Submitted:
31 December 2024
Posted:
31 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
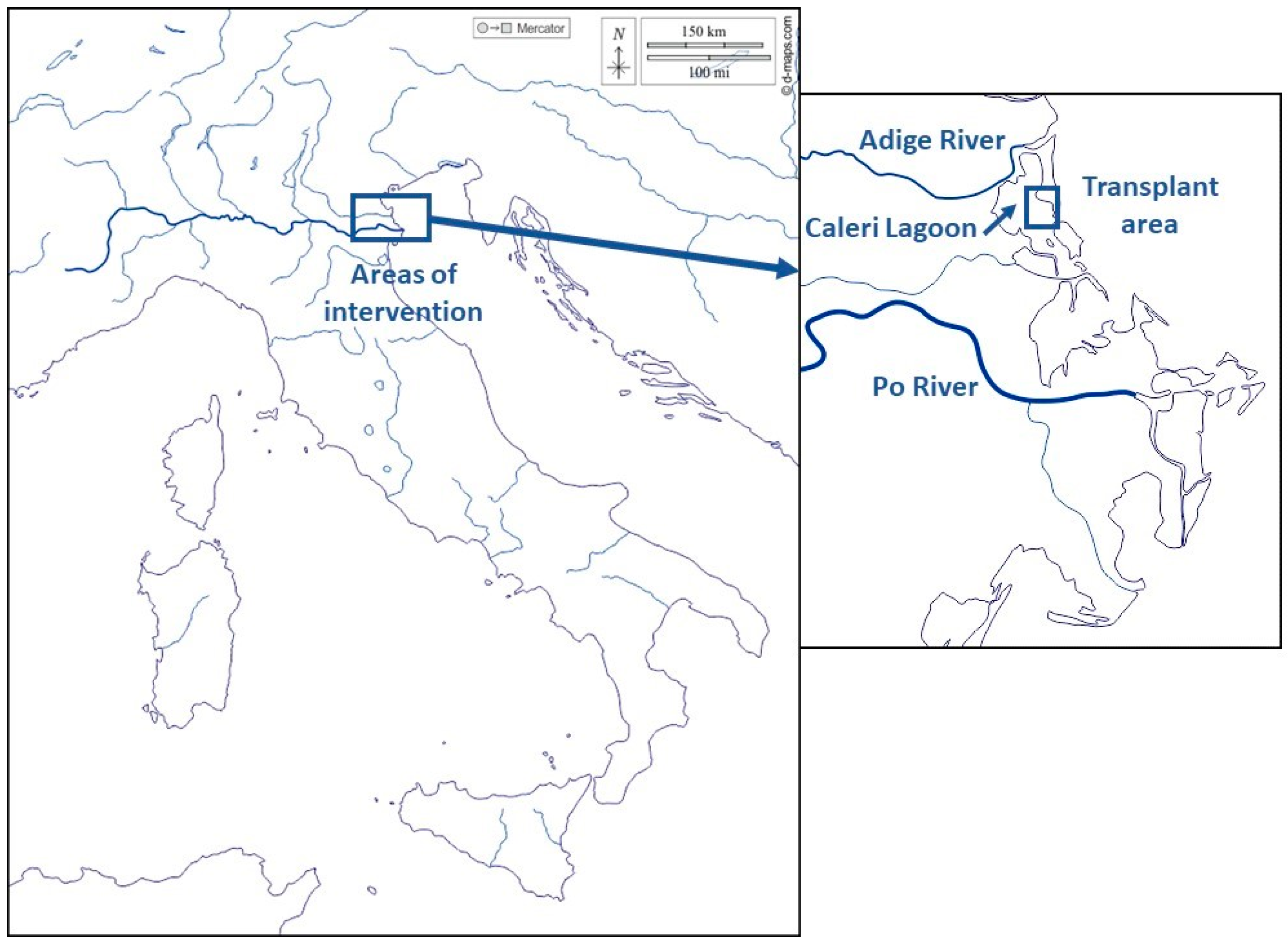
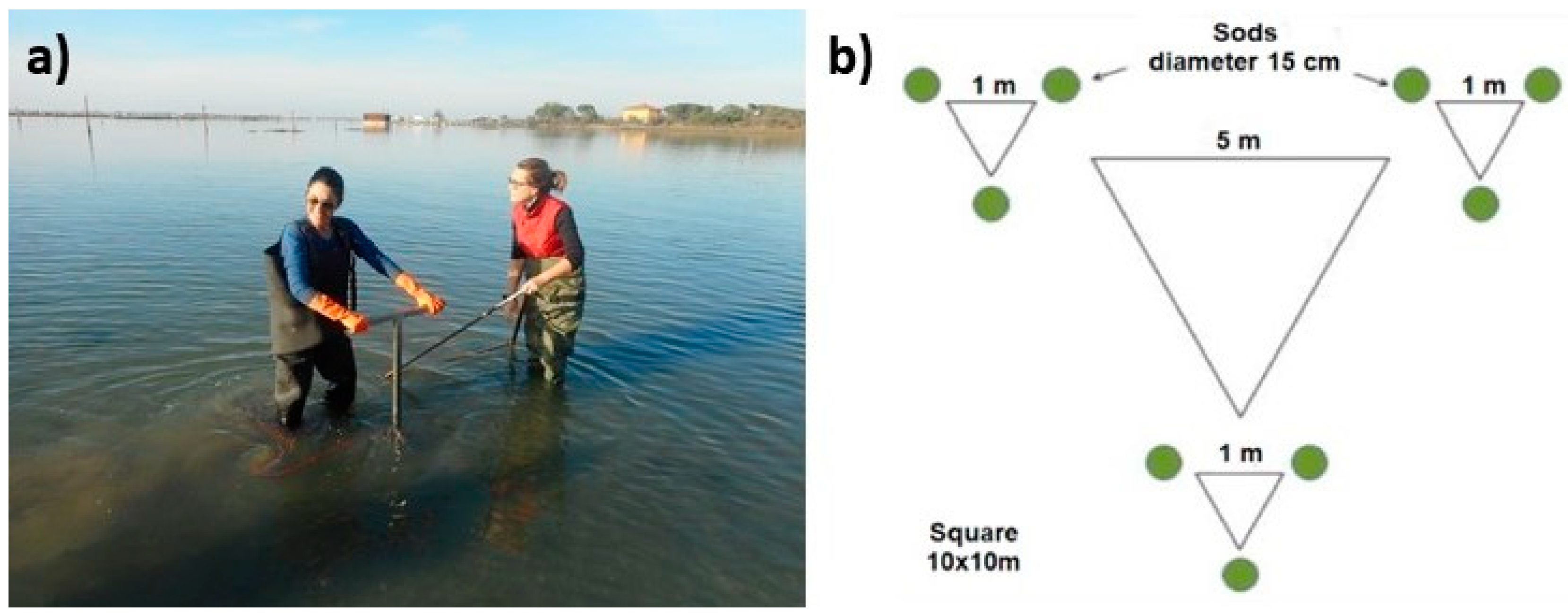
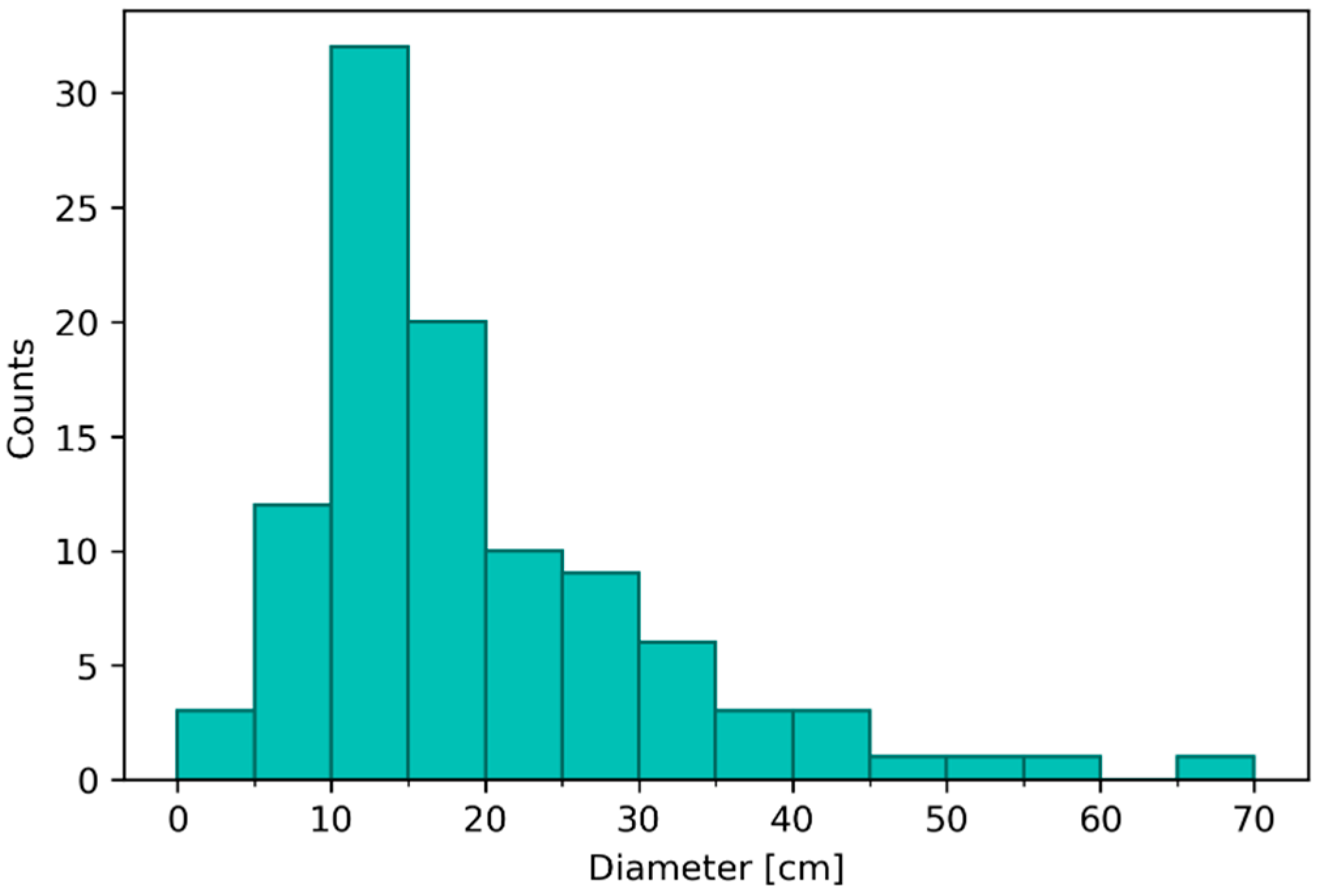
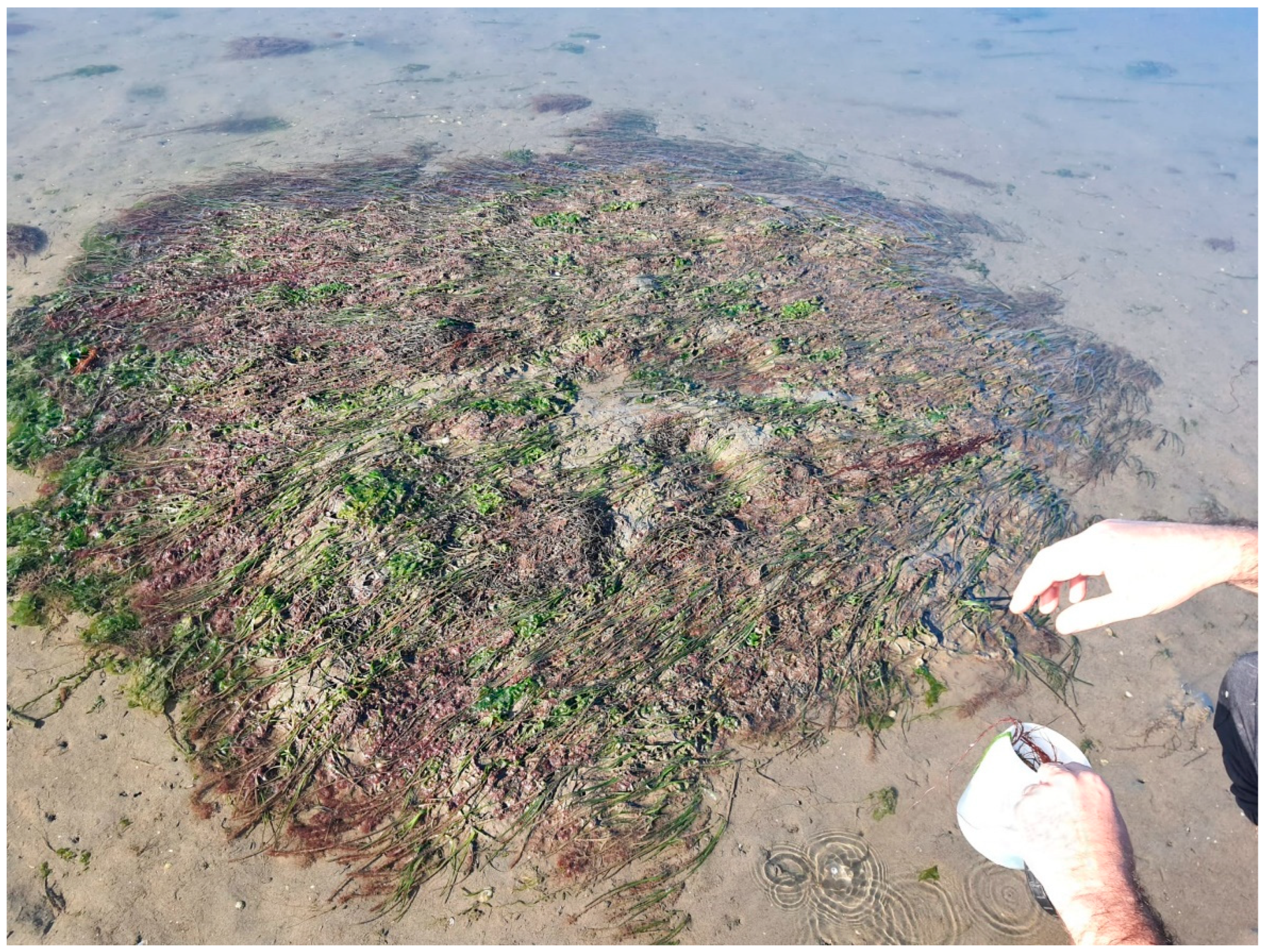
2.1. Study Area and Seagrass Transplantation
2.2. Sampling Environmental Parameters and the Biota
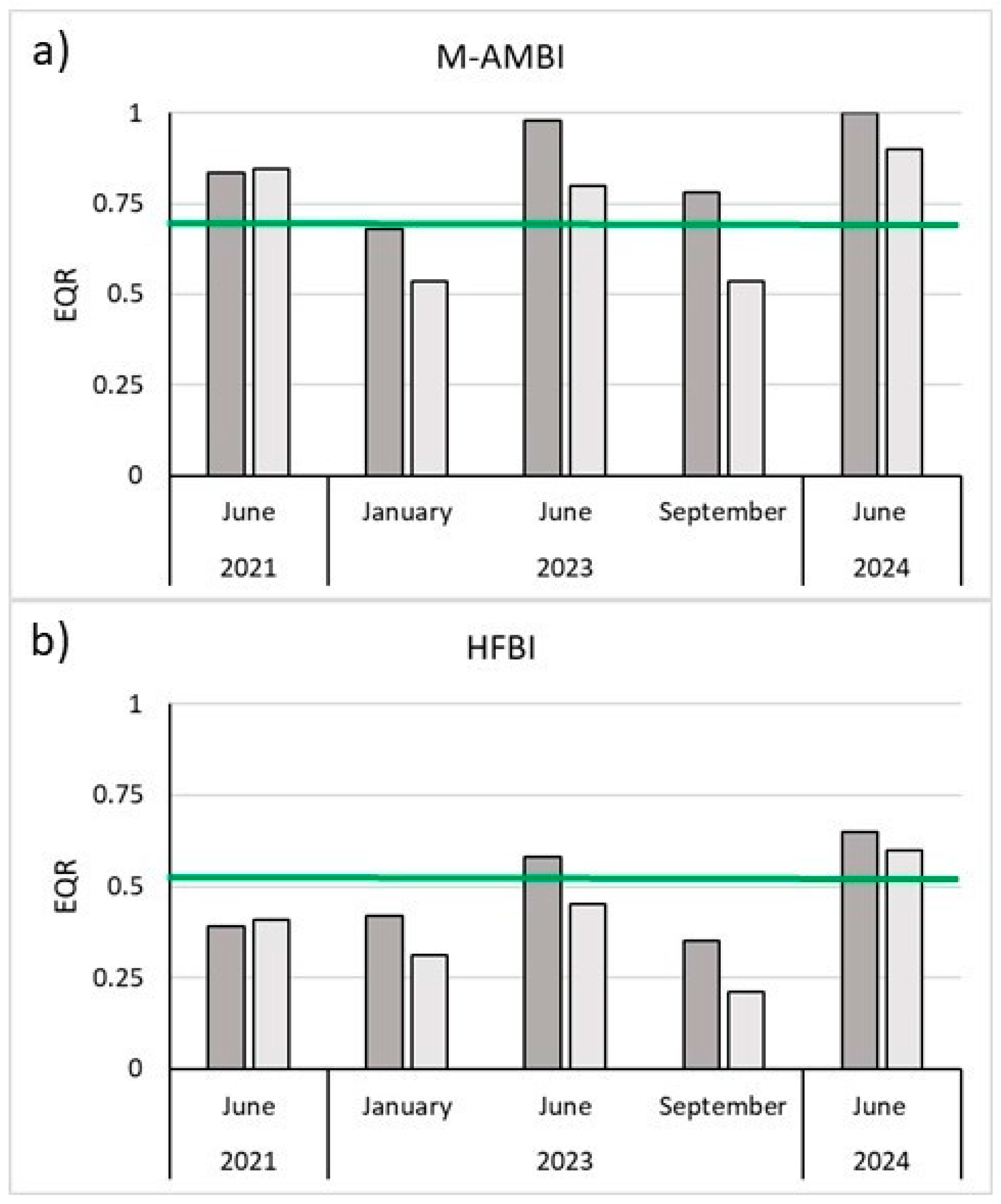
2.3. Data Analysis and Ecological Quality
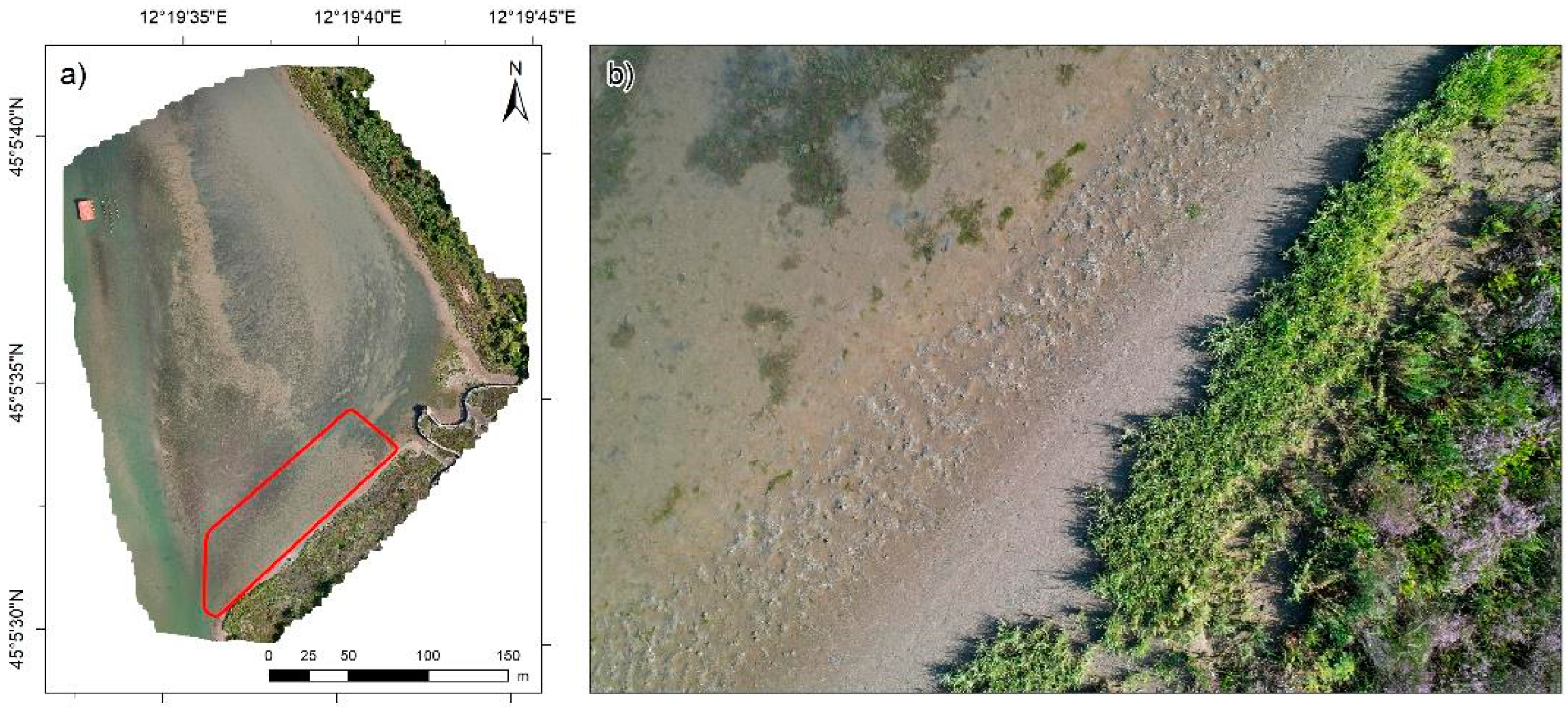
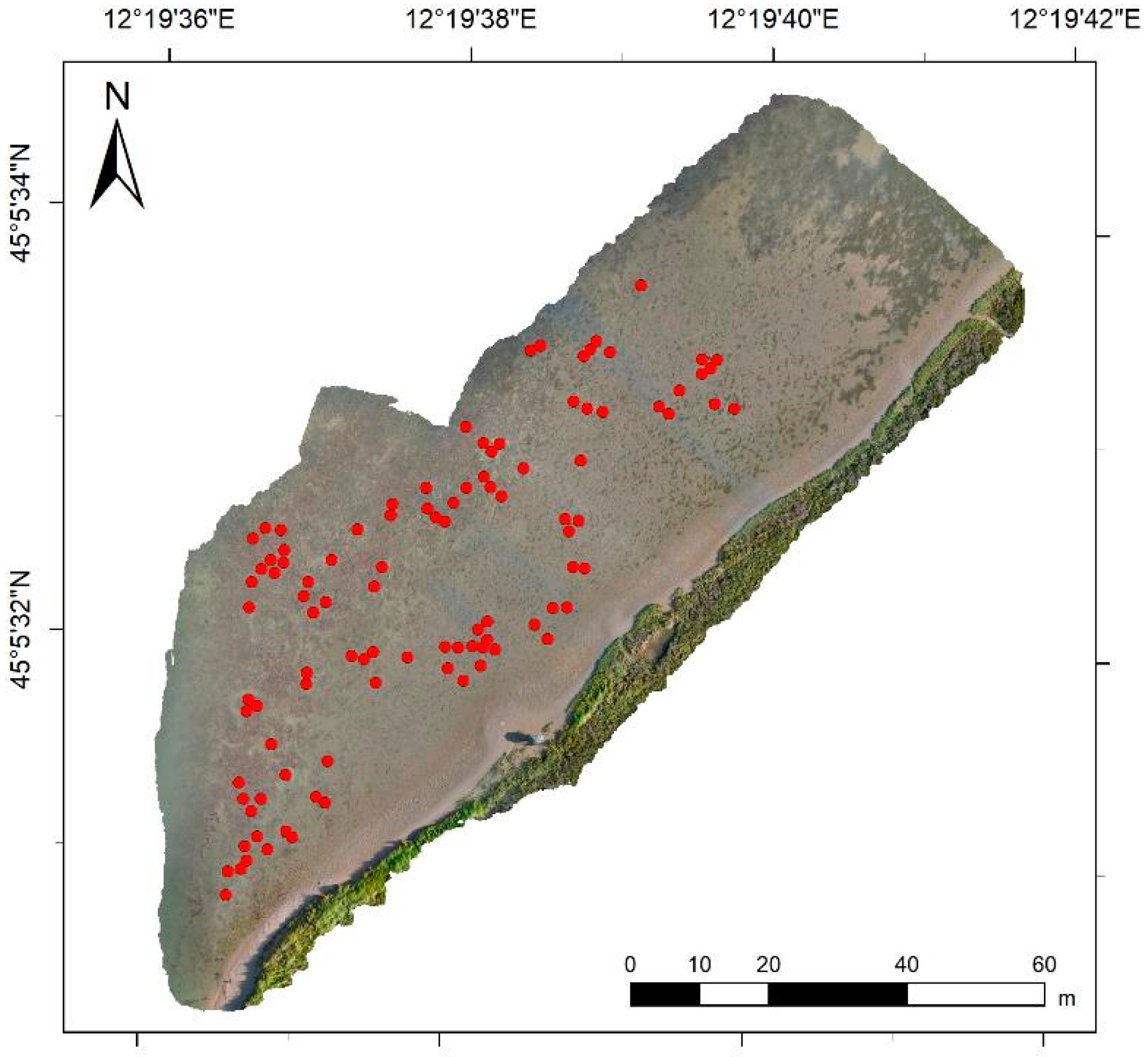
2.4. UAV Survey
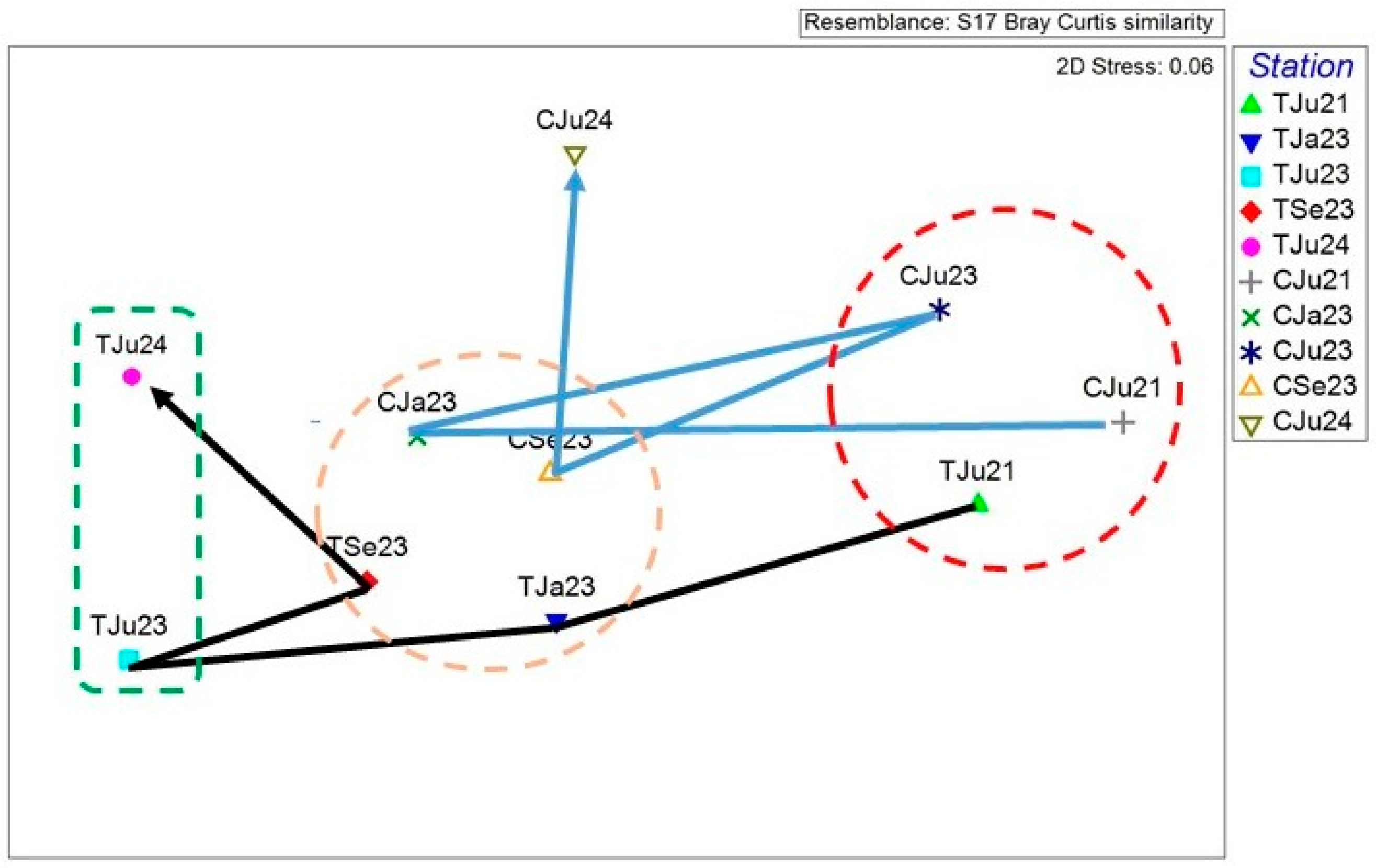
3. Results
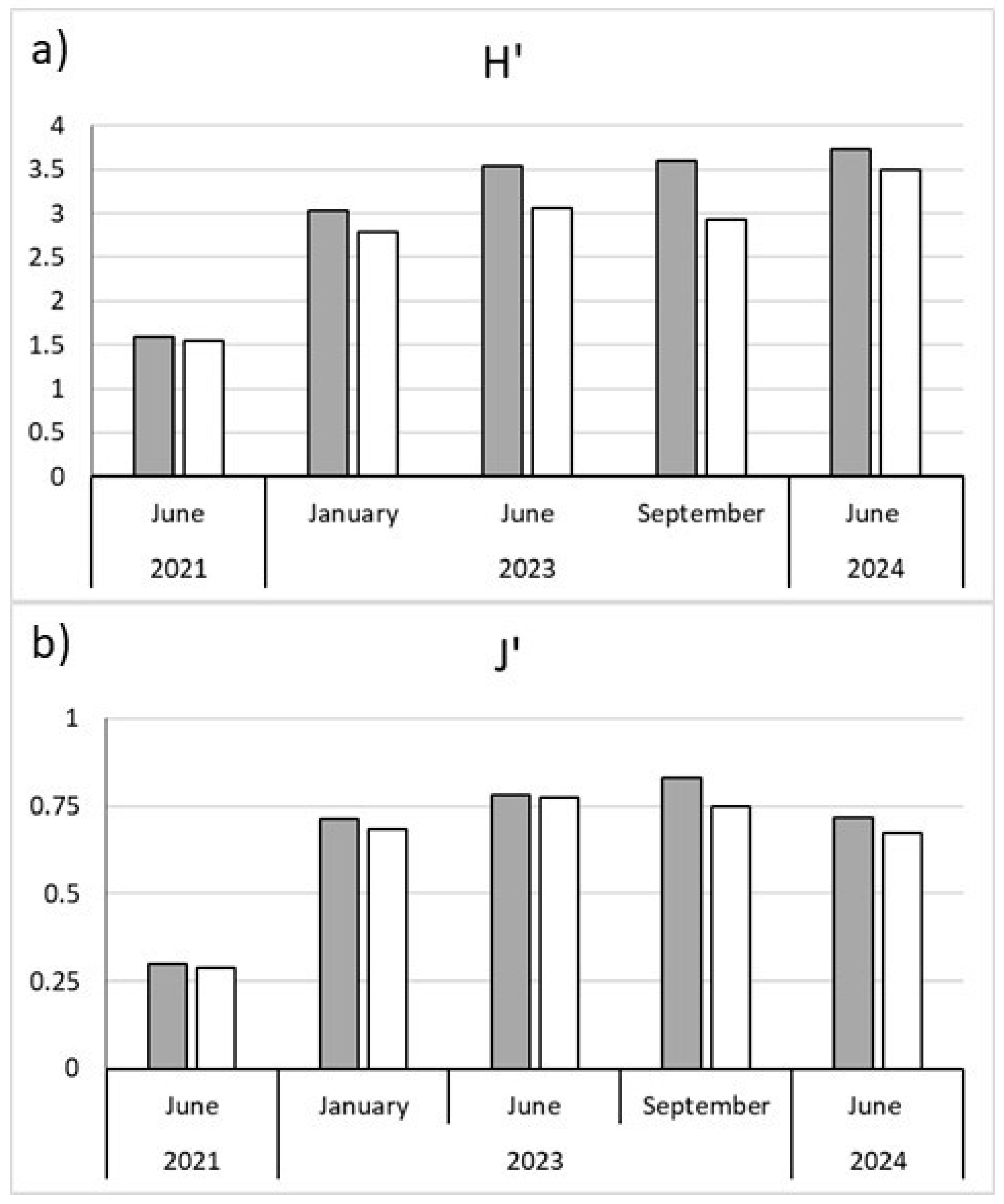
3.1. Environmental Parameters and Biota
| Date | Site | Ecological Groups | ||||
| I(%) | II(%) | III(%) | IV(%) | V(%) | ||
| June | C | 2.4 | 1.7 | 92.9 | 0.7 | 2.3 |
| 2021 | T | 4.1 | 1.6 | 90.8 | 1.2 | 2.4 |
| January | C | 0 | 3.1 | 20.6 | 21.6 | 54.7 |
| 2023 | T | 0.6 | 0.2 | 53.4 | 24.1 | 21.7 |
| June | C | 4.1 | 12.9 | 71 | 3.1 | 8.8 |
| 2023 | T | 25 | 23.7 | 41 | 9.6 | 0.6 |
| September | C | 0.0 | 0.6 | 20.4 | 30.9 | 48.1 |
| 2023 | T | 4.0 | 11.3 | 45.2 | 14.8 | 24.6 |
| June | C | 22.4 | 1.7 | 18.1 | 7.7 | 50.1 |
| 2024 | T | 26.1 | 5.2 | 35.5 | 15.2 | 18.1 |
3.2. Processing and Analysis of UAV Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orth, RJ, Carruthers, TJB, Dennison, WC, Duarte, CM, Fourqurean, JW, Heck, KL Jr., Hughes, AR, Kendrick, GA, Kenworthy, WJ, Olyamik, S, et al. A global crisis for seagrass ecosystems. BioScience 2006, 56:987–996.
- Sfriso, A., Buosi, A., Facca, C., Sfriso, A.A., Tomio, Y., Juhmani, A. S., et al. Environmental restoration by aquatic angiosperm transplants in transitional water systems: The Venice Lagoon as a case study. Sci. Total Environ. 2021, 795, 148859. [CrossRef]
- Sousa, A.I., da Silva, J.F., Azevedo, A., Lillebø, A.I. Blue Carbon stock in Zostera noltei meadows at Ria de Aveiro coastal lagoon (Portugal) over a decade. Sci. Rep. 2019, 9, 14387. [CrossRef]
- Curiel, D., Kraljevic Pavelic, S., Kovacev, A., Miotti, C., Rismondo, A. Marine Seagrasses Transplantation in Confined and Coastal Adriatic Environments: Methods and Results. Water 2021, 13, 2289. [CrossRef]
- Costa, V., Flindt, M.R., Lopes, M., Coelho, J.P., Costa, A.F., Lillebø, A.I., Sousa, A.I. Enhancing the resilience of Zostera noltei seagrass meadows against Arenicola spp. bio-invasion: a decision-making approach. J. Environ. Manag. 2022, 302. [CrossRef]
- Hossain, M.S.; Bujang, J.S.; Zakaria, M.H.; Hashim, M. The application of remote sensing to seagrass ecosystems: an overview and future research prospects. Intl. J. Remote Sensing 2014, 36, 61–114. [Google Scholar] [CrossRef]
- Veettil, B.K.; Ward, R.D.; Lima, M.D.A.C.; Stankovic, M.; Hoai, P.N.; Quang, N.X. Opportunities for seagrass research derived from remote sensing: A review of current methods. Ecol. Ind. 2020, 117. [Google Scholar] [CrossRef]
- Elma, E.; Gaulton, R.; Chudley, T.R.; Scott, C.L.; East, H.K.; Westoby, H.; Fitzsimmons, C. Evaluating UAV-based multispectral imagery for mapping an intertidal seagrass environment. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34. [Google Scholar] [CrossRef]
- Duffy, J.P.; Pratt, L.; Anderson, K.; Land, P.E.; Shutler, J.D. Spatial assessment of intertidal seagrass meadows using optical im-aging systems and a lightweight drone. Estuar. Coast. Shelf Sci. 2018, 200, 169–180. [Google Scholar] [CrossRef]
- Poluzzi, A. , Sabelli, B., Taviani, M. Auto-sinecologia del Molluschi dei fondi mobili del Delta settentrionale del Po (Estate 1980). Boll. Soc. Paloeont. It. 1981, 20, 169–178. [Google Scholar]
- Colombo, G., Ferrari, I., Rossi, R., Ceccherelli, V.U., Cavallini, G. Risorse biologiche di una sacca del delta del Po. Conv. Sc. Naz. Ocean. Fondi Mar. 1979, 199-214.
- Viaroli, P., Bartoli, M., Giordani, G., Naldi, M., Orfanidis, S., Zaldivar, J. Community shifts, alternative stable states, biogeochemical controls and feedbacks in eutrophic coastal lagoons: a brief overview. Aquatic Conserv: Mar. Freshw. Ecosyst. 2008, 18, S105–S117. [CrossRef]
- Marini, M.; Grilli, F. The Role of Nitrogen and Phosphorus in Eutrophication of the Northern Adriatic Sea: History and Future Scenarios. Appl. Sci. 2023, 13, 9267. [Google Scholar] [CrossRef]
- Sfriso, A.A., Sciuto, K., Mistri, M., Munari, C., Juhmani, A.-S., Buosi, A., Tomio, Y., Sfriso, A. Where, when, how and what seagrass to transplant for long lasting results in transitional water systems: the cases of Cymodocea nodosa, Zostera marina, Zostera noltei and Ruppia cirrhosa. Front. Mar. Sci. 2023, 1299428. [CrossRef]
- Sfriso, A., Buosi, A, Sciuto, K., Wolf, M., Tomio, Y., Juhmani A.-S., Sfriso,A.A. Effect of Ecological Recovery on Macrophyte Dominance and Production in the Venice Lagoon. Front. Mar. Sci. 2022, 9. [CrossRef]
- Sfriso, A., Bonometto, A., Boscolo, R., Bruno, L., Buosi, A., Facca, C., et al. Trapianto delle piante acquatiche per il ripristino dell’habitat «lagune costiere»—Linee guida dall’esperienza del progetto LIFE Natura SERESTO. 2017. Available at: http://www.lifeseresto.eu.
- Sfriso, A., Buosi, A., Tomio, Y., Juhmani, A.-S., Chiesa, S., Greco, M., et al. Sediment carbon variations in the Venice lagoon and other transitional water systems of the Northern Adriatic Sea. Water 2020, 12, 3430. [CrossRef]
- Sfriso, A., Buosi, A., Mistri, M., Munari, C., Franzoi, P., Sfriso, A.A. (2019b). Long-term changes of the trophic status in transitional ecosystems of the northern Adriatic Sea, key parameters and future expectations: The lagoon of Venice as a study case. Nat. Conserv. 2019, 34, 193–215. [CrossRef]
- Strickland, J.D.H., Parsons, T.R. A Practical Handbook of Seawater Analyses (2nd Ed.) 1984. Ottawa, Canada: Bulletin of Fishery Research Board of Canada.
- Clarke, K.R., Gorley, R.N., 2006. PRIMER V6: User Manual/Tutorial. PRIMER-E, Plymouth, UK.
- Marchini, A., Munari, C., Mistri, M. Functions and ecological status of eight Italian lagoons examined using biological traits analysis (BTA). Mar. Pollut. Bull. 2008, 56, 1076–1085.
- Borja, A., Franco J., Perez V. A marine biotic index to estabilish the ecological quality of soft bottom benthos within European estuarine and coastal environments. Mar. Poll. Bull. 2000, 40, 1100–1114. [CrossRef]
- Muxika, I., Borja, A., Bald, J. Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. Pollut. Bull 2007, 55, 16–29. [CrossRef] [PubMed]
- ISPRA. Manuale per la classificazione dell’Elemento di Qualità Biologica “Fauna Ittica” nelle lagune costiere italiane. 2017. Manuali e Linee Guida 168, 267 pp.
- Holling, C.S. Resilience and stability of ecological systems. Ann. Rev.. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Crespo D, Faião R, Freitas V, Oliveira VH, Sousa AI, Coelho JP, Dolbeth M. Using seagrass as a nature-based solution: Short-term effects of Zostera noltei transplant in benthic communities of a European Atlantic coastal lagoon. Mar Pollut Bull 2023, 197, 115762. [CrossRef]
- Fonseca, M., Bell, S. Influence of physical setting on seagrass landscapes near Beaufort, North Carolina, USA. Mar. Ecol. Prog. Ser. 1998; 171, 109–121.
- Grall, J., Glémarec, M. Using biotic indices to estimate macrobenthic community perturbations in the Bay of Brest. Estuar. Coast. Shelf Sci. 1997, 44, 43–53. [CrossRef]
- Leung, J.Y.S. Habitat heterogeneity affects ecological functions of macrobenthic communities in a mangrove: implication for the impact of restoration and afforestation. Glob. Ecol. Conserv. 2015, 4, 423–433. [Google Scholar] [CrossRef]
- Southwood, T. Habitat, the templet for ecological strategies? J. Anim. Ecol. 1977, 46, 337–365. [Google Scholar] [CrossRef]
- Townsend, C.R., Hildrew, A.G. Species traits in relation to a habitat templet for river systems. Freshw. Biol. 1994, 31, 265–275. [CrossRef]
- Franco, A.; Franzoi, P.; Malavasi, S.; Riccato, F.; Torricelli, P.; Mainardi, D. Use of shallow water habitats by fish assemblages in a Mediterranean coastal lagoon. Estuar. Coast. Shelf Sci. 2006, 66, 67–83. [Google Scholar] [CrossRef]
- Elliott, M., Dewailly, F. The structure and components of European estuarine fish assemblages. Neth. J. Aquat. Ecol, 1995; 29, 397–417.
- Mathieson, S., Cattrijsse, A., Costa, M.J., Drake, P., Elliott, M., Gardner, J., Marchand, J. Fish assemblages of European tidal marshes: a comparison based on species, families and functional guilds. Mar. Ecol., Prog. Ser, 2000; 204, 225–242.
- Barbedo, J.G.A. A review on the use of unmanned aerial vehicles and imaging sensors for monitoring and assessing plant stresses. Drones 2019, 3(2), 40. [Google Scholar] [CrossRef]
- Cassel, M., Piégay, H., Fantino, G., Lejot, J., Bultingaire, L., Michel, K., & Perret, F. Comparison of ground-based and UAV a-UHF artificial tracer mobility moni-toring methods on a braided river. Earth Surf. Proc. Land. 2020; 45, 5, 1123–1140.
- Casagli, N., Frodella, W., Morelli, S., Tofani, V., Ciampalini, A., Intrieri, E., Lu, P. Spaceborne, UAV and ground-based remote sensing techniques for land-slide mapping, monitoring and early warning. Geoenv. Disasters 2017, 4, 1–23.
- Zeybek, M., Taşkaya, S., Elkhrachy, I., Tarolli, P. Improving the Spatial Accuracy of UAV Platforms Using Direct Georeferencing Methods: An Application for Steep Slopes. Remote Sens 2003, 15, 2700.
- Kim, H., Hyun, C.-U., Park, H.-D., Cha, J. Image Mapping Accuracy Evaluation Using UAV with Standalone, Differential (RTK), and PPP GNSS Positioning Techniques in an Abandoned Mine Site. Sensors, 2003; 2, 5858.
- Agrawal, J., Arafat, M.Y. Transforming Farming: A Review of AI-Powered UAV Technologies in Precision Agriculture. Drones, 2024; 8, 664.
- Zhang, Z., Zhu, L. A Review on Unmanned Aerial Vehicle Remote Sensing: Platforms, Sensors, Data Processing Methods, and Applications. Drones, 2023; 7, 398.
- Mistri, M., Fano E.A., Rossi R. Redundancy of macrobenthos from lagoonal habitats in the Adriatic Sea. Mar. Ecol. Prog. Ser 2001, 215, 289–296. [CrossRef]
- Barbier, E.B., Hacker, S.D., Kennedy, C., Koch, E.W., Stier, A.C., Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 8, 169–193.
- Borja, A., Dauer, D.M., Elliott, M., Simenstad, C.A. Medium-and long-term recovery of estuarine and coastal ecosystems: patterns, rates and restoration effectiveness. Estuar. Coasts 2010, 33, 1249–1260. [CrossRef]
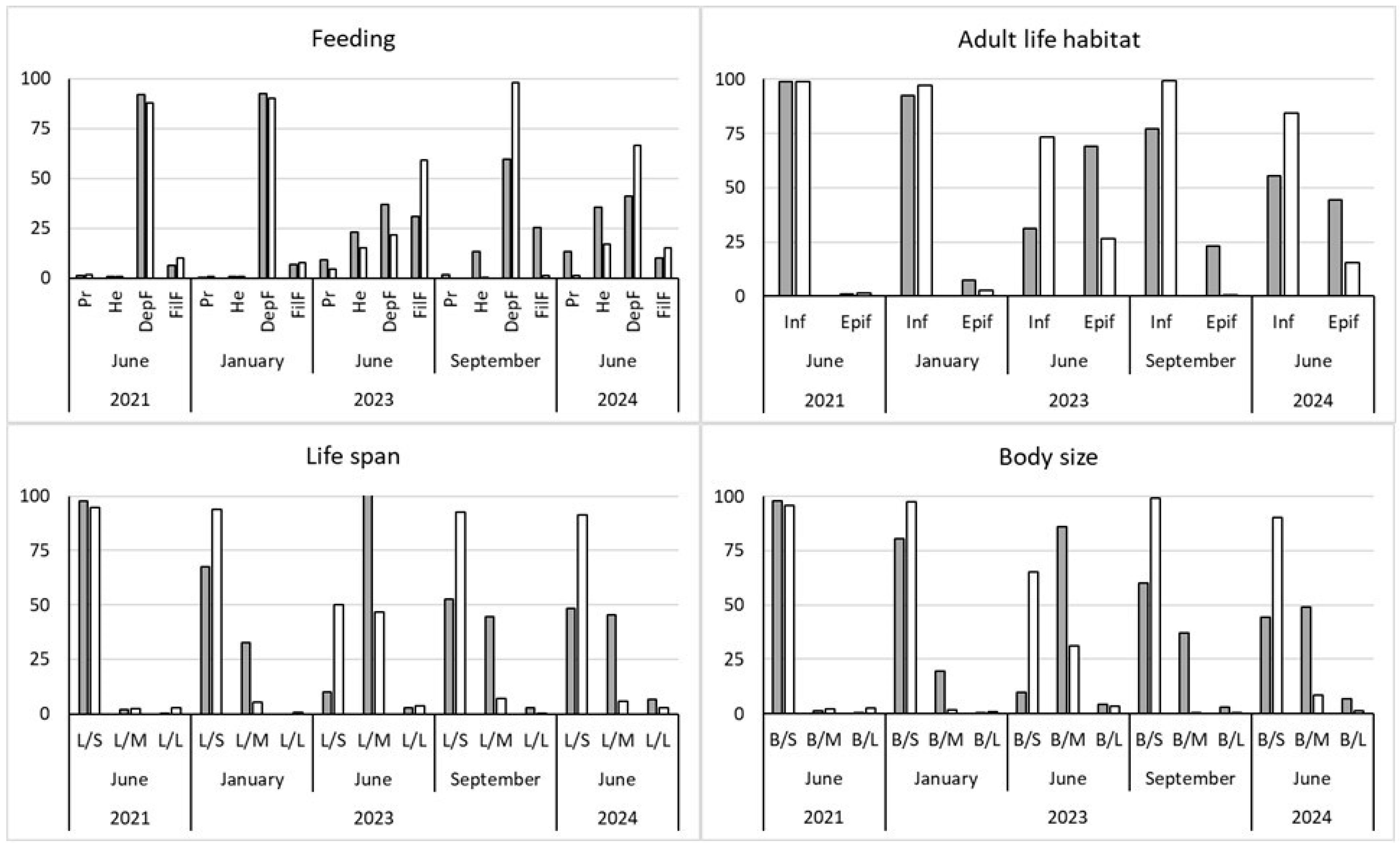

| (a) Biological traits | Traits modalities | Labels |
| Feeding mode | Predator | Pr |
| Herbivorous | He | |
| Deposit-feeder | DepF | |
| Filter-feeder | FilF | |
| Adult life habitat | Infauna | Inf |
| Epifauna | Epif | |
| Life span | Short (<1 year) | L/S |
| Medium (1–5 years) | L/M | |
| Long (>5 years) | L/L | |
| Body size (g) | Small (<0.001 g) | B/S |
| Medium (0.01–0.05 g) | B/M | |
| Large (>0.05 g) | B/L | |
| (b) Ecological groups | Sensitive | EG-I |
| Indifferent | EG-II | |
| Tolerant | EG-III | |
| 2nd order opportunists | EG-IV | |
| 1st order opportunists | EG-V |
| Mean | SD | Min | Max | |||
| Water | Temp | °C | 17.8 | 7.2 | 7.5 | 29 |
| pH | 8.3 | 0.2 | 8.1 | 8.6 | ||
| Eh | mV | 307.1 | 47.5 | 216.0 | 383.0 | |
| Salinity | psu | 18.7 | 2.8 | 15.0 | 23.2 | |
| DO | mg/L | 9.7 | 2.0 | 6.7 | 13.6 | |
| TSS | mg/L | 17.4 | 9.0 | 7.2 | 37.0 | |
| RP | µg/L | 0.4 | 0.1 | 0.2 | 0.6 | |
| DIN | µg/L | 12.5 | 5.7 | 5.4 | 23.9 | |
| RSI | µg/L | 24.0 | 14.5 | 2.6 | 44.2 | |
| Chl-a tot | µg/L | 2.5 | 1.2 | 0.9 | 4.7 | |
| Sediment | pH | 7.6 | 0.1 | 7.4 | 7.8 | |
| Eh | mV | -15.3 | 81.4 | -162.0 | 122.0 | |
| Ptot | µg/g | 616.7 | 82.7 | 507.0 | 734.0 | |
| Ntot | mg/g | 1.4 | 0.3 | 0.9 | 1.8 | |
| Ctot | mg/g | 34.5 | 3.6 | 27.3 | 39.0 | |
| Fines | % | 36.7 | 7.1 | 28.2 | 50.0 | |
| Density | g/cm3 | 0.8 | 0.2 | 0.4 | 1.0 | |
| Moisture | % | 38.0 | 6.4 | 27.1 | 48.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





