1. Introduction
Tumour malignancy defines the ability of tumour cells to metastasize and to form daughter tumours. Metastasis is the main cause of death in cancer patients. Most solid tumours arise from epithelial tissues. After undergoing extensive reprogramming initiated and supported by a multitude of external signals from the tumour micro-environment, tumour cells arise after undergoing the so-called epithelial to mesenchymal transition (EMT) leading to the re-establishment of the ability for active migration. Metastatic tumour cells migrate out from their original organ or tissue as single cells or clusters using either the amoeboid or mesenchymal (or fibroblastic) form of locomotion [
1,
2]. They traverse multiple barriers of the extracellular matrix (ECM) such as compact basement membranes or net-like structures formed by ECM fibrils in order to invade vessels and finally extravasate in distant organs, where they may form new solid tumours [for reviews see [
1,
2,
3]]. Independent of the mode of migration, metastasis of tumour cells depends on dynamic alterations of their cytoskeleton and in particular on the actin filament system.
Actin itself is a protein of 42 kDa molecular mass and cycles between two main states: the monomeric and filamentous form termed G-actin and F-actin, respectively [
4,
5]. In quiescent cells about 50% of the actin is maintained in monomeric state stabilised by G-actin binding proteins like thymosin beta4 (Tß4), profilin or transiently by cofilin/ADF [
6,
7,
8,
9,
10]. During active cell migration, however, the equilibrium between G- and F-actin is shifted towards the filamentous form. Actin filament bundles form the cores of specific protrusions at the cellular fronts to generate protrusive forces: invadopodia (invasive protrusions), filopodia, lamellipodia, and blebs [
11,
12,
13,
14,
15]. Invadopodia enable tumour cells to also secrete proteolytic enzymes, mostly matrix metalloproteases, which degradate extracellular matrix (ECM) components to facilitate tumour cell migration [
14]. Filopodia and lamellipodia are plasma membrane extensions formed in the direction of the movement by cells exhibiting the fibroblastic type of migration, whereas blebs are typical for amoeboid cells migrating through dense ECM networks [
16].
The directional movement of metastatic cells is determined by filopodia and lamellipodia. Their propulsive extension is solely achieved by actin treadmilling that depends on the different properties of the actin filament ends [
11,
13,
17]. Their plus ends are attached to the plasma membrane and elongate by addition of new actin subunits, which are simultaneously released from their minus ends [
11,
13]. Treadmilling generates the forces necessary for the propulsive translocation the plasma membrane of these extensions [
17]. These plasma membrane protrusions explore the surrounding of the migrating cell and attach to particular ECM components via integrin receptors [
2,
18]. Finally, the more stable F-actin networks at the posterior parts of the migrating cell interact with short myosin filaments to generate the forces necessary for the retraction of the trailing cell body [
2,
18].
In contrast, when cells move through a dense three-dimensional network of extracellular components, they switch to the amoeboid type of movement, which is independent of adhesions to ECM components and characterized by rapid shape changes of the migrating cell and the formation of bleb-like extensions caused by localized disassembly of the sub-membranous cortical F-actin and cytoplasmic pressure generated by force producing acto-myosin interactions [
2,
19]. The blebs seem to search for spaces within the ECM network allowing a squeezing passage powered by acto-myosin interactions. Many metastatic tumour cells can rapidly switch between these modes of migration [
2,
19]. Though both modes of metastatic cell migration (amoeboid or fibroblastic) may be differently regulated, they both depend on the basic dynamic behaviour of the actin filament system, whose dis- and re-assembly and treadmilling within the protrusions is regulated by a large number of actin-binding proteins (ABPs) [
4,
5,
20].
The members of the ADF/cofilin protein family stimulate actin cycling by their ability to fragment F-actin (at low relative concentration to F-actin) or promote actin subunit dissociation from the slow-growing minus ends of F-actin leading to stimulation of cell migration [
4,
21,
22]. A number of reports have also shown that the ubiquitous non-muscle cofilin-1 variant is overexpressed in metastatic tumour cells and by severing F-actin it may lead to numerous short actin filaments exposing free plus-ends that induce the formation of new actin filaments [
3,
23,
24].
The beta-thymosins are small peptides of 43 amino acid residues. In aequous solutions Tß4 behaves like an intrinsically unstructured protein, but when bound to G-actin it attains a conformation characterized by N- and C-terminal α-helices connected by a flexible loop allowing it to span from the bottom of the actin subdomain-1 to the top of subdomain-2 [
8,
25,
26]. Polymerization of actin complexed to Tß4 is inhibited, but due the high K
D of this complex (about 1 µM) it establishes an equilibrium between F-actin, Tß4-complexed and a “pool” of non-Tß4-complexed and reusable G-actin. Therefore, a reduction of the pool of reusable actin leads to its replenishing either by F-actin disassembly or the re-establishment of the equilibrium between free and Tß4-sequestered actin. Indeed, activated nucleating proteins (like the formins or Arp2/3 complex) that promote actin polymerization will consume and reduce the concentration of “reusable” G-actin and thereby indirectly induce the dissociation of the actin:Tß4 complex in order to maintain an equilibrium between free and Tß4-bound G-actin [
27]. If however, a large fraction of actin is sequestered by an increase of Tß4, the reusable actin pool will be diminished and the assembly of new filamentous structures will be reduced or even inhibited. Indeed, it has been demonstrated that HeLa cells overexpressing Tß4 have a reduced rate of migration due to a reduction of the amount of free G-actin necessary for active cycling (treadmilling), whereas cells with a lowered Tß4 content showed a higher rate of cell migration [
28].
Conflicting results have been reported about the effect of Tß4 on the migratory activity of carcinoma cells. Thus, it was reported that overexpression of Tß4 correlated for instance with increased metastasis of lung carcinoma cells [
29]. Furthermore, thyroid and bladder cancer cells were shown to overexpress Tß10 [
30,
31], a close relative of Tß4 with identical actin sequestering activity [
32]. In contrast, an inverse correlation of Tß4 expression and tumour cell metastasis was reported for colon carcinoma [
33,
34]. These contradictory results may be due to differing concentrations of cofilin/ADF, since Tß4 cooperates with these proteins during F-actin disassembly [
28].
Therefore, we investigated whether a modulation of the intracellular concentration of cofilin-1 (subsequently termed only cofilin) and in particular of Tß4 affected the migratory activity of tumour cells with different metastatic potential, i.e., of cells possessing a different intrinsic potential for active migration. To this end we selected three established tumour cell lines: the MDA-MB-231 cells derived from triple negative breast and the 3LNLN and EB3 lines derived from colon carcinoma [
35] possessing high, intermediate and low migratory activity, respectively. These cell lines were analysed by investigating the dependence of their migratory activity before and after modulation of their intracellular cofilin concentration by transfection with wild-type or mutant variants of cofilin. Our data show that overexpression of wild-type cofilin had only a small effect on the migration of the MDA-MB-231, 3LNLN and EB3 cells. Only transfecting the constitutively active S3A-cofilin mutant stimulated migration of the MDA-MB-231 cells.
In contrast, up- or down-regulation of Tß4 by transfection led to reduction or stimulation of tumour cell migration, respectively. Since previous reports indicated that the migratory activity of tumour cells was also modulated by extracellular Tß4, we analysed the effect of extracellular His-tagged-Tß4 and native Tß4 on the migratory activity of the selected tumour cells. Our data showed a biphasic response of their migratory activity when exposed to increasing Tß4: a stimulation of migration up to 0.24 µM Tß4 and an inhibition at higher (2.4 µM) Tß4 concentrations.
Furthermore, we demonstrated by immunostaining the colocalisation of Tß4 with the integrin linked kinase (ILK) that previous data had shown to stimulate the ILK-AKT/PKB (protein kinase B) signalling pathway [
36,
37]. Indeed, immunostaining of the 3LNLN colon carcinoma cells exposed to 0.24 µM Tß4 for phosphorylated AKT/PKB indicated an increase of P-Ser473-AKT-1 and P-Ser474-AKT-2 immunoreactivities. Activated AKT/PKB kinases are known to stimulate cell migration and the secretion of metalloproteases (MMPs [
38]). Indeed, at 0.24 µM extracellular Tß4 we detected increased MMP expression and secretion. In contrast, exposure of 3LNLN cells to 2.8 µM His-tagged-Tß4 led to cell rounding, inhibition of migration, and induction of apoptosis of many cells.
4. Discussion
The metastasis of cancer cells and the subsequent colonization in distant organs depends on cell intrinsic and extrinsic factors and are the main causes of death of cancer patients. Cancer cells originate by trans-differentiation of epithelial to mesenchymal cells (EMT), which after reorganisation of their actin cytoskeleton regain the motility necessary for metastatic invasiveness. However, it has not been possible to define a common trait or genetic signature for the metastatic behaviour of cancer cells [
1]. Even tumour cells originating form a particular tumour may contain cell subpopulations with differing metastatic activity. The dynamic rearrangements of the actin cytoskeleton, the transient formation of actin-rich membrane extensions and of attachment points to ECM components are essential intrinsic prerequisites for tumour cell metastasis. The dynamic behaviour of their actin cytoskeleton is regulated by a multitude of actin binding proteins (ABPs) and furthermore be modified by the tumour micro-environment formed by ECM components, cytokines and growth factors [
1,
58]. Here we investigated under cell culture conditions the effects of cofilin and in particular of the actin binding peptide thymosin ß4 on the migratory behaviour of three established cell lines derived from breast and colorectal cancer possessing different metastatic potential.
Cofilin is a member of the cofilin/ADF (actin depolymerizing factor) family and has been shown to stimulate F-actin severing and/or cycling by promoting actin subunit dissociation from the filament minus ends [
21,
22]. These activities have been shown to be increased in migrating tumour cells [
23]. Though the three selected tumour cells exhibited different migratory activities, they contained almost equal amounts of cofilin suggesting that their varying migratory activity was not due to differing cofilin content. Nevertheless, we investigated by transfection experiments the effects of wild-type, constitutively active or inactive cofilin variants assuming that differences in cofilin’s state activation might be responsible for the differences of their migratory activity. Our data, however, showed that only transfection with the constitutively active S3A-cofilin mutant led to a slight increase in migratory activity, whereas wild-type and the constitutively inactive S3D-cofilin mutant exerted only a small stimulation or no effect, respectively. These data suggested that de- or phosphorylation of the endogenous cofilin might have re-setted the concentration of active cofilin.
In contrast, a completely different effect on the migratory activity of the analysed tumour cells was observed when modulating the intracellular concentration of Tß4 by transfection. The Tß4-shRNA or Tß4-IRES vectors inducing a decrease or increase of intracellular Tß4 led to a clear stimulation or reduction, respectively, of their migratory activity. These results are in agreement with the inverse correlation of their migratory activity and endogenous Tß4 concentrations as determined by immunoblotting and also suggested that intracellular Tß4 is not regulated by post-translational modifications.
It should also be mentioned that the HPLC-analyses of the intracellular concentrations of ß-thymosins showed that these tumour cells contained in addition to Tß4 also Tß10, a closely related variant possessing 65% sequence identity to Tß4 and identical actin sequestering properties [
32]. Though no specific properties or activities are known for Tß10, it has been reported to be overexpressed in a number of tumour cells [
30,
31]. Transfection with the Tß4-shRNA vector might have reduced only the Tß4 isoform, but led to an increase of the migratory activity probably due to a decrease in total ß-thymosin concentration. Thus, the effects obtained after transfection with these vectors seemed to be in full agreement with their G-actin sequestering activities.
Transfection experiments usually affect only a small fraction of the targeted cells. Since previous reports had indicated that extracellular Tß4 supports survival of cardiomyocytes [
36] and is even taken up these cells, we exposed the 3LNLN and MDA-MB-231 tumour cells to increasing concentrations of His-tagged-Tß4 (varying from zero to 2.8 µM). The data obtained showed a biphasic response of their migratory activity with maximal stimulation of migration at 0.24 µM and inhibition at 2.8 µM His-Tß4. Immunostaining after His-Tß4 exposure with anti-His demonstrated the intracellular presence of His-Tß4 in all exposed cells indicating either its passive diffusion or active uptake.
The inhibition of 3LNLN (
Figure 5) and MDA-MB-231 (
Figure S5) cell migration at high extracellular Tß4 (2.4 to 2.8 µM) was most probably due to disassembly of their actin filaments as observed by Tß4 overexpression after Tß4-pIRES transfection. Assuming an intracellular Tß4 concentration of 0.33 µM in 3LNLN cells before His-Tß4 exposure (see
Table 1), it appears possible that at 2.4 to 2.8 µM extracellular His-Tß4 its intracellular concentration increased high enough to lead to an almost complete microfilament disassembly and thereby to inhibition of cell migration. Furthermore, we observed cell rounding and occasionally an accumulation of anti-His immunoreactivity in the nuclei of rounded cells (see
Figure 5B–D). The presence of Tß4 in cell nuclei has been reported previously [
59], however, the presumed accumulation of His-Tß4 in the nuclei and cytoplasm might have led to F-actin depolymerisation in both organelles and led to induction of apoptosis [
60].
The stimulatory effect up to 0.24 µM extracellular Tß4 on cell migration appears more difficult to explain, since the stimulating extracellular His-Tß4 concentrations were lower than the intracellular Tß4 (0.33 µM) except an active uptake of extracellular His-Tß4 occurred as observed at 2.8 µM. Indeed, anti-His immunostaining at 0.24 µM extracellular His-Tß4 showed intracellular anti-His immunoreactivity concentrated at presumed focal adhesion points (see
Figure 4C). Alternatively, it has been suggested that Tß4 interacted with a putative receptor protein [
52], however, a beta-thymosin specific receptor has not yet been identified.
Nevertheless, tumour cell exposure to 0.24 µM extracellular His-Tß4 appeared to initiate the formation of the ILK-Tß4-PINCH-parvin complex leading to activation of the integrin-linked kinase (ILK) that activated the ILK-AKT/PKB pathway by phosphorylation of Ser473 of AKT1 and Ser474 of AKT2 (also termed phosphokinases B; PKBs) as demonstrated by immunostaining phospho-specific anti-AKT antibodies. Their phosphorylation is known to lead to increased cell survival by inhibition of apoptosis, elevated migratory activity and expression and secretion of matrix-metalloproteases (as shown in Figs. 7and 8) further supporting migration [
37,
38].
Thus, our more system biological approach shows a concentration dependent effect of extracellular Tß4 on cell migration and survival and might explain the divergent reports of the effect of increasing Tß4 concentration of tumour cell migration. Immunohistochemical analyses of biopsy material of colon tumour areas have shown a higher Tß4 immunoreactivity of the tumour cells in comparison to non-transformed cells [
30,
55]. We assume that these tumour cells corresponded to the tumour cells exposed to 0.24 µM extracellular His-Tß4 in this study. Indeed, stimulation of ILK and AKT/PBK was also shown for the ex-vivo colon tumour cells [
30,
55].
So far, no reports have given evidence that in vivo tumour cell migration was stimulated by extracellular Tß4. Furthermore, the serum Tß4 concentration was determined to be only about 0.04 mg/ml (= 8 nM) [
43], i.e., far below the Tß4 concentration found in this report to stimulate tumour cell migration. However, after blood clotting Tß4 was found to increase to 16.3 mg/ml (= 3.3 µM) [
49] that may be due to Tß4 release from platelets and/or during the formation of extracellular traps (NETs) by neutrophils, which possess also a high Tß4 content of Tß4 [
61,
62]. Therefore, it is possible that under different regimes of chemotherapy neutrophils are induced to form NETs increasing the extracellular concentration of Tß4 to a level that supports tumour cell migration. Recent publications have reported contradictory effects of NETs on tumour cell migration [see reviews 63,64], which might again be due to varying concentrations of the extracellular Tß4 attained by different extents or number of NET-forming neutrophils. Therefore, more investigations are necessary to elucidate the in vivo “dual” role of the beta-thymosins possibly released by neutrophils. In addition, it might be worth considering the possibility to inhibit tumour cell migration/metastasis by applying Tß4 or its active fragments as a therapeutic measure.
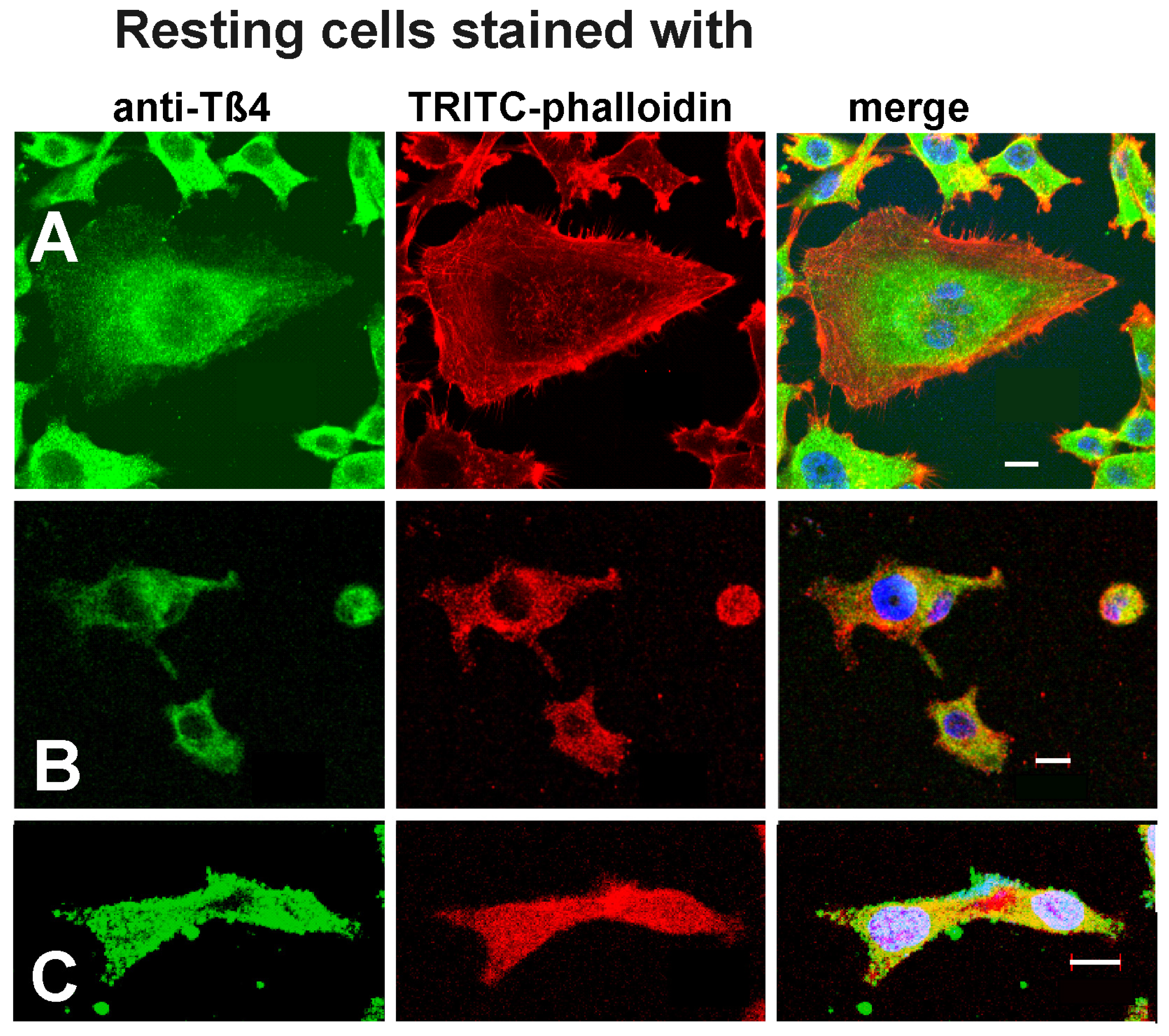
Figure 1.
Immunostaining of (A) MDA-MB-231, (B) 3LNLN and (C) EB3 cells with anti-Tß4 and TRITC-phalloidin. Bars represent 10 µm.
Figure 1.
Immunostaining of (A) MDA-MB-231, (B) 3LNLN and (C) EB3 cells with anti-Tß4 and TRITC-phalloidin. Bars represent 10 µm.
Figure 2.
Effect of the modulation of the Tß4 concentration by transfection on tumour cell migration. (A) Anti-Tß4 Western (boxed) and an immunodot blot using the mouse monoclonal anti-Tß4 of homogenates of the MDA-MB-231, EB 3, and 3LNLN cells (immunodot analysis was repeated five times). Note the differences in immunoreactivity between Western and immunodot blots (for further details see text). (B–D) Quantitation of cell migration of the three tumour cell lines by using the transwell assay (Boyden chamber). Cells were transfected with vectors leading to expression of only EGFP, with pIRES-Tß4 leading to overexpression of Tß4 and with Tß4-specific-siRNA as described in Materials and methods. 24 hours after transfection 50,000 cells were loaded on the transwell insert and further treated as described. After further 24 h the number of cells migrated through the transwell membrane were counted. Transfected cells were identified by EGFP-fluorescence and non-transfected cells by Hoechst 33342 nuclear stain using confocal microscopy. Images give representative areas showing migrated cells: (B) MDA-MB-231 cells; (C) 3LNLN cells and (D) EB3 cells. The percental evaluation (% migrated cells of total number of cells applied: 50,000) together with the standard deviations from four independent experiments of the different conditions is shown in the graphs below the confocal images. Note the different scaling of the ordinates showing percental migration. Note that transfection with a vector leading to expression of only EGFP led only a slight reduction of the migration of control cells. (E) Table compiling the percental migration activity (± standard deviations as given in the figures from four different experiments) of transfected, i.e., EGFP expressing cells.
Figure 2.
Effect of the modulation of the Tß4 concentration by transfection on tumour cell migration. (A) Anti-Tß4 Western (boxed) and an immunodot blot using the mouse monoclonal anti-Tß4 of homogenates of the MDA-MB-231, EB 3, and 3LNLN cells (immunodot analysis was repeated five times). Note the differences in immunoreactivity between Western and immunodot blots (for further details see text). (B–D) Quantitation of cell migration of the three tumour cell lines by using the transwell assay (Boyden chamber). Cells were transfected with vectors leading to expression of only EGFP, with pIRES-Tß4 leading to overexpression of Tß4 and with Tß4-specific-siRNA as described in Materials and methods. 24 hours after transfection 50,000 cells were loaded on the transwell insert and further treated as described. After further 24 h the number of cells migrated through the transwell membrane were counted. Transfected cells were identified by EGFP-fluorescence and non-transfected cells by Hoechst 33342 nuclear stain using confocal microscopy. Images give representative areas showing migrated cells: (B) MDA-MB-231 cells; (C) 3LNLN cells and (D) EB3 cells. The percental evaluation (% migrated cells of total number of cells applied: 50,000) together with the standard deviations from four independent experiments of the different conditions is shown in the graphs below the confocal images. Note the different scaling of the ordinates showing percental migration. Note that transfection with a vector leading to expression of only EGFP led only a slight reduction of the migration of control cells. (E) Table compiling the percental migration activity (± standard deviations as given in the figures from four different experiments) of transfected, i.e., EGFP expressing cells.
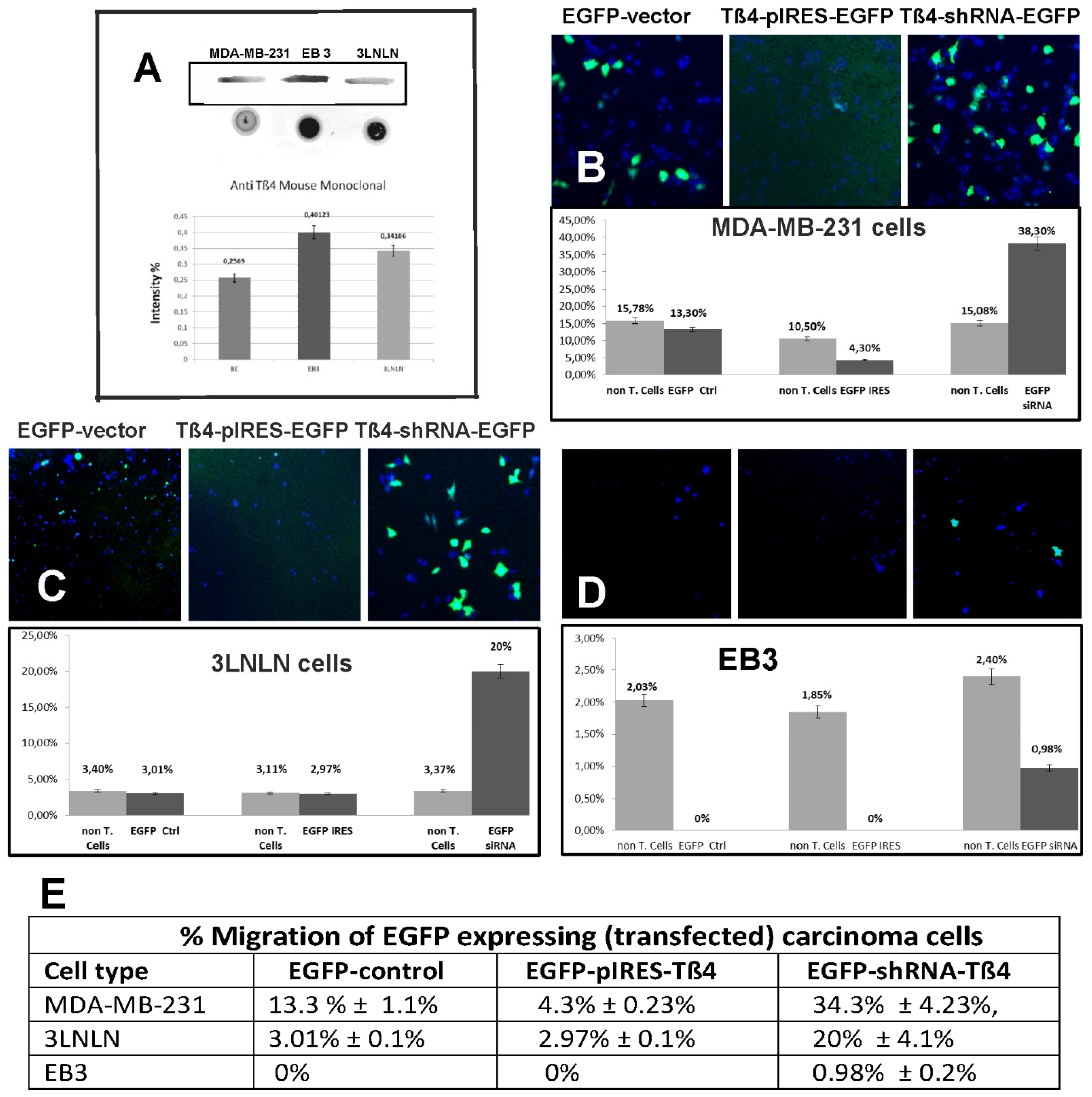
Figure 3.
Migration of the tumour cells after transfection with different Tß4-vectors out the agarose drops. (A) MBA-MB-231, (B) 3LNLN, and (C) EB3 cells after transfection with EGFP-control clone out of agarose drops. For further details see text. Red arrows point to the margin of the agarose drops and yellow arrows to the agarose free zone, into which the cells migrated. These cells were counted either as non-transfected or as transfected identified by their EGFP-fluorescence. (D to G) 50,000 3LNLN cells were included in agarose drops 24 h after transfection with the Tß4-vectors indicated (non-transfected, EGFP-vector, pIRES-EGFP-Tß4, and Tß4-shRNA-EGFP vector) and after further 72 h the cells migrating out the agarose were photographed using the confocal microscopy and counted. Yellow lines give the boundaries of the agarose drops. Yellow arrows point to non-transfected and red arrows to transfected cells. (H,I) Staining of transfected 3LNLN cells that migrated out the agarose with TRITC-phalloidin. (H) 3LNLN cells transfected with pIRES-Tß4 leading to additional expression of EGFP-Tß4 (EGFP-fluorescence). (H’) TRITC-phalloidin staining; note the partial disassembly of the F-actin cytoskeleton in the transfected (EGFP-positive) cells (arrow). (I) 3LNLN cells transfected with Tß4-shRNA-EGFP vector. (I’) TRITC-phalloidin staining; note the broader lamellipodial structure containing densely packed actin filaments (arrows). Merged images give also the nuclear staining with Hoechst 33342. Bars correspond to 20 µm.
Figure 3.
Migration of the tumour cells after transfection with different Tß4-vectors out the agarose drops. (A) MBA-MB-231, (B) 3LNLN, and (C) EB3 cells after transfection with EGFP-control clone out of agarose drops. For further details see text. Red arrows point to the margin of the agarose drops and yellow arrows to the agarose free zone, into which the cells migrated. These cells were counted either as non-transfected or as transfected identified by their EGFP-fluorescence. (D to G) 50,000 3LNLN cells were included in agarose drops 24 h after transfection with the Tß4-vectors indicated (non-transfected, EGFP-vector, pIRES-EGFP-Tß4, and Tß4-shRNA-EGFP vector) and after further 72 h the cells migrating out the agarose were photographed using the confocal microscopy and counted. Yellow lines give the boundaries of the agarose drops. Yellow arrows point to non-transfected and red arrows to transfected cells. (H,I) Staining of transfected 3LNLN cells that migrated out the agarose with TRITC-phalloidin. (H) 3LNLN cells transfected with pIRES-Tß4 leading to additional expression of EGFP-Tß4 (EGFP-fluorescence). (H’) TRITC-phalloidin staining; note the partial disassembly of the F-actin cytoskeleton in the transfected (EGFP-positive) cells (arrow). (I) 3LNLN cells transfected with Tß4-shRNA-EGFP vector. (I’) TRITC-phalloidin staining; note the broader lamellipodial structure containing densely packed actin filaments (arrows). Merged images give also the nuclear staining with Hoechst 33342. Bars correspond to 20 µm.

Figure 4.
Effect of extracellularly added His-Tß4 or His-scTß4 (scrambled peptide, negative control) on the migration of 3LNLN cells out the agarose drops. (A) Gives the percentages of cells migrated in dependence on the extracellular concentration of His-Tß4 (green bars) and His-scTß4 (red bars). Note the clear biphasic response on His-Tß4, whereas His-scTß4 did not elicit any effect. (B) Gives the sequences of native Tß4 and the scrambled version of Tß4. (C-E) Immunostaining of 3LNLN cells having migrated out of agarose drops at 0.24 µM His-Tß4 with anti-Tß4 (green) and anti-His (red). (C) Note high Tß4 concentration within the cytoplasm and the tailing rear (arrow heads), but a less intense anti-Tß4 immunoreactivity within a lamellipodium. (C’and C’’) Similar anti-His staining, however, localisation clear nuclear presence as also visible in the merged image (arrow heads); arrows point to colocalisation of anti-Tß4 and -His-staining. (D) Anti-Tß4 and TRITC-phalloidin staining shows punctate accumulation of anti-Tß4 staining (arrows) that at least partially colocalize with TRITC-phalloidin staining, which also shows a prominent F-actin cytoskeleton concentrating around the cell periphery. Arrow heads point to presumed areas of cell contacts, which are also Tß4-positive. (E) Merged image of a single cell, which clearly shows peripheral Tß4-immunoreactivity colocalizing with TRITC-phalloidin staining (arrow head). (F) Immunostaining of 3LNLN cells exposed 2.4 µM His-Tß4 with anti-His (green) and TRITC-phalloidin after removal of the agarose drop. Note that most of the cells have become rounded and show an intense cytoplasmic anti-His staining. Within the rounded cells cytoplasmic and possibly nuclear anti-His and phalloidin positive dots (aggregates) are visible. Bars in (C-E) correspond to 20 µm and (F) to 50 µm.
Figure 4.
Effect of extracellularly added His-Tß4 or His-scTß4 (scrambled peptide, negative control) on the migration of 3LNLN cells out the agarose drops. (A) Gives the percentages of cells migrated in dependence on the extracellular concentration of His-Tß4 (green bars) and His-scTß4 (red bars). Note the clear biphasic response on His-Tß4, whereas His-scTß4 did not elicit any effect. (B) Gives the sequences of native Tß4 and the scrambled version of Tß4. (C-E) Immunostaining of 3LNLN cells having migrated out of agarose drops at 0.24 µM His-Tß4 with anti-Tß4 (green) and anti-His (red). (C) Note high Tß4 concentration within the cytoplasm and the tailing rear (arrow heads), but a less intense anti-Tß4 immunoreactivity within a lamellipodium. (C’and C’’) Similar anti-His staining, however, localisation clear nuclear presence as also visible in the merged image (arrow heads); arrows point to colocalisation of anti-Tß4 and -His-staining. (D) Anti-Tß4 and TRITC-phalloidin staining shows punctate accumulation of anti-Tß4 staining (arrows) that at least partially colocalize with TRITC-phalloidin staining, which also shows a prominent F-actin cytoskeleton concentrating around the cell periphery. Arrow heads point to presumed areas of cell contacts, which are also Tß4-positive. (E) Merged image of a single cell, which clearly shows peripheral Tß4-immunoreactivity colocalizing with TRITC-phalloidin staining (arrow head). (F) Immunostaining of 3LNLN cells exposed 2.4 µM His-Tß4 with anti-His (green) and TRITC-phalloidin after removal of the agarose drop. Note that most of the cells have become rounded and show an intense cytoplasmic anti-His staining. Within the rounded cells cytoplasmic and possibly nuclear anti-His and phalloidin positive dots (aggregates) are visible. Bars in (C-E) correspond to 20 µm and (F) to 50 µm.
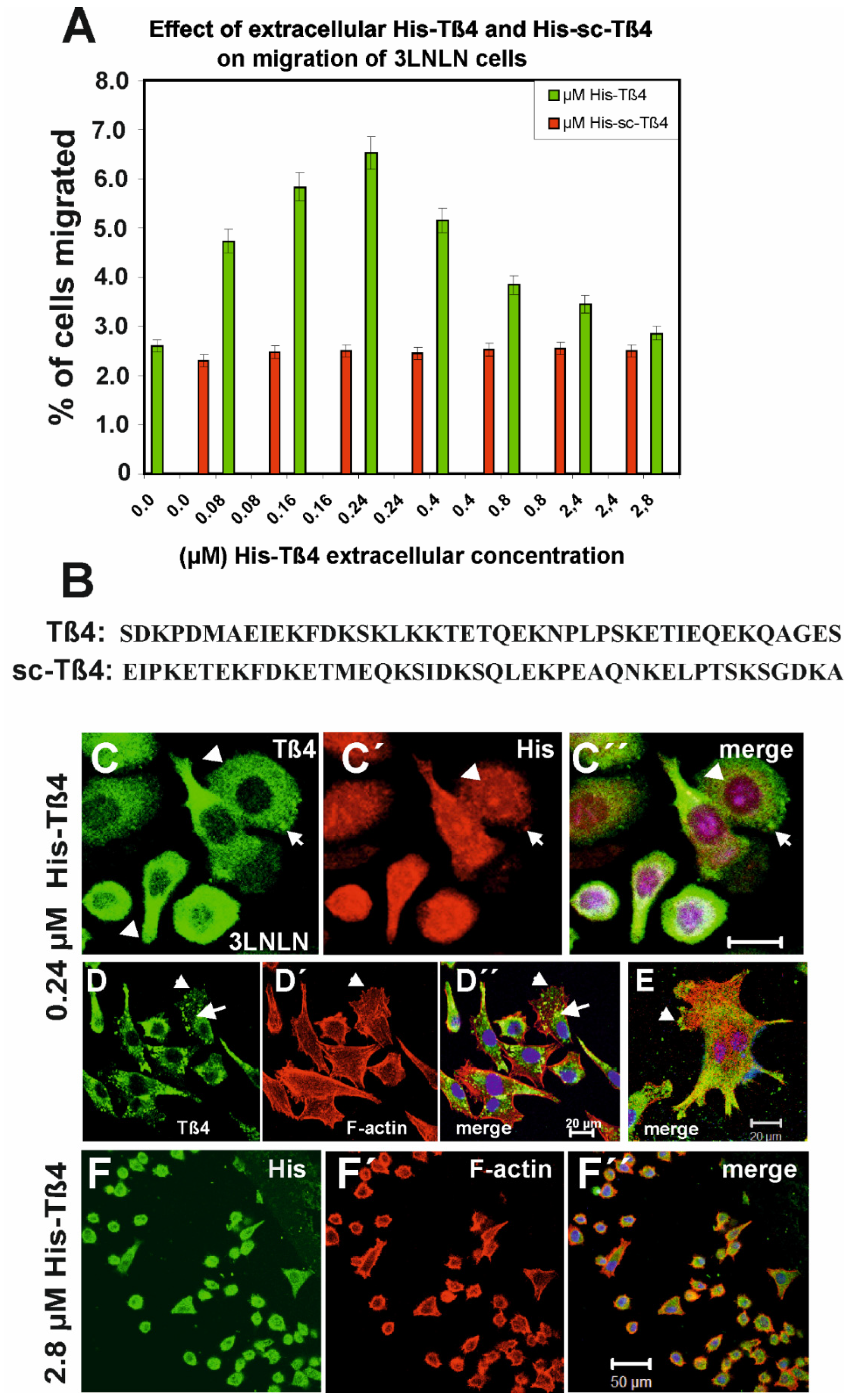
Figure 5.
Imunostaining of 3LNLN cells exposed to 2.8 µM His-Tß4. (A,B) Imunostaining of 3LNLN cells exposed to 2.8 µM His-Tß4 with anti-His (green) and TRITC-.phalloidin. (A) Note that all cells are rounded. (B) Single cell showing aggregated mainly cytoplasmic actin and an accumulation of the anti-His immunoreactivity within the nucleus. (C,C’) Phase contrast image of a single cells with many peripheral membrane blebs reminiscent of apoptosis as verified by anti-active caspase-3 staining (C’). (D) 3LNLN cells identically exposed to 2.8 µM His-sc-Tß4. Note the absence of cell rounding and an intact appearing actin cytoskeleton, but a prominent anti-His staining of the cell nuclei. (E) 3LNLN cells transfected with pIRES-Tß4-EGFP vector leading to overexpression of Tß4. Note that a number of EGFP-Tß4 expressing cells have rounded and formed plasma membrane blebs as also indicated by phase contrast (E’’). These cells are positively stained by anti-active caspase-3 (E’). (E’’’) Gives merged image additionally stained by Hoechst 33342. Bars correspond 10 µm (B), 20 µm (C,E), and 50 µm (A,D).
Figure 5.
Imunostaining of 3LNLN cells exposed to 2.8 µM His-Tß4. (A,B) Imunostaining of 3LNLN cells exposed to 2.8 µM His-Tß4 with anti-His (green) and TRITC-.phalloidin. (A) Note that all cells are rounded. (B) Single cell showing aggregated mainly cytoplasmic actin and an accumulation of the anti-His immunoreactivity within the nucleus. (C,C’) Phase contrast image of a single cells with many peripheral membrane blebs reminiscent of apoptosis as verified by anti-active caspase-3 staining (C’). (D) 3LNLN cells identically exposed to 2.8 µM His-sc-Tß4. Note the absence of cell rounding and an intact appearing actin cytoskeleton, but a prominent anti-His staining of the cell nuclei. (E) 3LNLN cells transfected with pIRES-Tß4-EGFP vector leading to overexpression of Tß4. Note that a number of EGFP-Tß4 expressing cells have rounded and formed plasma membrane blebs as also indicated by phase contrast (E’’). These cells are positively stained by anti-active caspase-3 (E’). (E’’’) Gives merged image additionally stained by Hoechst 33342. Bars correspond 10 µm (B), 20 µm (C,E), and 50 µm (A,D).
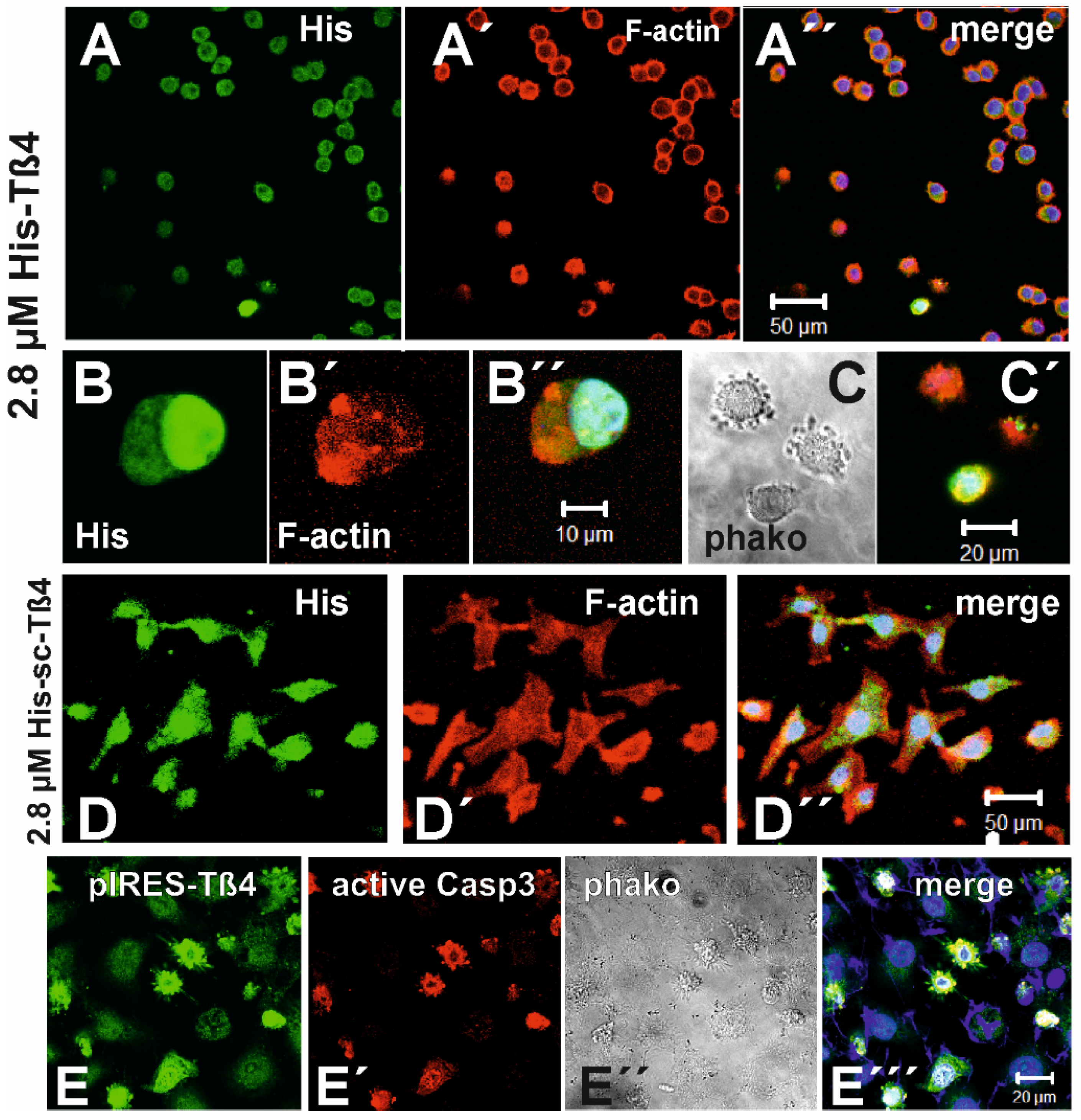
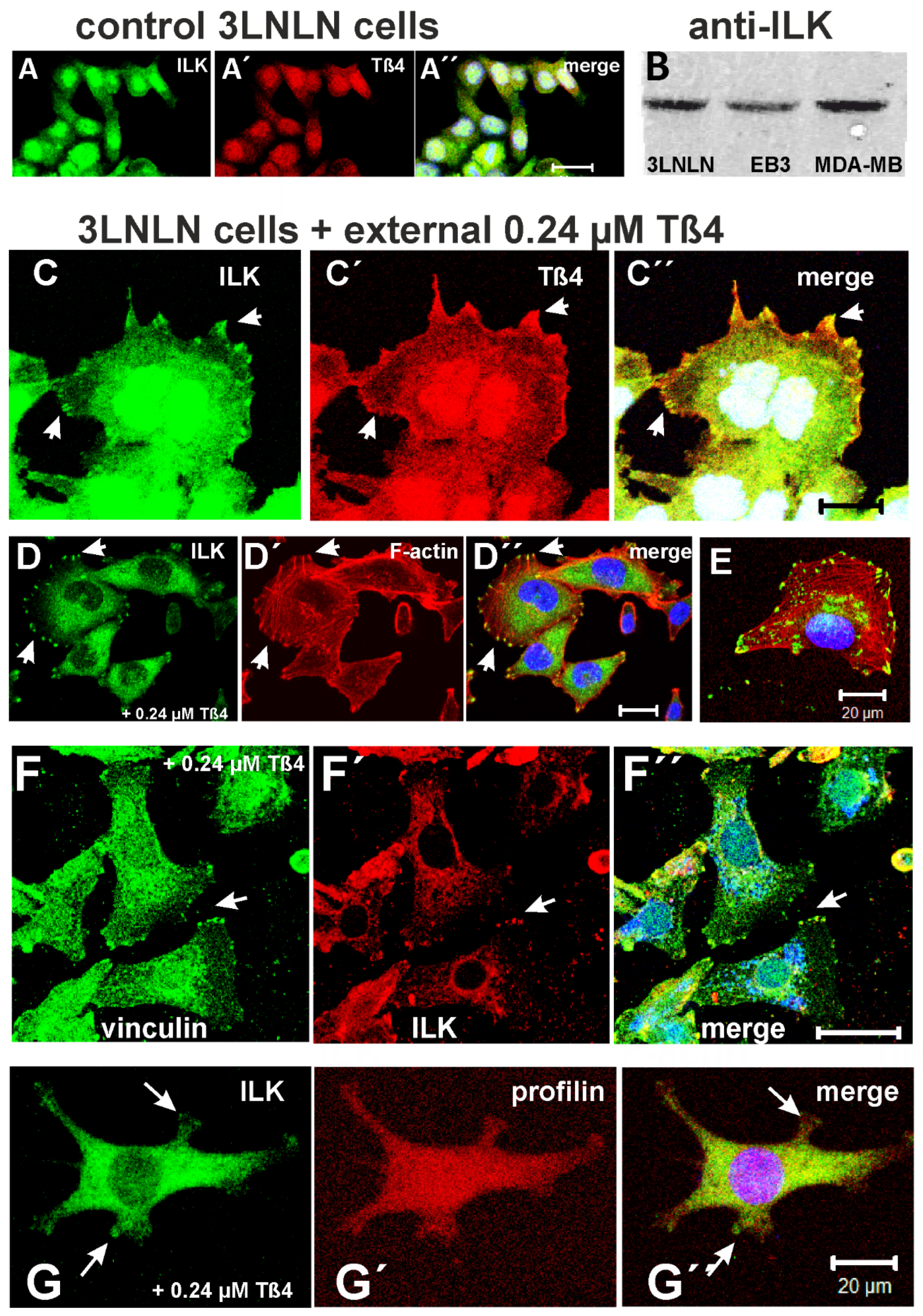
Figure 6.
Immunostaining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-ILK. (A) Control 3LNLN cells immunostained with anti-ILK and anti-Tß4. Note very little peripheral staining of anti-ILK and -Tß4. (B) Western blot of resting 50 µg of 3LNLN, EB3, and MDA-MB-231 cell homogenates with anti-ILK antibody showing a single band at about 55kDa. (C-F) after exposure to 0.24 µM Tß4 for 24 h were immunostained with anti-ILK and together (A,C) with anti-Tß4, (D,E) by TRITC-phalloidin for F-actin, (F) anti-vinculin, and (G) anti-profilin. (B) Western blot of resting 3LNLN, EB3, and MDA-MB-231 cell homogenates with anti-ILK antibody showing a single band at about 55kDa. For further details see text. All bars correspond to 20 µM µm.
Figure 6.
Immunostaining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-ILK. (A) Control 3LNLN cells immunostained with anti-ILK and anti-Tß4. Note very little peripheral staining of anti-ILK and -Tß4. (B) Western blot of resting 50 µg of 3LNLN, EB3, and MDA-MB-231 cell homogenates with anti-ILK antibody showing a single band at about 55kDa. (C-F) after exposure to 0.24 µM Tß4 for 24 h were immunostained with anti-ILK and together (A,C) with anti-Tß4, (D,E) by TRITC-phalloidin for F-actin, (F) anti-vinculin, and (G) anti-profilin. (B) Western blot of resting 3LNLN, EB3, and MDA-MB-231 cell homogenates with anti-ILK antibody showing a single band at about 55kDa. For further details see text. All bars correspond to 20 µM µm.
Figure 7.
Phosphorylation of AKT/PKB by exposure to 0.24 µM Tß4. (A) Dot blots of cell homogenates of 3LNLN cells before and after exposure for 24 h to 0.24 µM or 2.4 µM extracellular Tß4 using the anti-ILK antibody, or antibodies specific for P-Ser473-AKT1 or for P-Ser474-AKT2. (B) Immunostaining of control 3LNLN cells with anti-AKT antibody and Hoechst 33342 (see merged image). Note the presence of AKT proteins within the cytoplasm. (C) Double immunostaining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-Tß4 and anti-P-Ser473-AKT1. Note their peripheral colocalisation (also indicated by arrows). (D) Staining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-P-Ser474-AKT2 and with TRITC-phalloidin. Note peripheral colocalisation also indicated by an arrow. (E) Staining of 3LNLN cells exposed to 2.4 µM Tß4 with anti-P-Ser474-AKT2 and with TRITC-phalloidin. Note no clear peripheral AKT-staining, instead dotted AKT-staining within the cytoplasm and weaker peripheral F-actin staining. All bars correspond to 20 µm.
Figure 7.
Phosphorylation of AKT/PKB by exposure to 0.24 µM Tß4. (A) Dot blots of cell homogenates of 3LNLN cells before and after exposure for 24 h to 0.24 µM or 2.4 µM extracellular Tß4 using the anti-ILK antibody, or antibodies specific for P-Ser473-AKT1 or for P-Ser474-AKT2. (B) Immunostaining of control 3LNLN cells with anti-AKT antibody and Hoechst 33342 (see merged image). Note the presence of AKT proteins within the cytoplasm. (C) Double immunostaining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-Tß4 and anti-P-Ser473-AKT1. Note their peripheral colocalisation (also indicated by arrows). (D) Staining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-P-Ser474-AKT2 and with TRITC-phalloidin. Note peripheral colocalisation also indicated by an arrow. (E) Staining of 3LNLN cells exposed to 2.4 µM Tß4 with anti-P-Ser474-AKT2 and with TRITC-phalloidin. Note no clear peripheral AKT-staining, instead dotted AKT-staining within the cytoplasm and weaker peripheral F-actin staining. All bars correspond to 20 µm.
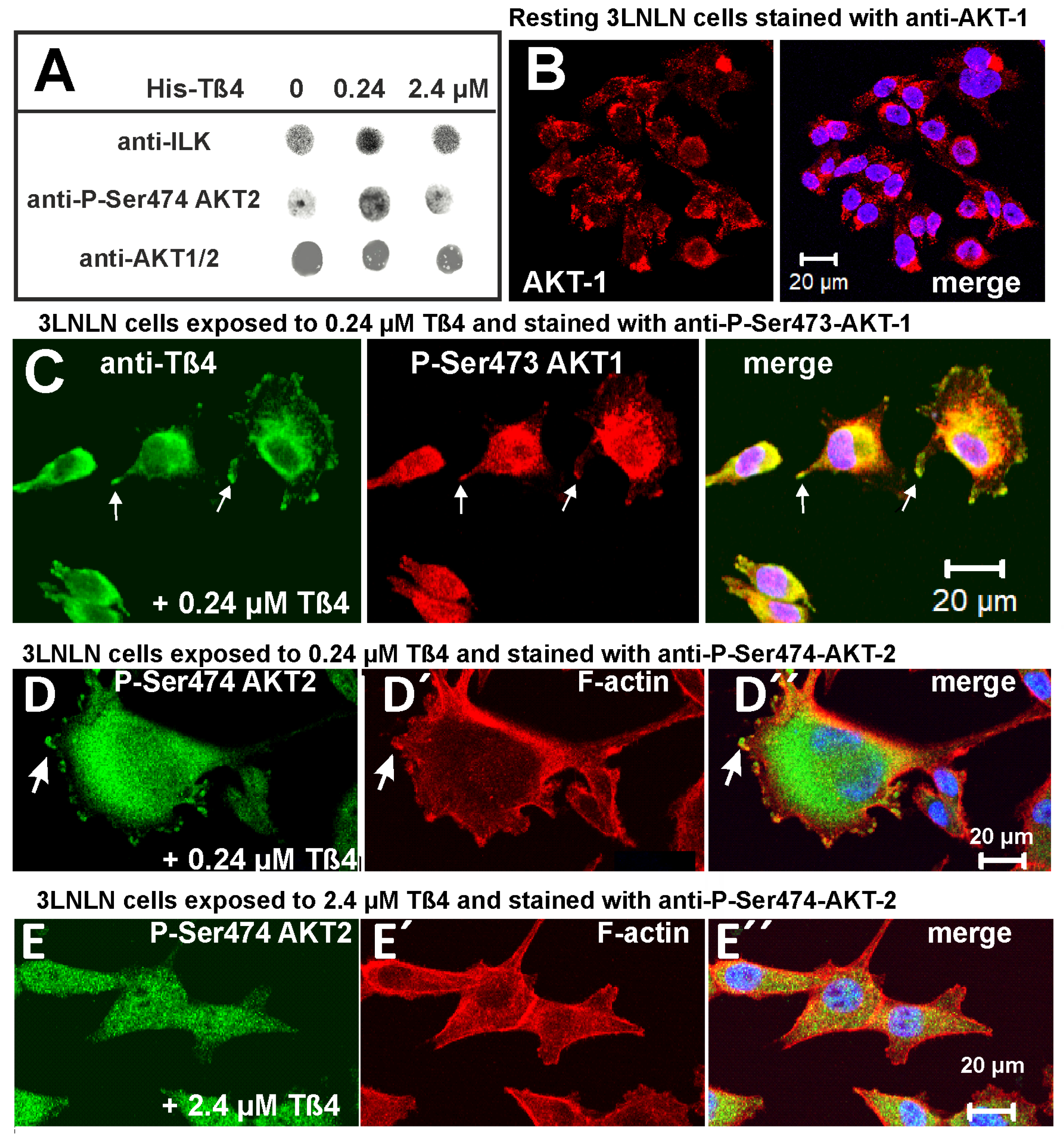
Figure 8.
Expression of TIMP2, MMP2 and MMP9 in the different tumour cell lines in the absence of extracellular Tß4. (A) Dot immunoblots of homogenates and cell medium (50 µg protein/dot) of the three cell lines immunostained with antibodies against MMP2 and TIMP2. (B) Table giving percental evaluation by densitometry (for details see text). (C,D) Double immunostaining of (C) MDA-MB-231 and (D) EB3 cells with anti-TIMP2 and -MMP2. Merged images together with Hoechst 33342 staining (for details see text). All bars correspond to 10 µm. (E to I) Expression of MMP2, MMP9 and TIMP2 by 3LNLN cells exposed to increasing Tß4 concentrations. (E) Table of densitometric evaluation of immunodot blots of 3LNLN cell homogenates and respective culture media stained with anti-MMP2 and -MMP9. The values obtained at zero (no exposure to) Tß4 were set as 100%. Note the intracellular increase in MMP2 and MMP9 expression by exposure to extracellular Tß4. (F to H) Immunostaining of 3LNLN cells with anti-TIMP2 and -MMP2 after exposure to zero Tß4 (F), to 0.24 µM Tß4 (G) and to 2.4 µM Tß4 (H). Merged images with Hoechst 33342 staining. (I) Immunostaining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-Tß4 and -MMP9. Bars correspond to 10 µm.
Figure 8.
Expression of TIMP2, MMP2 and MMP9 in the different tumour cell lines in the absence of extracellular Tß4. (A) Dot immunoblots of homogenates and cell medium (50 µg protein/dot) of the three cell lines immunostained with antibodies against MMP2 and TIMP2. (B) Table giving percental evaluation by densitometry (for details see text). (C,D) Double immunostaining of (C) MDA-MB-231 and (D) EB3 cells with anti-TIMP2 and -MMP2. Merged images together with Hoechst 33342 staining (for details see text). All bars correspond to 10 µm. (E to I) Expression of MMP2, MMP9 and TIMP2 by 3LNLN cells exposed to increasing Tß4 concentrations. (E) Table of densitometric evaluation of immunodot blots of 3LNLN cell homogenates and respective culture media stained with anti-MMP2 and -MMP9. The values obtained at zero (no exposure to) Tß4 were set as 100%. Note the intracellular increase in MMP2 and MMP9 expression by exposure to extracellular Tß4. (F to H) Immunostaining of 3LNLN cells with anti-TIMP2 and -MMP2 after exposure to zero Tß4 (F), to 0.24 µM Tß4 (G) and to 2.4 µM Tß4 (H). Merged images with Hoechst 33342 staining. (I) Immunostaining of 3LNLN cells exposed to 0.24 µM Tß4 with anti-Tß4 and -MMP9. Bars correspond to 10 µm.
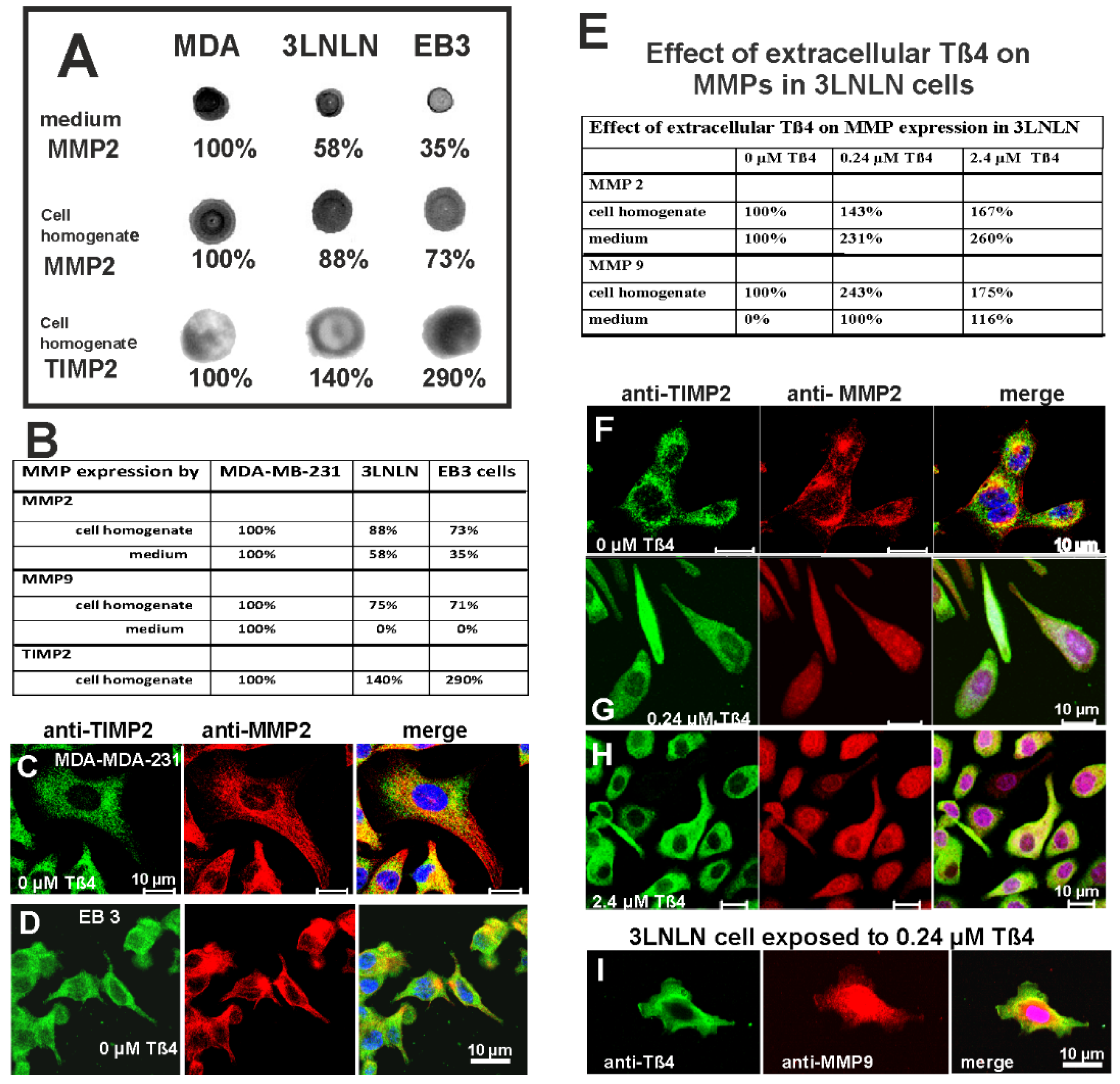
Table 1.
Calculation of the intracellular beta-thymosin concentrations in the three cell lines as determined by HPLC (mean values from two measurements). For original HPLC data see Supplementary data (
Figure S3).
Table 1.
Calculation of the intracellular beta-thymosin concentrations in the three cell lines as determined by HPLC (mean values from two measurements). For original HPLC data see Supplementary data (
Figure S3).
| Cell type |
MDA-MB-231 |
3LNLN |
EB3 |
| Intracellular Tß4 |
0.306 µM |
0.33 µM |
0.466 µM |
| Intracellular Tß10
|
0.353 µM |
0.62 µM |
0.66 µM |
Table 2.
Changes of the relative concentrations of the proteins indicated in cell homogenates of 3LNLN cells before and after exposure to His-Tß4 as determined by dot immunoblotting (see also
Figure 8A).
Table 2.
Changes of the relative concentrations of the proteins indicated in cell homogenates of 3LNLN cells before and after exposure to His-Tß4 as determined by dot immunoblotting (see also
Figure 8A).
| |
Control |
0.24 µM His-Tß4 |
2.4 µM His-Tß4 |
| anti-ILK |
100% |
141% |
107% |
| anti-P-Ser474-AKT2 |
100% |
208% |
121% |
| anti-AKT1,2 |
100% |
85% |
72% |
| anti-actin |
100% |
97% |
99% |
| anti-tubulin |
100% |
98% |
98% |