Submitted:
24 December 2024
Posted:
25 December 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and methods
Insects
Guinea Pigs
Parasite Culture
Experimental Infections
Confirmation of Infection Status
Feeding and Defecation Behavioral Trials
Statistical Analyses
Results
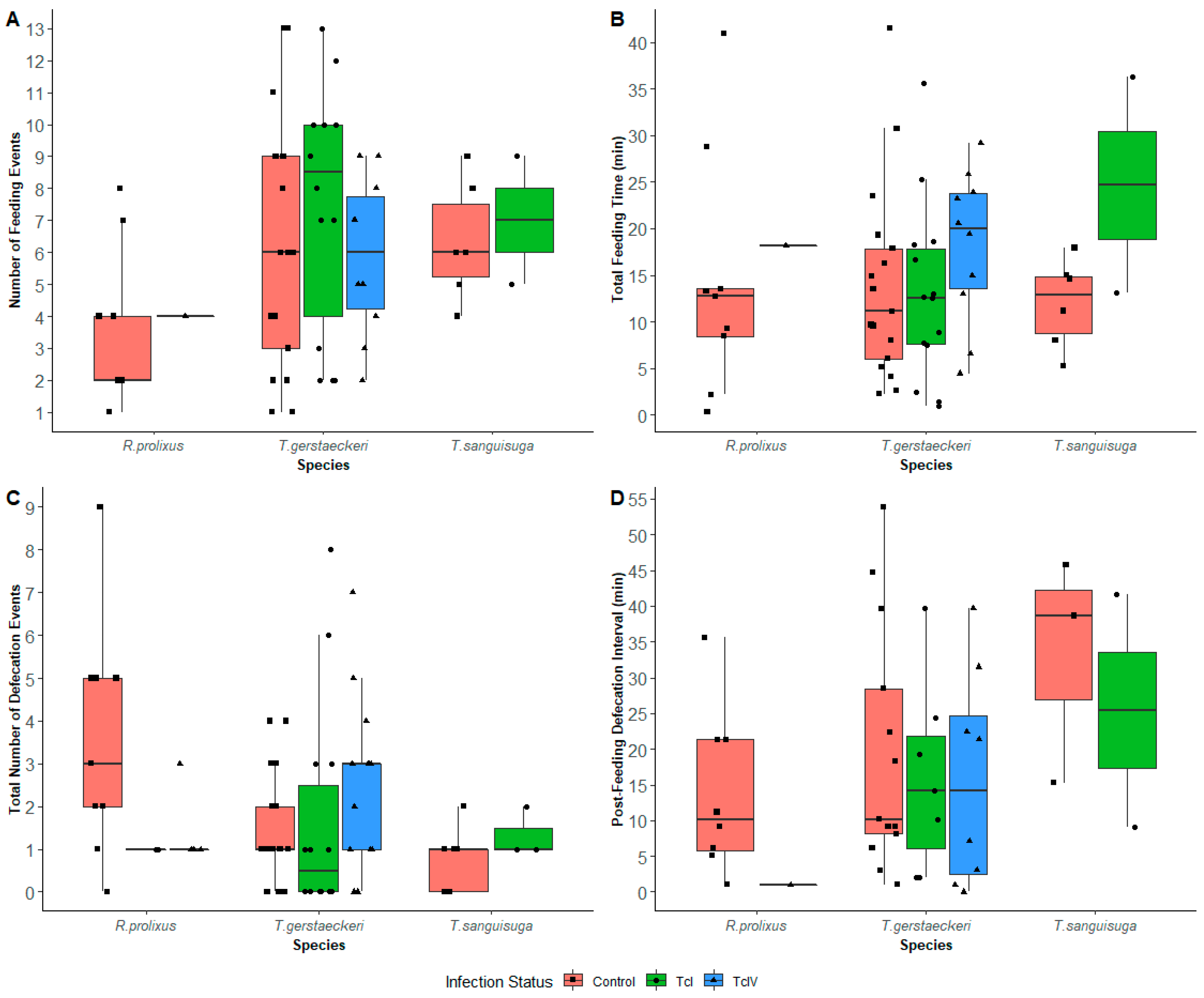
Feeding Results
Defecation Results
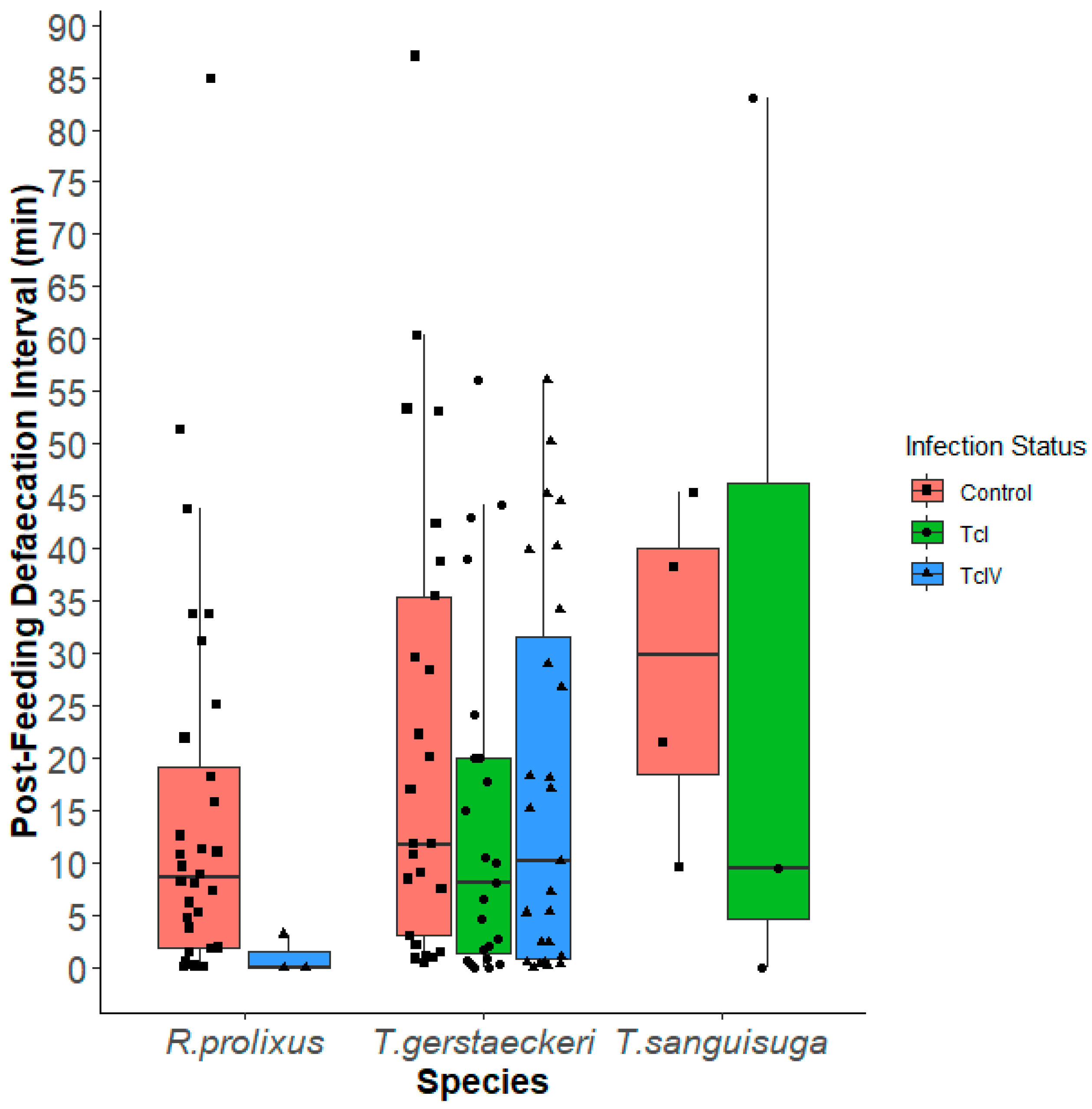
Post-Feeding Defecation Intervals
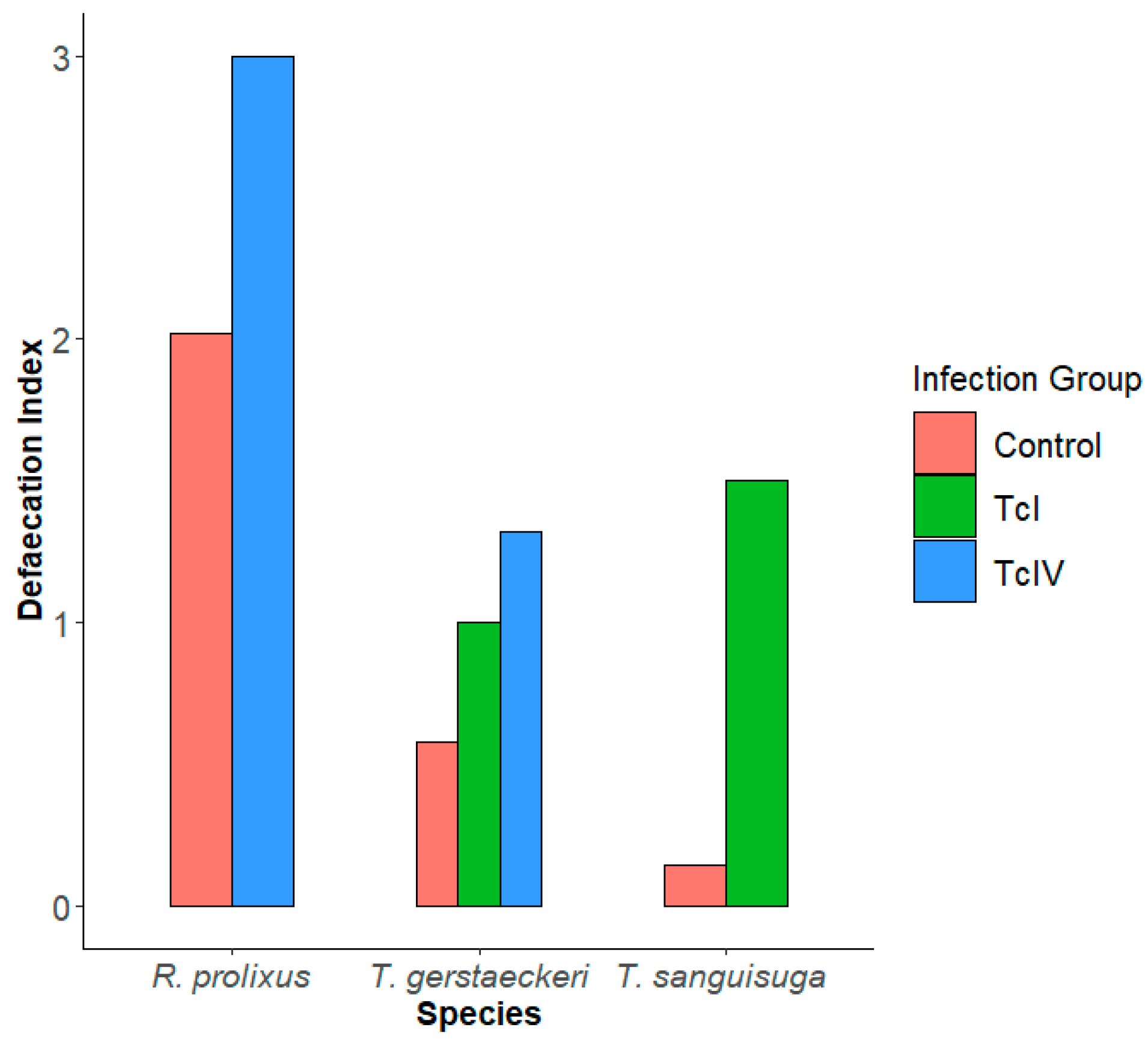
Defecation Index
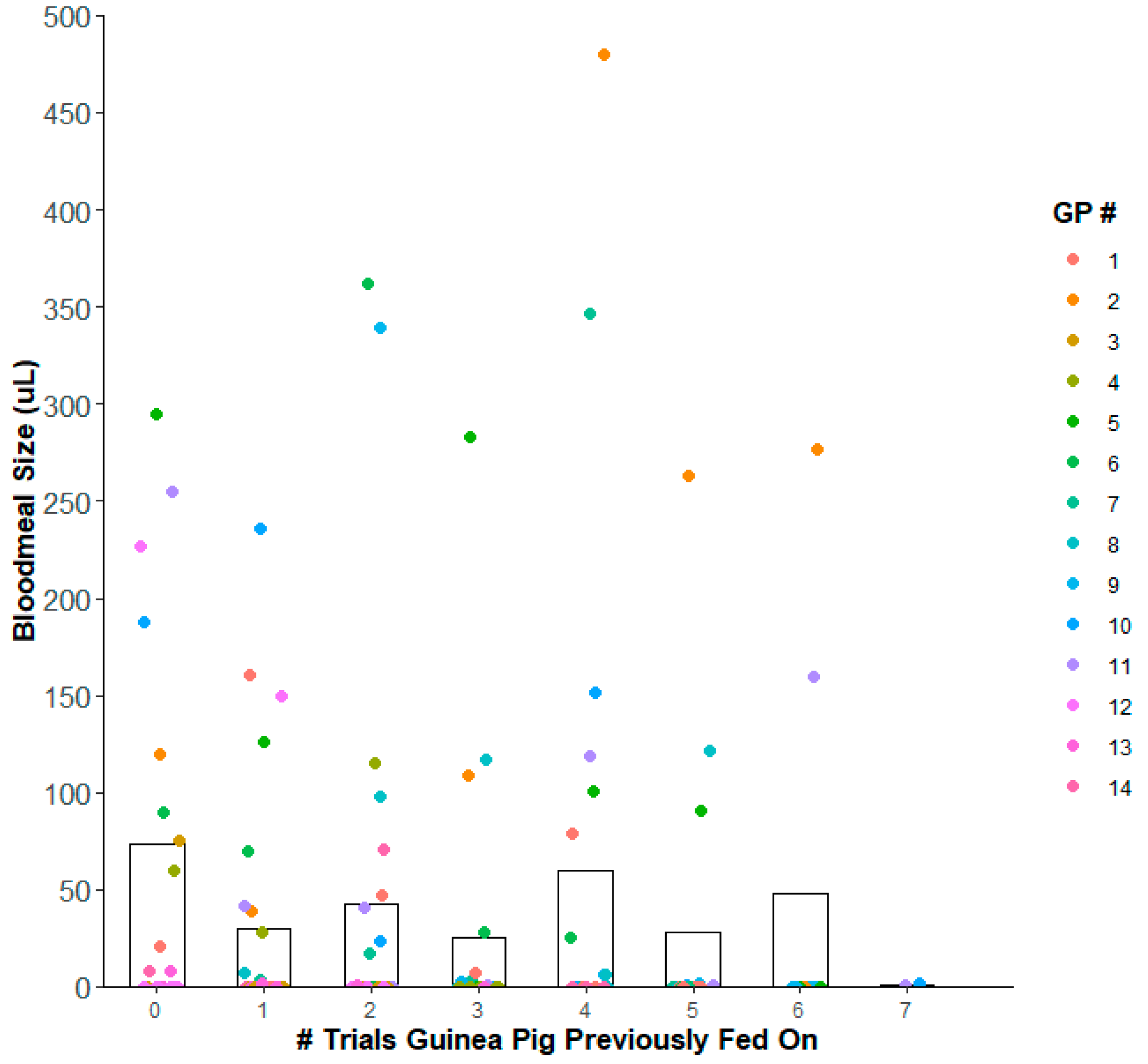
Weight gain & Bloodmeal Size
Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bulletin of the AMNH ; v. 163, article 3; 1979.
- Galvão, C. Taxonomy. In Triatominae - The Biology of Chagas Disease Vectors, Guarneri, A., Lorenzo, M., Eds.; Springer International Publishing: Cham, 2021; pp. 15–38. [Google Scholar]
- Kirk, M.L.; Schofield, C.J. Density-dependent timing of defaecation by Rhodnius prolixus, and its implications for the transmission of Trypanosoma cruzi. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 348–349. [Google Scholar] [CrossRef]
- Zeledón, R.; Alvarado, R.; Jiron, L.F. Observations on the feeding and defecation patterns of three triatomine species (Hemiptera: Reduviidae). Acta Trop. 1977, 34, 65–77. [Google Scholar]
- Canals, M.; Solis, R.; Tapia, C.; Ehrenfeld, M.; Cattan, P. Comparison of some behavioral and physiological feeding parameters of Triatoma infestans Klug, 1834 and Mepraia spinolai Porter, 1934, vectors of Chagas disease in Chile. Mem. Inst. Oswaldo Cruz 1999, 94, 687–692. [Google Scholar] [CrossRef]
- Klotz, S.A.; Dorn, P.L.; Klotz, J.H.; Pinnas, J.L.; Weirauch, C.; Kurtz, J.R.; Schmidt, J. Feeding behavior of triatomines from the southwestern United States: an update on potential risk for transmission of Chagas disease. Acta Trop. 2009, 111, 114–118. [Google Scholar] [CrossRef]
- Reisenman, C.E.; Gregory, T.; Guerenstein, P.G.; Hildebrand, J.G. Feeding and defecation behavior of Triatoma rubida (Uhler, 1894) (Hemiptera: Reduviidae) under laboratory conditions, and its potential role as a vector of Chagas disease in Arizona, USA. Am. J. Trop. Med. Hyg. 2011, 85, 648–656. [Google Scholar] [CrossRef]
- Almeida, C.E.; Francischetti, C.N.; Pacheco, R.S.; Costa, J. Triatoma rubrovaria (Blanchard, 1843) (Hemiptera-Reduviidae-Triatominae) III: patterns of feeding, defecation and resistance to starvation. Mem. Inst. Oswaldo Cruz 2003, 98, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.F. Importance of feeding and defecation times of insect vectors in transmission of Chagas' Disease. J. Econ. Entomol. 1951, 44, 52–54. [Google Scholar] [CrossRef]
- Aldana, E.; Lizano, E.; Rodríguez, M.; Valderrama, A. Alimentación y defecación en triatominos del género Rhodnius (Hemiptera: Reduviidae) alimentados con sangre humana. Rev. Biol. Trop. 2001, 49, 693–696. [Google Scholar] [PubMed]
- Botto-Mahan, C.; Cattan, P.E.; Medel, R. Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop. 2006, 98, 219–223. [Google Scholar] [CrossRef]
- Pippin, W.F. The biology and vector capability of Triatoma sanguisuga Texana Usinger and Triatoma gerstaeckeri (StÅL) compared with Rhodnius prolixus (StÅL) (Hemiptera: Triatominae)1. J. Med. Entomol. 1970, 7, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ibarra, J.A.; Alejandre-Aguilar, R.; Paredes-Gonzalez, E.; Martinez-Silva, M.A.; Solorio-Cibrian, M.; Nogueda-Torres, B.; Trujillo-Contreras, F.; Novelo-Lopez, M. Biology of three species of North American Triatominae (Hemiptera: Reduviidae: Triatominae) fed on rabbits. Mem. Inst. Oswaldo Cruz 2007, 102, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.B.; Praça, Y.R.; Bentes, K.L.d.S.; Santiago, P.B.; Silva, S.M.M.; Silva, G.D.S.; Motta, F.N.; Bastos, I.M.D.; de Santana, J.M.; de Araújo, C.N. Triatomines: Trypanosomatids, Bacteria, and Viruses Potential Vectors? Frontiers in cellular and infection microbiology 2018, 8, 405–405. [Google Scholar] [CrossRef] [PubMed]
- Chacón, F.; Bacigalupo, A.; Álvarez-Duhart, B.; Cattan, P.E.; Solís, R.; Muñoz-San Martín, C. The Parasite Load of Trypanosoma cruzi Modulates Feeding and Defecation Patterns of the Chagas Disease Vector Triatoma infestans. Microorganisms 2022, 10, 1003. [Google Scholar] [CrossRef]
- Rabinovich, J.E.; Leal, J.A.; Feliciangeli de Piñero, D. Domiciliary biting frequency and blood ingestion of the Chagas's disease vector Rhodnius prolixus Ståhl (Hemiptera: Reduviidae), in Venezuela. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 272–283. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Segovia, V.; García, A.; Botto-Mahan, C.; Ortiz, S.; Solari, A.; Acuna-Retamar, M.; Torres-Pérez, F.; Cattan, P.E. Differential Pattern of Infection of Sylvatic Nymphs and Domiciliary Adults of Triatoma infestans with Trypanosoma cruzi Genotypes in Chile. The American Society of Tropical Medicine and Hygiene 2012, 87, 473–480. [Google Scholar] [CrossRef]
- Roellig, D.M.; Ellis, A.E.; Yabsley, M.J. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. The Journal of Parasitology 2009, 95, 360–364. [Google Scholar] [CrossRef]
- Nóbrega, A.A.; Garcia, M.H.; Tatto, E.; Obara, M.T.; Costa, E.; Sobel, J.; Araujo, W.N. Oral transmission of Chagas disease by consumption of açaí palm fruit, Brazil. Emerg. Infect. Dis. 2009, 15, 653–655. [Google Scholar] [CrossRef]
- Dias, E. Observations on defecation and contact feeding time of several South American Triatoma. Mem. Inst. Oswaldo Cruz 1956, 54, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, N.; Lobbia, P.A.; Mougabure-Cueto, G. Effects of the infection with Trypanosoma cruzi on the feeding and excretion/defecation patterns of Triatoma infestans. Bull. Entomol. Res. 2020, 110, 169–176. [Google Scholar] [CrossRef]
- Pipkin, A.C., Sr. Domiciliary reduviid bugs and the epidemiology of Chagas' disease in Panama (Hemiptera: Reduviidae: Triatominae). J. Med. Entomol. 1968, 5, 107–124. [Google Scholar] [CrossRef]
- Mosquera, K.D.; Villacís, A.G.; Grijalva, M.J. Life Cycle, Feeding, and Defecation Patterns of Panstrongylus chinai (Hemiptera: Reduviidae: Triatominae) Under Laboratory Conditions. J. Med. Entomol. 2016, 53, 776–781. [Google Scholar] [CrossRef]
- Shields, T.L.; Walsh, E.N. Kissing bug bite. AMA Arch Derm 1956, 74, 14–21. [Google Scholar] [CrossRef]
- Vetter, R. Kissing bugs (Triatoma) and the skin. Dermatol. Online J. 2001, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, J.E.; Kitron, U.D.; Obed, Y.; Yoshioka, M.; Gottdenker, N.; Chaves, L.F. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 2011, 106, 479–494. [Google Scholar] [CrossRef]
- Lynn, M.K.; Bossak, B.H.; Sandifer, P.A.; Watson, A.; Nolan, M.S. Contemporary autochthonous human Chagas disease in the USA. Acta Trop. 2020, 205, 105361. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas Disease in the United States: a Public Health Approach. Clinical microbiology reviews 2019, 33, e00023–00019. [Google Scholar] [CrossRef] [PubMed]
- Kjos, S.A.; Snowden, K.F.; Olson, J.K. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector borne and zoonotic diseases 2009, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Kjos, S.; Yabsley, M.J.; Montgomery, S.P. Trypanosoma cruzi and Chagas' Disease in the United States. Clin. Microbiol. Rev. 2011, 24, 655–681. [Google Scholar] [CrossRef]
- Sandoval-Ruiz, C.A.; Cervantesperedo, L.M.-P., Fredy Severo; Ibanez-Bernal, S. The Triatominae (Hemiptera: Heteroptera: Reduviidae) of Veracruz, Mexico: geographic distribution, taxonomic redescriptions, and a key. Zootaxa 2012, 3487, 1–23. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Auckland, L.D.; Snowden, K.F.; Hamer, G.L.; Hamer, S.A. Analysis of over 1500 triatomine vectors from across the US, predominantly Texas, for Trypanosoma cruzi infection and discrete typing units. Infect Genet Evol 2018, 58, 171–180. [Google Scholar] [CrossRef]
- Kjos, S.A.; Marcet, P.L.; Yabsley, M.J.; Kitron, U.; Snowden, K.F.; Logan, K.S.; Barnes, J.C.; Dotson, E.M. Identification of bloodmeal sources and Trypanosoma cruzi infection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. J. Med. Entomol. 2013, 50, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Waleckx, E.; Suarez, J.; Richards, B.; Dorn, P.L. Triatoma sanguisuga blood meals and potential for Chagas disease, Louisiana, USA. Emerg. Infect. Dis. 2014, 20, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.N.; Burroughs, H.; Gorchakov, R.; Gunter, S.M.; Dumonteil, E.; Murray, K.O.; Herrera, C.P. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infection, Genetics and Evolution 2017, 49, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, C.A.; Mitchell, E.A.; Reisenman, C.E.; Sarkar, S.; Williamson, P.C.; Machado, C.A. Phylogenetic diversity of two common Trypanosoma cruzi lineages in the Southwestern United States. Infection, Genetics and Evolution 2022, 99, 105251. [Google Scholar] [CrossRef]
- Zeledón, R.; Rabinovich, J.E. Chagas Disease: an Ecological Appraisal With Special Emphasis on its Insect Vectors. Annu. Rev. Entomol. 1981, 26, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas disease. The Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Waleckx, E.; Gourbière, S.; Dumonteil, E. Intrusive <i>versus</i> domiciliated triatomines and the challenge of adapting vector control practices against Chagas disease. Mem. Inst. Oswaldo Cruz 2015, 110. [Google Scholar]
- Buxton, P.A. The biology of a blood-sucking bug, Rhodnius prolixus. Transactions of the Royal Entomological Society of London 1930, 78, 227–256. [Google Scholar] [CrossRef]
- Uribe, C. On the biology and life history of Rhodnius prolixus Stahl. The Journal of Parasitology 1926, 13, 129–136. [Google Scholar] [CrossRef]
- Wormington, J.D.; Gillum, C.; Meyers, A.C.; Hamer, G.L.; Hamer, S.A. Daily activity patterns of movement and refuge use in Triatoma gerstaeckeri and Rhodnius prolixus (Hemiptera: Reduviidae), vectors of the Chagas disease parasite. Acta Trop 2018, 185, 301–306. [Google Scholar] [CrossRef]
- Hodo, C.L.; Bertolini, N.R.; Bernal, J.C.; VandeBerg, J.L.; Hamer, S.A. Lack of Trypanosoma cruzi infection in urban roof rats (Rattus rattus) at a Texas facility housing naturally infected nonhuman primates. J Am Assoc Lab Anim Sci 2017, 56, 57–62. [Google Scholar]
- Hodo, C.L.; Wilkerson, G.K.; Birkner, E.C.; Gray, S.B.; Hamer, S.A. Trypanosoma cruzi Transmission Among Captive Nonhuman Primates, Wildlife, and Vectors. Ecohealth 2018, 15, 426–436. [Google Scholar] [CrossRef]
- Fernandes, J.F.; Castellani, O. Growth characteristics and chemical composition of Trypanosoma cruzi. Exp. Parasitol. 1966, 18, 195–202. [Google Scholar] [CrossRef]
- Sadigursky, M.; Brodskyn, C.I. A new liquid medium without blood and serum for culture of hemoflagellates. Am. J. Trop. Med. Hyg. 1986, 35, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.P. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 1964, 6, 93–100. [Google Scholar] [PubMed]
- Cura, C.I.; Duffy, T.; Lucero, R.H.; Bisio, M.; Péneau, J.; Jimenez-Coello, M.; Calabuig, E.; Gimenez, M.J.; Valencia Ayala, E.; Kjos, S.A.; et al. Multiplex real-time PCR assay using TaqMan probes for the identification of Trypanosoma cruzi DTUs in biological and clinical samples. Plos Neglect. Trop. Dis. 2015, 9, e0003765. [Google Scholar] [CrossRef] [PubMed]
- Bice, D.E.; Zeledon, R. Comparison of infectivity of strains of Trypanosoma cruzi (Chagas, 1909). J. Parasitol. 1970, 56, 663–670. [Google Scholar] [CrossRef]
- Mejia-Jaramillo, A.M.; Pena, V.H.; Triana-Chavez, O. Trypanosoma cruzi: Biological characterization of lineages I and II supports the predominance of lineage I in Colombia. Exp. Parasitol. 2009, 121, 83–91. [Google Scholar] [CrossRef]
- Peterson, J.K.; Graham, A.L.; Dobson, A.P.; Chavez, O.T. Rhodnius prolixus life history outcomes differ when infected with different Trypanosoma cruzi I strains. Am. J. Trop. Med. Hyg. 2015, 93, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Añez, N.; Martens, M.L.; Romero, M.; Crisante, G. Trypanosoma cruzi primary infection prevents severe re-infection in mice. Boletín de Malariología y Salud Ambiental 2011, 51, 177–186. [Google Scholar]
- Fellet, M.R.; Lorenzo, M.G.; Elliot, S.L.; Carrasco, D.; Guarneri, A.A. Effects of infection by Trypanosoma cruzi and Trypanosoma rangeli on the reproductive performance of the vector Rhodnius prolixus. PLoS One 2014, 9, e105255. [Google Scholar] [CrossRef] [PubMed]
- Verly, T.; Costa, S.; Lima, N.; Mallet, J.; Odencio, F.; Pereira, M.; Moreira, C.J.C.; Britto, C.; Pavan, M.G. Vector competence and feeding-excretion behavior of Triatoma rubrovaria (Blanchard, 1843) (Hemiptera: Reduviidae) infected with Trypanosoma cruzi TcVI. PLoS Negl Trop Dis 2020, 14, e0008712. [Google Scholar] [CrossRef] [PubMed]
- Nogueda-Torres, B.; Martinez-Ibarra, J.A.; Barboza-Lopez, M.; Montanez-Valdez, O.D.; Michel-Parra, J.G. Biological Parameters of Two Triatoma protracta Subspecies (Hemiptera: Reduviidae). J. Med. Entomol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern applied statistics with S; Springer: New York, 2002. [Google Scholar]
- Chaves, L.F. An entomologist guide to demystify pseudoreplication: data analysis of field studies with design constraints. J. Med. Entomol. 2010, 47, 291–298. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models CRC Press: Boca Raton, 2006.
- Pan, W. Akaike's Information Criterion in Generalized Estimating Equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef]
- Schwarz, A.; Sternberg, J.M.; Johnston, V.; Medrano-Mercado, N.; Anderson, J.M.; Hume, J.C.; Valenzuela, J.G.; Schaub, G.A.; Billingsley, P.F. Antibody responses of domestic animals to salivary antigens of Triatoma infestans as biomarkers for low-level infestation of triatomines. Int. J. Parasitol. 2009, 39, 1021–1029. [Google Scholar] [CrossRef]
- Dornakova, V.; Salazar-Sanchez, R.; Borrini-Mayori, K.; Carrion-Navarro, O.; Levy, M.Z.; Schaub, G.A.; Schwarz, A. Characterization of guinea pig antibody responses to salivary proteins of Triatoma infestans for the development of a triatomine exposure marker. PLoS Negl Trop Dis 2014, 8, e2783. [Google Scholar] [CrossRef]
- Martinez-Ibarra, J.A.; Paredes-Gonzalez, E.; Licon-Trillo, A.; Montanez-Valdez, O.D.; Rocha-Chavez, G.; Nogueda-Torres, B. The biology of three Mexican-American species of Triatominae (Hemiptera: Reduviidae): Triatoma recurva, Triatoma protracta and Triatoma rubida. Mem. Inst. Oswaldo Cruz 2012, 107, 659–663. [Google Scholar] [CrossRef]
- Cordero-Montoya, G.; Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Vences-Blanco, M.O.; Rocha-Ortega, M.; Gutierrez-Cabrera, A.E.; Rojas-Ortega, E.; Cordoba-Aguilar, A. The cost of being a killer's accomplice: Trypanosoma cruzi impairs the fitness of kissing bugs. Parasitol. Res. 2019, 118, 2523–2529. [Google Scholar] [CrossRef]
- Elliot, S.L.; Rodrigues Jde, O.; Lorenzo, M.G.; Martins-Filho, O.A.; Guarneri, A.A. Trypanosoma cruzi, etiological agent of Chagas disease, is virulent to its triatomine vector Rhodnius prolixus in a temperature-dependent manner. PLoS Negl Trop Dis 2015, 9, e0003646. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, G.; Nava-Bolanos, A.; De Fuentes-Vicente, J.A.; Tellez-Rendon, J.L.; Huerta, H.; Martinez-Hernandez, F.; Rocha-Ortega, M.; Gutierrez-Cabrera, A.E.; Ibarra-Cerdena, C.N.; Cordoba-Aguilar, A. A reduction in ecological niche for Trypanosoma cruzi-infected triatomine bugs. Parasit Vectors 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A. Developmental time and mortality of larvae of Triatoma infestans infected with Trypanosoma cruzi. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 94–96. [Google Scholar] [CrossRef]
- Ramirez-Gonzalez, M.G.; Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Gutierrez-Cabrera, A.E.; Rojas-Ortega, E.; Cordoba-Aguilar, A. Zombie bugs? Manipulation of kissing bug behavior by the parasite Trypanosoma cruzi. Acta Trop. 2019, 200, 105177. [Google Scholar] [CrossRef]
- Trumper, E.V.; Gorla, D.E. Density-dependent timing of defaecation by Triatoma infestans. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Zeledón, *!!! REPLACE !!!*; Beard, C.; Dias, J.C.; Leiby, D.A.; Dorn, P.; Coura, J.R. Zeledón; Beard, C.; Dias, J.C.; Leiby, D.A.; Dorn, P.; Coura, J.R. An Appraisal of the Status of Chagas Disease in the United States, 2012. [Google Scholar]
- Sant’Anna, M.R.V.; Soares, A.C.; Araujo, R.N.; Gontijo, N.F.; Pereira, M.H. Triatomines (Hemiptera, Reduviidae) blood intake: Physical constraints and biological adaptations. J. Insect Physiol. 2017, 97, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. Population regulation in ticks: the role of acquired resistance in natural and unnatural hosts. Parasitology 1979, 79, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Hays, K.L. Some habitat requirements for Triatoma sanguisuga (Le Conte) (Hemiptera; Reduviidae). J. Alabama Acad. Sci. 1966, 37, 8–14. [Google Scholar]
- Hays, K.L. Longevity, Fecundity, and Food Intake of Adult Triatoma Sanguisuga (Leconte) (Hemiptera: Triatominae)1. J. Med. Entomol. 1965, 2, 200–202. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. Memoirs: The Physiology of Ecdysis in Rhodnius Prolixus (Hemiptera). II. Factors controlling Moulting and ‘Metamorphosis’. J. Cell Sci. 1934, s2-77, 191–222. [Google Scholar] [CrossRef]
- Soares, R.P.; das Graças Evangelista, L.; Laranja, L.S.; Diotaiuti, L. Population dynamics and feeding behavior of Triatoma brasiliensis and Triatoma pseudomaculata, main vectors of chagas disease in Northeastern Brazil. Mem. Inst. Oswaldo Cruz 2000, 95, 151–155. [Google Scholar] [CrossRef]
- de Azambuja, P.; Garcia, E.S. Care and maintenance of triatomine colonies. In The Molecular Biology of Insect Disease Vectors: A Methods Manual, Crampton, J.M., Beard, C.B., Louis, C., Eds.; Springer Netherlands: Dordrecht, 1997; pp. 56–64. [Google Scholar]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol 2012, 12, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, H.J.; Segovia, M.; Llewellyn, M.S.; Morocoima, A.; Urdaneta-Morales, S.; Martínez, C.; Martínez, C.E.; Garcia, C.; Rodríguez, M.; Espinosa, R.; et al. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. Plos Neglect. Trop. Dis. 2012, 6, e1707–e1707. [Google Scholar] [CrossRef]
- Takano-Lee, M.; Edman, J.D. Lack of manipulation of Rhodnius prolixus (Hemiptera: Reduviidae) vector competence by Trypanosoma cruzi. J. Med. Entomol. 2002, 39, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Curtis-Robles, R.; Wozniak, E.J.; Auckland, L.D.; Hamer, G.L.; Hamer, S.A. Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. PLoS Negl Trop Dis 2015, 9, e0004235. [Google Scholar] [CrossRef]
- Edward, J.W.; Gena, L.; Rodion, G.; Hasanat, A.; Ellen, D.; Blake, S.; Sahotra, S.; Kristy, O.M. The Biology of the Triatomine Bugs Native to South Central Texas and Assessment of the Risk They Pose for Autochthonous Chagas Disease Exposure. J. Parasitol. 2015, 101, 520–528. [Google Scholar] [CrossRef]
- Ryckman, R.E. Recent observations of cannibalism in Triatoma (Hemiptera: Reduviidae). J. Parasitol. 1951, 37, 433–434. [Google Scholar] [CrossRef]
- Schaub, G.A. Direct transmission of Trypanosoma cruzi between vectors of Chagas' disease. Acta Trop. 1988, 45, 11–19. [Google Scholar] [PubMed]
- Balasubramanian, S.; Curtis-Robles, R.; Chirra, B.; Auckland, L.D.; Mai, A.; Bocanegra-Garcia, V.; Clark, P.; Clark, W.; Cottingham, M.; Fleurie, G.; et al. Characterization of triatomine bloodmeal sources using direct Sanger sequencing and amplicon deep sequencing methods. Scientific Reports 2022, 12, 10234. [Google Scholar] [CrossRef]
- Gorchakov, R.; Trosclair, L.P.; Wozniak, E.J.; Feria, P.T.; Garcia, M.N.; Gunter, S.M.; Murray, K.O. Trypanosoma cruzi Infection Prevalence and Bloodmeal Analysis in Triatomine Vectors of Chagas Disease From Rural Peridomestic Locations in Texas, 2013–2014. J. Med. Entomol. 2016, 53, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, R.; Fernández, M.; Cardinal, M.V. Eco-Epidemiology of Vector-Borne Transmission of Trypanosoma cruzi in Domestic Habitats. 2021; pp. 447–489.

| Species | Infection Group | No. of Insects | No. Fed (%) | No. Defecated (%)a | No. Fed + Defecated (%)b | % Fed Insects that Defecated in 120 min |
| T. gerstaeckeri | Control | 26 | 17 (65) | 15 (58) | 13 (50) | 76 |
| TcI | 21 | 14 (67) | 7 (33) | 7 (33) | 50 | |
| TcIV | 17 | 10 (58) | 11 (65) | 8 (47) | 80 | |
| T. sanguisuga | Control | 16 | 6 (38) | 5 (31) | 3 (19) | 50 |
| TcI | 11 | 2 (17) | 3 (25) | 2 (17) | 100 | |
| R. prolixus | Control | 21 | 9 (47) | 8 (42) | 8 (42) | 89 |
| TcI | 19 | 0 (0) | 2 (11) | 0 (0) | 0 | |
| TcIV | 17 | 1 (6) | 5 (29) | 1 (6) | 100 | |
| Overall | 148 | 59 (40) | 56 (38) | 42 (28) | 71 | |
| aThis is the number of insects that defecated at least once in the 2-hour period, including insects that defecated before feeding or did not feed at all. bThis number represents insects that fed on a guinea pig either with simultaneous defecation or any defecation following feeding. | ||||||
| Species | Infection Group | Nymphal Instar | No. Insects that Gained Weight | Mean ± S.E. Weight Gain (mg) | Mean Blood Volume Ingested (µL) | % Weight Gain ± S.E. |
| T. gerstaeckeri | Control | 5th | 15 | 121 ± 26.5 | 120.7 | 98.8 ± 23.2 |
| TcI | 5th | 13 | 102 ± 27.9 | 102.2 | 104.8 ± 35.2 | |
| TcIV | 5th | 8 | 265 ± 46.1 | 264.9 | 164.0 ± 37.0 | |
| T. sanguisuga | Control | 4th | 4 | 14 ± 9.0 | 14.2 | 44.9 ± 33.9 |
| 5th | 1 | 39 | 39.0 | 46.9 | ||
| TcI | 4th | 1 | 1.4 | 1.4 | 2.8 | |
| 5th | 1 | 126 | 126.0 | 175 | ||
| R. prolixus | Control | 5th | 9 | 71 ± 18.5 | 70.7 | 222.3 ± 44.4 |
| TcI | n/a | 0 | 0 | 0 | 0 | |
| TcIV | 5th | 1 | 160 | 159.5 | 406.9 | |
| a There were six insects that fed on guinea pigs but did not gain any weight. These data points were excluded from the calculations. b Some infection groups only had 5th instar triatomines that fed and gained weight. R. prolixus (TcI) group did not have any insects that fed and gained weight, but the data is still shown in this table. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





