Submitted:
30 December 2024
Posted:
31 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. A Summary
2.2. Preamble to the Long Primary Afferent System’s Development
2.3. The Impact of Hypoxia during the Critical Period
2.4. A Watershed Delineates the Critical Period
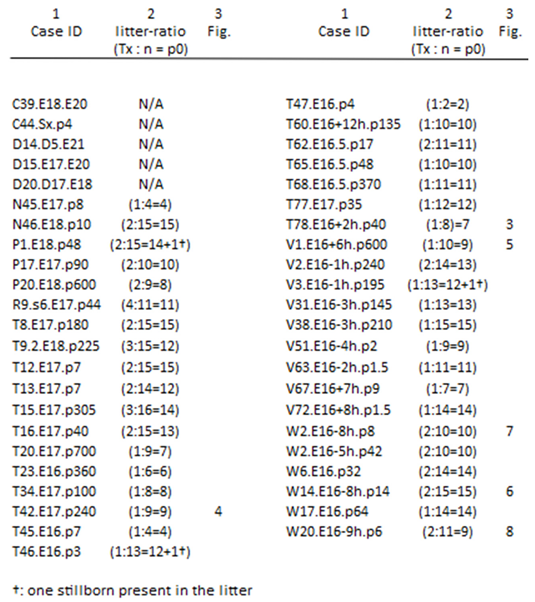
3. Results
3.1. Regeneration Comes to a Halt before Neap Tide
3.2. Six Bell Ringer Cases Exhibiting All Dynamic Features
| */** | asterisk/s | a single asterisk indicates a TH.1-stage i-FS / a tandem asterisk indicates colls |
| g.nu | gracile nucleus | |
| 4th V | fourth ventricle | |
| L|Th|C | Lumbar|Thoracic|Cervical segment of the spinal cord | |
| c <== ==> r | caudal <== ==> rostral direction of the spinal cord | |
| d <== ==> v | dorsal <== ==> ventral in a sagittal section of the spinal cord | |
| L/R | left side/right side | of the spinal cord in a horizontal section |
| N | fix pinhole
|
an artifact from tissue processing; a number identifies the spinal cord's level derived from the gelatine block's count spinal level of Tx / depicted level in the Figure |
4. Discussion
Competing Interests
Ethical Approval
Funding
Glossary
| a-FS | abrupt front stop | WM-facing medullary bundle of fetal cut high-tide primary afferent i-FSs blocked at their reprogrammed TH.1 stage. Frequently, the a-FS abuts the lesion site caudally [12]. |
| CP | critical period | Upstream time slot(s) delineating elongation in each (and all) TH.0 stage pioneering long primary afferent axon(s). |
| CG | central gray | neuropil |
| colls | TH.2-staged collaterals = collateral sprouting following T.H.2 transit and illustrating unrestrained development had been accomplished in gracile nuclei. | |
| DRG | dorsal root ganglion | involving the lumbar segments L4, L5, and L6 (HRP-tracing at the left side, only) |
| DC | dorsal column | |
| E16-8h | 15th day of gestation | M0 is scheduled 15 days + 16 h after mating, restricted to 1 h |
| E16 | day of conception without surveillance of mating time | |
| f-ES | fast Elongation Stop | Figuring a hypothetical configuration where the elongating TH.0-staged pioneering axons arriving at the medulla swiftly slow down. |
| fringe | the CG, adjacent to the DC white matter | |
| high tide | in the rostral DC, pioneering axons target the gracile nuclei in high-tide waves at spring tide | |
| i-FS | individual front stop | The TH.1 staged axon mimicry generates a blunt fiber termination, i.e., a regenerated axon substitute. At high tide, the axon re-elongates towards the medulla. At low tide, the fiber tip remains caudally distanced from the lesion site, forming the look-alike of the terminal club. |
| HRP | low tide | in the lower thoracic DC, pioneering axons target Clarke's nucleus in low-tide waves at neap tide #break#horse radish peroxidase |
| M0 | The moment of Tx is referenced to the hour (or day) of conception and serves the ID in combination with survival time (Table 1). M0 has a temporospatial link with a location on the assembly line determined by the axon features, which demonstrate variability. This underlines that the development between fetuses may differ. | |
| neap tide | see low tide | |
| s-ES | slow Elongation Switch | Figuring the transition of the hypothetical TH.1 staged axon before creating collateral sproutings, i.e., TH.2 staged colls. |
| spring tide | see high tide | |
| tc | terminal club, see i-FS | |
| THs | transition hubs | TH.0, TH.1, TH.2, and TH.3 are hypothetically situated on the developmental cascade, figuring unknown phenotypes that disclose a mimicry of TH.1, TH.2, and TH.3 stage axon features after Tx. |
| TH.0 | staged axon | pioneering primary afferent axon in a high tide wave |
| TH.1 | staged axons | Mimicked by a bundle of reprogrammed regenerated axons featuring the a-FS rostrally at spring tide or the multilevel i-FSs at a caudal Tx at low tide. |
| Tx | dorsal myelotomy | the microsurgical procedure |
References
- Bourguignon, L.; Tong, B. International surveillance study in acute spinal cord injury confirms viability of multinational clinical trials. BMC Medicine 2022, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.A.; Roberts, N. Fighting for recovery on multiple fronts: The past, present, and future of clinical trials for spinal cord injury. Front Cell Neurosci 2022, 16, 1–18. [Google Scholar] [CrossRef]
- Jagrit, V.; Koffler, J. Combinatorial strategies for cell transplantation in traumatic spinal cord injury. Front Neurosci 2024, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Zhang, F. Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learned. Dev Dynamics 2003, 226, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Rodemer, W.; Hu, J. Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others? Neur Reg Res 2020, 15, 996–1005. [Google Scholar]
- Norris, V. Successive paradigm shifts in the bacterial cell cycle and related subjects. Life 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Illis, LS. Central nervous system regeneration does not occur. Spinal Cord 2012, 50, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tator, C.H. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Devl Neurosci 2013, 31, 701–713. [Google Scholar] [CrossRef]
- Olivero, R.S.; Bello, S. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol Dis 2014, 62, 338–353. [Google Scholar] [CrossRef]
- Xu X-M. Breaking news in spinal cord injury research. Neur Reg Res 2012, 7, 1685–1687. [Google Scholar]
- Ahuja, C.S.; Mothe, A. The leading edge: emerging neuroprotective cell-based therapies for spinal cord injury. Stem Cells Transl Med 2020, 9, 1509–1530. [Google Scholar] [CrossRef] [PubMed]
- De Beer, F.C.; Steinbusch, H.W. On the blueprint of the long primary afferent axons and the dichotomous axon trajectory of Clarke's nucleus. A morphological tracing study on the effect of hypoxia during development. Anatomia 2023, 2, 414–49. [Google Scholar] [CrossRef]
- Schwab, M.E.; Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 1996, 76, 319–370. [Google Scholar] [CrossRef]
- Quraishe, S.; Forbes, L.H. The extracellular environment of the CNS: Influence on plasticity, sprouting, and axonal regeneration after spinal cord injury. Neural Plast 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Induced pluripotent stem cells: Past, Present, and Future. Cell Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Gearhart, J.; Oster-Granite, M.L. Histological changes after transection of the spinal cord of fetal and neonatal mice. Exp Neurol 1979, 66, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Buyan Dent, L.J.; McCasland, J.S. Attempts to facilitate dorsal column axonal regeneration in a neonatal spinal environment. J Comp Neurol 1996, 372, 435–456. [Google Scholar] [CrossRef]
- Wree, A.; Fiekas, D. Lokale zerebrale Glukose-Utilisation des Riickenmarkes und der Hinterstrangkerne der Ratte nach pränataler dorsaler Myelotomie in Höhe Thl2: Eine [14C]2-Desoxyglukose-Studie. Eur J Neurosci 1998, 10, 62. [Google Scholar]
- Steward, O.; Zheng, B. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol 2003, 459, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mesulam MM, Brushart TM. Transganglionic and anterograde transport of horseradish peroxidase across dorsal root ganglia: a tetramethylbenzidine method for tracing central sensory connections of muscles and peripheral nerves. Neuroscience. 1979; 4:1107-17.
- Cox-Limpens, K.; Strackx, E. Fetal Asphyctic Preconditioning Protects Against Perinatal Asphyxia-Induced Apoptosis and Astrogliosis in Neonatal Brain. CNS Neurol Disord Drug Targets 2015, 14, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A.; Wendler, C.C. Long-term consequences of disrupting adenosine signaling during embryonic development. Mol Aspects Med 2017, 55, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bustelo, M.; Barkhuizen, M. Clinical Implications of Epigenetic Dysregulation in Perinatal Hypoxic-Ischemic Brain Damage. Front Neurol 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- De Beer, F.C. Dorsal myelotomy in E15-E16 fetal rat: A promising paradigm in regeneration research with serendipitous transcriptomic effects on development of the long primary afferent system. J Neurosci Methods 2022, 366, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, MV. Dissecting spinal cord regeneration. Nature 2018, 557, 344. [Google Scholar] [CrossRef] [PubMed]
- Pires Monteiro, S.; Voogd, E. Neuroprotective effect of hypoxic preconditioning and neuronal activation in an in vitro human model of the ischemic penumbra. J Neural Eng 2021, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, E.E.; Scott, A.L. Purinergic signaling systems across comparative models of spinal cord injury. Neural Regen Res 2022, 17, 2391–2398. [Google Scholar] [PubMed]
- Lanfranchi, M.; Yandiev, S. The AMPK-related kinase NUAK1 controls cortical axon branching by locally modulating mitochondrial metabolic functions. Nat Commun 2024, 15, 2487–2504. [Google Scholar] [CrossRef]
- Liu, K.; Tedeschi, A. Neuronal intrinsic mechanisms of axon regeneration. Ann Rev Neurosci 2011, 34, 131–152. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Pradhan, B. New paradigms in purinergic receptor ligand discovery. Neuropharmacology 2023, 230, 109503. [Google Scholar] [CrossRef]
- Stepien, B.K.; Wielockx, B. From vessels to neurons – The role of hypoxia pathway proteins in embryonic neurogenesis. Cells 2024, 13, 621–651. [Google Scholar] [CrossRef]
- Vissers, C.; Sinha, A. The epitranscriptome in stem cell biology and neural development. Neurobiol Dis 2020, 146, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Coquand, L.; Avalos, C.B. A cell fate decision map reveals abundant direct neurogenesis bypassing intermediate progenitors in the human developing neocortex. Nat Cell Biol 2024, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, J.; Belin, S. Customization of the translational complex regulates mRNA-specific translation to control CNS regeneration. Neuron 2023, 111, 2881–2898. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y. Intrinsic control of axon regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef]
- Tedeschi, A.; Dupraz, S. ADF/Cofilin-mediated actin turnover promotes axon regeneration in the adult CNS. Neuron 2019, 103, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Martins-Costa, C.; Wiegers, A. ARID1B controls transcriptional programs of axon projection in an organoid model of the human corpus callosum. Cell Stem Cell 2024, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.C.; Pinto-Costa, R. Actin dynamics in the growth cone: a key player in axon regeneration. Curr Opin Neurobiol 2021, 69, 11–18. [Google Scholar] [CrossRef]
- Marichal, N.; Fabbiani, G. Purinergic signaling in a latent stem cell niche of the rat spinal cord. Purinergic Signal 2016, 12, 331–341. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic nerves. Pharmacol Rev 1972, 24, 509–557. [Google Scholar] [PubMed]
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arias, J.C.; Van der Slagt, E. Purinergic signaling in nervous system health and disease: Focus on pannexin 1. Pharmacol Ther 2021, 225, 107840. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W. Why has it been so difficult to make CNS axons regenerate? Neurochem Res 2020, 45, 144–158. [Google Scholar] [CrossRef] [PubMed]
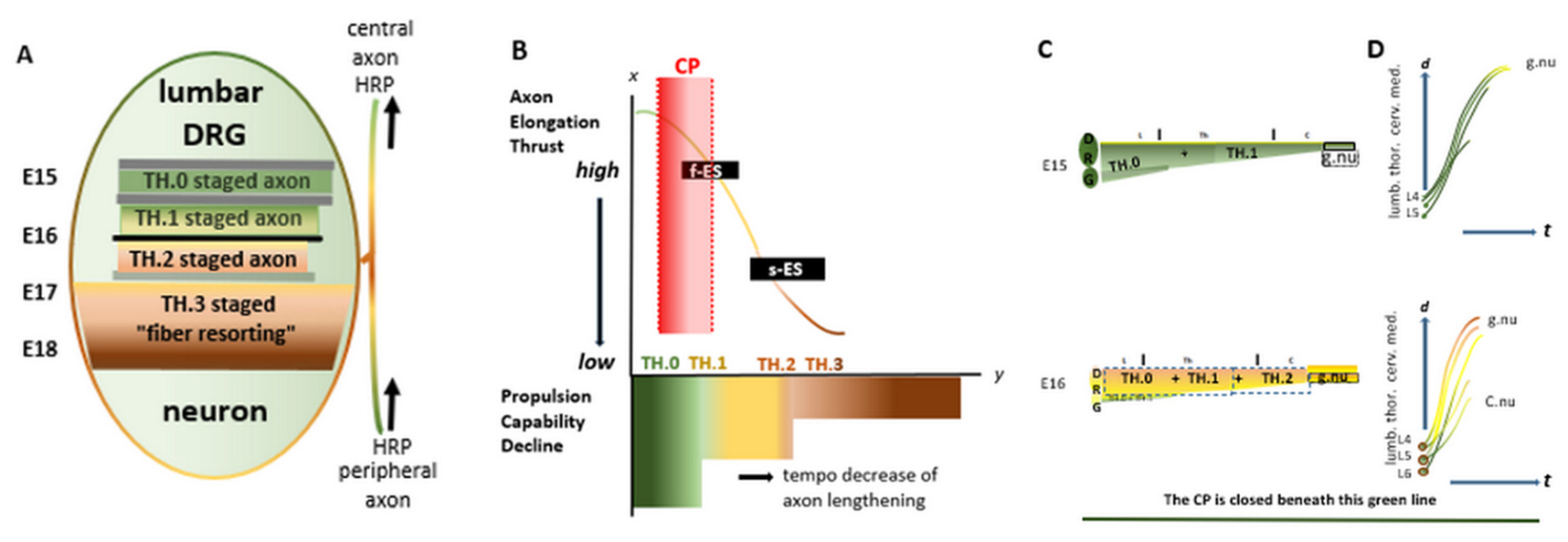
 and
and  respectively, the axon lengths might parallel the available energy for elongation. The reprogrammed i-FS’s phenotype is yellow-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla enabled by
respectively, the axon lengths might parallel the available energy for elongation. The reprogrammed i-FS’s phenotype is yellow-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla enabled by  . The a-FS of joined i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)-(8) denote the six cases with Txs at their estimated M0 spots on the assembly line.
. The a-FS of joined i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)-(8) denote the six cases with Txs at their estimated M0 spots on the assembly line.
 and
and  respectively, the axon lengths might parallel the available energy for elongation. The reprogrammed i-FS’s phenotype is yellow-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla enabled by
respectively, the axon lengths might parallel the available energy for elongation. The reprogrammed i-FS’s phenotype is yellow-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla enabled by  . The a-FS of joined i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)-(8) denote the six cases with Txs at their estimated M0 spots on the assembly line.
. The a-FS of joined i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)-(8) denote the six cases with Txs at their estimated M0 spots on the assembly line.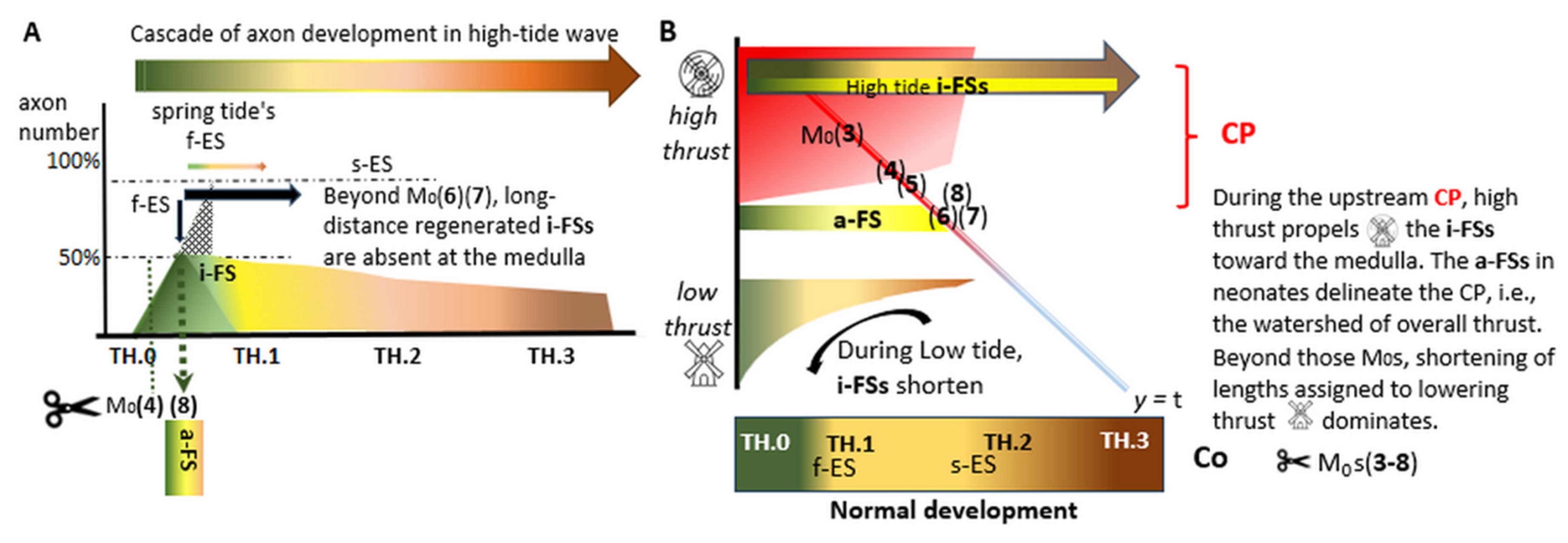
 : Denotes the spinal levels at C and D (horizontal sections). (B) The lines represent the TH.1 axons mimicry (in yellow), and TH.2 colls (in brown) originating from parent axons at the medulla (C).
: Denotes the spinal levels at C and D (horizontal sections). (B) The lines represent the TH.1 axons mimicry (in yellow), and TH.2 colls (in brown) originating from parent axons at the medulla (C).  : The p40 male exhibited a medulla with multistage axons in both gracile nuclei. The dominance of TH.2 colls in the left gracile nucleus (noted with**) indicated accomplished development due to sustained high thrust levels. Phenotypically altered i-FSs outnumbered in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)
: The p40 male exhibited a medulla with multistage axons in both gracile nuclei. The dominance of TH.2 colls in the left gracile nucleus (noted with**) indicated accomplished development due to sustained high thrust levels. Phenotypically altered i-FSs outnumbered in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)  : Caudally from the medulla, occasional i-FSs (noted with *) were present in both DCs. t = time. N: iron fix pinhole. Bars: 100 µm.
: Caudally from the medulla, occasional i-FSs (noted with *) were present in both DCs. t = time. N: iron fix pinhole. Bars: 100 µm.
 : Denotes the spinal levels at C and D (horizontal sections). (B) The lines represent the TH.1 axons mimicry (in yellow), and TH.2 colls (in brown) originating from parent axons at the medulla (C).
: Denotes the spinal levels at C and D (horizontal sections). (B) The lines represent the TH.1 axons mimicry (in yellow), and TH.2 colls (in brown) originating from parent axons at the medulla (C).  : The p40 male exhibited a medulla with multistage axons in both gracile nuclei. The dominance of TH.2 colls in the left gracile nucleus (noted with**) indicated accomplished development due to sustained high thrust levels. Phenotypically altered i-FSs outnumbered in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)
: The p40 male exhibited a medulla with multistage axons in both gracile nuclei. The dominance of TH.2 colls in the left gracile nucleus (noted with**) indicated accomplished development due to sustained high thrust levels. Phenotypically altered i-FSs outnumbered in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)  : Caudally from the medulla, occasional i-FSs (noted with *) were present in both DCs. t = time. N: iron fix pinhole. Bars: 100 µm.
: Caudally from the medulla, occasional i-FSs (noted with *) were present in both DCs. t = time. N: iron fix pinhole. Bars: 100 µm.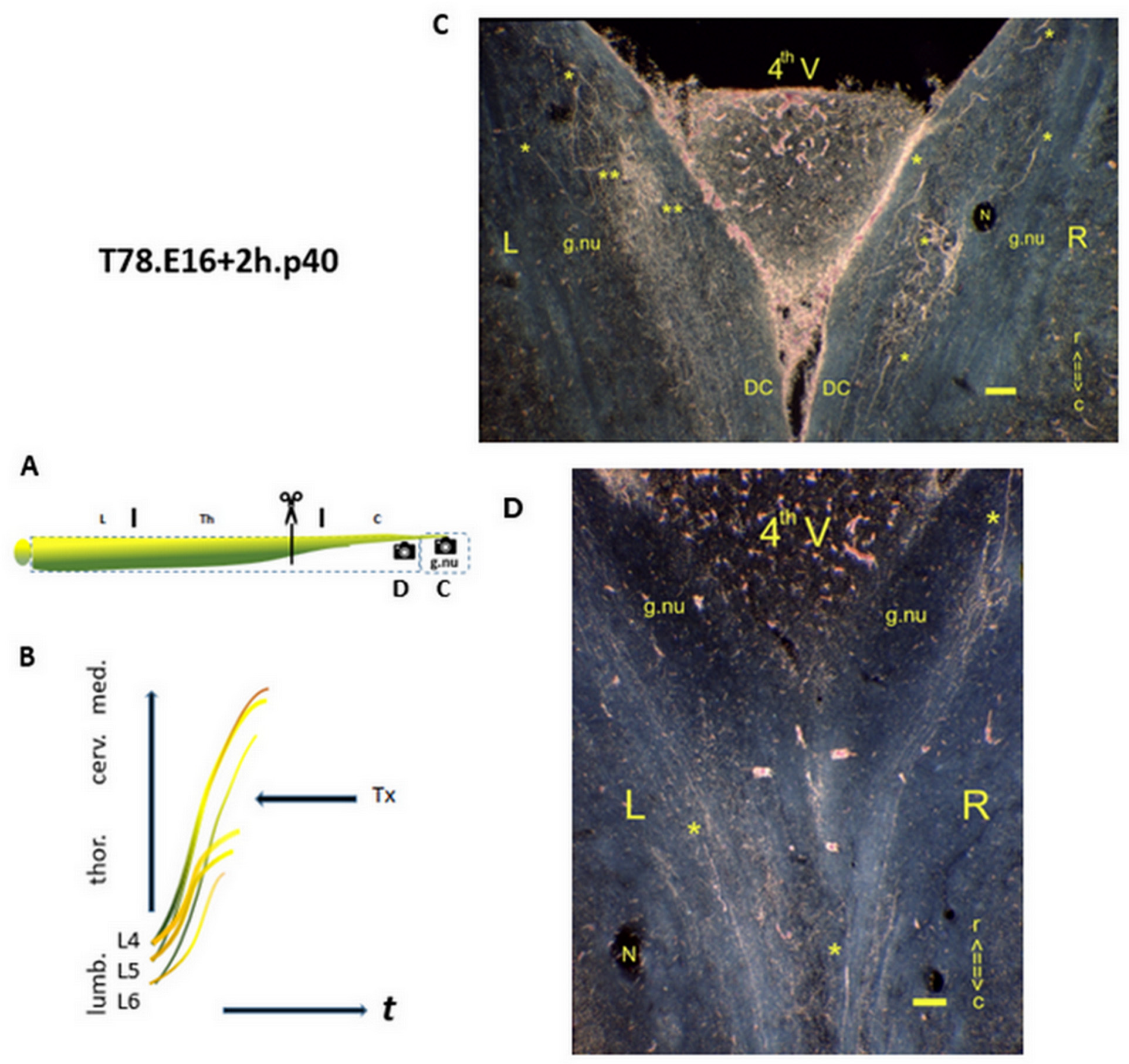
 : Denotes the spinal levels at C and D. (B) Various axons crossed the lesion site. (C)
: Denotes the spinal levels at C and D. (B) Various axons crossed the lesion site. (C)  : Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled with TH.2 staged colls (noted: **). The covering DC contained a few i-FSs (noted: *). (D)
: Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled with TH.2 staged colls (noted: **). The covering DC contained a few i-FSs (noted: *). (D)  : Sagital section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed i-FSs (*) abutted the lesion site caudally. These dispersed i-FSs reflected a neonatal a-FS, which converted into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing ventrally the lesion site, a few parent axons (TH.0 staged) were localized. t = time. N: iron fix pinhole. Bars: 100 µm.
: Sagital section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed i-FSs (*) abutted the lesion site caudally. These dispersed i-FSs reflected a neonatal a-FS, which converted into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing ventrally the lesion site, a few parent axons (TH.0 staged) were localized. t = time. N: iron fix pinhole. Bars: 100 µm.
 : Denotes the spinal levels at C and D. (B) Various axons crossed the lesion site. (C)
: Denotes the spinal levels at C and D. (B) Various axons crossed the lesion site. (C)  : Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled with TH.2 staged colls (noted: **). The covering DC contained a few i-FSs (noted: *). (D)
: Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled with TH.2 staged colls (noted: **). The covering DC contained a few i-FSs (noted: *). (D)  : Sagital section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed i-FSs (*) abutted the lesion site caudally. These dispersed i-FSs reflected a neonatal a-FS, which converted into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing ventrally the lesion site, a few parent axons (TH.0 staged) were localized. t = time. N: iron fix pinhole. Bars: 100 µm.
: Sagital section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed i-FSs (*) abutted the lesion site caudally. These dispersed i-FSs reflected a neonatal a-FS, which converted into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing ventrally the lesion site, a few parent axons (TH.0 staged) were localized. t = time. N: iron fix pinhole. Bars: 100 µm.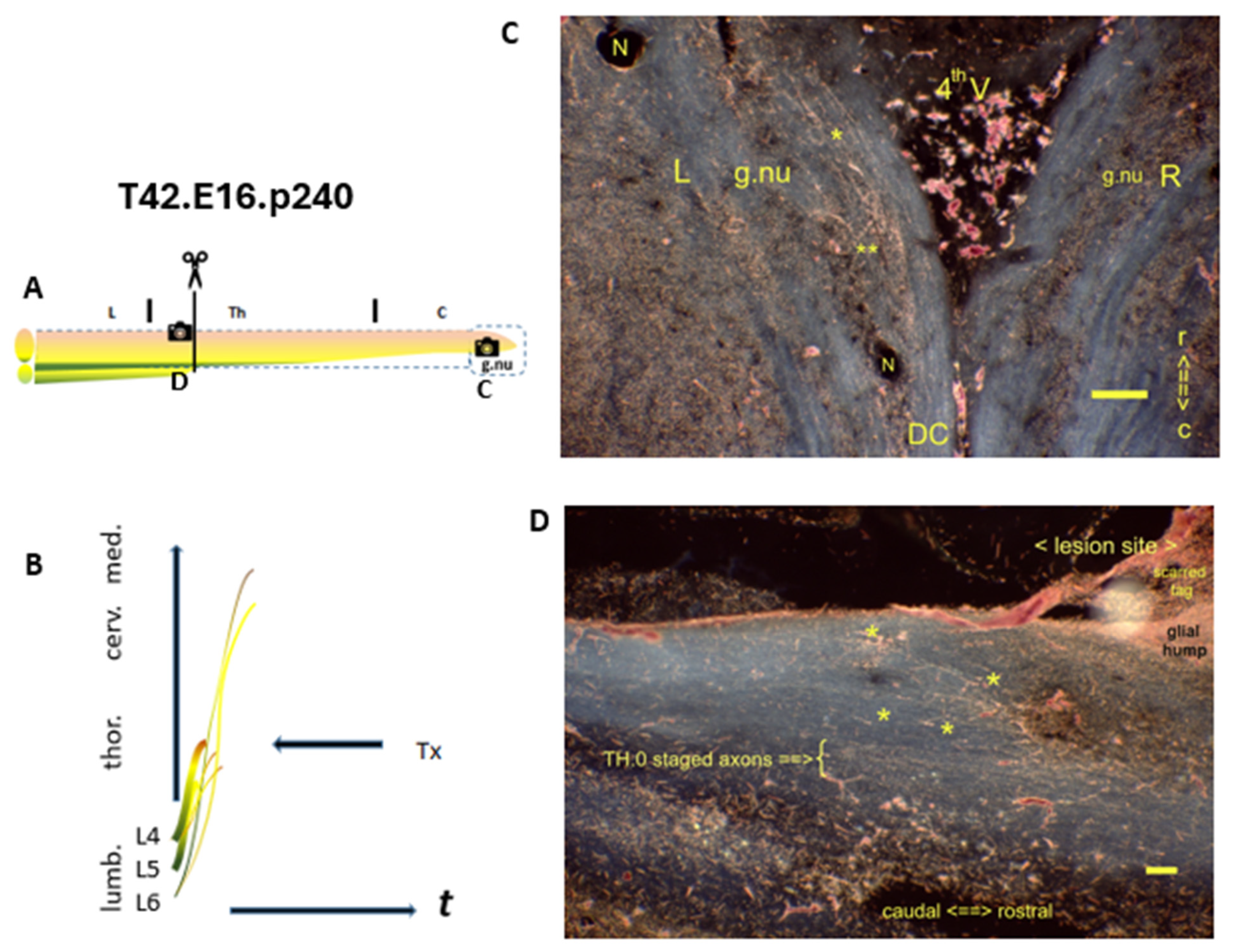
 : Denotes the spinal levels at C and D. (B) The axons at the medulla demonstrate the CP had not been closed. (C)
: Denotes the spinal levels at C and D. (B) The axons at the medulla demonstrate the CP had not been closed. (C)  : Horizontal section of gracile nuclei. The p600 male showed various i-FSs in the left gracile nucleus and a few colls. (D)
: Horizontal section of gracile nuclei. The p600 male showed various i-FSs in the left gracile nucleus and a few colls. (D) : Sagittal section of the mid-thoracic spinal cord. The multilevel i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine the lengths of axons. t = time. N: iron fix pinhole. Bars: 100 µm.
: Sagittal section of the mid-thoracic spinal cord. The multilevel i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine the lengths of axons. t = time. N: iron fix pinhole. Bars: 100 µm.
 : Denotes the spinal levels at C and D. (B) The axons at the medulla demonstrate the CP had not been closed. (C)
: Denotes the spinal levels at C and D. (B) The axons at the medulla demonstrate the CP had not been closed. (C)  : Horizontal section of gracile nuclei. The p600 male showed various i-FSs in the left gracile nucleus and a few colls. (D)
: Horizontal section of gracile nuclei. The p600 male showed various i-FSs in the left gracile nucleus and a few colls. (D) : Sagittal section of the mid-thoracic spinal cord. The multilevel i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine the lengths of axons. t = time. N: iron fix pinhole. Bars: 100 µm.
: Sagittal section of the mid-thoracic spinal cord. The multilevel i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine the lengths of axons. t = time. N: iron fix pinhole. Bars: 100 µm.
 : Denotes the spinal levels at C (transverse section) and D (sagital section). (B) A few TH.2 axons have reached the medulla. (C) The left gracile nucleus had been labeled. (D) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various i-FSs (noted: *) and colls (noted: **) were visible in the neuropil. The parent axons might be inferred from their presence in the medulla. t = time. Bars: 100 µm.
: Denotes the spinal levels at C (transverse section) and D (sagital section). (B) A few TH.2 axons have reached the medulla. (C) The left gracile nucleus had been labeled. (D) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various i-FSs (noted: *) and colls (noted: **) were visible in the neuropil. The parent axons might be inferred from their presence in the medulla. t = time. Bars: 100 µm.
 : Denotes the spinal levels at C (transverse section) and D (sagital section). (B) A few TH.2 axons have reached the medulla. (C) The left gracile nucleus had been labeled. (D) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various i-FSs (noted: *) and colls (noted: **) were visible in the neuropil. The parent axons might be inferred from their presence in the medulla. t = time. Bars: 100 µm.
: Denotes the spinal levels at C (transverse section) and D (sagital section). (B) A few TH.2 axons have reached the medulla. (C) The left gracile nucleus had been labeled. (D) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various i-FSs (noted: *) and colls (noted: **) were visible in the neuropil. The parent axons might be inferred from their presence in the medulla. t = time. Bars: 100 µm.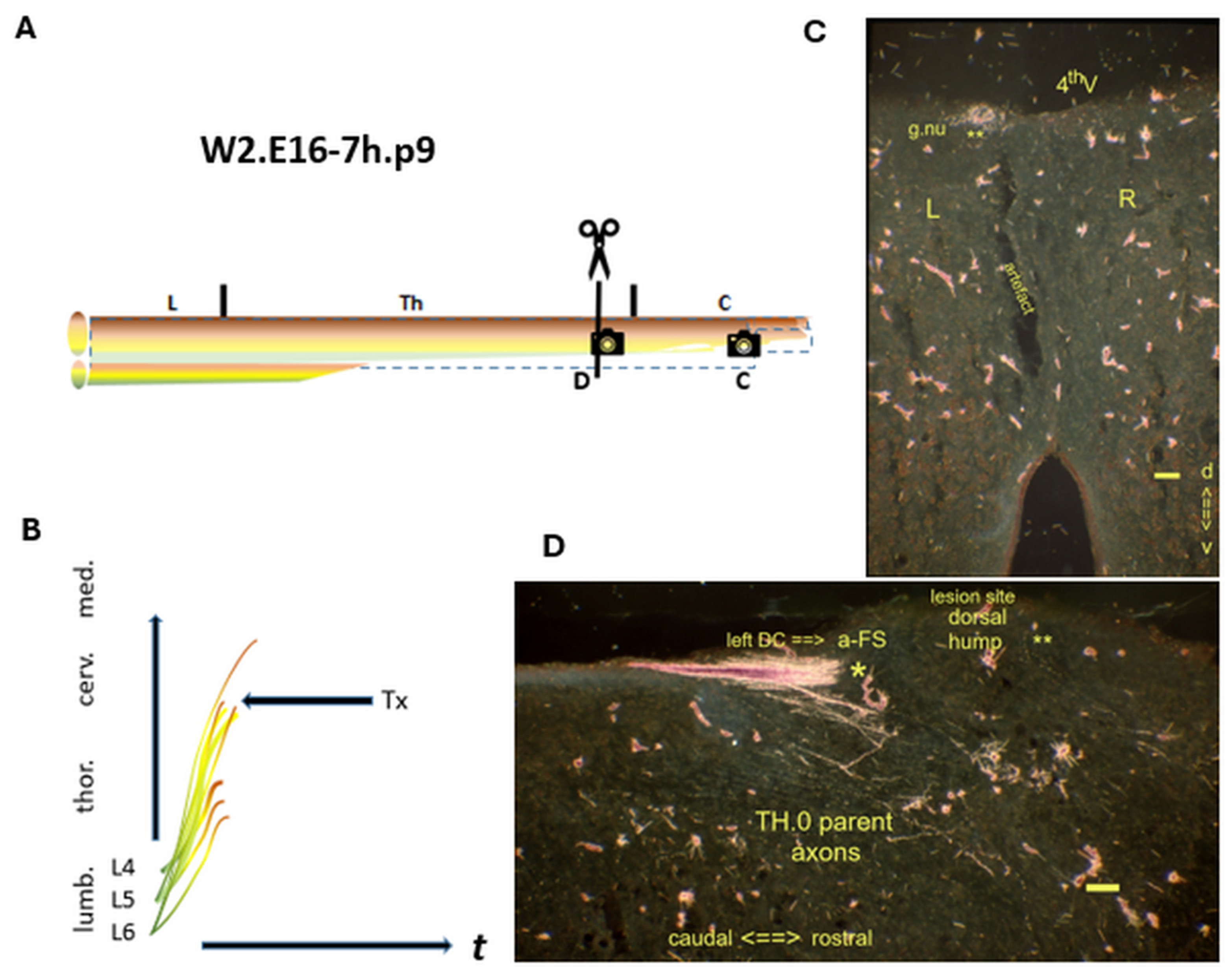
 : The spinal levels at C and D (horizontal sections). (B) A final i-FS crossed the lesion site. (C)
: The spinal levels at C and D (horizontal sections). (B) A final i-FS crossed the lesion site. (C) : In the left rostral DC, the final i-FS has not yet reached the medulla. (D)
: In the left rostral DC, the final i-FS has not yet reached the medulla. (D) : The lesion site’s level is identified first and foremost by the midline cyste. The a-FS is the typical feature of bundled i-FSs in a neonate. The colls (noted: **) exhibited an unusual growth pattern into the CG far beyond the natural superficially located gracile nucleus. These dystopic TH.2 stage colls in the CG exemplified the features complying with the downstream phenotype. Colls have always been qualified mistakenly in the literature as CNS regeneration. t = time. Bars: 100 µm.
: The lesion site’s level is identified first and foremost by the midline cyste. The a-FS is the typical feature of bundled i-FSs in a neonate. The colls (noted: **) exhibited an unusual growth pattern into the CG far beyond the natural superficially located gracile nucleus. These dystopic TH.2 stage colls in the CG exemplified the features complying with the downstream phenotype. Colls have always been qualified mistakenly in the literature as CNS regeneration. t = time. Bars: 100 µm.
 : The spinal levels at C and D (horizontal sections). (B) A final i-FS crossed the lesion site. (C)
: The spinal levels at C and D (horizontal sections). (B) A final i-FS crossed the lesion site. (C) : In the left rostral DC, the final i-FS has not yet reached the medulla. (D)
: In the left rostral DC, the final i-FS has not yet reached the medulla. (D) : The lesion site’s level is identified first and foremost by the midline cyste. The a-FS is the typical feature of bundled i-FSs in a neonate. The colls (noted: **) exhibited an unusual growth pattern into the CG far beyond the natural superficially located gracile nucleus. These dystopic TH.2 stage colls in the CG exemplified the features complying with the downstream phenotype. Colls have always been qualified mistakenly in the literature as CNS regeneration. t = time. Bars: 100 µm.
: The lesion site’s level is identified first and foremost by the midline cyste. The a-FS is the typical feature of bundled i-FSs in a neonate. The colls (noted: **) exhibited an unusual growth pattern into the CG far beyond the natural superficially located gracile nucleus. These dystopic TH.2 stage colls in the CG exemplified the features complying with the downstream phenotype. Colls have always been qualified mistakenly in the literature as CNS regeneration. t = time. Bars: 100 µm.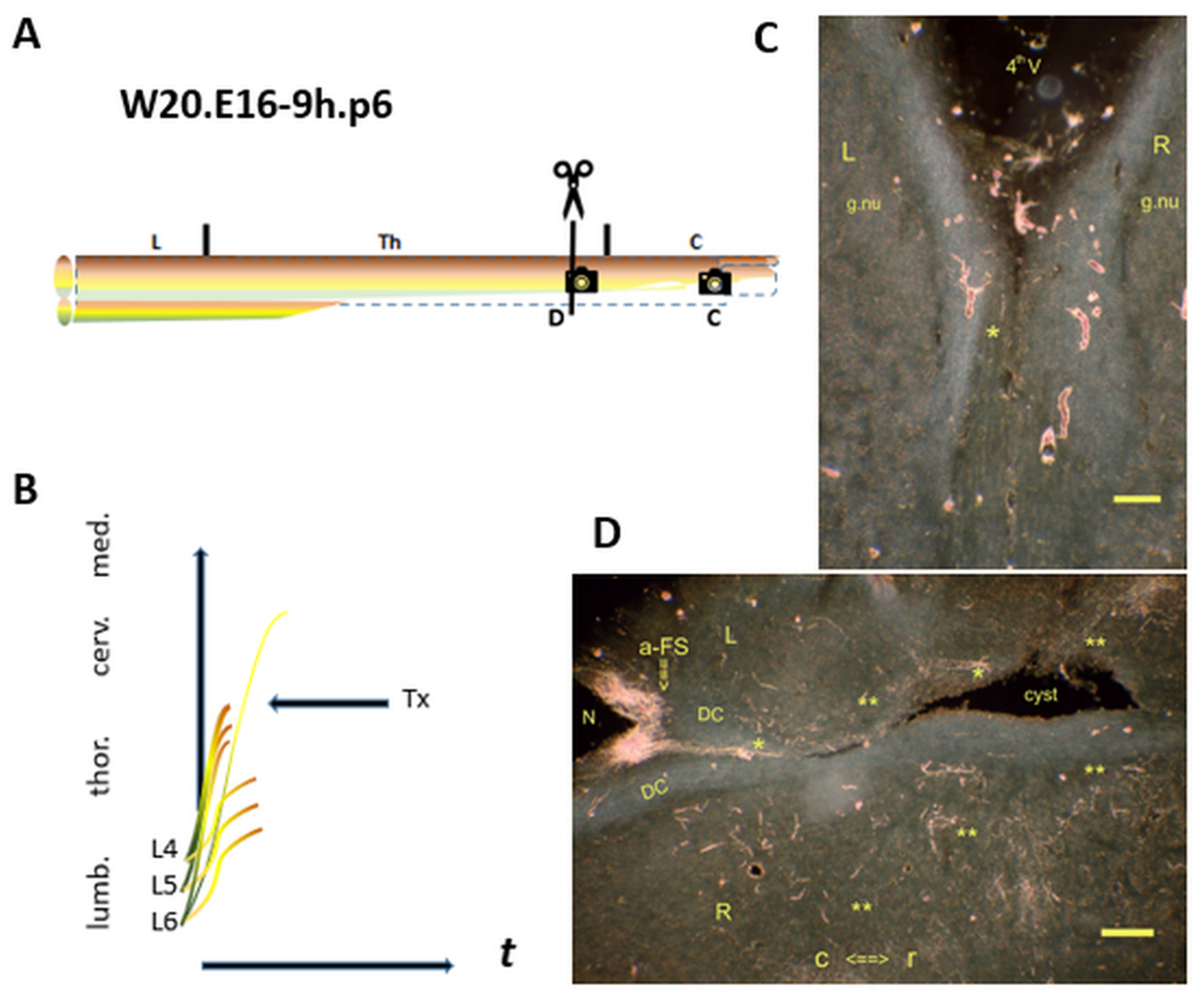
 : The spinal levels at C and D. (B) The yellow i-FSs regenerate into the medulla. (C)
: The spinal levels at C and D. (B) The yellow i-FSs regenerate into the medulla. (C)  : Horizontal slide at the medulla. In the p14 female, the left DC (open arrow) covering the gracile nucleus harbored i-FSs (noted: *). They joined the a-FS feature at a rather rostral medullary level. The axons might have bypassed the gracile nucleus. (D) The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained i-FSs (noted:*), which had been re-elongated into the medulla. t = time. Bar: 100 µm.
: Horizontal slide at the medulla. In the p14 female, the left DC (open arrow) covering the gracile nucleus harbored i-FSs (noted: *). They joined the a-FS feature at a rather rostral medullary level. The axons might have bypassed the gracile nucleus. (D) The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained i-FSs (noted:*), which had been re-elongated into the medulla. t = time. Bar: 100 µm.
 : The spinal levels at C and D. (B) The yellow i-FSs regenerate into the medulla. (C)
: The spinal levels at C and D. (B) The yellow i-FSs regenerate into the medulla. (C)  : Horizontal slide at the medulla. In the p14 female, the left DC (open arrow) covering the gracile nucleus harbored i-FSs (noted: *). They joined the a-FS feature at a rather rostral medullary level. The axons might have bypassed the gracile nucleus. (D) The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained i-FSs (noted:*), which had been re-elongated into the medulla. t = time. Bar: 100 µm.
: Horizontal slide at the medulla. In the p14 female, the left DC (open arrow) covering the gracile nucleus harbored i-FSs (noted: *). They joined the a-FS feature at a rather rostral medullary level. The axons might have bypassed the gracile nucleus. (D) The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained i-FSs (noted:*), which had been re-elongated into the medulla. t = time. Bar: 100 µm.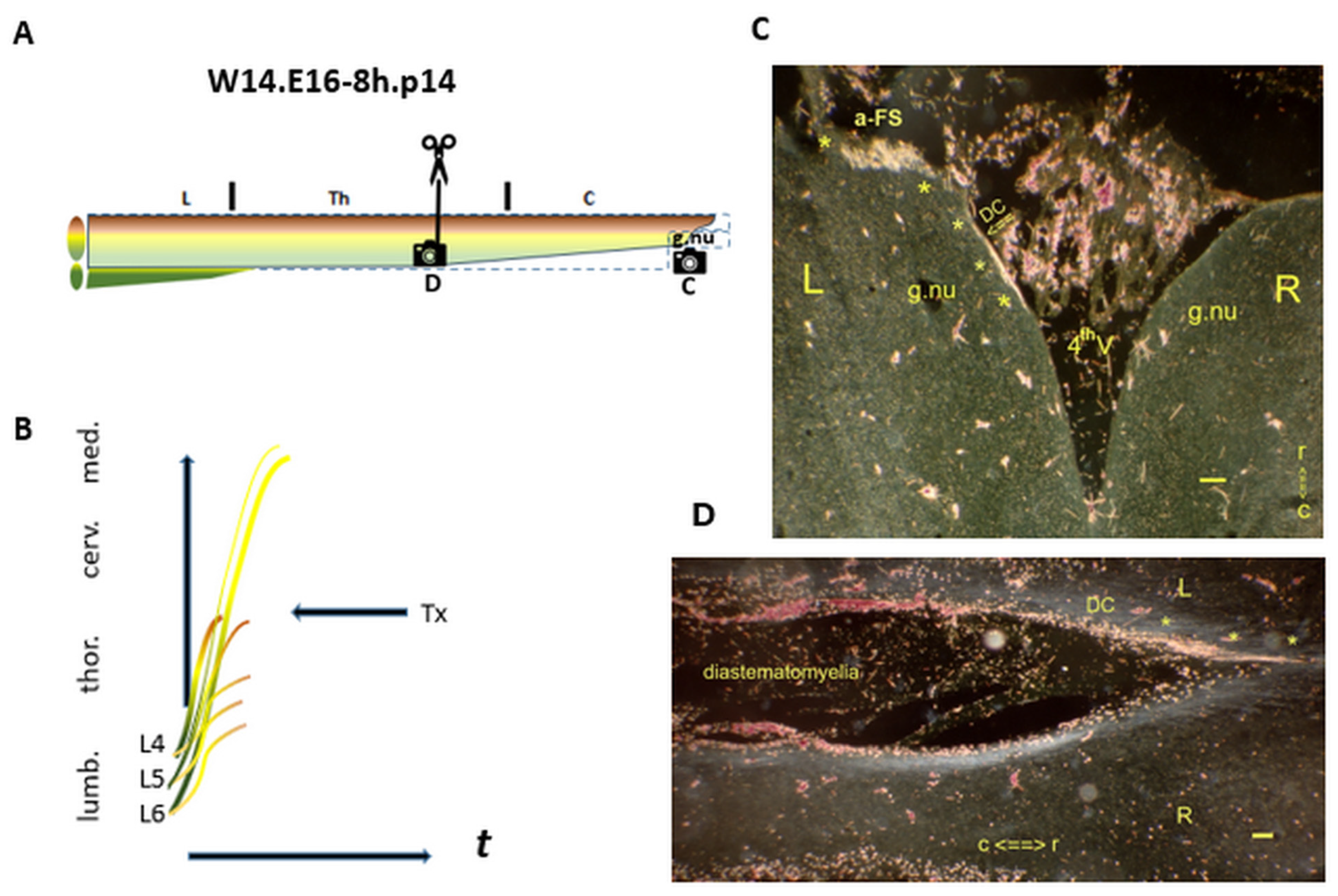
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





