Submitted:
31 December 2024
Posted:
02 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructure Evolution
3.2. Electrochemical analysis
4. Discussion
5. Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohapatra, S.; Poojari, G.; Das, S.; Das, K. Insights into the dynamic impact behavior of intercritically annealed automotive-grade Fe–7Mn–4Al−0.18C steel. Mat. Sci. Eng. A 2023, 887, 145769.
- Leták, R.; Jirková, H.; Kučerová, L.; Jeníček, Š.; Volák, J. Effect of forming and heat treatment parameters on the mechanical properties of medium manganese steel with 5% Mn. Materials 2023, 16, 4340. [CrossRef]
- Dykas, J.; Samek, L.; Grajcar, A.; Kozłowska A. Modelling of phase diagrams and continuous cooling transformation diagrams of medium manganese steels. Symmetry 2023, 15, 381. [CrossRef]
- Liu, T.; Dong, Y.; Qin, D.; Wu, H. Gao, X. Du, L. Effect of rolling temperature on microstructure and mechanical properties of medium manganese steel. Mat Sci Eng A. 2023, 863, 144547. [CrossRef]
- Mou, Y.; Li, Z.; Zhang, X.; Misra, D.; He, L.; Li, H. Design of an effective heat treatment involving intercritical hardening for high strength/high elongation of 0.2C–3Al–(6–8.5)Mn–Fe TRIP steels: microstructural evolution and deformation behavior. Metals 2019, 9, 1275. [CrossRef]
- Hu, Z.; Fu, H. Effect of Si Content on Microstructure and Properties of Low-Carbon Medium-Manganese Steel after Intercritical Heat Treatment. Metals 2024, 14(6), 675. [CrossRef]
- Li, Z.; Li, X.; Mou, Y.; Cai, Z.; Misra, D.; Zhang, X.; Li, H. The significance of microstructural evolution on governing impact toughness of Fe–0.2C–6Mn–3Al medium-Mn TRIP steel studied by a novel heat treatment. Int. J. Mater. Res. 2021, 112(4), 271–279.
- Qiao, Y.; Zheng, Z.; Yang, H.; Long, J.; Han, P. Recent progress in microstructural evolution, mechanical and corrosion properties of medium-Mn steel. J. Iron. Steel. Res. Int. 2023, 30, 1463–1476. [CrossRef]
- Li, Z.; Zhang, X.; Mou, Y.; Cai, Z.; Misra, D.; He, L.; Li, H.; Ding, H. Design of an effective heat treatment involving intercritical hardening for high-strength–high elongation of 0.2C–1.5Al–(6–8.5)Mn-Fe TRIP steels: microstructural evolution and deformation behaviour Mater Sci Tech 2020, 36(4), 500–510.
- Yan, X.; Kang, Shumei.; Xu M.; Li, P.; Corrosion Product Film of a Medium-Mn Steel Exposed to Simulated Marine Splash Zone Environment. Materials 2021, 14(19), 5652. [CrossRef]
- Mohapatra, S.; Palai, D.; Satpathy, B.; Das, S.; Das, K. Electrochemical study of intercritically annealed Fe–0.18C–7Mn–4Al steel. Mater Today Commun. 2023, 34, 105282.
- Su, G.; Yu, C.; Zheng, H.; Gao, X.; Xie, H.; Huo, M.; Wu, H.; Xu, J.; Du, L.; Jiang, Z. The wet–dry cycling corrosion behavior of low-carbon medium manganese steel exposed to a 3.5% NaCl solution environment. J. Mater. Eng. Perform. 2022, 31, 7856–7869.
- Choudhary, S.; Nanda, V.; Shekhar, S.; Garg, A.; Mondal, K. Effect of microstructural anisotropy on the electrochemical behavior of rolled mild steel. J. Mater. Eng. Perform. 2017, 26(1), 185–194. [CrossRef]
- Wang, J.; Zhang, L. Effects of cold deformation on electrochemical corrosion behaviors of 304 stainless steel. Anti-Corros Method M. 2017, 64(2), 252–262. [CrossRef]
- Su, G.; Gao, X.; Du, L.; Zhang, D.; Hu, J.; Liu, Z. Influence of Mn on the corrosion behaviour of medium manganese steels in a simulated seawater environment. Int. J. Electrochem. Sci. 2016, 11, 9447–9461. [CrossRef]
- Poling, W.A.; Moor, D.E.; Speer, J.G.; Findley, K.O. Temperature effects on tensile deformation behavior of a medium manganese TRIP Steel and a quenched and partitioned steel. Metals 2021, 11, 375. [CrossRef]
- Chen, J.; Zhu, Y.; Chen, X.; Ma, X.; Chen, B. Interfacial Microstructure and Cladding Corrosion Resistance of Stainless Steel/Carbon Steel Clad Plates at Different Rolling Reduction Ratios. Metals 2025, 15, 16. [CrossRef]
- Arafin, M.A.; Szpunar, J.A. A new understanding of intergranular stress corrosion cracking resistance of pipeline steel through grain boundary character and crystallographic texture studies. Corros. Sci. 2009, 51, 119–128. [CrossRef]
- Zhang, L.; Lin, N.; Zou, J.; et al. Super-hydrophobicity and corrosion resistance of laser surface textured AISI 304 stainless steel decorated with Hexadecyltrimethoxysilane (HDTMS). Opt. Laser. Technol. 2020, 127, 106146. [CrossRef]
- Shkatulyak, N.M.; Tkachuk, O.M. A role played by the crystallographic texture in the process of corrosion of hot-rolled rods made of carbon steel. Mater. Sci. 2012, 48, 153–161. [CrossRef]
- Wang, P.; Ma, L.; Cheng, X.; Li, X. Comparative effect of (111) and (110) crystallographic orientation on the passive behavior of low alloy steels in bicarbonate solution. Appl. Surf. Sci. 2021, 561, 150066. [CrossRef]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Effect of grain size on the corrosion resistance of low carbon steel. Mater Res Express 2020, 7, 016522. [CrossRef]
- Mishra, R.; Balasubramaniam, R. Effect of nanocrystalline grain size on the electrochemical and corrosion behavior of nickel. Corros Sci. 2004, 46(12), 3019–3029. [CrossRef]
- Li, Q.; Sun, Y.; Zuo, H.; Feng, J.; Li, Z.; Cai, Z.; He, L.; Li, H. Microstructure evolution and mechanical properties of light medium manganese steel: different rolling directions during warm stamping. J. Mater. Eng. Perform. 2023, 1–15. [CrossRef]
- Li, Z.; Ding, H.; Misra, R.D.K.; Cai, Z.; Li, H. Microstructural evolution and deformation behavior in the Fe–(6, 8.5)Mn–3Al–0.2C TRIP steels. Mat. Sci. Eng. A 2016, 672, 161–169.
- Tsai, W.; Chen, J. Galvanic corrosion between the constituent phases in duplex stainless steel. Corros Sci. 2007, 49(9), 3659–3668. [CrossRef]
- Wang, P.; Zheng, W.; Dai, X.; Zhang, P.; Wang, Y. Prominent role of reversed austenite on corrosion property of super 13Cr martensitic stainless steel. J. Mater. Res. Technol. 2023, 22, 1753–1767. [CrossRef]
- Hu, B.; Zheng, Q.; Lu, Y.; Jia, C.; Liang, T.; Zheng, C. Stabilizing austenite via intercritical Mn partitioning in a medium Mn steel. Scripta Mater. 2023, 225, 115162. [CrossRef]
- Ding, R.; Zhang, C.; Wang, Y.; Liu, C.; Yao, Y.; Zhang, J.; Yang, Z.; Zhang, C.; Liu, Y.; Chen, H. Mechanistic role of Mn heterogeneity in austenite decomposition and stabilization in a commercial quenching and partitioning steel. Acta Mater. 2023, 250, 118869. [CrossRef]
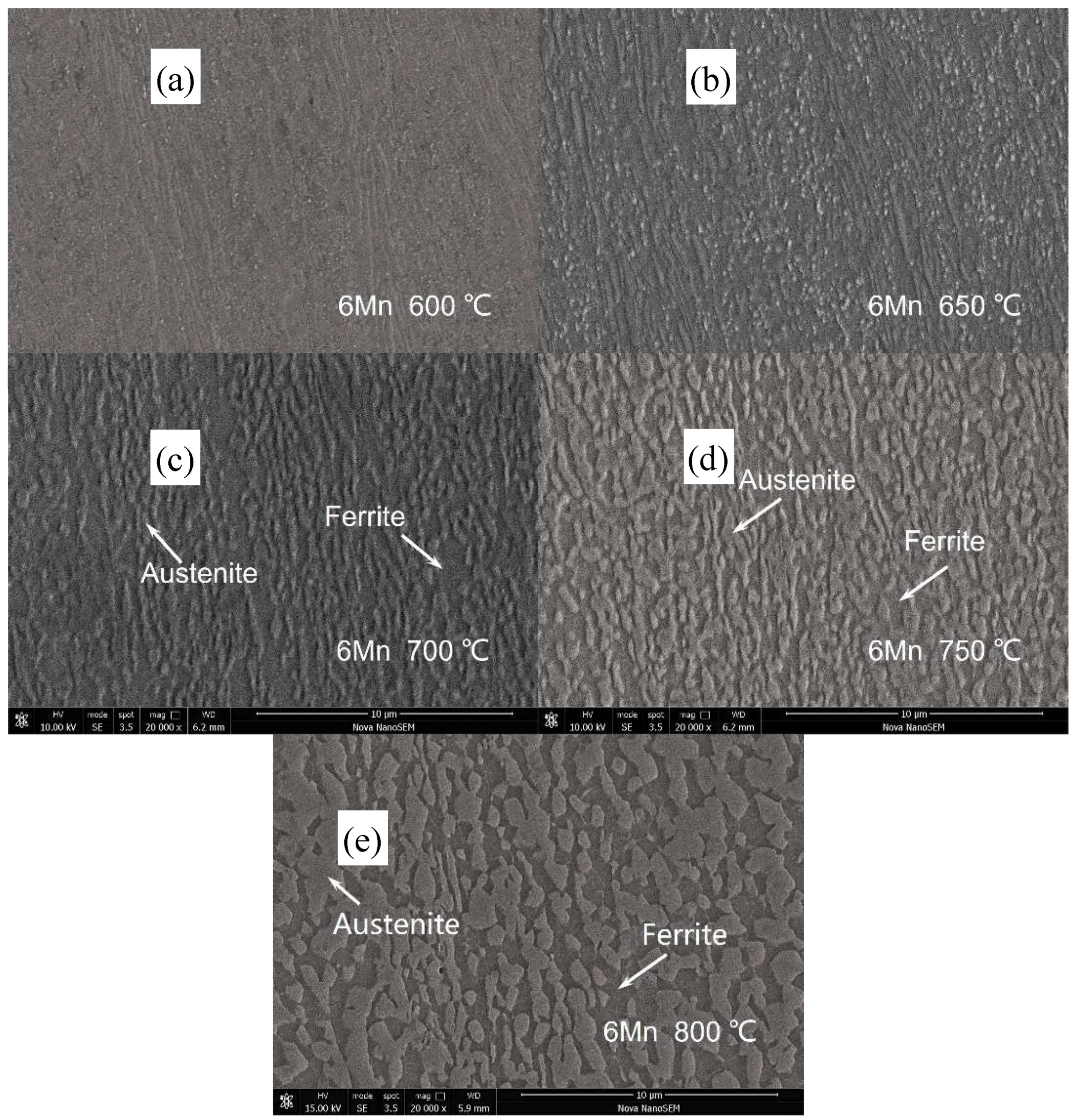
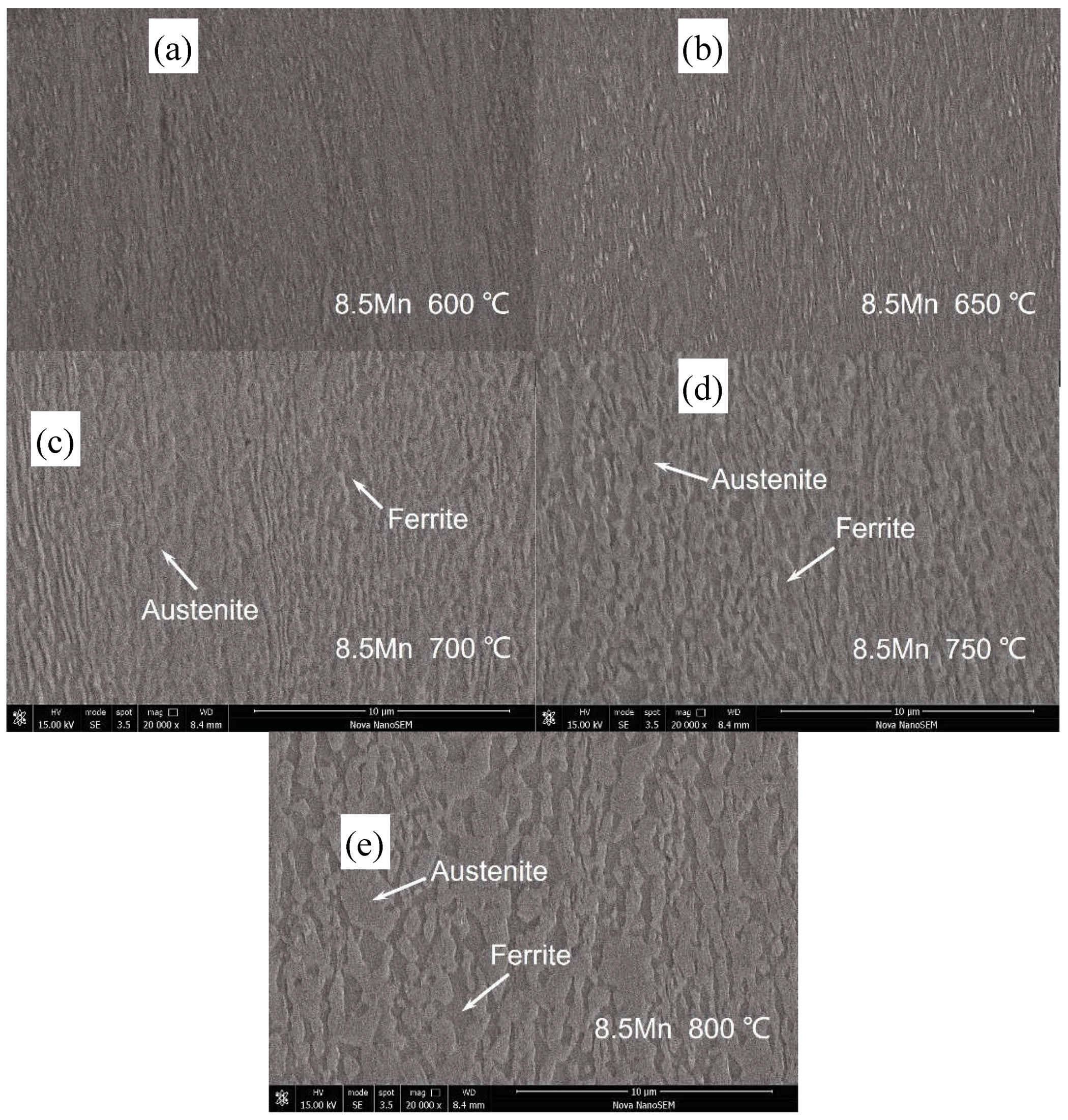
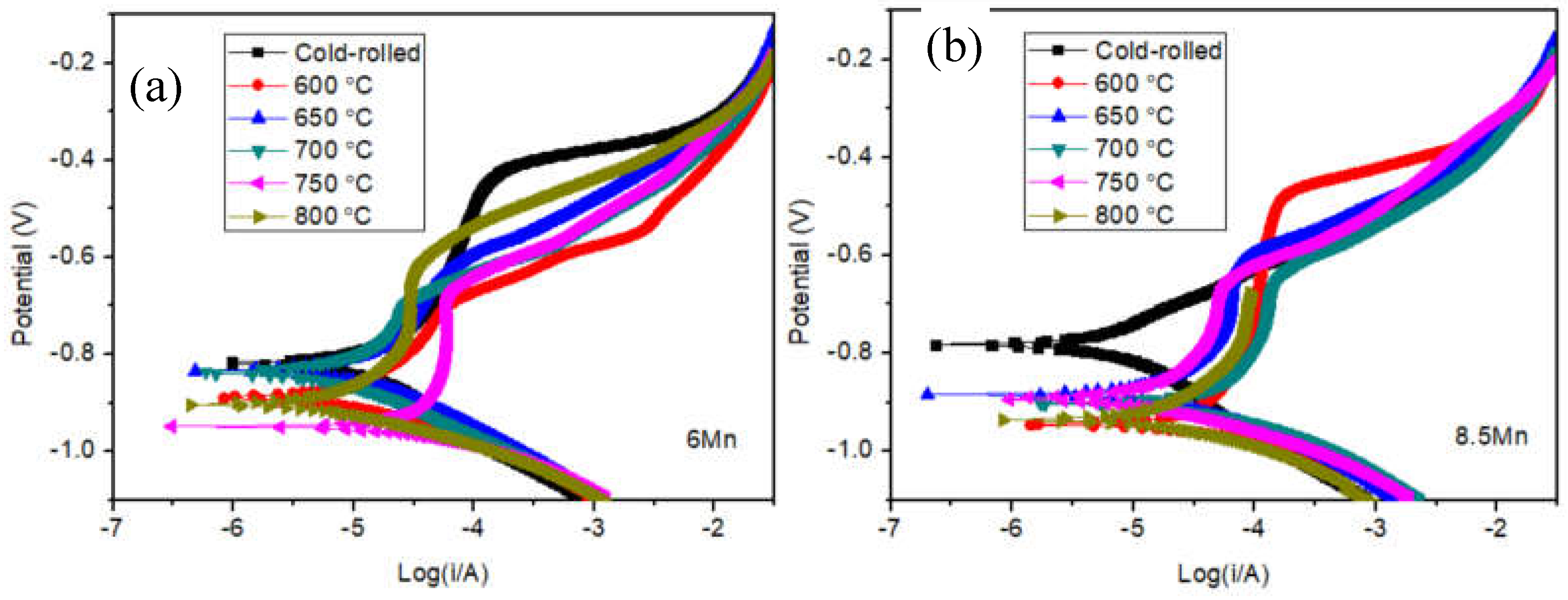
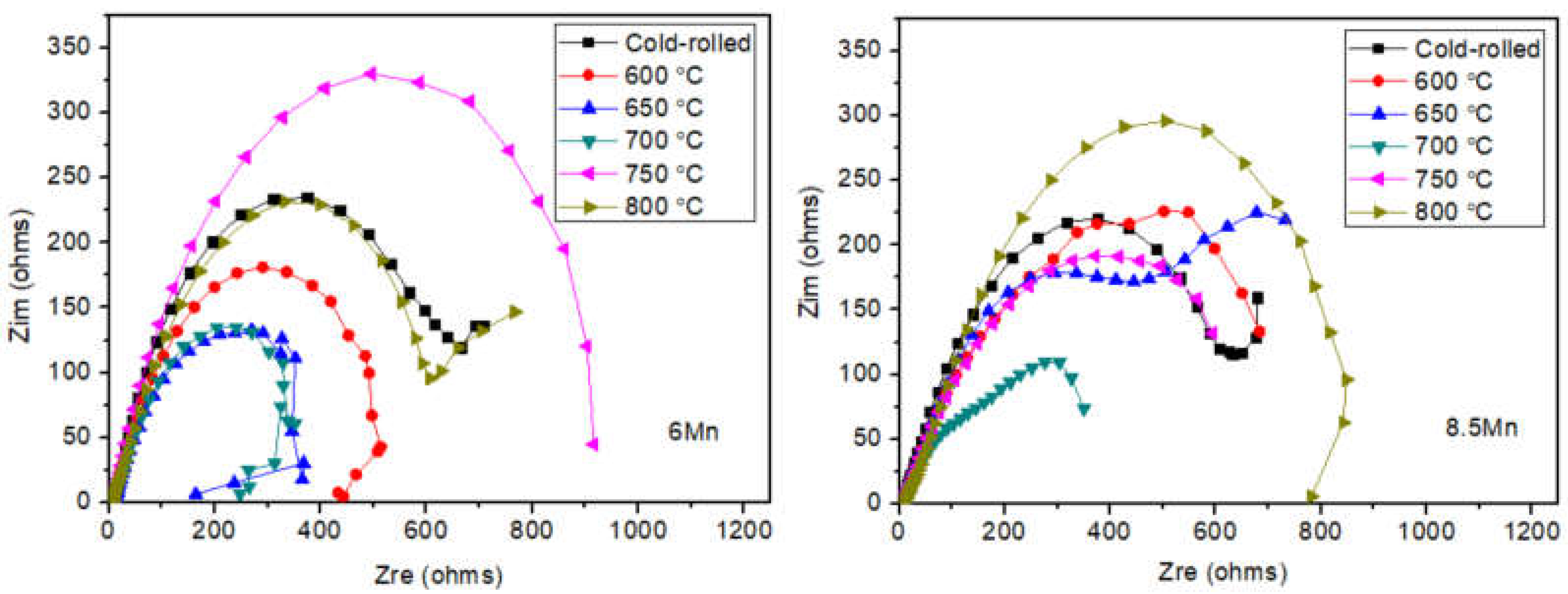
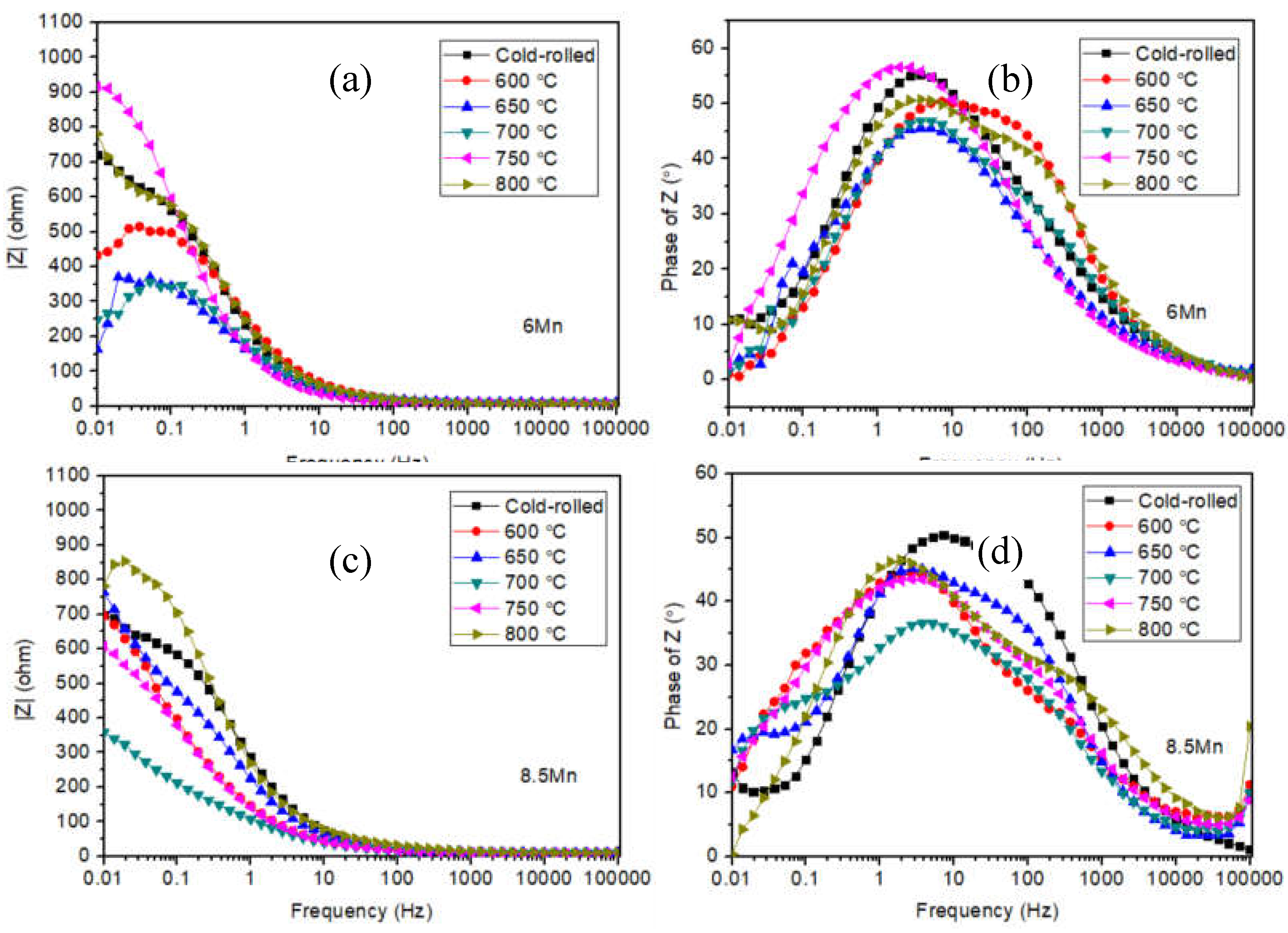
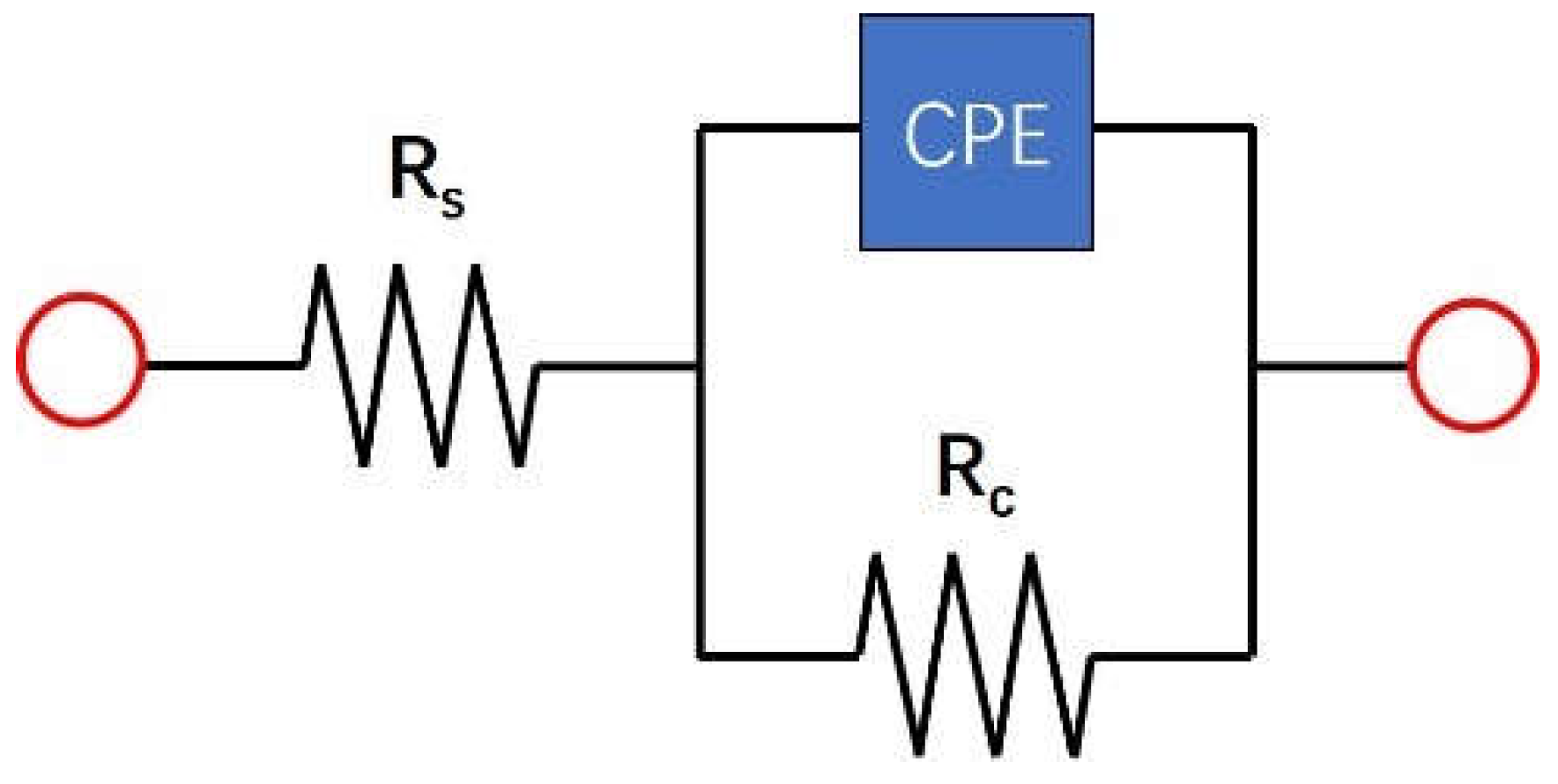
| Steel | C | Al | Mn | Fe |
| 6Mn | 0.20 | 3.20 | 6.0 | 90.60 |
| 8.5Mn | 0.19 | 3.11 | 8.4 | 88.30 |
| Samples | Ecorr (V) | Icorr (A/cm2) | Epit (V) |
| Cold-rolled | -0.82 | 1.349×10-5 | -0.44 |
| 600 ℃ | -0.89 | 1.0×10-5 | -0.70 |
| 650 ℃ | -0.84 | 1.288×10-5 | -0.65 |
| 700 ℃ | -0.86 | 1.023×10-5 | -0.70 |
| 750 ℃ | -0.93 | 3.548×10-5 | -0.68 |
| 800 ℃ | -0.90 | 9.120×10-6 | -0.64 |
| Samples | Ecorr (V) | Icorr (A/cm2) | Epit (V) |
| Cold-rolled | -0.76 | 6.025×10-6 | - |
| 600 ℃ | -0.92 | 7.943×10-5 | -0.49 |
| 650 ℃ | -0.88 | 1.995×10-5 | -0.62 |
| 700 ℃ | -0.90 | 4.786×10-5 | -0.65 |
| 750 ℃ | -0.89 | 1.445×10-5 | -0.65 |
| 800 ℃ | -0.92 | 2.238×10-5 | - |
| Samples | Rs(Ω·cm2) | CPE | Rc(Ω·cm2) | % error | |
| P(Ω-1·s-n·cm-2) | n | ||||
| Cold-rolled | 7.6322 | 1.0864×10-3 | 0.69098 | 760.51 | ≤4.6577 |
| 600 ℃ | 6.5467 | 7.5823×10-4 | 0.69721 | 521.97 | ≤3.6619 |
| 650 ℃ | 9.9172 | 1.5758×10-3 | 0.63731 | 433.78 | ≤4.2213 |
| 700 ℃ | 8.0861 | 1.201×10-3 | 0.65489 | 338.74 | ≤7.5118 |
| 750 ℃ | 7.5513 | 1.5256×10-3 | 0.7112 | 1041.1 | ≤4.3011 |
| 800 ℃ | 6.1727 | 1.015×10-3 | 0.65329 | 766.51 | ≤4.4369 |
| Samples | Rs(Ω·cm2) | CPE | Rc(Ω·cm2) | % error | |
| P(Ω-1·s-n·cm-2) | n | ||||
| Cold-rolled | 6.7926 | 8.1371×10-4 | 0.66234 | 730.95 | ≤2.6804 |
| 600 ℃ | 8.8095 | 2.3881×10-3 | 0.73174 | 974.33 | ≤7.9957 |
| 650 ℃ | 9.6177 | 1.3098×10-3 | 0.59995 | 772.31 | ≤6.2676 |
| 700 ℃ | 7.9054 | 3.4156×10-3 | 0.50568 | 436.55 | ≤5.5204 |
| 750 ℃ | 7.2716 | 2.3419×10-3 | 0.55676 | 813.37 | ≤4.9458 |
| 800 ℃ | 9.8619 | 1.1333×10-3 | 0.5655 | 1073.4 | ≤10.313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





