Introduction
Autoimmune diseases affect millions globally, presenting as chronic and often debilitating conditions resulting from immune attacks on self-tissues. While traditional treatments, including immunosuppressants and biologics, offer temporary symptom relief, they fall short in addressing the root causes of immune dysregulation. Over the past decade, cell immunotherapy has emerged as a transformative approach, offering tools to precisely modulate immune activity. This review focuses on CAR-T cell and CAR-Treg therapies, exploring their mechanisms of action, clinical implications, and the potential to shift autoimmune disease management from symptom control to long-term remission.
CAR-T Cell and Regulatory T-Cell (Treg) Therapy for Autoimmune Diseases
Mechanism of Action
Chimeric antigen receptor (CAR)-T cells are advanced immunotherapeutic agents engineered to specifically target and neutralize autoreactive immune cells implicated in autoimmune diseases. The engineering process involves isolating T cells from the patient’s peripheral blood, genetically modifying them to express a CAR capable of recognizing antigens uniquely or predominantly expressed on pathogenic cells, and reintroducing these modified cells back into the patient. Unlike their oncological counterparts that primarily target tumor-associated antigens, CAR-T cells for autoimmunity are designed to identify and eliminate autoreactive B cells, plasma cells producing autoantibodies, and other inflammatory cellular subsets. Recent developments include fine-tuning CAR constructs to improve specificity, such as incorporating co-stimulatory domains that enhance T-cell activation only upon interaction with specific autoimmune targets. Furthermore, the incorporation of safety switches, such as suicide genes or pharmacologically controlled activation domains, adds an additional layer of control, reducing the risk of off-target effects and cytokine release syndrome.
CAR-Tregs represent an evolution of this approach, integrating the chimeric antigen receptor technology with the immunosuppressive capabilities of regulatory T cells (Tregs). CAR-Tregs are designed to suppress excessive immune responses by targeting specific antigens on autoreactive immune cells or within inflamed tissues. By engineering Tregs to express CARs, these cells gain enhanced specificity and functionality, enabling precise modulation of immune activity. Unlike conventional Treg therapies, CAR-Tregs can be tailored to address localized immune dysregulation by targeting antigens present only in diseased tissues, thus minimizing systemic immunosuppression.
Applications
CAR-T cell therapy has demonstrated remarkable potential in treating refractory autoimmune diseases such as systemic lupus erythematosus (SLE) and systemic sclerosis (SSc). Anti-CD19 CAR-T cells, which specifically target CD19-expressing B cells, have shown efficacy in depleting autoreactive B cells and reducing the production of autoantibodies, a key pathological feature in these diseases. Clinical trials have documented instances of sustained clinical remission, highlighting the potential of this approach to not only alleviate symptoms but also modify the underlying disease course. Additionally, CAR-T cells are being investigated for their ability to target plasma cells in cases where long-lived autoantibody-secreting cells perpetuate inflammation.
Similarly, CAR-Tregs are emerging as a promising therapeutic avenue for autoimmune diseases characterized by excessive inflammation and tissue-specific immune attacks, such as type 1 diabetes, multiple sclerosis, and inflammatory bowel disease. Early preclinical models have demonstrated that CAR-Tregs can migrate to inflamed tissues, suppress autoreactive immune responses, and promote tissue repair. Unlike conventional Tregs, CAR-Tregs’ antigen specificity allows them to focus their immunosuppressive effects on disease sites, enhancing efficacy while reducing off-target effects.
Challenges
Antigen Heterogeneity: Autoimmune diseases are highly complex, involving a wide variety of autoreactive immune cell populations and dynamic interactions within the immune system. This heterogeneity makes it difficult to design CAR-T and CAR-Treg therapies that can effectively target all pathogenic cells without affecting healthy ones. For instance, some patients may exhibit different dominant autoantigens or even shift their immune profiles during disease progression, necessitating personalized approaches to therapy.
Safety Concerns: One of the primary risks associated with CAR-T cell therapy in autoimmune diseases is cytokine release syndrome (CRS), a potentially life-threatening immune reaction characterized by excessive inflammation and systemic symptoms such as fever, hypotension, and organ dysfunction. Additionally, off-target effects, where engineered T cells attack healthy tissues expressing low levels of the target antigen, remain a significant challenge. For CAR-Tregs, ensuring long-term stability post-transfer is critical, as these cells can lose their suppressive function or even convert into pathogenic Th17 cells under inflammatory conditions.
Manufacturing and Scalability: Scaling up the production of CAR-T and CAR-Treg therapies for widespread clinical applications is complex and resource-intensive. These therapies require patient-specific engineering, which increases costs and limits scalability. Advances in automation and allogeneic approaches may help address these challenges in the future.
Table 1.
Comparison of CAR-T and Treg Therapies for Autoimmune Diseases.
Table 1.
Comparison of CAR-T and Treg Therapies for Autoimmune Diseases.
| Therapy |
Mechanism of Action |
Targeted Immune Components |
Applications in Autoimmune Diseases |
Challenges |
Clinical Outcomes/Progress |
| CAR-T Cells |
Genetically engineered T cells that express a chimeric receptor to recognize and target specific antigens on autoreactive cells. |
Autoreactive B cells, plasma cells, autoantibodies. |
Systemic lupus erythematosus (SLE), systemic sclerosis (SSc), rheumatoid arthritis (RA) |
Antigen heterogeneity, cytokine release syndrome (CRS), off-target effects |
Documented clinical remission in SLE and SSc. Ongoing trials. |
| Regulatory T Cells (Tregs) |
Isolated Tregs are expanded and infused to restore immune tolerance by suppressing autoreactive immune responses. |
Treg cells themselves target various immune cells and pathways. |
Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) |
Ensuring long-term stability of infused Tregs, potential conversion into pathogenic Th17 cells |
Reduced disease activity and biomarkers of immune tolerance. |
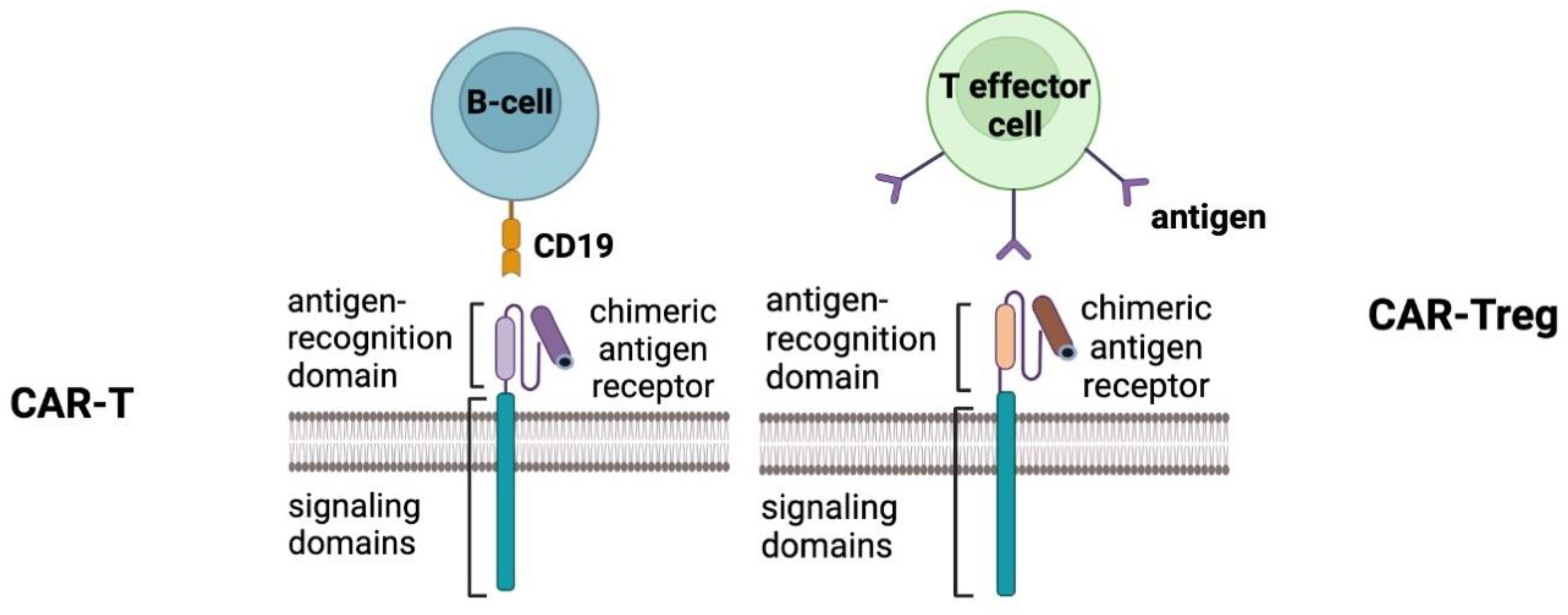
Figure 1.
Comparison of CAR-T and CAR-Treg therapies in autoimmune diseases. On the left, CAR-T cells are engineered to recognize and target autoreactive B cells through CD19 antigen binding, using a chimeric antigen receptor with signaling and antigen-recognition domains. On the right, CAR-Treg cells are engineered to suppress immune responses by recognizing antigens on T effector cells. Both approaches utilize distinct chimeric antigen receptor designs tailored to their respective mechanisms of action in managing autoimmune conditions.
Figure 1.
Comparison of CAR-T and CAR-Treg therapies in autoimmune diseases. On the left, CAR-T cells are engineered to recognize and target autoreactive B cells through CD19 antigen binding, using a chimeric antigen receptor with signaling and antigen-recognition domains. On the right, CAR-Treg cells are engineered to suppress immune responses by recognizing antigens on T effector cells. Both approaches utilize distinct chimeric antigen receptor designs tailored to their respective mechanisms of action in managing autoimmune conditions.
Conclusion
Cell immunotherapy represents a transformative approach to treating autoimmune diseases by directly addressing the root causes of immune dysregulation rather than merely alleviating symptoms. This paradigm shift has been catalyzed by innovations such as CAR-T cells and CAR-Treg therapies. CAR-T cells offer targeted interventions for autoreactive immune cells, while CAR-Tregs aim to restore immune homeostasis with enhanced precision and localized effects.
However, the journey toward integrating these advanced therapies into clinical practice requires overcoming significant hurdles, including scalability, cost, and regulatory complexities. The role of interdisciplinary collaboration among immunologists, geneticists, and clinicians cannot be overstated in accelerating translational research and ensuring the safe deployment of these therapies. As research efforts and clinical trials continue to expand, the promise of cell immunotherapy heralds a new era of precision medicine, offering tailored solutions for individuals with complex autoimmune disorders.
Funding
This work was funded by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities (project number FZSM-2023-0011).
Acknowledgments
The study has been performed according to the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vukovic J, Abazovic D, Vucetic D, Medenica S. CAR-engineered T cell therapy as an emerging strategy for treating autoimmune diseases. Front. Med. 2024, 11, 1447147. [Google Scholar]
- Chasov, V.; Ganeeva, I.; Zmievskaya, E.; Davletshin, D.; Gilyazova, E.; Valiullina, A.; Bulatov, E. Cell-Based Therapy and Genome Editing as Emerging Therapeutic Approaches to Treat Rheumatoid Arthritis. Cells 2024, 13, 1282. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhao, H. Chimeric antigen receptor T-cell therapy in autoimmune diseases. Front. Immunol. 2024, 15, 1492552. [Google Scholar] [CrossRef] [PubMed]
- Arjomandnejad M, Kopec AL, Keeler AM. CAR-T regulatory (CAR-Treg) cells: engineering and applications. Biomedicines 2022, 10, 287. [Google Scholar] [CrossRef]
- Yu J, Yang Y, Gu Z, Shi M, La Cava A, Liu A. CAR immunotherapy in autoimmune diseases: promises and challenges. Front. Immunol. 2024, 15, 1461102. [Google Scholar]
- Requejo Cier, C.J.; Valentini, N.; Lamarche, C. Unlocking the potential of Tregs: innovations in CAR technology. Front. Mol. Biosci. 2023, 10, 1267762. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).