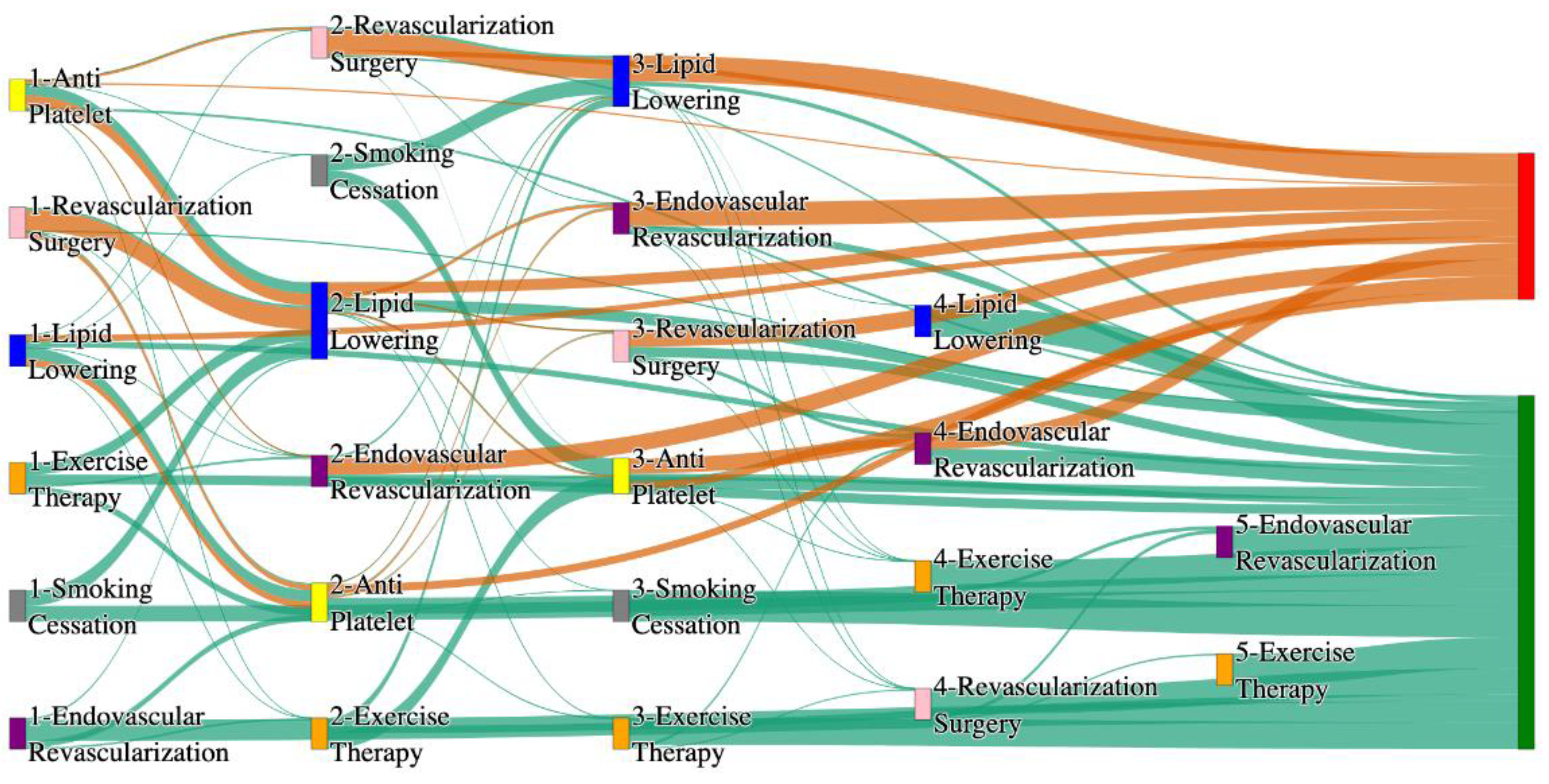
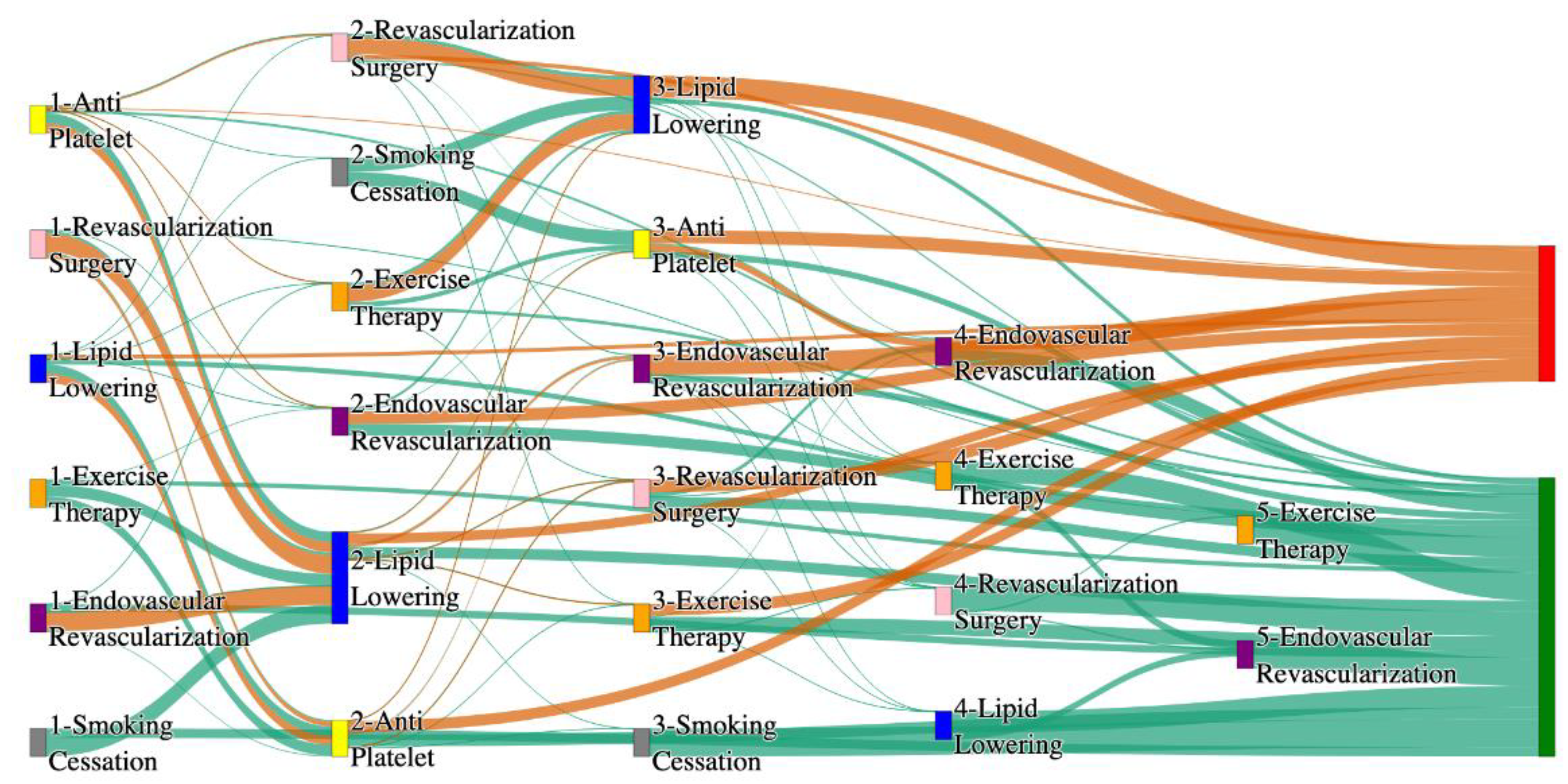
Figure 1.
Sankey plot for the STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section.
Figure 1.
Sankey plot for the STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section.
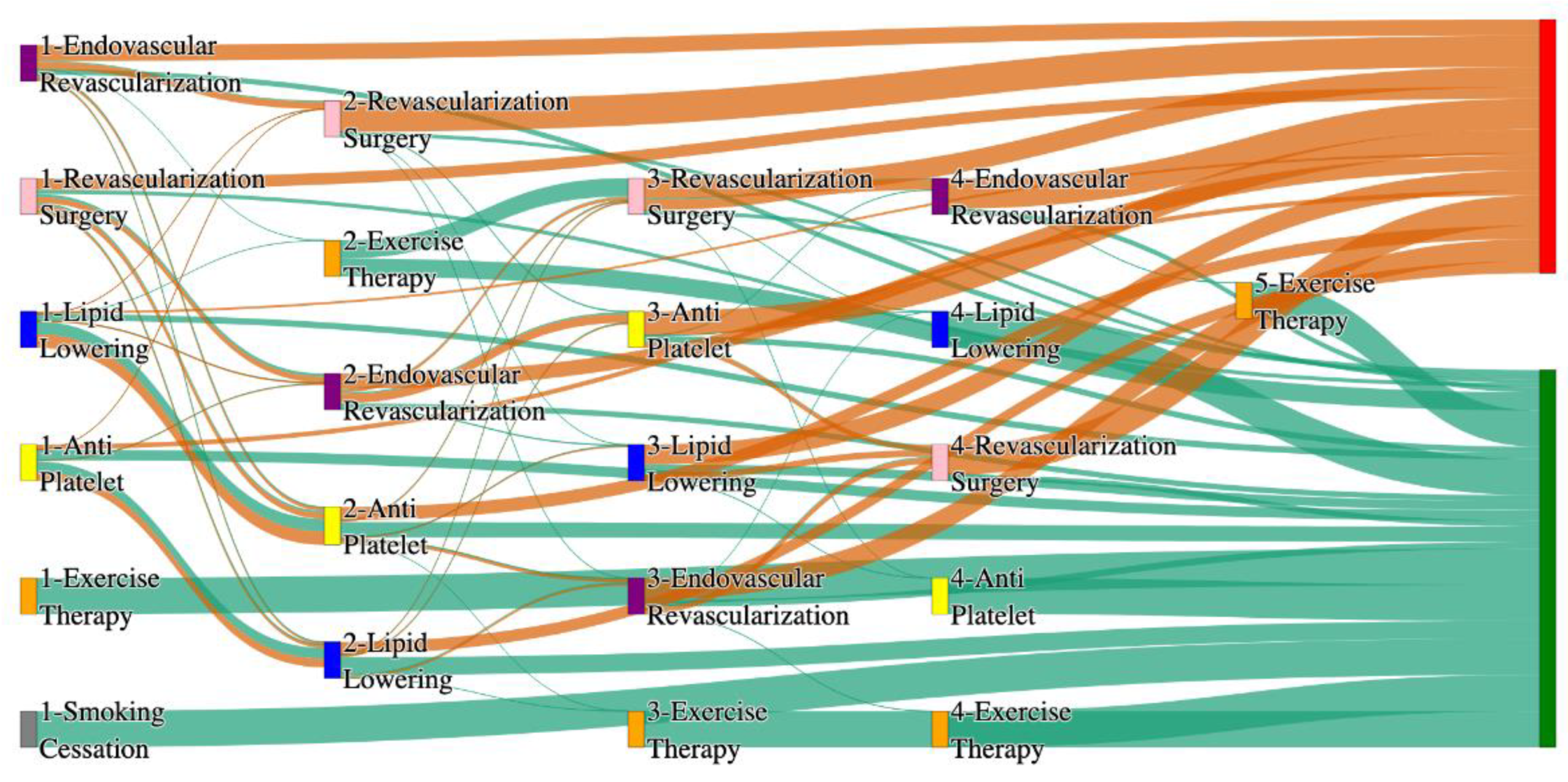
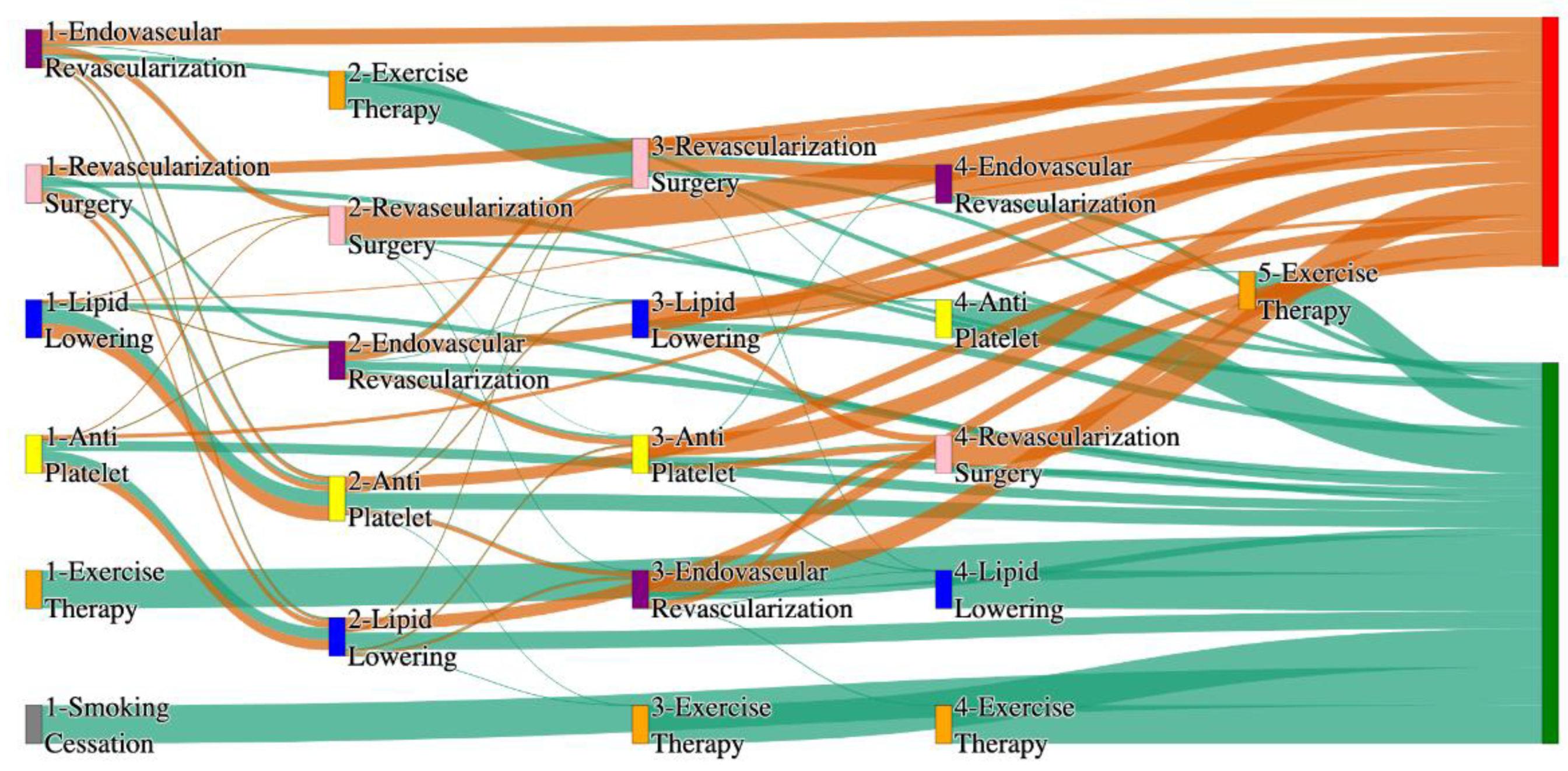
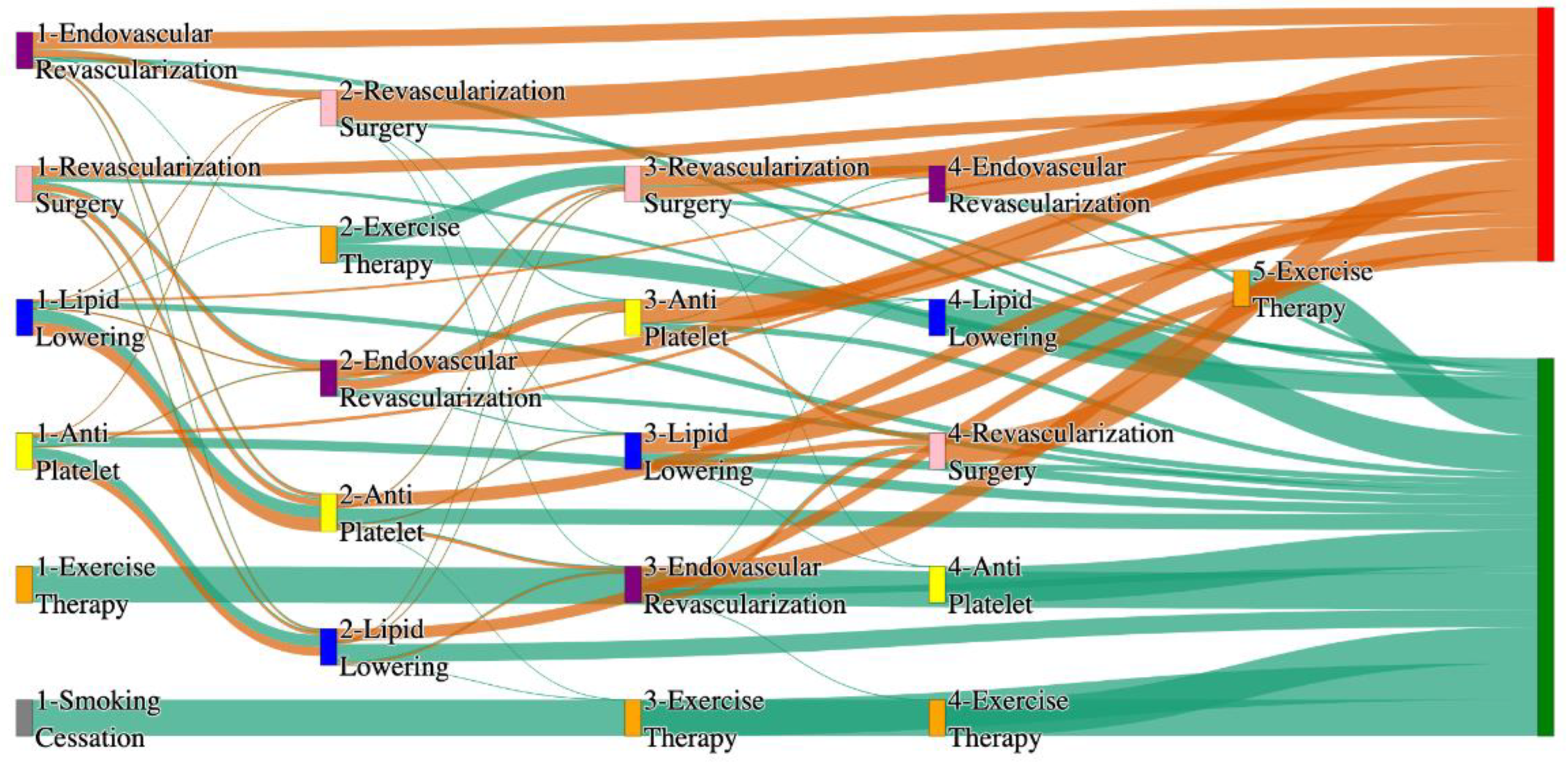
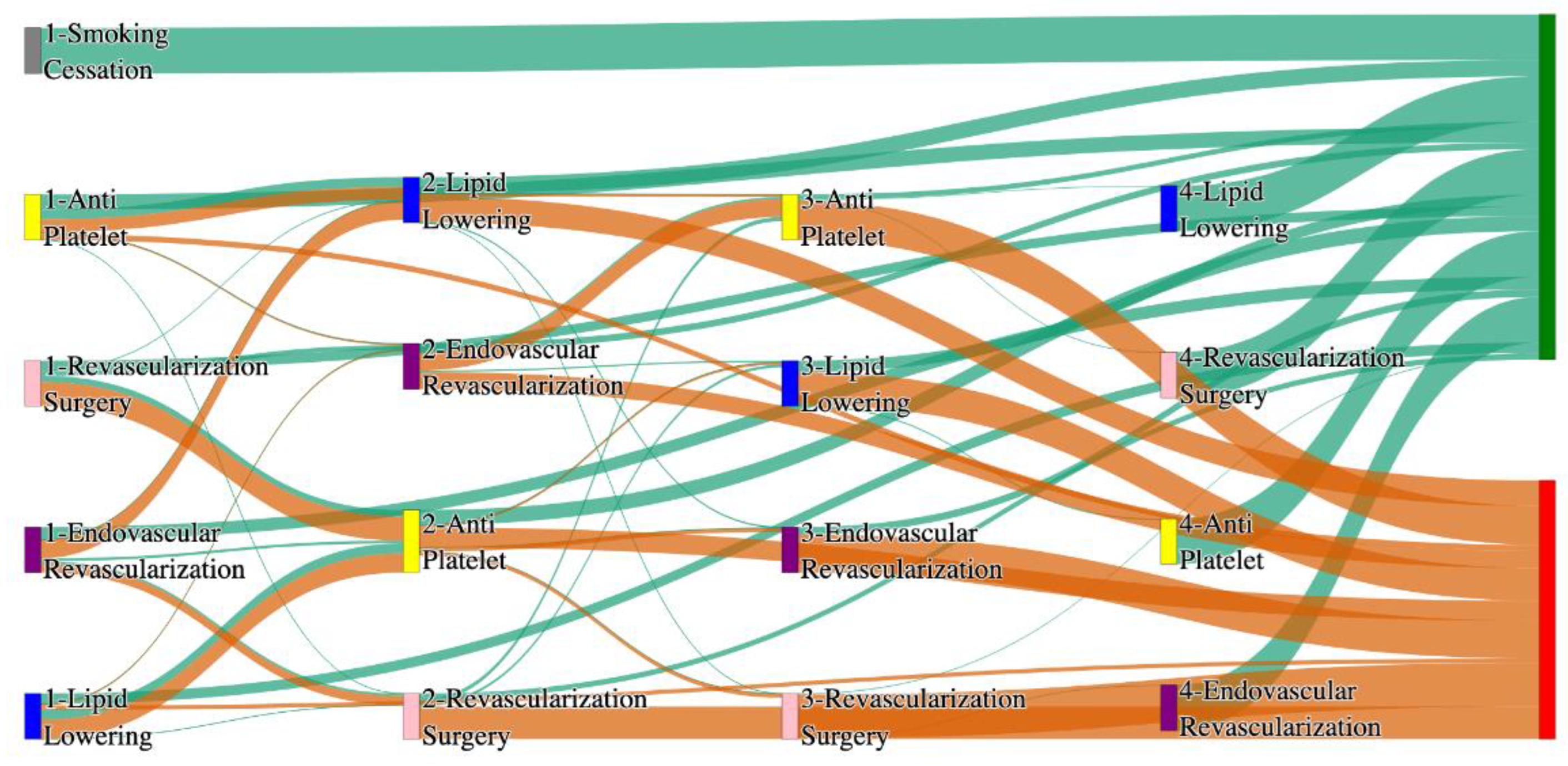
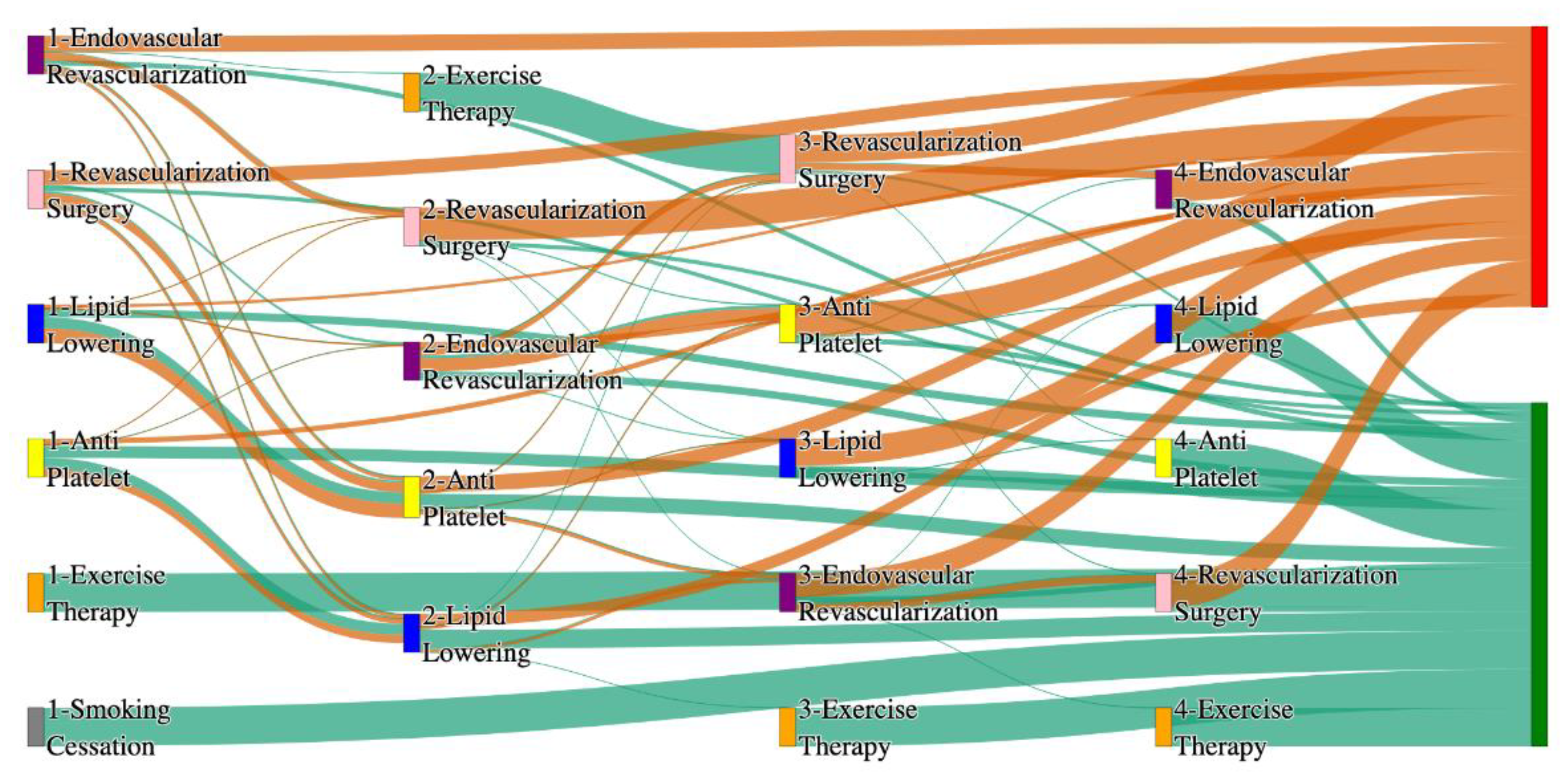
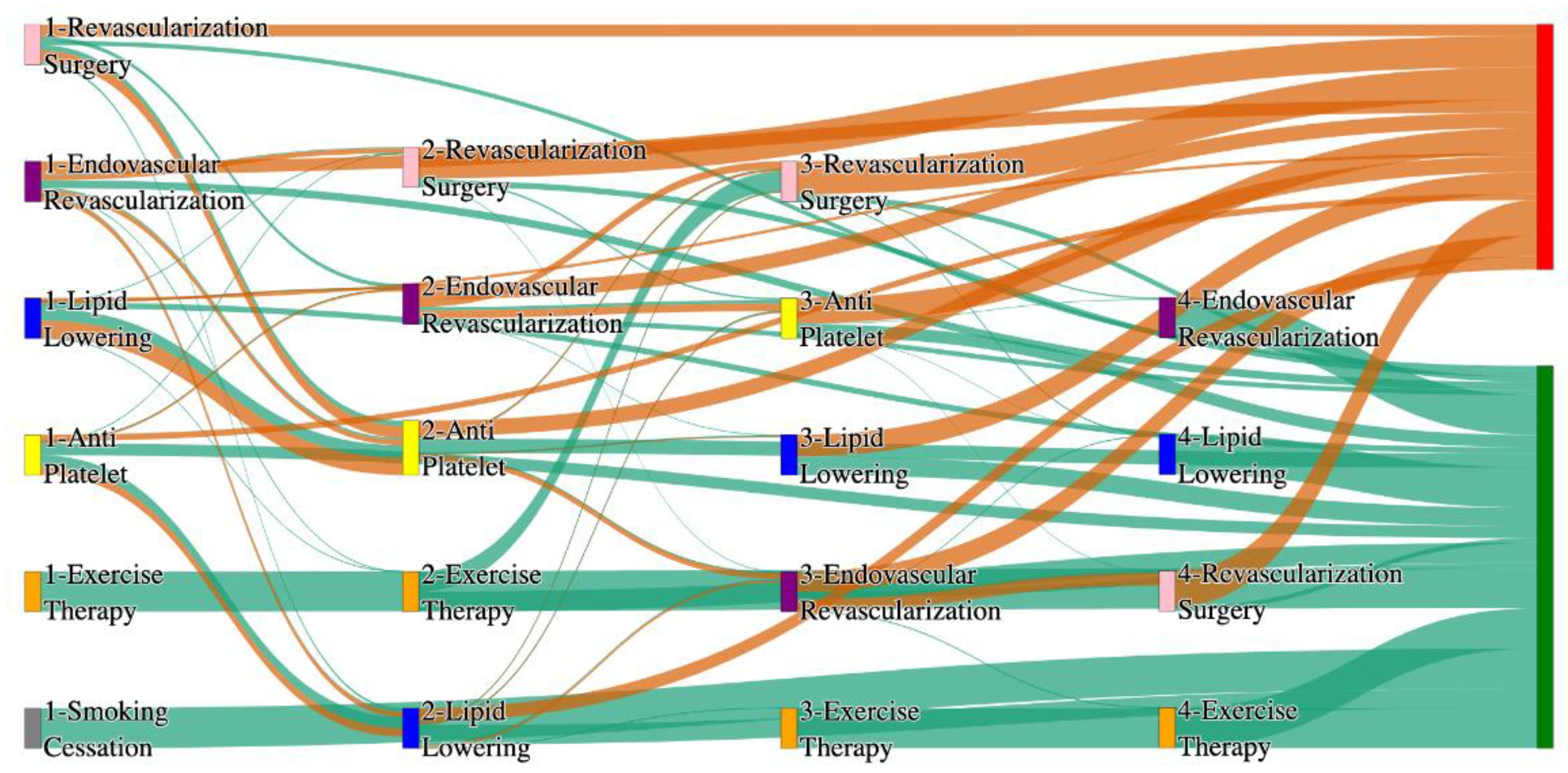
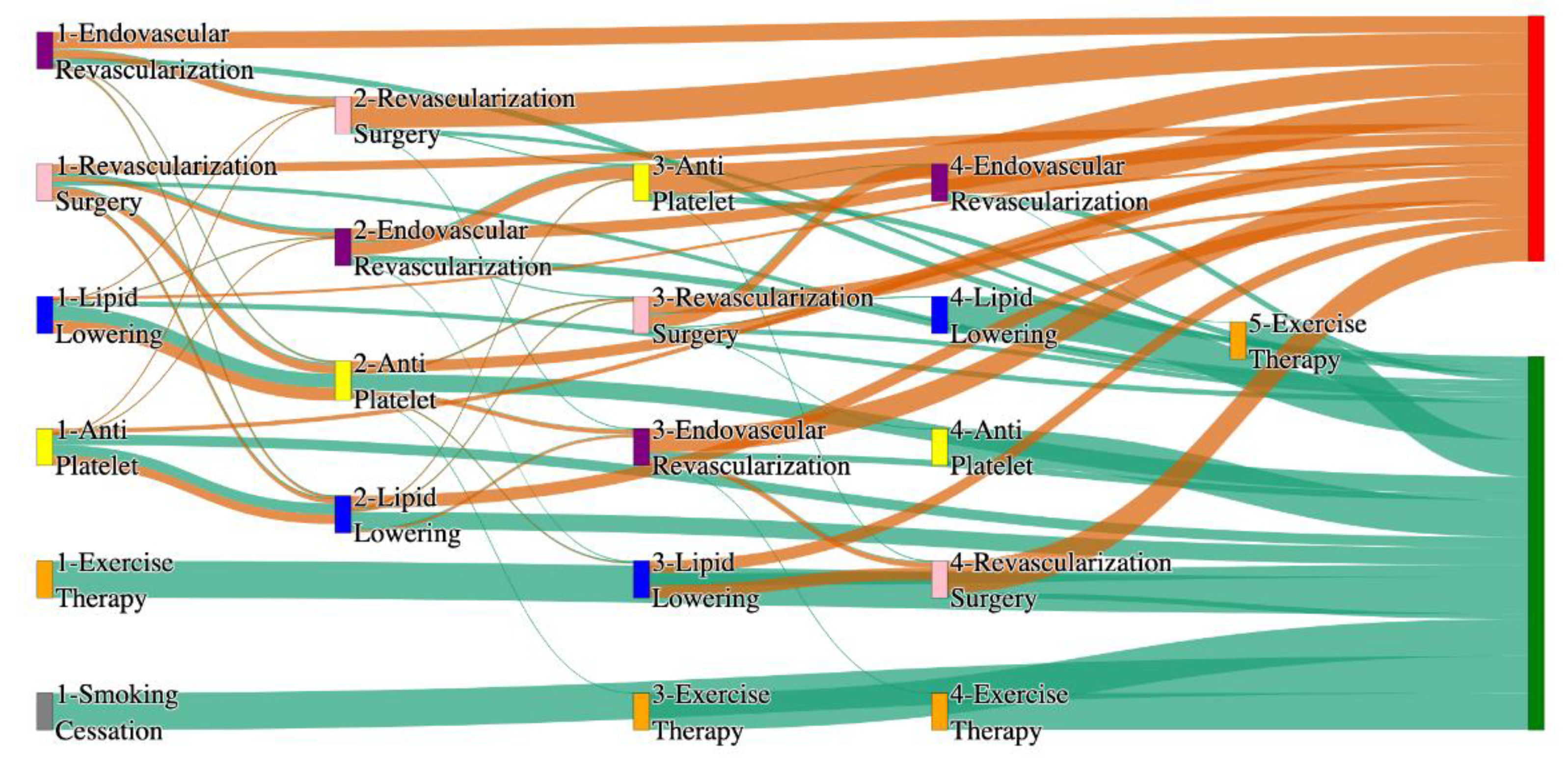
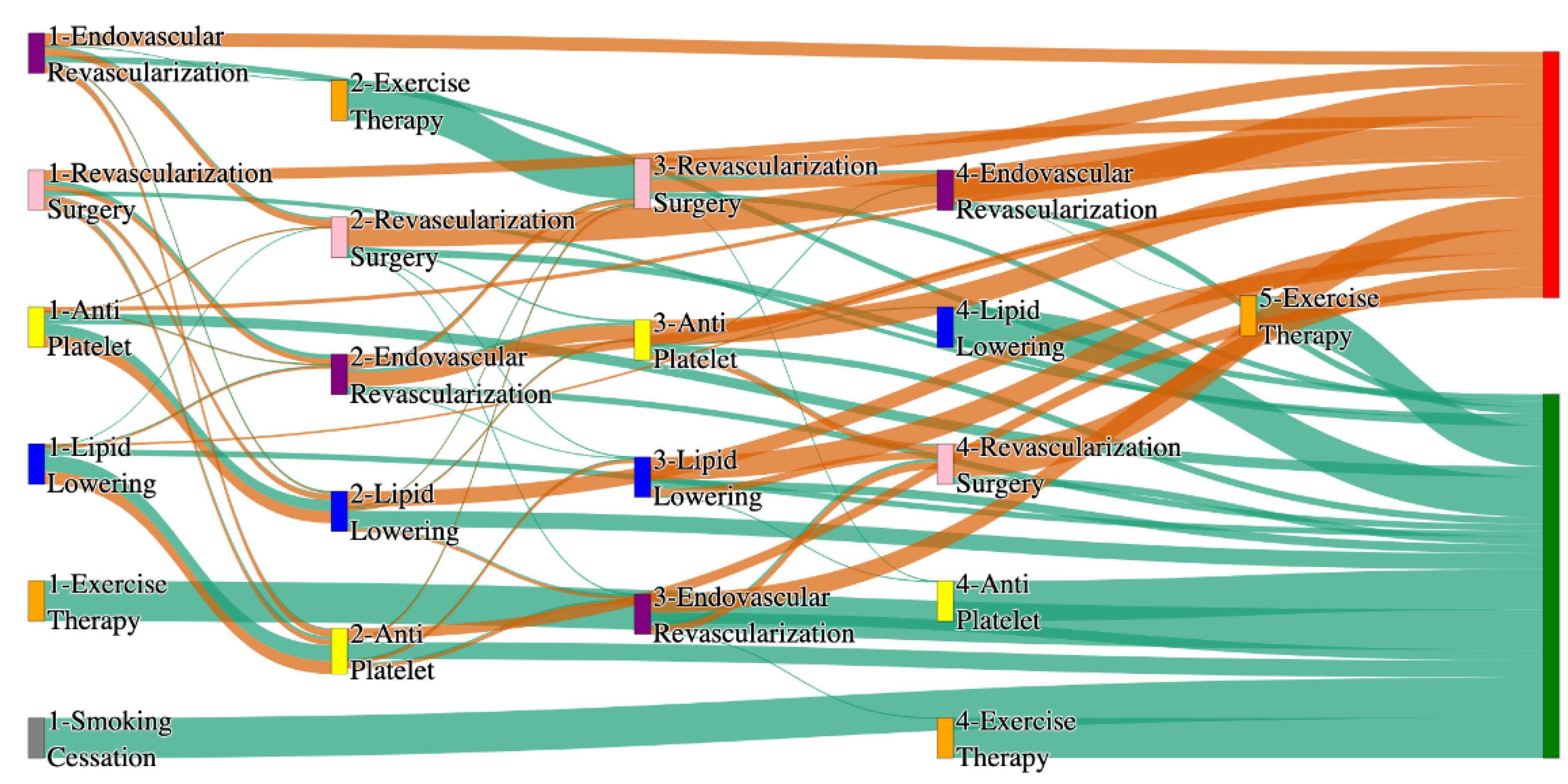
Figure 2.
Sankey plot for the All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 2.
Sankey plot for the All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
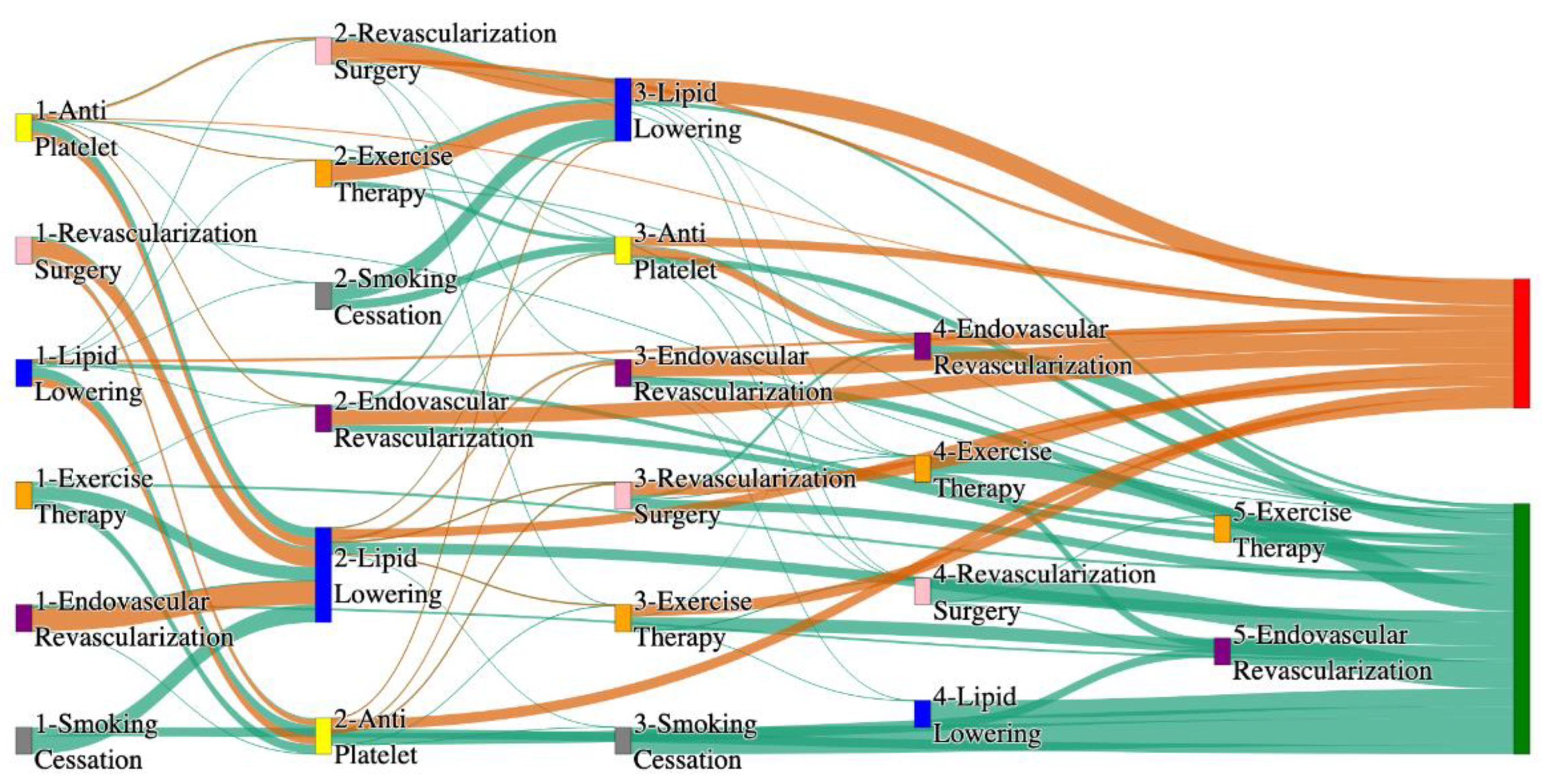
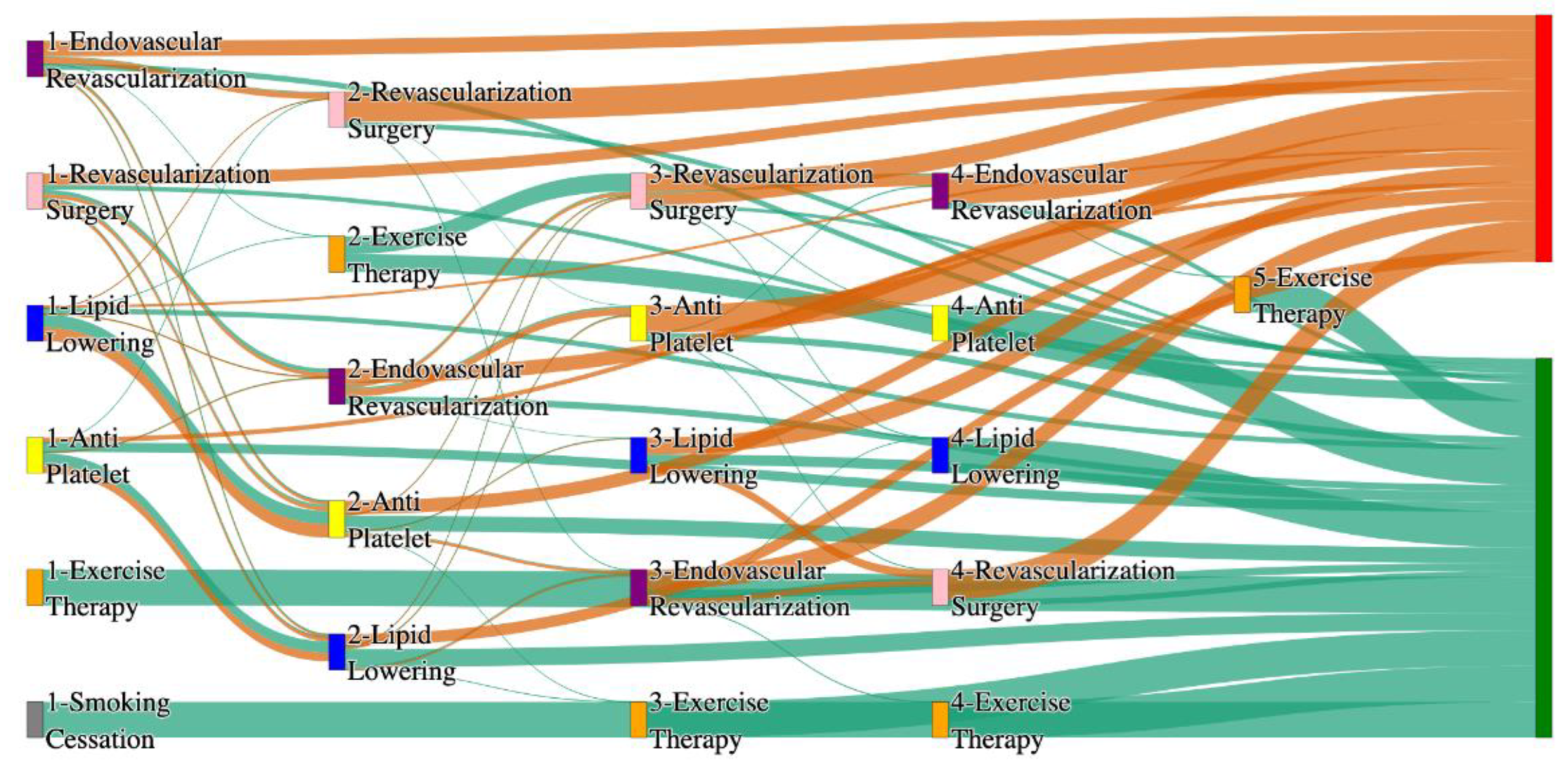
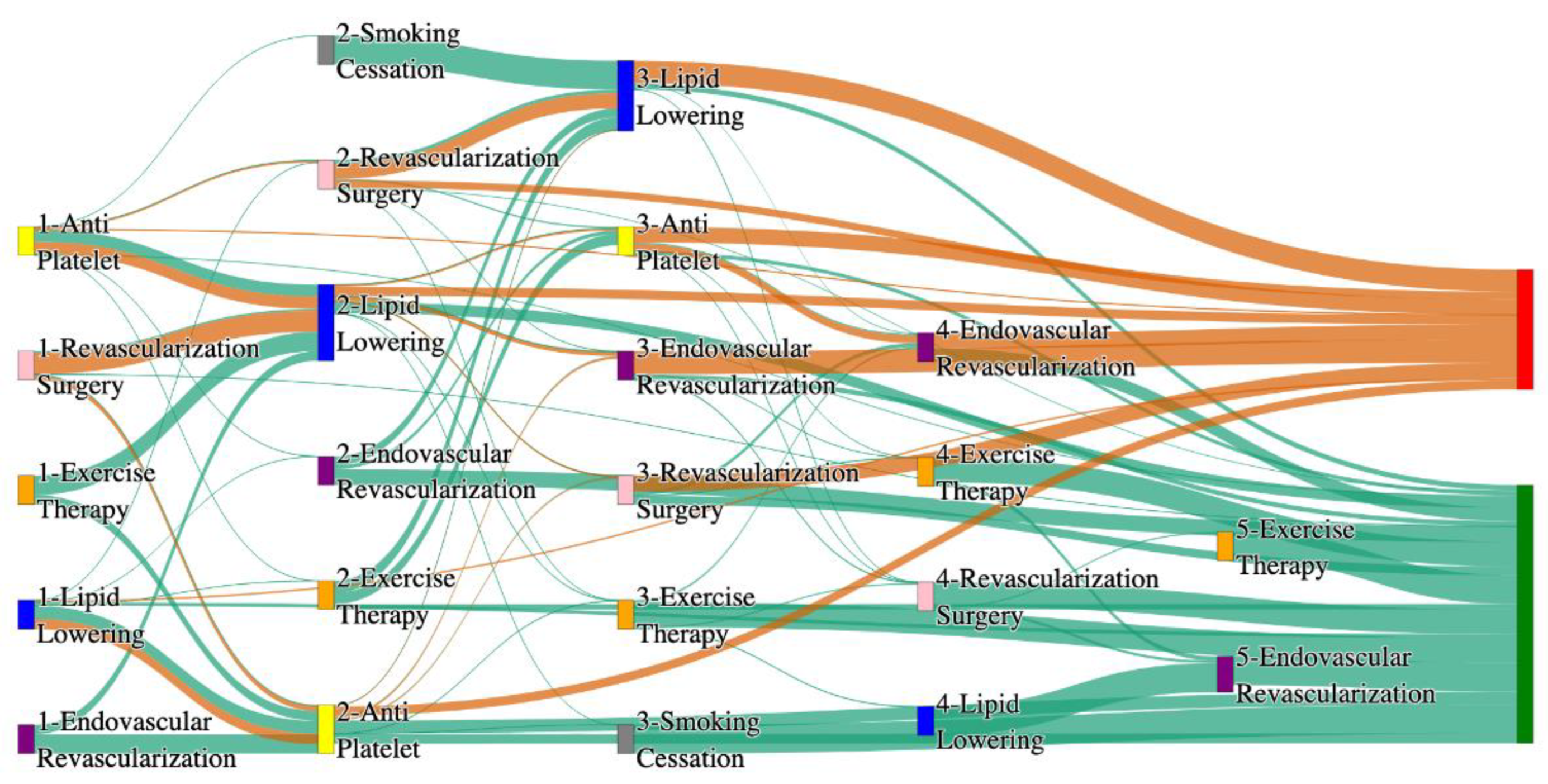
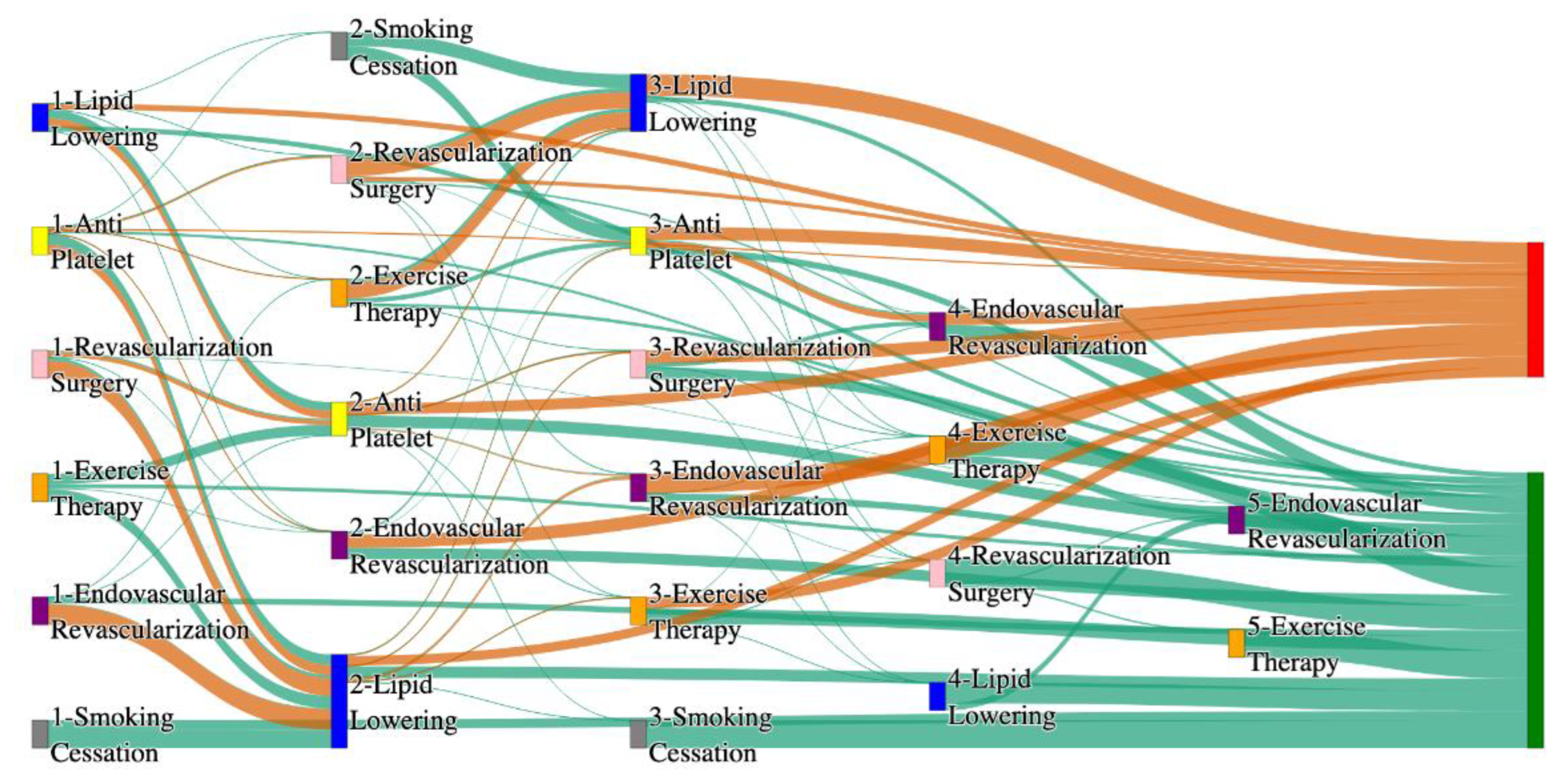
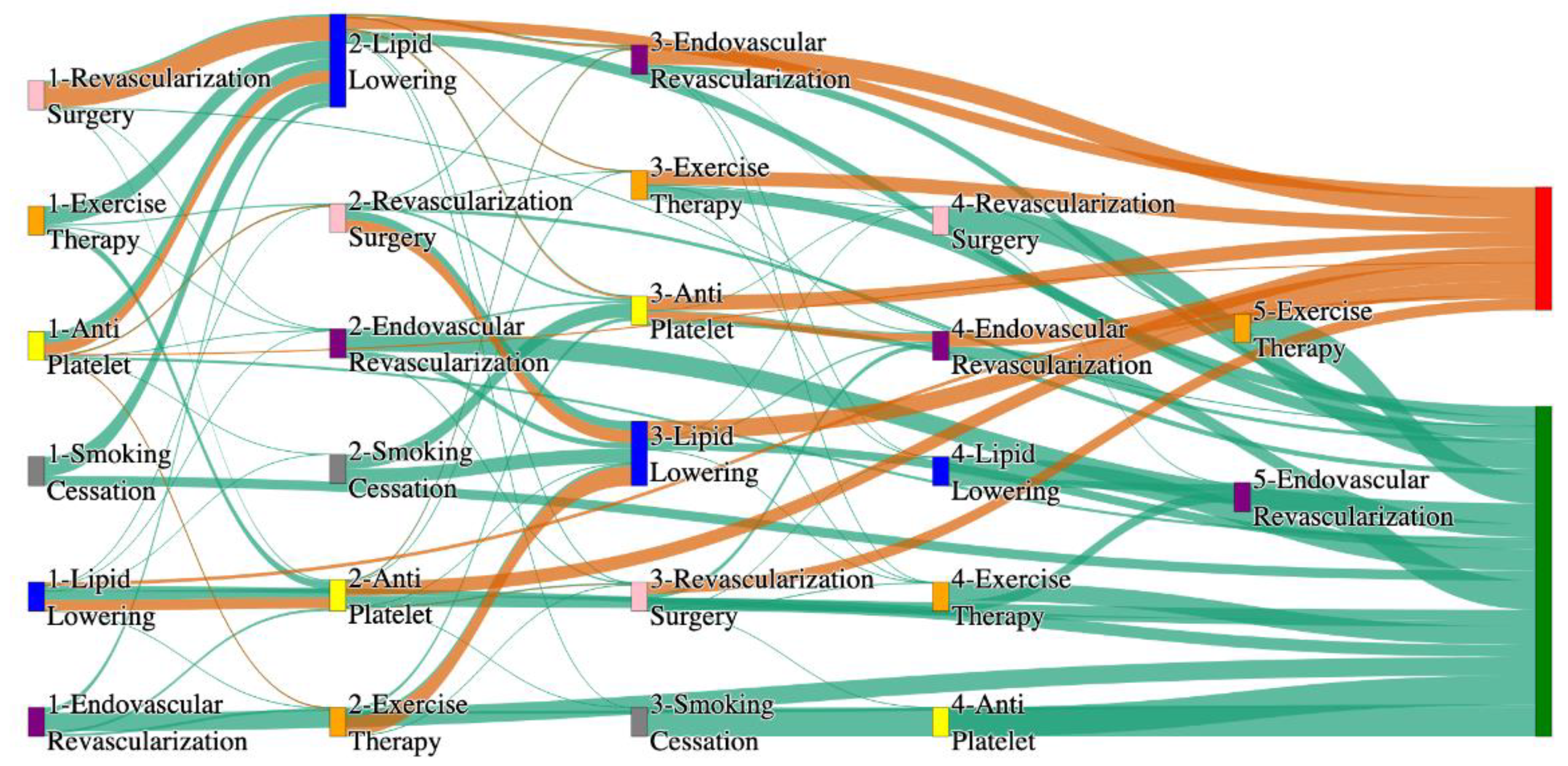
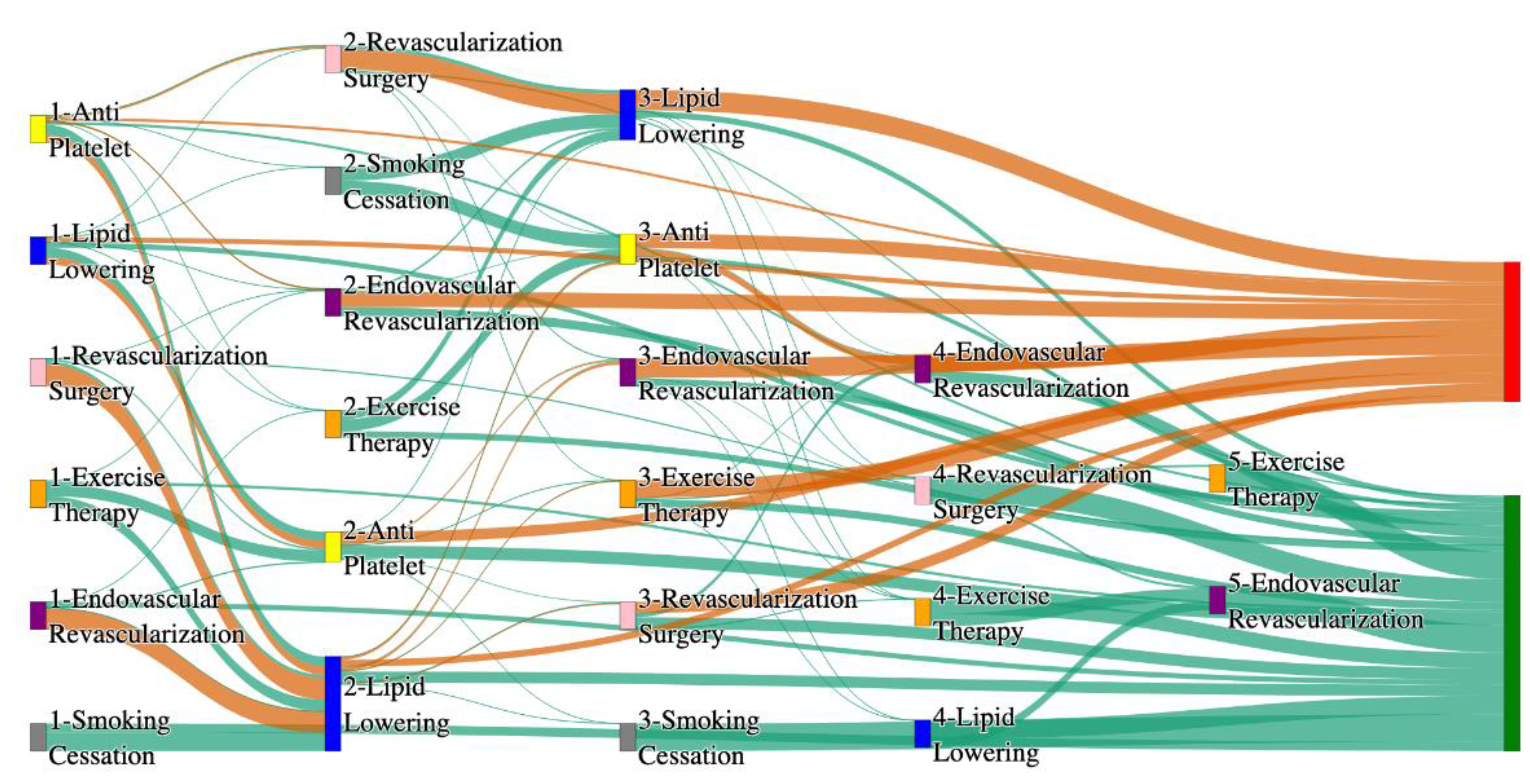
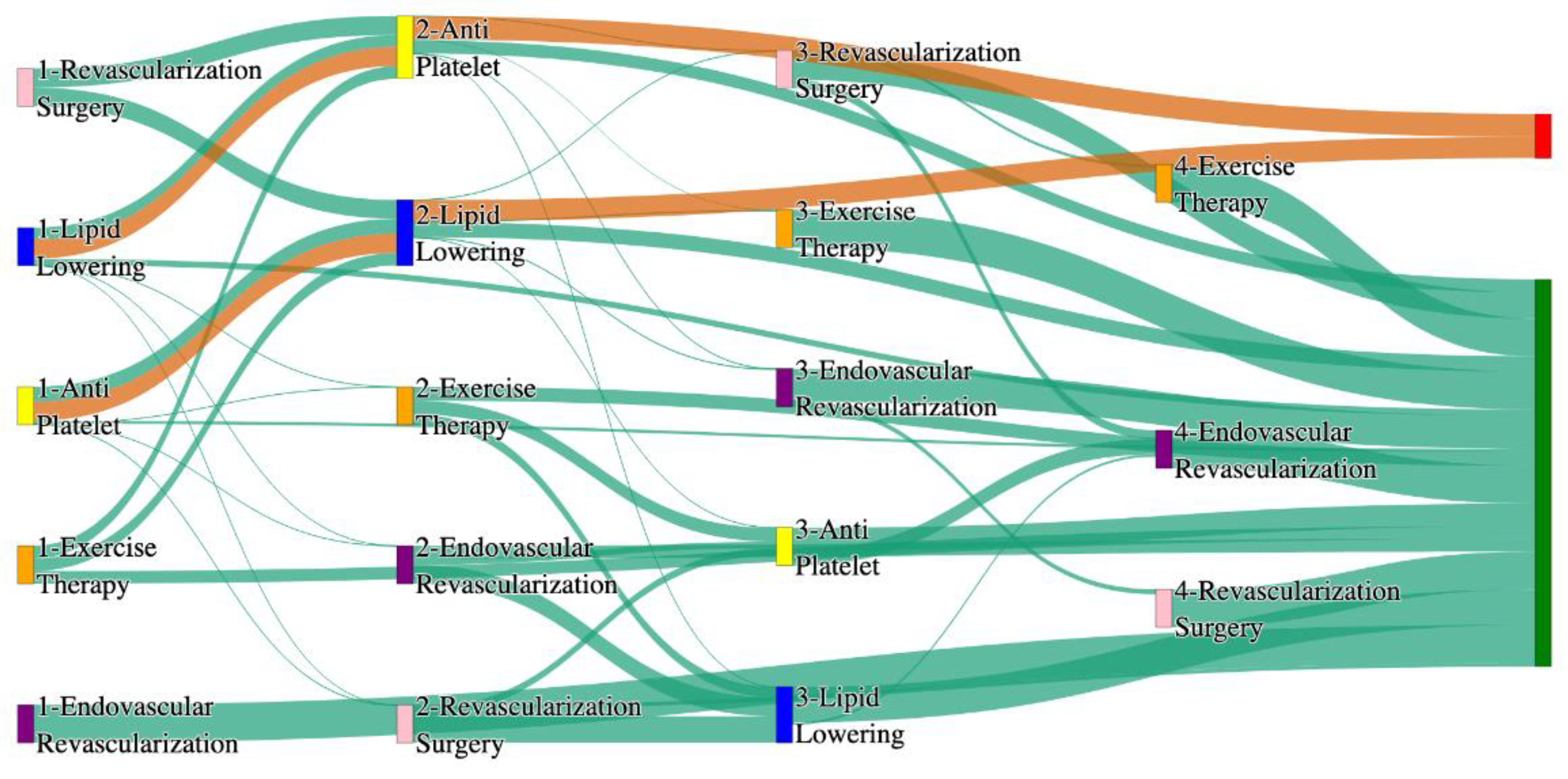
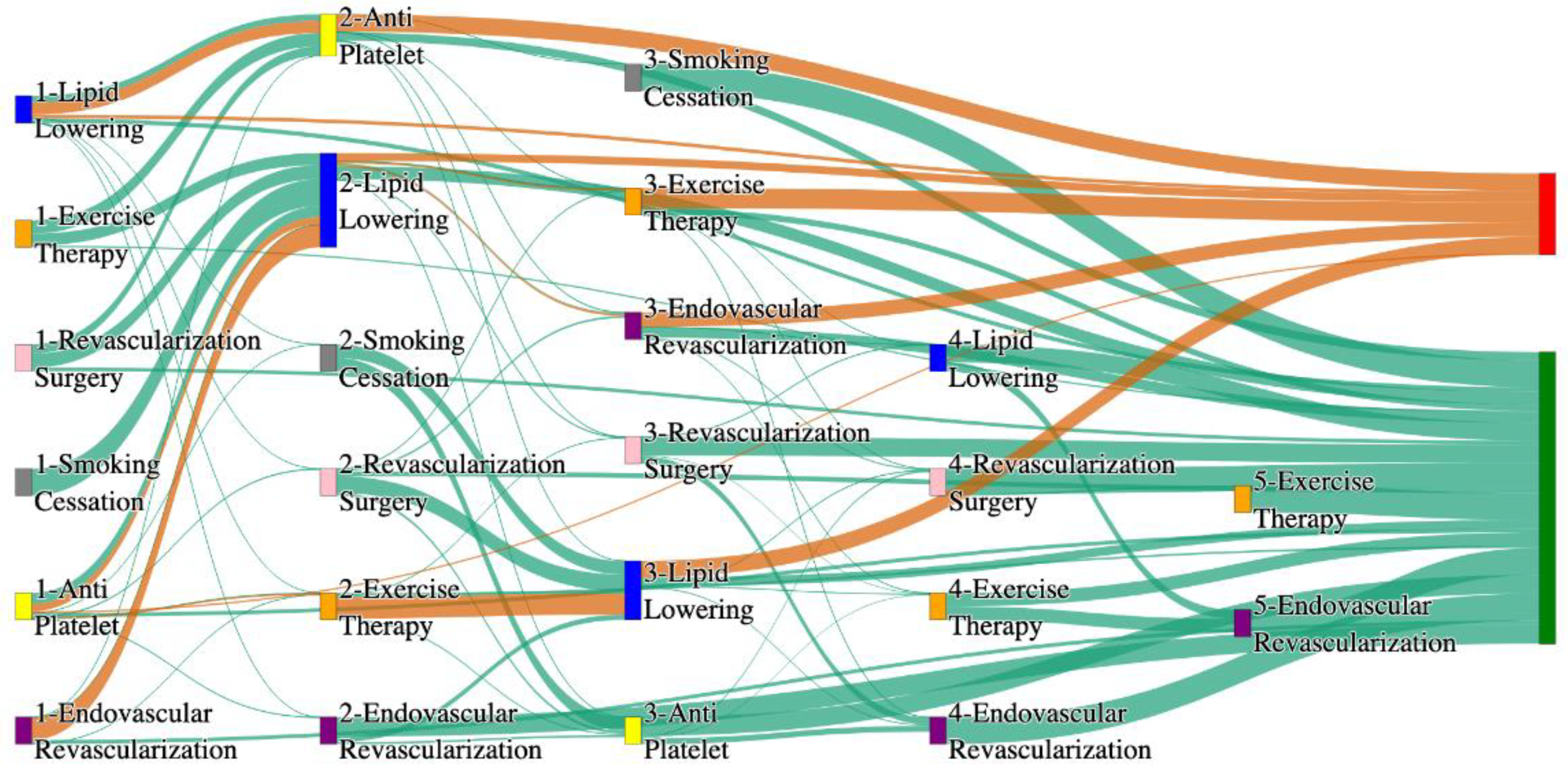
Figure 3.
Sankey plot for the PAD with Hypertension in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 3.
Sankey plot for the PAD with Hypertension in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
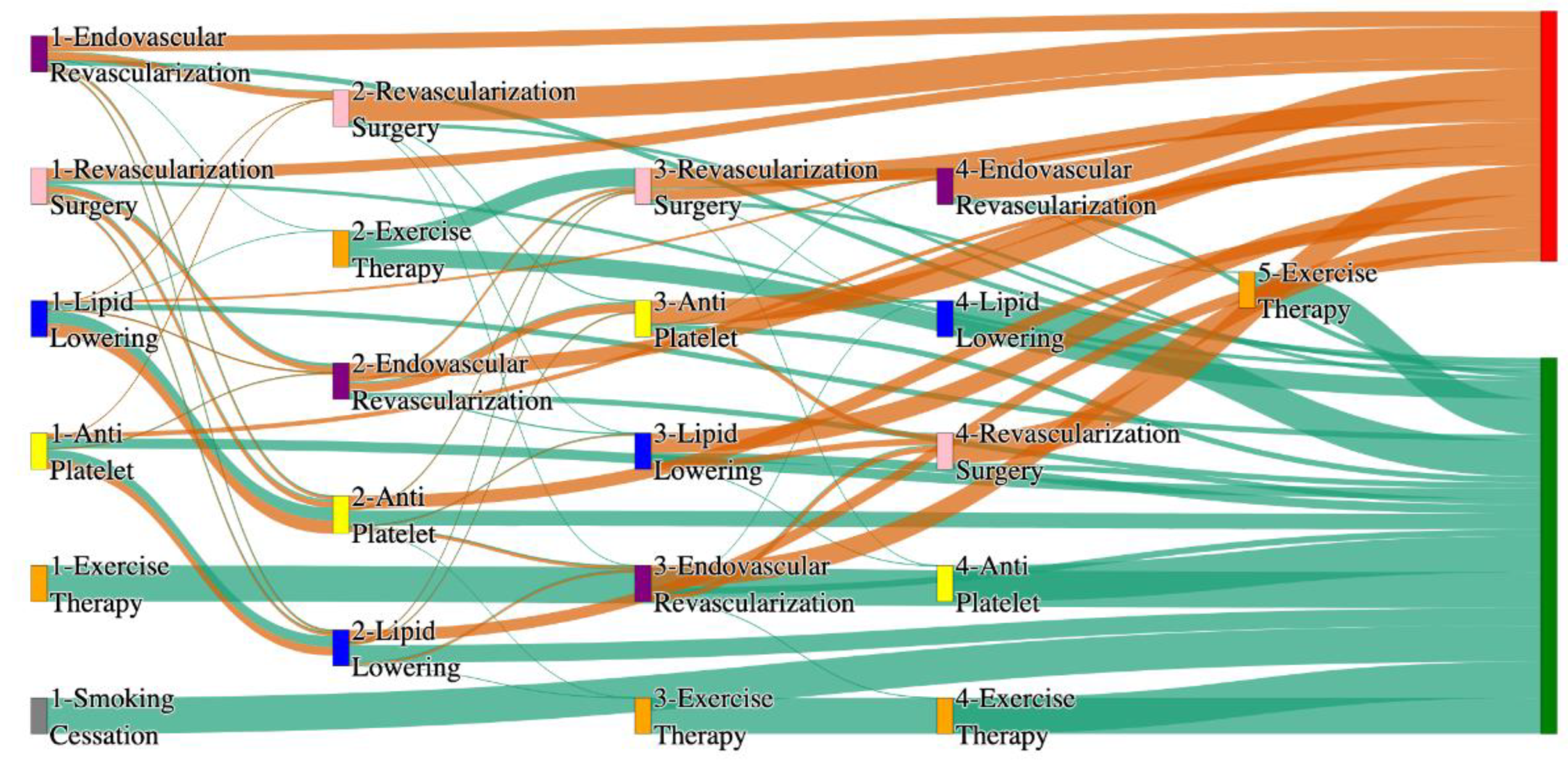
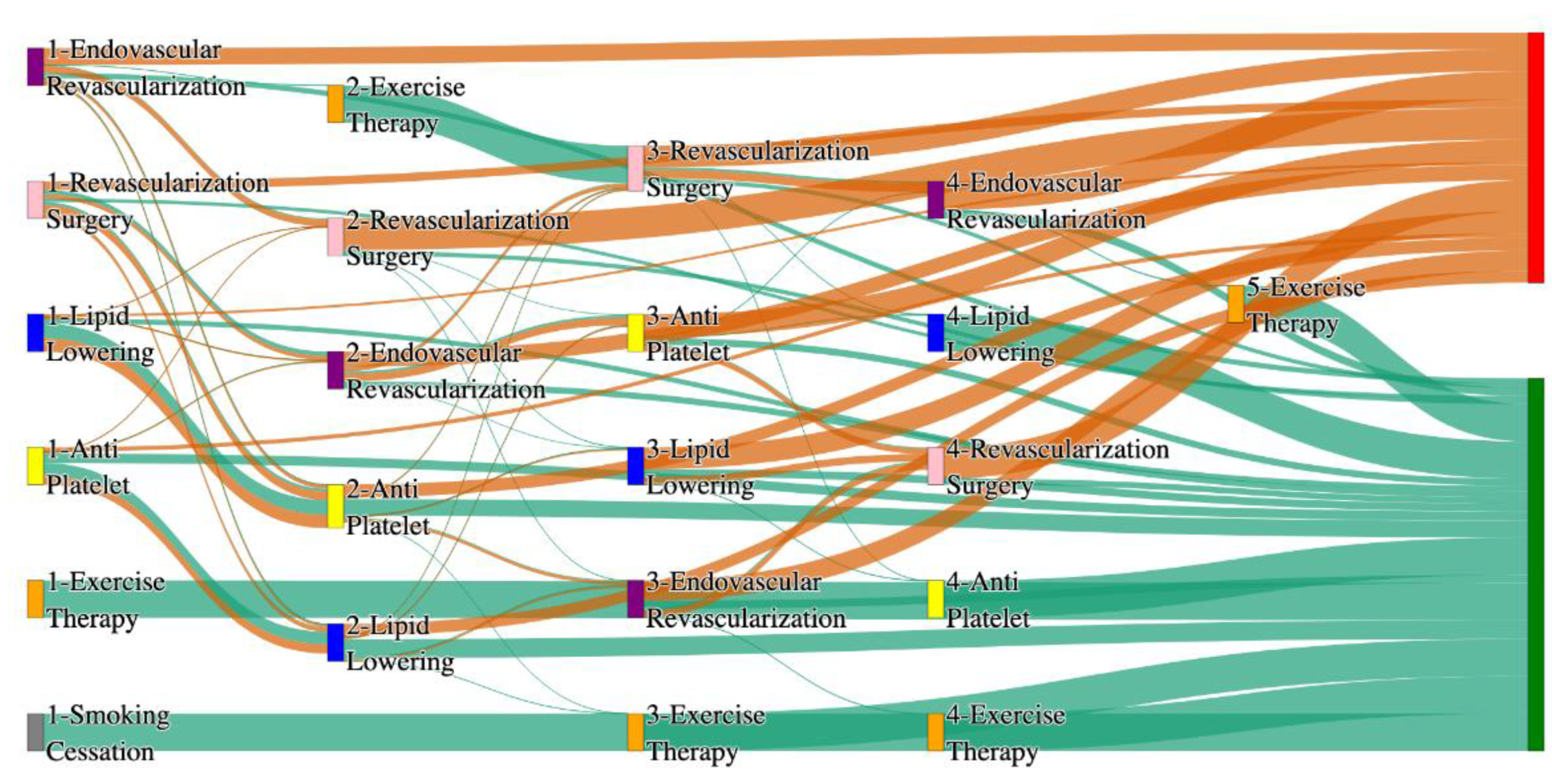
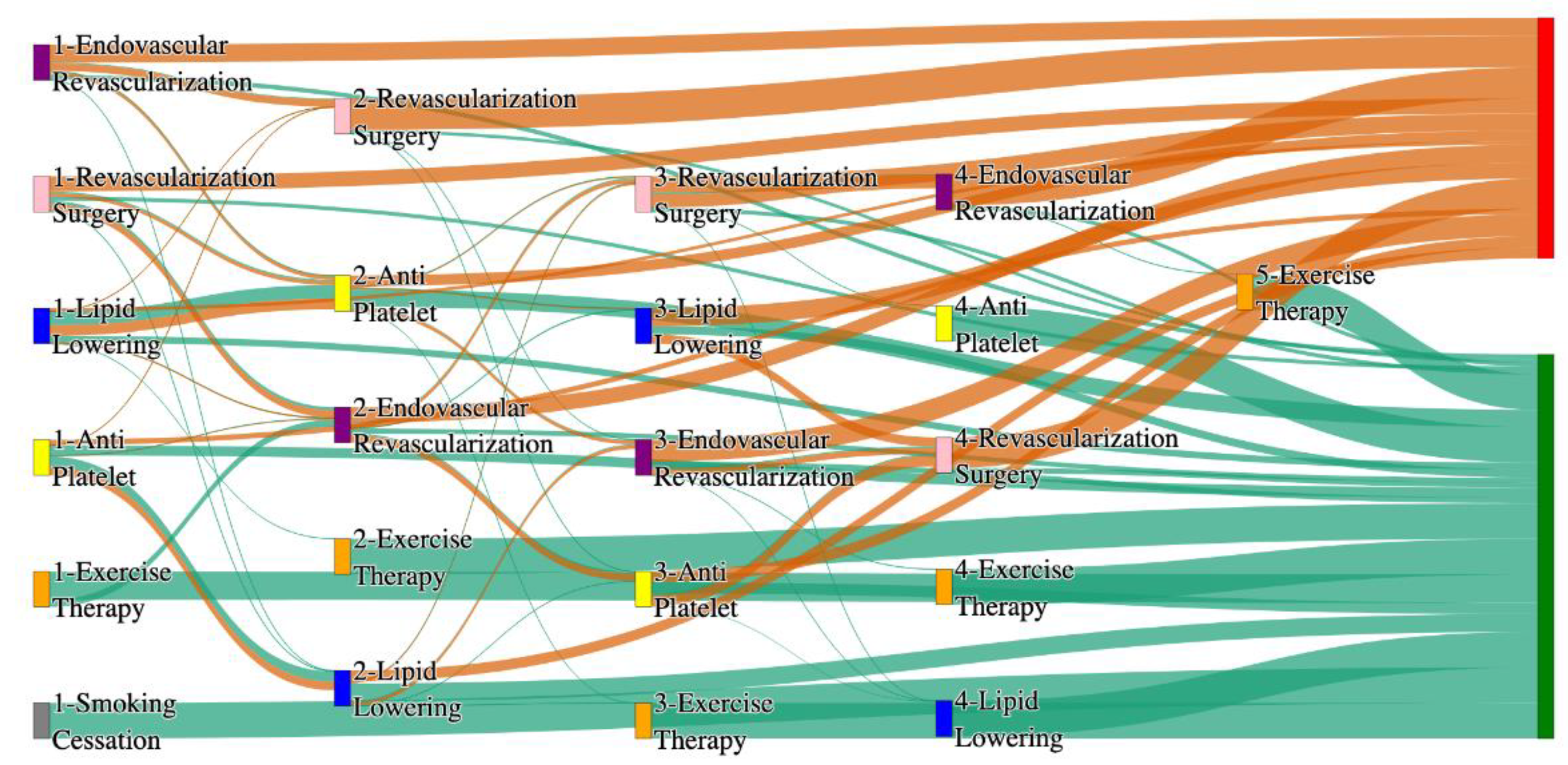
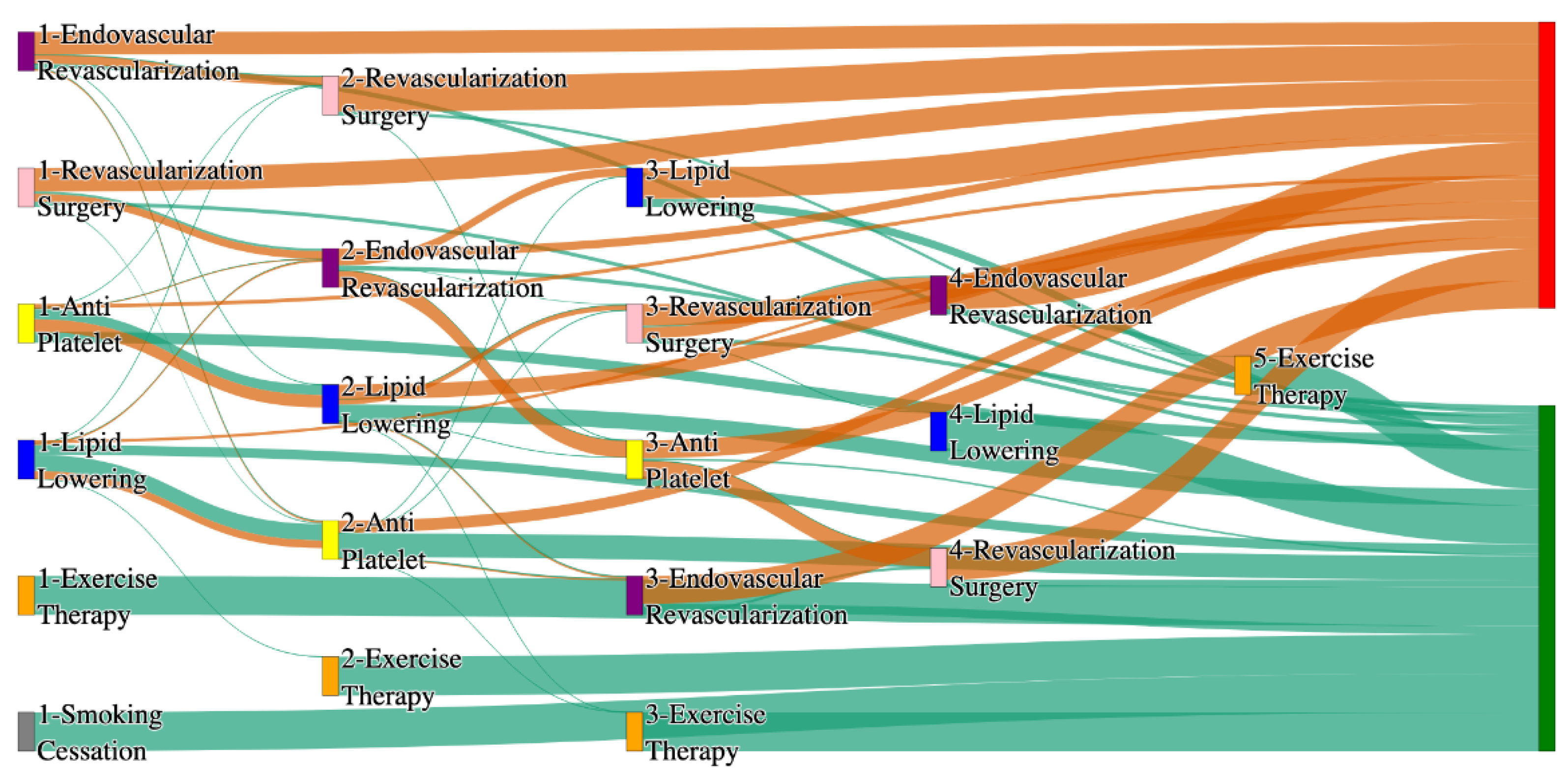
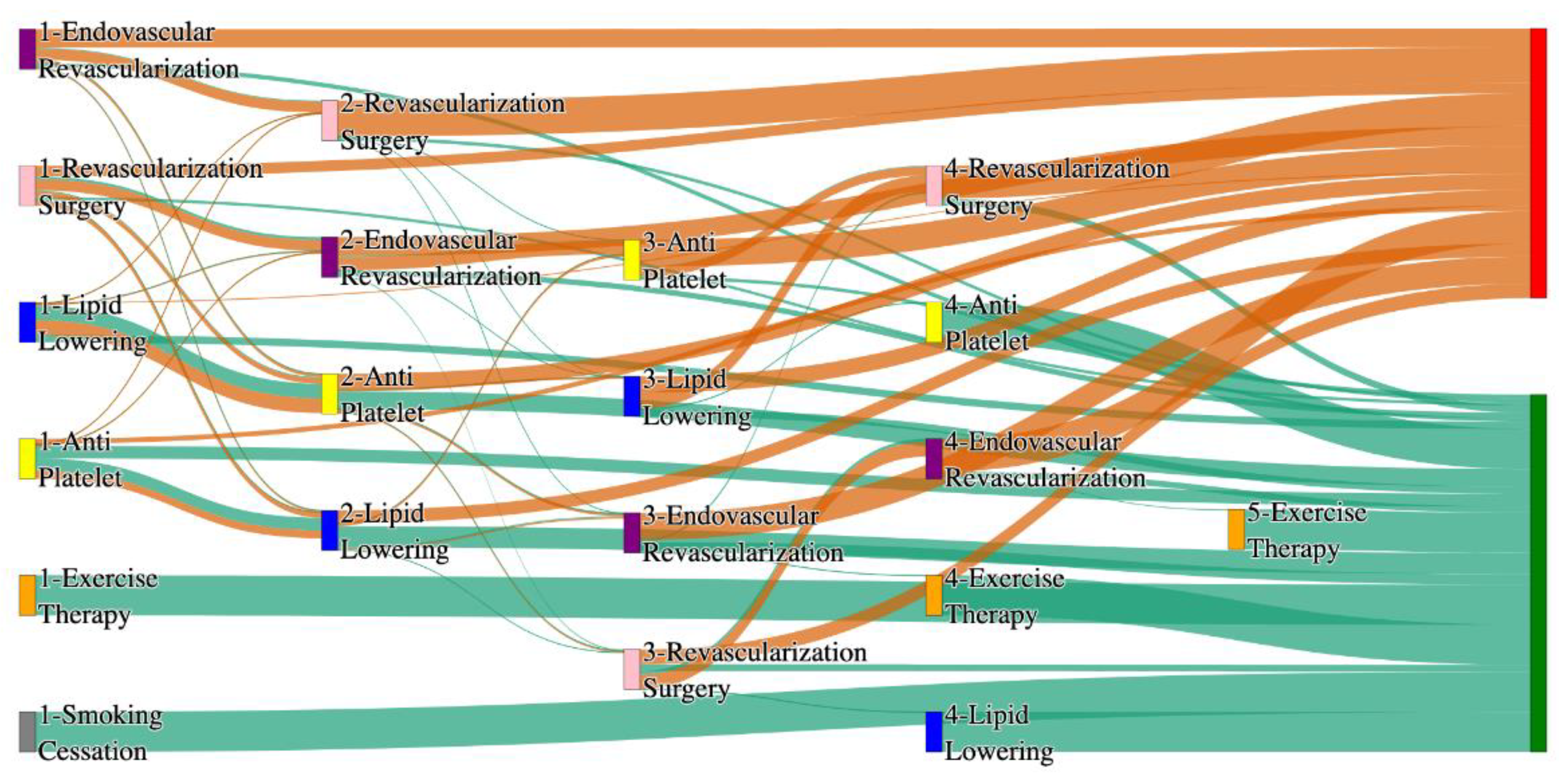
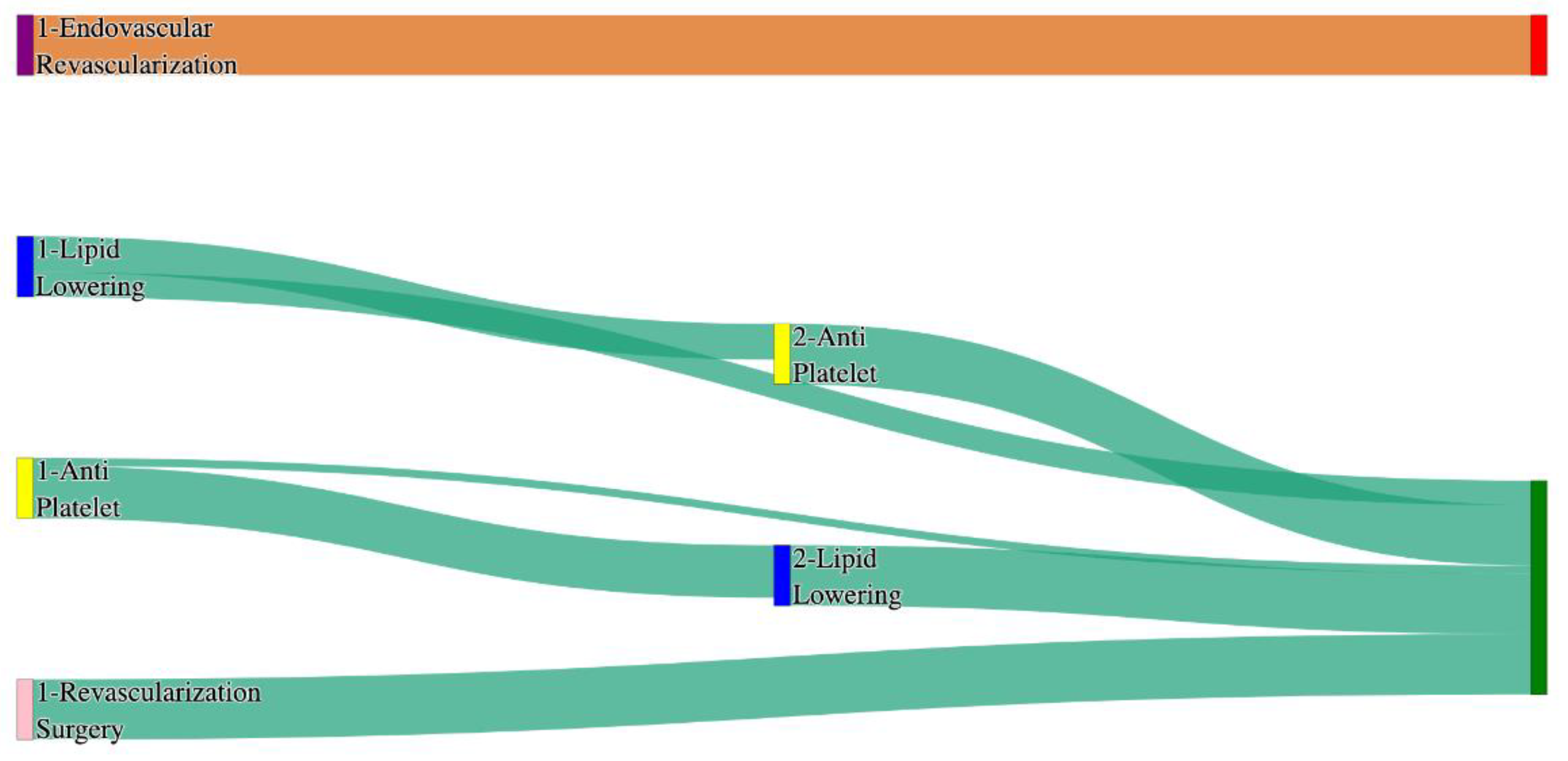
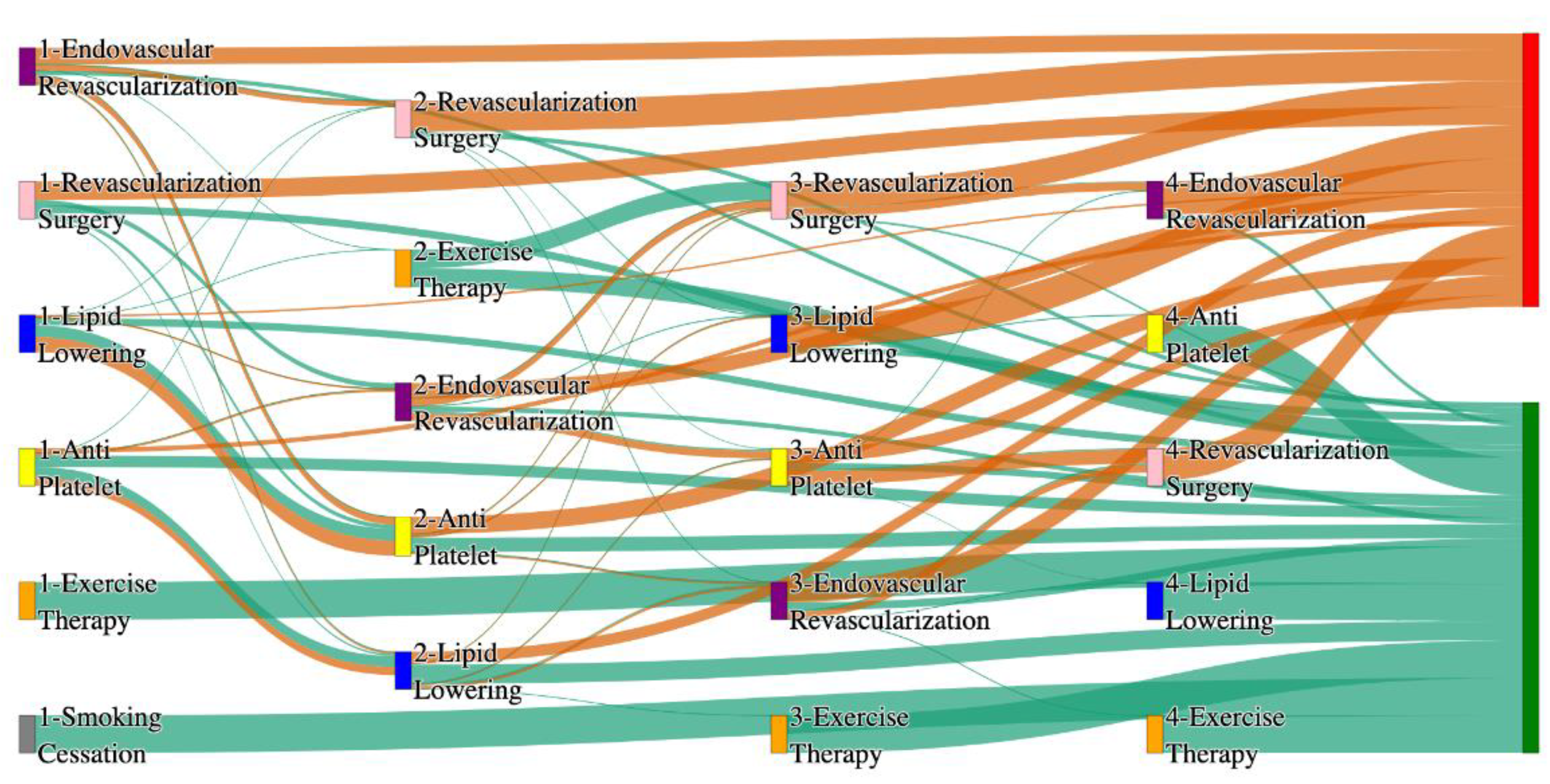
Figure 4.
Sankey plot for the PAD with Hypertension in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section . Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 4.
Sankey plot for the PAD with Hypertension in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section . Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
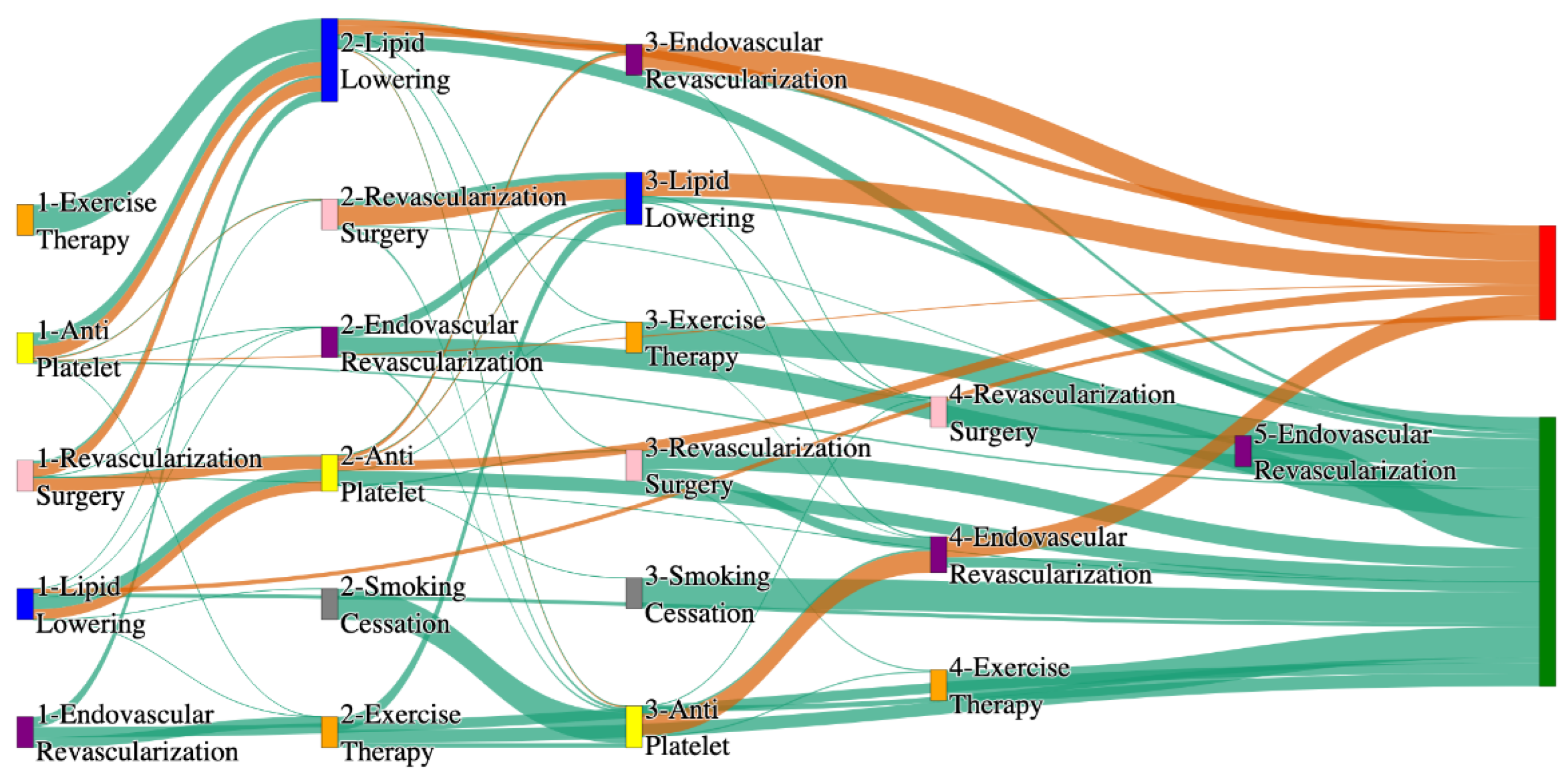
Figure 5.
Sankey plot for the PAD with Diabetes in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 5.
Sankey plot for the PAD with Diabetes in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 6.
Sankey plot for the PAD with Diabetes in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 6.
Sankey plot for the PAD with Diabetes in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
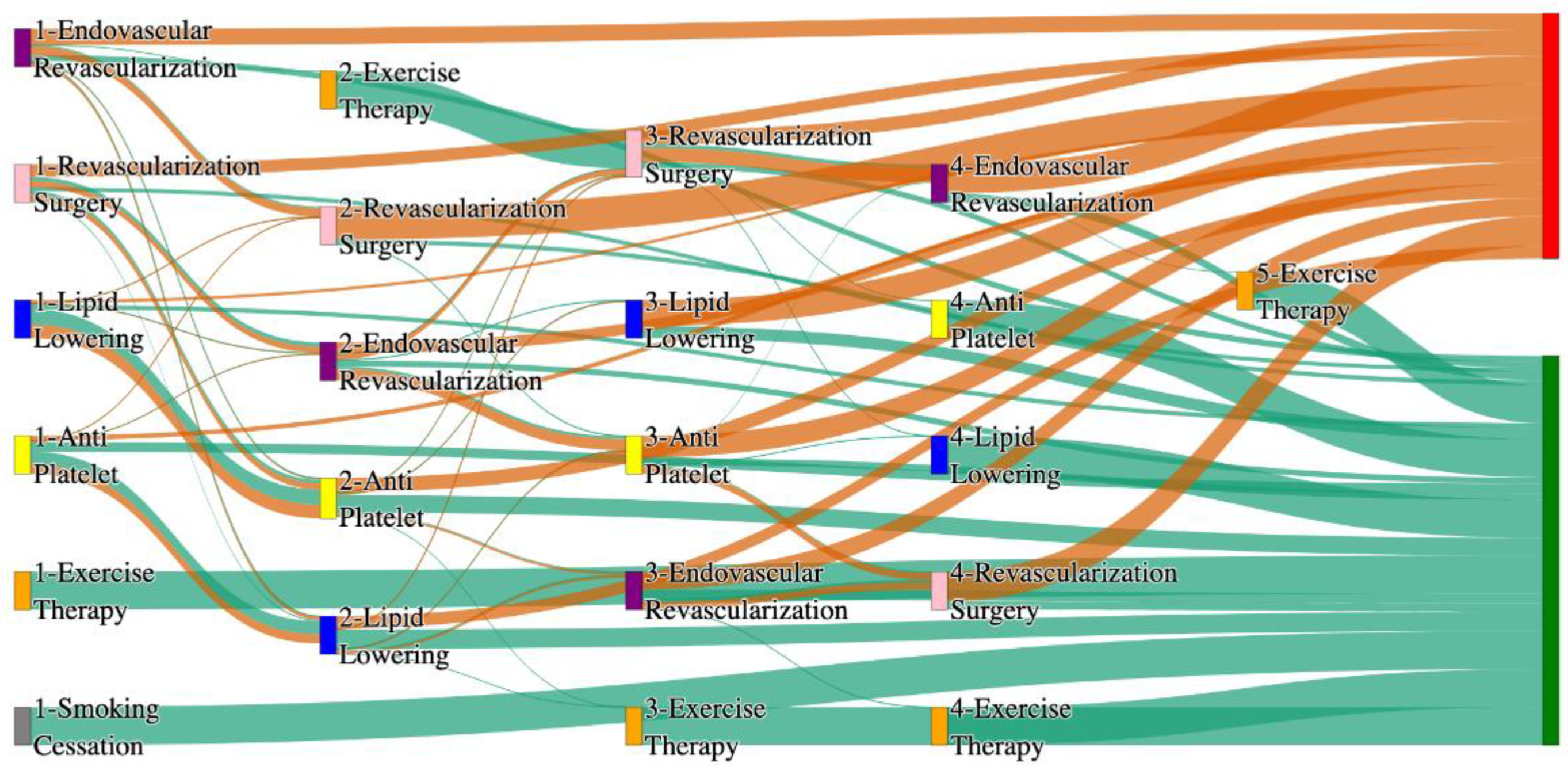
Figure 7.
Sankey plot for the PAD with Heart Failure in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 7.
Sankey plot for the PAD with Heart Failure in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 8.
Sankey plot for the PAD with Heart Failure in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 8.
Sankey plot for the PAD with Heart Failure in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 9.
Sankey plot for the PAD with Cerebrovascular disease in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 9.
Sankey plot for the PAD with Cerebrovascular disease in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 10.
Sankey plot for the PAD with Cerebrovascular disease in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 10.
Sankey plot for the PAD with Cerebrovascular disease in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 11.
Sankey plot for the PAD with Coronary artery disease in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 11.
Sankey plot for the PAD with Coronary artery disease in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 12.
Sankey plot for the PAD with Coronary artery disease in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 12.
Sankey plot for the PAD with Coronary artery disease in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 13.
Sankey plot for the PAD with Hyperlipidemia in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 13.
Sankey plot for the PAD with Hyperlipidemia in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 14.
Sankey plot for the PAD with Hyperlipidemia in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 14.
Sankey plot for the PAD with Hyperlipidemia in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 15.
Sankey plot for the PAD with age > 50 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 15.
Sankey plot for the PAD with age > 50 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 16.
Sankey plot for the PAD with age > 50 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 16.
Sankey plot for the PAD with age > 50 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 17.
Sankey plot for the PAD with age <= 50 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 17.
Sankey plot for the PAD with age <= 50 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 18.
Sankey plot for the PAD with age <= 50 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 18.
Sankey plot for the PAD with age <= 50 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 19.
Sankey plot for the PAD with age > 65 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 19.
Sankey plot for the PAD with age > 65 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 20.
Sankey plot for the PAD with age > 65 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 20.
Sankey plot for the PAD with age > 65 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 21.
Sankey plot for the PAD with age <= 65 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 21.
Sankey plot for the PAD with age <= 65 in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 22.
Sankey plot for the PAD with age <= 65 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 22.
Sankey plot for the PAD with age <= 65 in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 23.
Sankey plot for the PAD filtered for patients who reported their race as ‘white’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 23.
Sankey plot for the PAD filtered for patients who reported their race as ‘white’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 24.
Sankey plot for the PAD filtered for patients who reported their race as ‘white’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 24.
Sankey plot for the PAD filtered for patients who reported their race as ‘white’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 25.
Sankey plot for the PAD patients who reported their race as ‘black’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 25.
Sankey plot for the PAD patients who reported their race as ‘black’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 26.
Sankey plot for the PAD patients who reported their race as ‘black’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 26.
Sankey plot for the PAD patients who reported their race as ‘black’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 27.
Sankey plot for the PAD patients who reported their race as ‘Asian’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 27.
Sankey plot for the PAD patients who reported their race as ‘Asian’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 28.
Sankey plot for the PAD patients who reported their race as ‘Asian’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 28.
Sankey plot for the PAD patients who reported their race as ‘Asian’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 29.
Sankey plot for the PAD patients who reported their gender as ‘male’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 29.
Sankey plot for the PAD patients who reported their gender as ‘male’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 30.
Sankey plot for the PAD patients who reported their gender as ‘male’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 30.
Sankey plot for the PAD patients who reported their gender as ‘male’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 31.
Sankey plot for the PAD patients who reported their gender as ‘Female’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 31.
Sankey plot for the PAD patients who reported their gender as ‘Female’ cohort in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 32.
Sankey plot for the PAD patients who reported their gender as ‘Female’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 32.
Sankey plot for the PAD patients who reported their gender as ‘Female’ cohort in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 33.
Sankey plot for the PAD patients who reportedly smoke in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 33.
Sankey plot for the PAD patients who reportedly smoke in STARR data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 34.
Sankey plot for the PAD patients who reportedly smoke in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Figure 34.
Sankey plot for the PAD patients who reportedly smoke in All of Us data, depicting the flow of treatments between the patients that experienced amputation and those who did not experience any amputation. Thickness of the pathway indicates the percentage of patients experiencing the treatment, the size of the amputation cohort is visually larger here due to the Data normalized at the cohort and node levels: cohort normalization adjusts sequence counts by total cohort size, while node normalization ensures flow values sum to 1 at each node, enabling visual comparison of pathway proportions as stated in “2.3 Data Preprocessing” section. Pathways colored in deep amber are the amputation pathways and those colored in pine green are non-amputation pathways.
Table 1.
Counts of amputations vs non-amputations in both the PAD datasets.
Table 1.
Counts of amputations vs non-amputations in both the PAD datasets.
| Cohort |
Patients with at least one Treatment before Amputation |
Non-Amputations |
| STARR Data |
77 |
5504 |
|
All of Us Data |
176 |
4085 |
Table 2.
Pathways in the STARR Dataset, that exhibit higher prevalence in the amputation cohort than in the non-amputation cohort.
Table 2.
Pathways in the STARR Dataset, that exhibit higher prevalence in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| anti platelet |
lipid lowering |
Endovascular revascularization |
7.79 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
5.19 |
| Revascularization surgery |
Lipid lowering |
Anti platelet |
2.6 |
| Endovascular revascularization |
Lipid lowering |
- |
1.3 |
Table 3.
Pathways in the STARR Dataset, that exhibit higher prevalence in the non-amputation cohort than in the amputation cohort.
Table 3.
Pathways in the STARR Dataset, that exhibit higher prevalence in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
32 |
| lipid lowering |
anti platelet |
24.1 |
| lipid lowering |
- |
16.8 |
| anti platelet |
- |
10.2 |
Table 4.
Pathways in the All of Us Dataset, that exhibit higher prevalence in the amputation cohort than in the non-amputation cohort.
Table 4.
Pathways in the All of Us Dataset, that exhibit higher prevalence in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
13.6 |
| Endovascular revascularization |
Revascularization surgery |
- |
6.25 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
3.98 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
2.84 |
Table 5.
Pathways in the All of Us Dataset, that exhibit higher prevalence in the non-amputation cohort than in the amputation cohort.
Table 5.
Pathways in the All of Us Dataset, that exhibit higher prevalence in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
27.29 |
| anti platelet |
- |
23 |
| Anti platelet |
Lipid lowering |
22 |
| Lipid lowering |
- |
14 |
Table 6.
comparative analysis in non-amputation cohort in both the Datasets for the sequence of Anti-platelet → Lipid-lowering.
Table 6.
comparative analysis in non-amputation cohort in both the Datasets for the sequence of Anti-platelet → Lipid-lowering.
| Dataset |
Treatment Sequence |
Non-Amputation Rate (%) |
| STARR |
Anti-platelet → Lipid-lowering |
32.29 |
| All of Us |
Anti-platelet → Lipid-lowering |
21.93 |
Table 7.
Treatment sequences for amputation outcomes in both datasets.
Table 7.
Treatment sequences for amputation outcomes in both datasets.
| Dataset |
Treatment Sequence |
Amputation Rate (%) |
| STARR |
Anti-platelet → Lipid-lowering |
25.97 |
| STARR |
Lipid-lowering |
15.58 |
| All of Us |
Endovascular Revascularization |
13.64 |
| All of Us |
Endovascular Revascularization → Revascularization Surgery |
6.25 |
Table 8.
Comparative Analysis of Revascularization Interventions in amputation cohorts of both datasets.
Table 8.
Comparative Analysis of Revascularization Interventions in amputation cohorts of both datasets.
| Dataset |
Revascularization Sequence |
Amputation Rate (%) |
| STARR |
Occurrence of revascularization procedures alone |
0.0 |
| All of Us |
Endovascular Revascularization → Revascularization Surgery |
6.43 |
Table 9.
Peripheral Overview of variations in treatment pathways in both datasets.
Table 9.
Peripheral Overview of variations in treatment pathways in both datasets.
| Dataset |
Common Treatment Patterns in Amputation cohort |
| STARR |
Anti-platelet and lipid-lowering therapies |
| All of Us |
Revascularization Procedures |
Table 10.
Treatment pathways from odds ratio analysis in the STARR data with odds ratio between 0 and 1, lowering the amputation risk.
Table 10.
Treatment pathways from odds ratio analysis in the STARR data with odds ratio between 0 and 1, lowering the amputation risk.
| Pathway |
Odds Ratio |
| Lipid-lowering, Anti-platelet, Revascularization Surgery |
0.83 |
| Anti-platelet, Lipid-lowering, Revascularization Surgery |
0.81 |
| Lipid-lowering, Anti-platelet |
0.70 |
| Anti-platelet, Lipid-lowering |
0.62 |
| Lipid-lowering, Anti-platelet, Endovascular Revascularization |
0.60 |
Table 11.
Treatment pathways from odds ratio analysis in the All of Us data with odds ratio between 0 and 1, lowering the amputation risk.
Table 11.
Treatment pathways from odds ratio analysis in the All of Us data with odds ratio between 0 and 1, lowering the amputation risk.
| Pathway |
Odds Ratio |
| Anti-platelet |
0.35 |
| Anti-platelet, Lipid-lowering |
0.53 |
| Lipid-lowering |
0.34 |
Table 12.
Patient counts in STARR data - risk factors.
Table 12.
Patient counts in STARR data - risk factors.
| STARR data with PAD |
| cohort |
patients count with amputation |
Patient count with non-amputation |
| STARR PAD cohort |
77 |
5504 |
| PAD with diabetes |
54 |
1936 |
| PAD with heart failure |
26 |
1270 |
| PAD with hypertension |
56 |
3812 |
| PAD with cerebrovascular disease |
26 |
1749 |
| PAD with coronary artery disease |
43 |
2500 |
| PAD with Hyperlipidemia |
76 |
4941 |
| Patients with age > 50 |
74 |
5239 |
| Patients with age <= 50 |
3 |
265 |
| Patients with age > 65 |
51 |
3649 |
| Patients with age <= 65 |
26 |
1855 |
| Male Patient cohort |
56 |
2195 |
| Female Patient cohort |
21 |
2116 |
| Patients who reported their race as white |
39 |
3304 |
| Patients who reported their race as black |
14 |
297 |
| Patients who reported their race as Asian |
4 |
704 |
| Patients who smoke |
72 |
5143 |
Table 13.
Patient counts in All of Us data - risk factors.
Table 13.
Patient counts in All of Us data - risk factors.
|
All of Usdata with PAD
|
| cohort |
patients count with amputation |
Patient count with non-amputation |
| All of Us PAD cohort |
176 |
4085 |
| PAD with diabetes |
160 |
2780 |
| PAD with heart failure |
106 |
1812 |
| PAD with hypertension |
173 |
3853 |
| PAD with cerebrovascular disease |
117 |
2454 |
| PAD with coronary artery disease |
143 |
2947 |
| PAD with Hyperlipidemia |
170 |
3902 |
| Patients with age > 50 |
140 |
3276 |
| Patients with age <= 50 |
36 |
809 |
| Patients with age > 65 |
54 |
1152 |
| Patients with age <= 65 |
122 |
2933 |
| Male Patient cohort |
105 |
1889 |
| Female Patient cohort |
66 |
2063 |
| Patients who reported their race as white |
78 |
2437 |
| Patients who reported their race as black |
67 |
1009 |
| Patients who reported their race as Asian |
1 |
39 |
| Patients who smoke |
78 |
1205 |
Table 14.
Pathways in PAD with Hypertension cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 14.
Pathways in PAD with Hypertension cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Stage 4 Treatment |
Amputation Rate (%) |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
- |
7.14 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
- |
7.14 |
| Revascularization surgery |
Lipid lowering |
- |
- |
1.79 |
| Revascularization surgery |
Lipid lowering |
Anti platelet |
Endovascular revascularization |
1.79 |
Table 15.
Pathways in PAD with Hypertension cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 15.
Pathways in PAD with Hypertension cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
33 |
| lipid lowering |
anti platelet |
27 |
| lipid lowering |
- |
15 |
| Anti platelet |
- |
7.28 |
Table 16.
Pathways in PAD with Hypertension cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 16.
Pathways in PAD with Hypertension cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
13.29 |
| Endovascular revascularization |
Revascularization surgery |
- |
6.36 |
| Lipid lowering |
Anti platelet |
Endovascular Revascularization |
4.05 |
| Lipid lowering |
Anti platelet |
Revascularization surgery |
2.31 |
Table 17.
Pathways in PAD with Hypertension cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 17.
Pathways in PAD with Hypertension cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
28 |
| Anti platelet |
- |
22 |
| Anti platelet |
Lipid lowering |
22 |
| Lipid lowering |
- |
14 |
Table 18.
Pathways in PAD with Diabetes cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 18.
Pathways in PAD with Diabetes cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
9.26 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
5.56 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
3.7 |
| Revascularization surgery |
Lipid lowering |
Anti platelet |
3.7 |
Table 19.
Pathways in PAD with Diabetes cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 19.
Pathways in PAD with Diabetes cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
33 |
| lipid lowering |
anti platelet |
31 |
| lipid lowering |
- |
15 |
| Anti platelet |
- |
4.7 |
Table 20.
Pathways in PAD with Diabetes cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 20.
Pathways in PAD with Diabetes cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
13.75 |
| Endovascular revascularization |
Revascularization surgery |
- |
5.62 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
3.7 |
| Lipid lowering |
antiplatelet |
Anti platelet |
1.8 |
Table 21.
Pathways in PAD with Diabetes cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 21.
Pathways in PAD with Diabetes cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
29 |
| Anti platelet |
Lipid lowering |
23 |
| Anti platelet |
- |
21 |
| Lipid lowering |
- |
13 |
Table 22.
Pathways in PAD with Heart Failure cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 22.
Pathways in PAD with Heart Failure cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
19.23 |
| Lipid lowering |
- |
- |
11.54 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
7.69 |
Table 23.
Pathways in PAD with Heart Failure cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 23.
Pathways in PAD with Heart Failure cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
39 |
| lipid lowering |
anti platelet |
28 |
| Anti platelet |
- |
7.43 |
Table 24.
Pathways in PAD with Heart Failure cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 24.
Pathways in PAD with Heart Failure cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
14.15 |
| Endovascular revascularization |
Revascularization surgery |
- |
7.55 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
2.83 |
Table 25.
Pathways in PAD with Heart Failure cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 25.
Pathways in PAD with Heart Failure cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
30 |
| Anti platelet |
Lipid lowering |
25 |
| Anti platelet |
- |
20 |
Table 26.
Pathways in PAD with Cerebrovascular disease cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 26.
Pathways in PAD with Cerebrovascular disease cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
- |
30.77 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
11.54 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
7.69 |
| Anti platelet |
- |
- |
7.69 |
Table 27.
Pathways in PAD with Cerebrovascular disease cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 27.
Pathways in PAD with Cerebrovascular disease cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
35 |
| lipid lowering |
- |
8.5 |
Table 28.
Pathways in PAD with Cerebrovascular disease cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 28.
Pathways in PAD with Cerebrovascular disease cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
14.53 |
| Endovascular revascularization |
Revascularization surgery |
- |
6.84 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
5.13 |
Table 29.
Pathways in PAD with Cerebrovascular disease cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 29.
Pathways in PAD with Cerebrovascular disease cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| lipid lowering |
anti platelet |
27 |
| Anti platelet |
Lipid lowering |
23 |
| Anti platelet |
- |
21 |
| Lipid lowering |
- |
12 |
Table 30.
Pathways in PAD with coronary artery disease cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 30.
Pathways in PAD with coronary artery disease cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
13.95 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
4.65 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
4.65 |
| Revascularization surgery |
Lipid lowering |
Anti platelet |
4.65 |
Table 31.
Pathways in PAD with coronary artery disease cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 31.
Pathways in PAD with coronary artery disease cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
40 |
| lipid lowering |
anti platelet |
30 |
Table 32.
Pathways in PAD with Coronary artery disease cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 32.
Pathways in PAD with Coronary artery disease cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
15.38 |
| Endovascular revascularization |
Revascularization surgery |
- |
6.36 |
| Lipid lowering |
Anti platelet |
Endovascular Revascularization |
3.5 |
| Anti platelet |
Endovascular revascularization |
- |
2.1 |
Table 33.
Pathways in PAD with Coronary artery disease cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 33.
Pathways in PAD with Coronary artery disease cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
29 |
| Anti platelet |
Lipid lowering |
25 |
| Anti platelet |
- |
20 |
| Lipid lowering |
- |
11 |
Table 34.
Pathways in PAD with Hyperlipidemia cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 34.
Pathways in PAD with Hyperlipidemia cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
7.89 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
5.26 |
| Anti platelet |
- |
- |
3.95 |
| Revascularization surgery |
Lipid lowering |
Anti platelet |
2.63 |
Table 35.
Pathways in PAD with Hyperlipidemia cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 35.
Pathways in PAD with Hyperlipidemia cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
35.80 |
| lipid lowering |
anti platelet |
26.72 |
Table 36.
Pathways in PAD with Hyperlipidemia cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 36.
Pathways in PAD with Hyperlipidemia cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
14.12 |
| Endovascular revascularization |
Revascularization surgery |
- |
5.88 |
| Lipid lowering |
Anti platelet |
Endovascular Revascularization |
4.12 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
2.94 |
Table 37.
Pathways in PAD with Hyperlipidemia cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 37.
Pathways in PAD with Hyperlipidemia cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
29 |
| Anti platelet |
Lipid lowering |
23 |
| Anti platelet |
- |
20 |
| Lipid lowering |
- |
15 |
Table 38.
Pathways in PAD with age > 50 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 38.
Pathways in PAD with age > 50 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Lipid lowering |
- |
- |
20.25 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
7.59 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
5.06 |
| Revascularization surgery |
Lipid lowering |
Anti platelet |
2.53 |
Table 39.
Pathways in PAD with age > 50 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 39.
Pathways in PAD with age > 50 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| anti platelet |
lipid lowering |
32.43 |
| lipid lowering |
anti platelet |
24.31 |
Table 40.
Pathways in PAD with age > 50 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 40.
Pathways in PAD with age > 50 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
17.14 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
4.29 |
| Anti platelet |
Lipid lowering |
Endovascular Revascularization |
3.57 |
| Revascularization surgery |
- |
- |
2.86 |
Table 41.
Pathways in PAD with age > 50 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 41.
Pathways in PAD with age > 50 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
28.41 |
| Anti platelet |
Lipid lowering |
21.05 |
| Anti platelet |
- |
22.6 |
| Lipid lowering |
- |
15.35 |
Table 42.
Pathways in PAD with age <= 50 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 42.
Pathways in PAD with age <= 50 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
66.67 |
| Anti platelet |
- |
33.33 |
Table 43.
Pathways in PAD with age <= 50 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 43.
Pathways in PAD with age <= 50 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non-Amputation Rate (%) |
| Lipid lowering |
- |
18.49 |
| Lipid lowering |
Anti platelet |
17.36 |
| Anti platelet |
Endovascular revascularization |
5.28 |
Table 44.
Pathways in PAD with age <= 50 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 44.
Pathways in PAD with age <= 50 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
27.78 |
| Anti platelet |
Lipid lowering |
25 |
Table 45.
Pathways in PAD with age <= 50 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 45.
Pathways in PAD with age <= 50 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Non-Amputation Rate (%) |
| Anti platelet |
29 |
| Lipid lowering |
21.51 |
Table 46.
Pathways in PAD with age > 65 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 46.
Pathways in PAD with age > 65 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
- |
31.37 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
5.88 |
Table 47.
Pathways in PAD with age > 65 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 47.
Pathways in PAD with age > 65 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
31.64 |
| Lipid lowering |
- |
18.21 |
| Anti platelet |
- |
9.2 |
Table 48.
Pathways in PAD with age > 65 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 48.
Pathways in PAD with age > 65 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
24.07 |
| Endovascular revascularization |
Revascularization surgery |
9.26 |
| Revascularization surgery |
- |
5.56 |
Table 49.
Pathways in PAD with age > 65 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 49.
Pathways in PAD with age > 65 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
29.89 |
| Anti platelet |
- |
21.73 |
| Anti platelet |
Lipid lowering |
18.54 |
| Lipid lowering |
- |
18.27 |
Table 50.
Pathways in PAD with age <= 65 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 50.
Pathways in PAD with age <= 65 cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Lipid lowering |
- |
- |
24.14 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
6.9 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
6.9 |
Table 51.
Pathways in PAD with age <= 65 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 51.
Pathways in PAD with age <= 65 cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
31.86 |
| Lipid lowering |
Anti platelet |
18.87 |
| Anti platelet |
- |
14.34 |
Table 52.
Pathways in PAD with age <= 65 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 52.
Pathways in PAD with age <= 65 cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
10.92 |
| Endovascular revascularization |
Revascularization surgery |
- |
5.04 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
4.2 |
Table 53.
Pathways in PAD with age <= 65 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 53.
Pathways in PAD with age <= 65 cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
24.92 |
| Anti platelet |
- |
24.48 |
| Anti platelet |
Lipid lowering |
21.1 |
| Lipid lowering |
- |
18.51 |
Table 54.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 54.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
7.69 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
7.69 |
| Revascularization surgery |
Lipid lowering |
Anti platelet |
5.13 |
Table 55.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 55.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti Platelet |
Lipid lowering |
31.84 |
| Lipid lowering |
Anti platelet |
23.15 |
| Lipid lowering |
- |
15.38 |
| Anti platelet |
- |
10.26 |
Table 56.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 56.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
15.38 |
| Endovascular revascularization |
Revascularization surgery |
- |
8.97 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
3.85 |
Table 57.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 57.
Pathways in PAD filtered for patients who reported their race as ‘white’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
29.59 |
| Anti platelet |
- |
22.61 |
| Anti platelet |
Lipid lowering |
20.72 |
| Lipid lowering |
- |
15.80 |
Table 58.
Pathways in PAD patients who reported their race as ‘black’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 58.
Pathways in PAD patients who reported their race as ‘black’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Lipid lowering |
- |
- |
21.43 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
14.29 |
Table 59.
Pathways in PAD patients who reported their race as ‘black’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 59.
Pathways in PAD patients who reported their race as ‘black’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
31.65 |
| Anti platelet |
Lipid lowering |
29.29 |
| Anti platelet |
- |
10.77 |
Table 60.
Pathways in PAD patients who reported their race as ‘black’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 60.
Pathways in PAD patients who reported their race as ‘black’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Stage 4 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
- |
7.46 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
- |
4.48 |
| Lipid lowering |
Anti platelet |
Endovascular revascularization |
Revascularization surgery |
4.48 |
Table 61.
Pathways in PAD patients who reported their race as ‘black’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 61.
Pathways in PAD patients who reported their race as ‘black’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti platelet |
- |
27.65 |
| Anti platelet |
Lipid lowering |
24.18 |
| Lipid lowering |
Anti platelet |
22.70 |
| Lipid lowering |
- |
11.69 |
Table 62.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 62.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
50 |
| Anti platelet |
Lipid lowering |
50 |
Table 63.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 63.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
16.76 |
| Anti platelet |
7.67 |
Table 64.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 64.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 2 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
100 |
Table 65.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 65.
Pathways in PAD patients who reported their race as ‘Asian’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
33.33 |
| Anti platelet |
Lipid lowering |
33.33 |
| Lipid lowering |
- |
23.08 |
Table 66.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 66.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
8.93 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
7.14 |
Table 67.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 67.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
33.45 |
| Lipid lowering |
Anti platelet |
25.32 |
| Lipid lowering |
- |
16.40 |
Table 68.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 68.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
14.29 |
| Endovascular revascularization |
Revascularization surgery |
7.62 |
Table 69.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort
Table 69.
Pathways in PAD patients who reported their gender as ‘male’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
29.89 |
| Anti platelet |
Lipid lowering |
21.33 |
| Anti platelet |
- |
21.44 |
Table 70.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 70.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
- |
47.62 |
| Anti platelet |
Exercise therapy |
Lipid lowering |
4.76 |
| Endovascular revascularization |
Lipid lowering |
- |
4.76 |
Table 71.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 71.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
30.86 |
| Lipid lowering |
- |
17.77 |
| Anti platelet |
- |
12.71 |
Table 72.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 72.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
27.27 |
| Endovascular revascularization |
- |
13.68 |
Table 73.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 73.
Pathways in PAD patients who reported their gender as ‘Female’ cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti platelet |
- |
25.06 |
| Anti platelet |
Lipid lowering |
22.78 |
| Lipid lowering |
- |
16.48 |
Table 74.
Pathways in PAD patients who reportedly smoke cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 74.
Pathways in PAD patients who reportedly smoke cohort in the STARR Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
8.33 |
| Anti platelet |
Revascularization surgery |
Lipid lowering |
5.56 |
Table 75.
Pathways in PAD patients who reportedly smoke cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 75.
Pathways in PAD patients who reportedly smoke cohort in the STARR Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Anti platelet |
Lipid lowering |
32.14 |
| Lipid lowering |
Anti platelet |
24.62 |
| Lipid lowering |
- |
16.45 |
| Anti platelet |
- |
9.64 |
Table 76.
Pathways in PAD patients who reportedly smoke cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
Table 76.
Pathways in PAD patients who reportedly smoke cohort in the All of Us Dataset, whose prevalence is higher in the amputation cohort than in the non-amputation cohort.
| Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Stage 3 Treatment |
Amputation Rate (%) |
| Endovascular revascularization |
- |
- |
11.54 |
| Endovascular revascularization |
Revascularization surgery |
- |
6.41 |
| Anti platelet |
Lipid lowering |
Endovascular revascularization |
3.85 |
Table 77.
Pathways in PAD patients who reportedly smoke cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
Table 77.
Pathways in PAD patients who reportedly smoke cohort in the All of Us Dataset, whose prevalence is higher in the non-amputation cohort than in the amputation cohort.
| Non - Amputation Pathways |
| Stage 1 Treatment |
Stage 2 Treatment |
Non - Amputation Rate (%) |
| Lipid lowering |
Anti platelet |
24.90 |
| Anti platelet |
- |
22.78 |
| Anti platelet |
Lipid lowering |
21 |