Submitted:
24 December 2024
Posted:
25 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
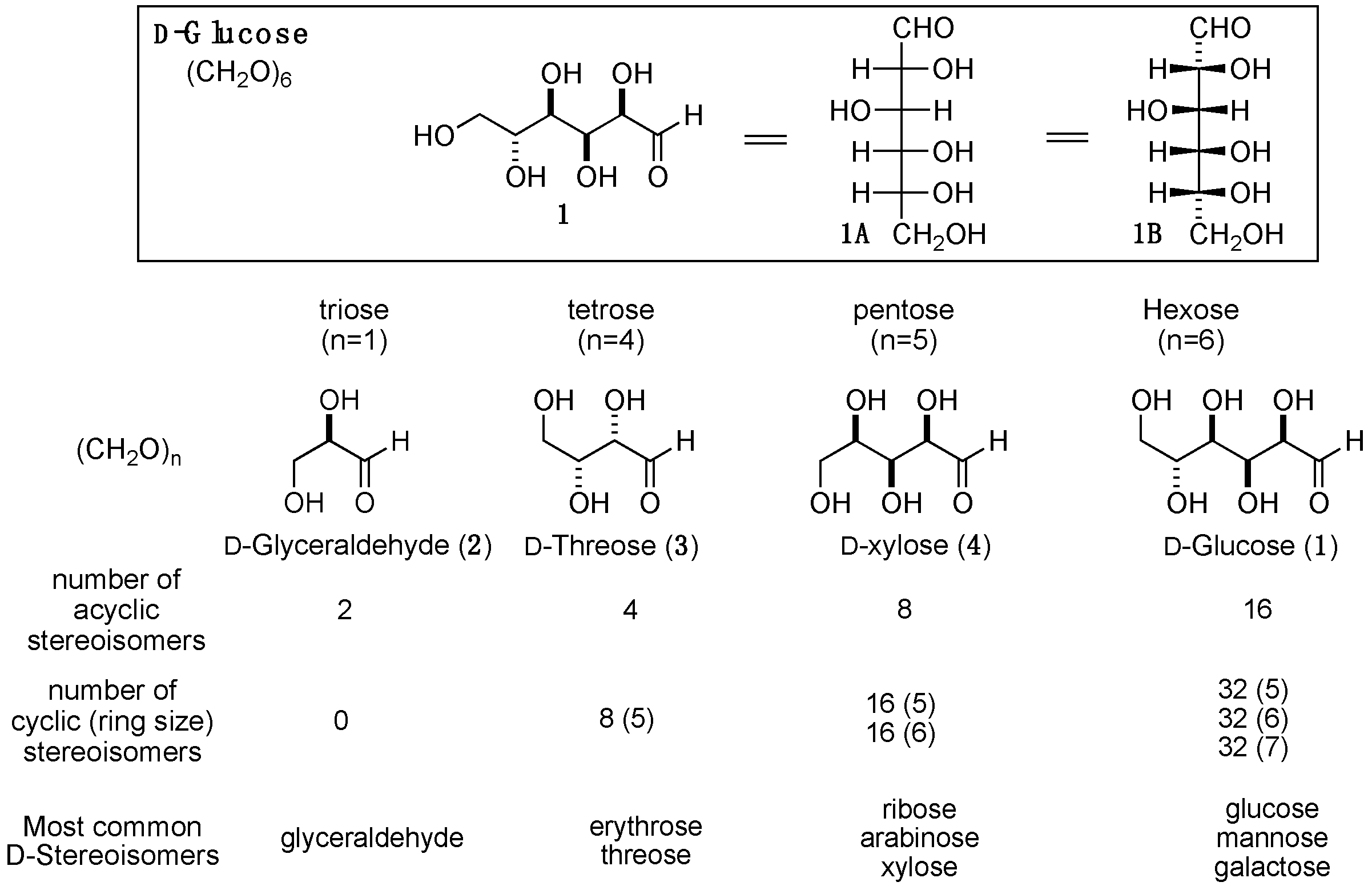
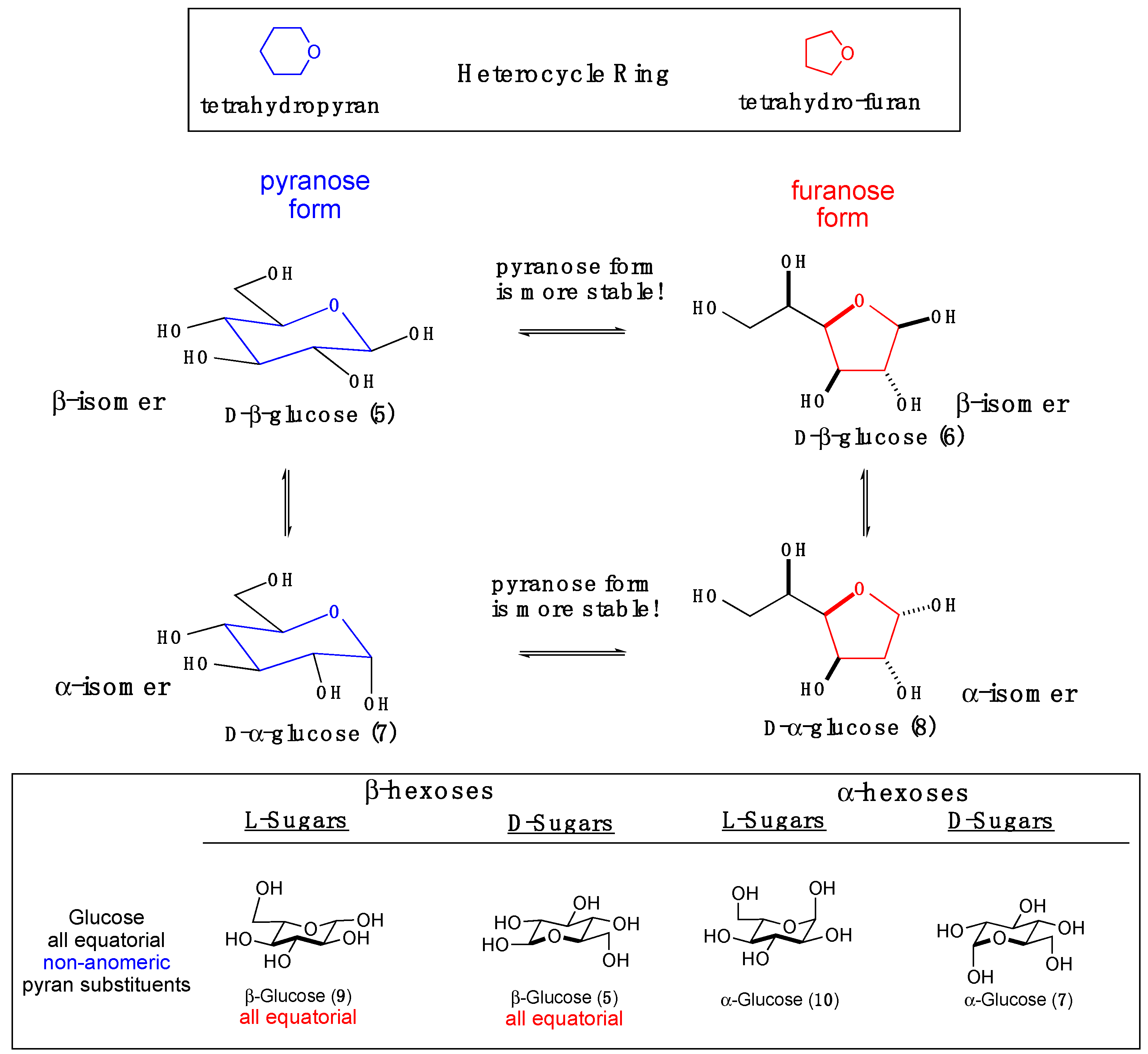
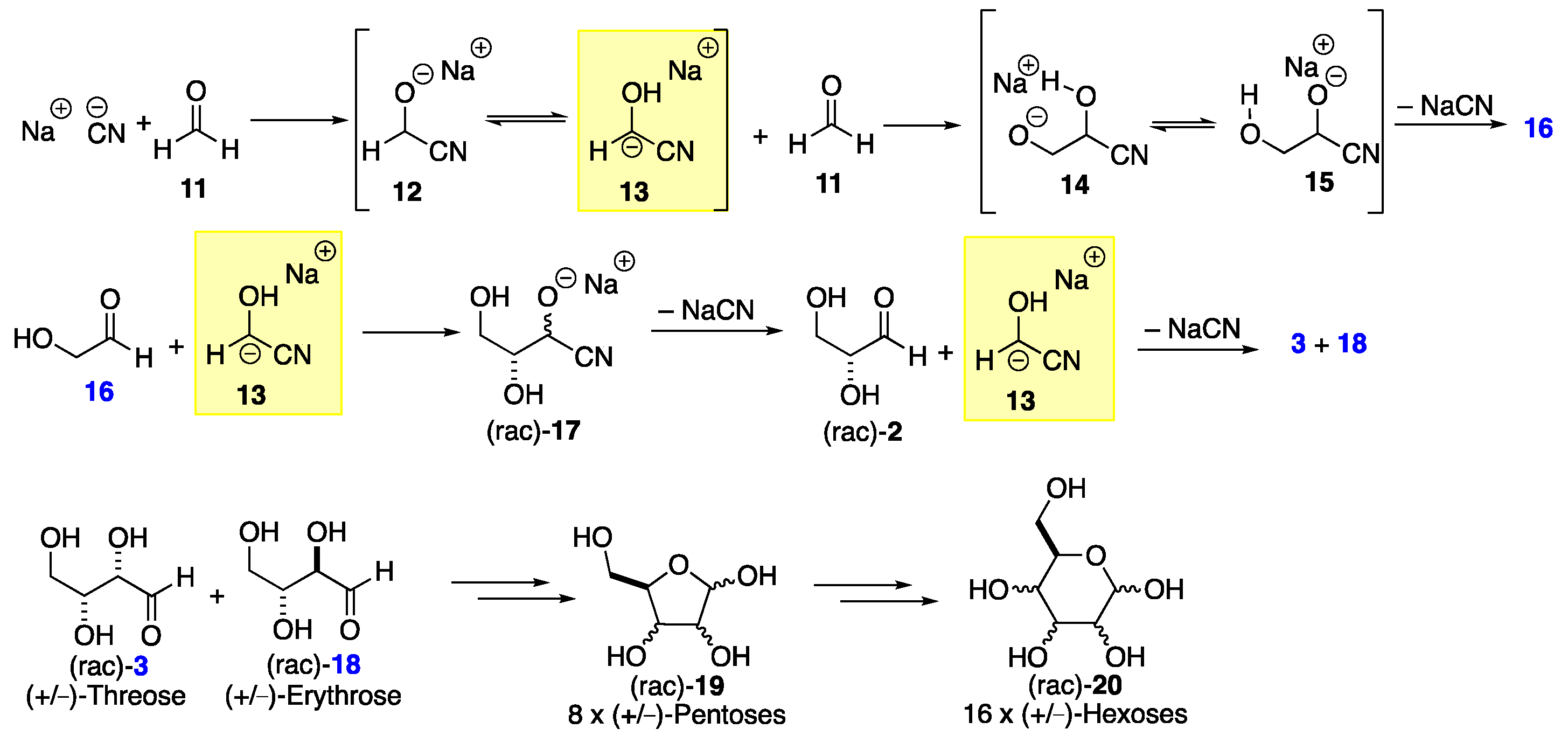
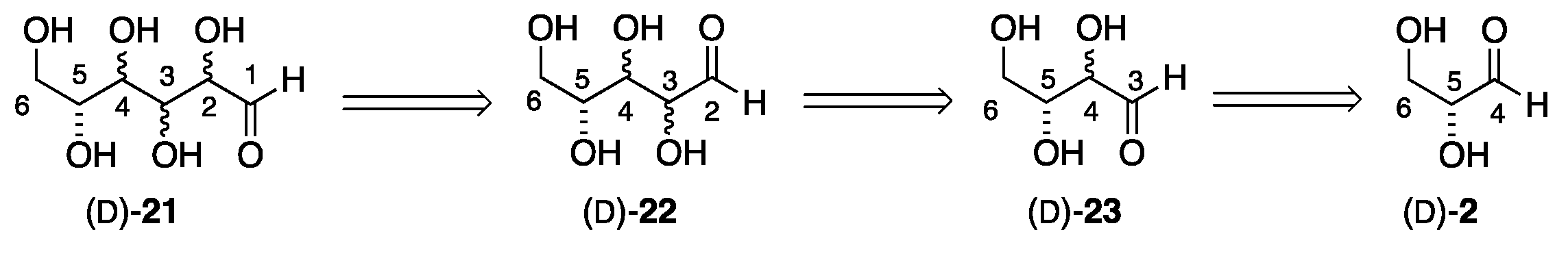
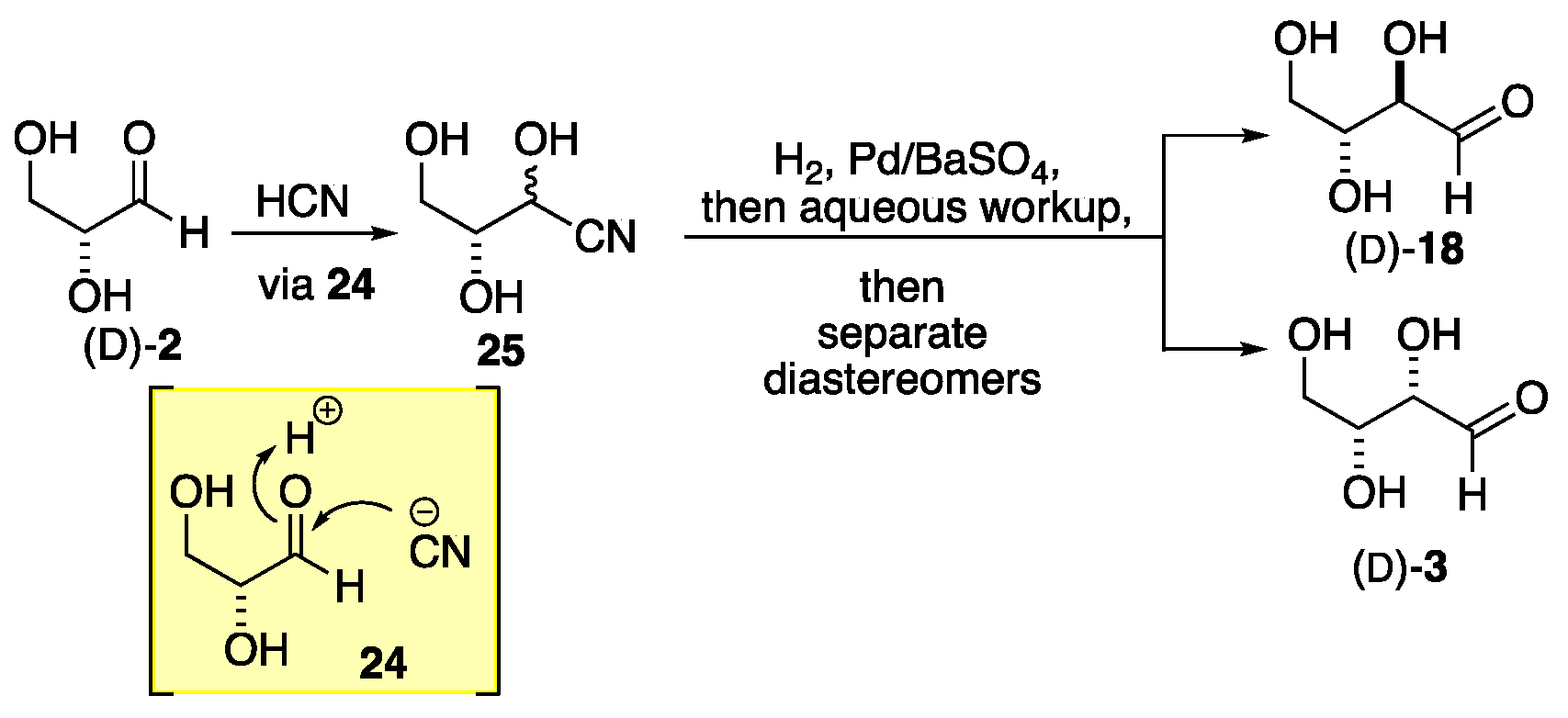
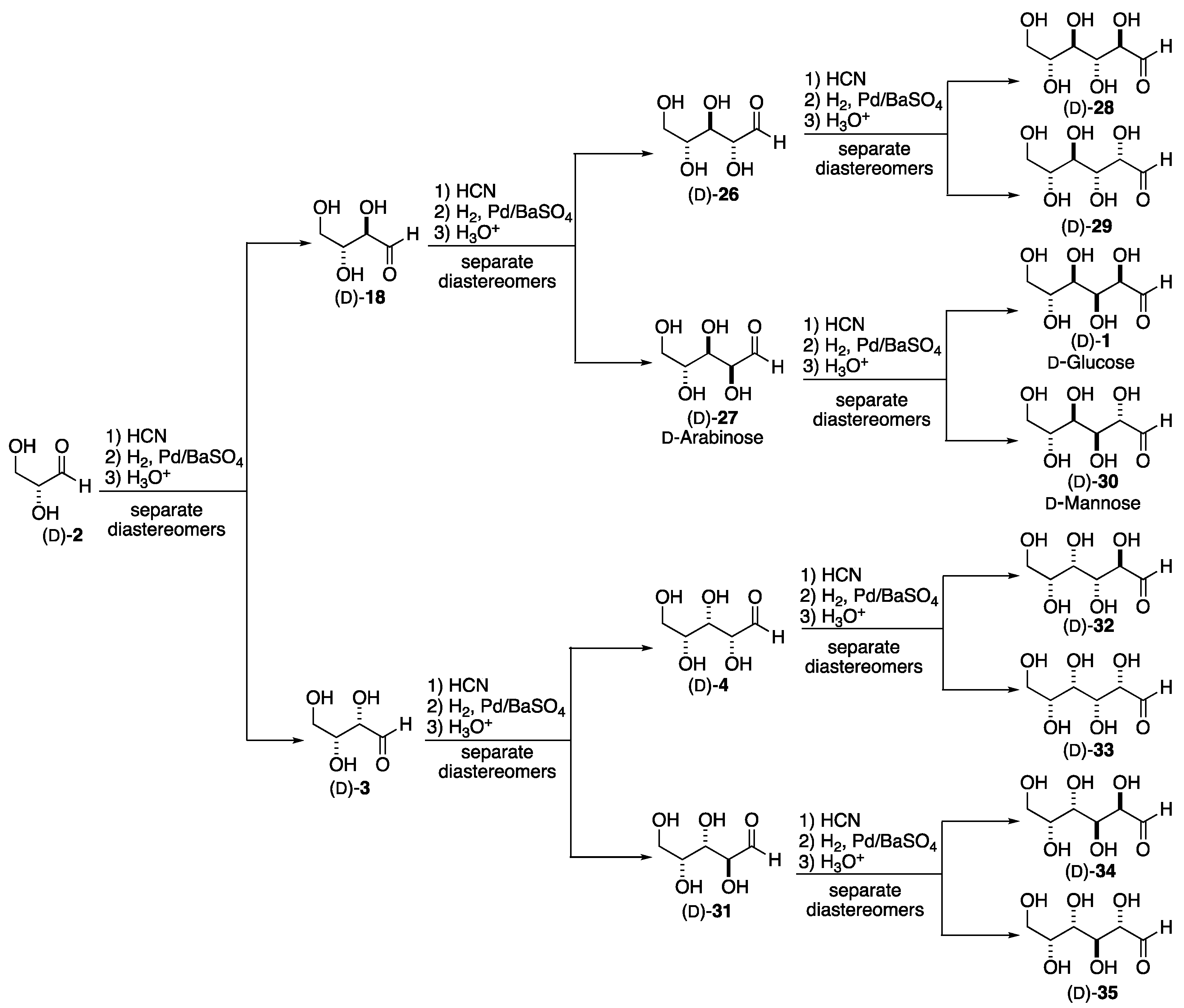
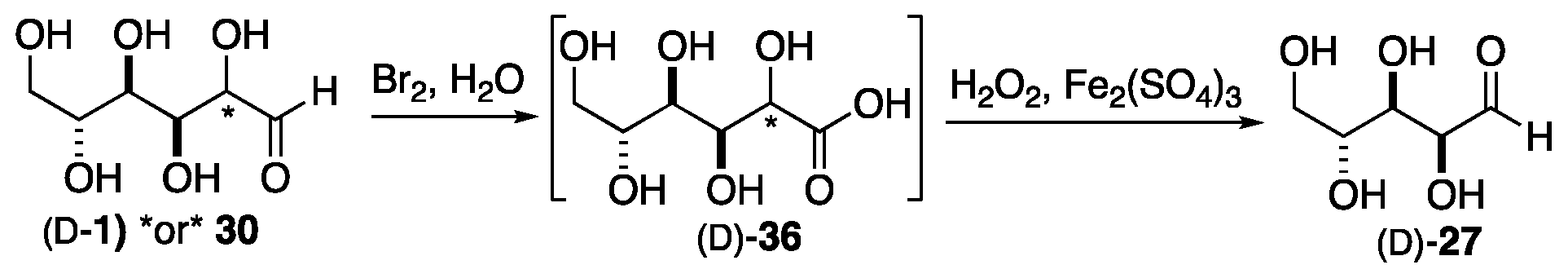
2. Formose Synthesis
3. Fischer Synthesis of the Hexoses
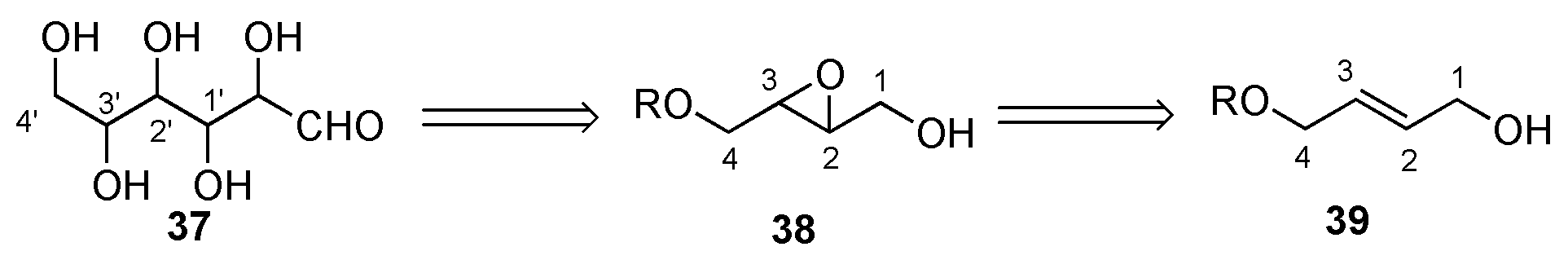
4. Sharpless De Novo Asymmetric Synthesis of the Hexoses
5. Other De Novo Asymmetric Carbohydrate Syntheses
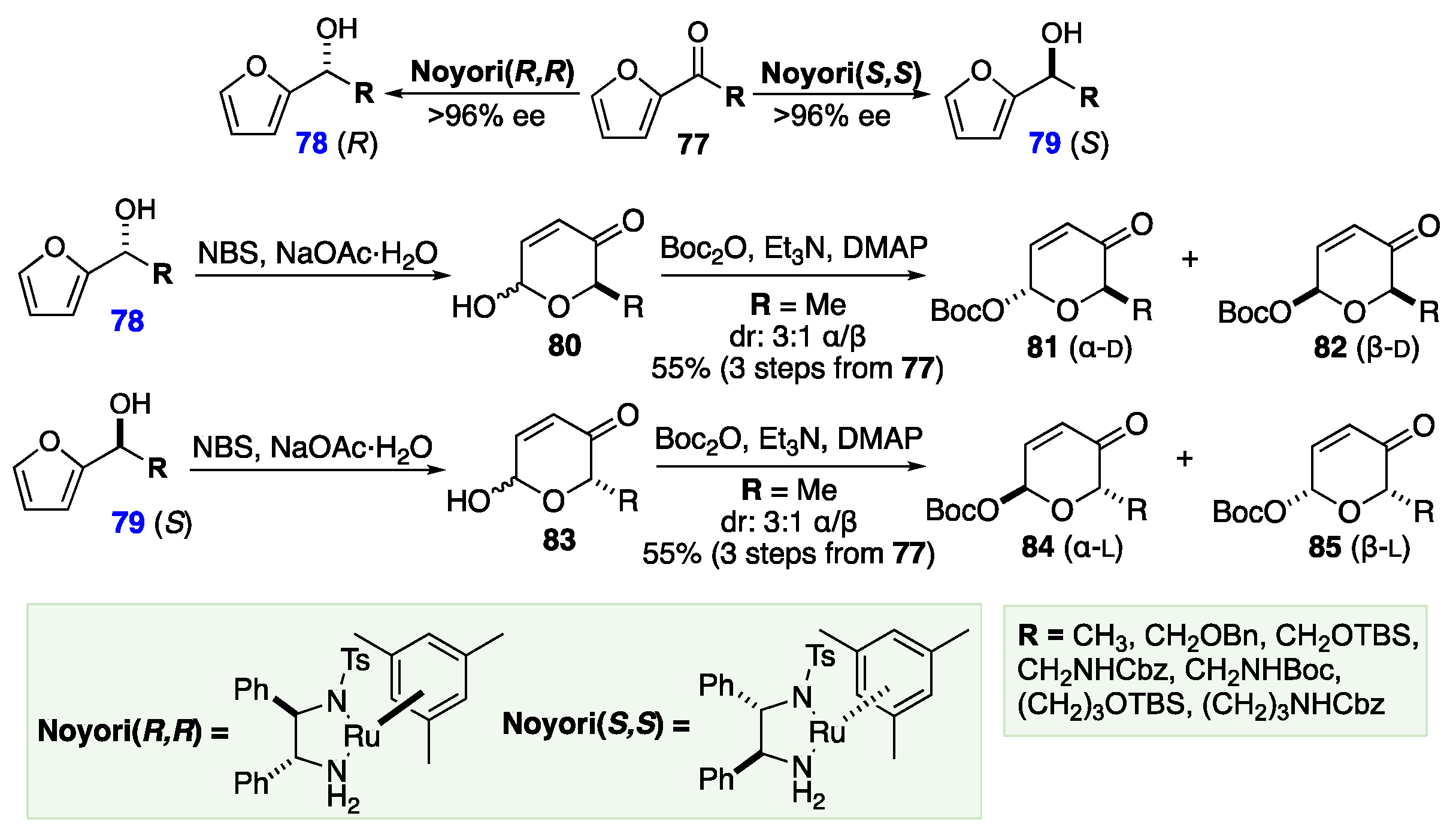
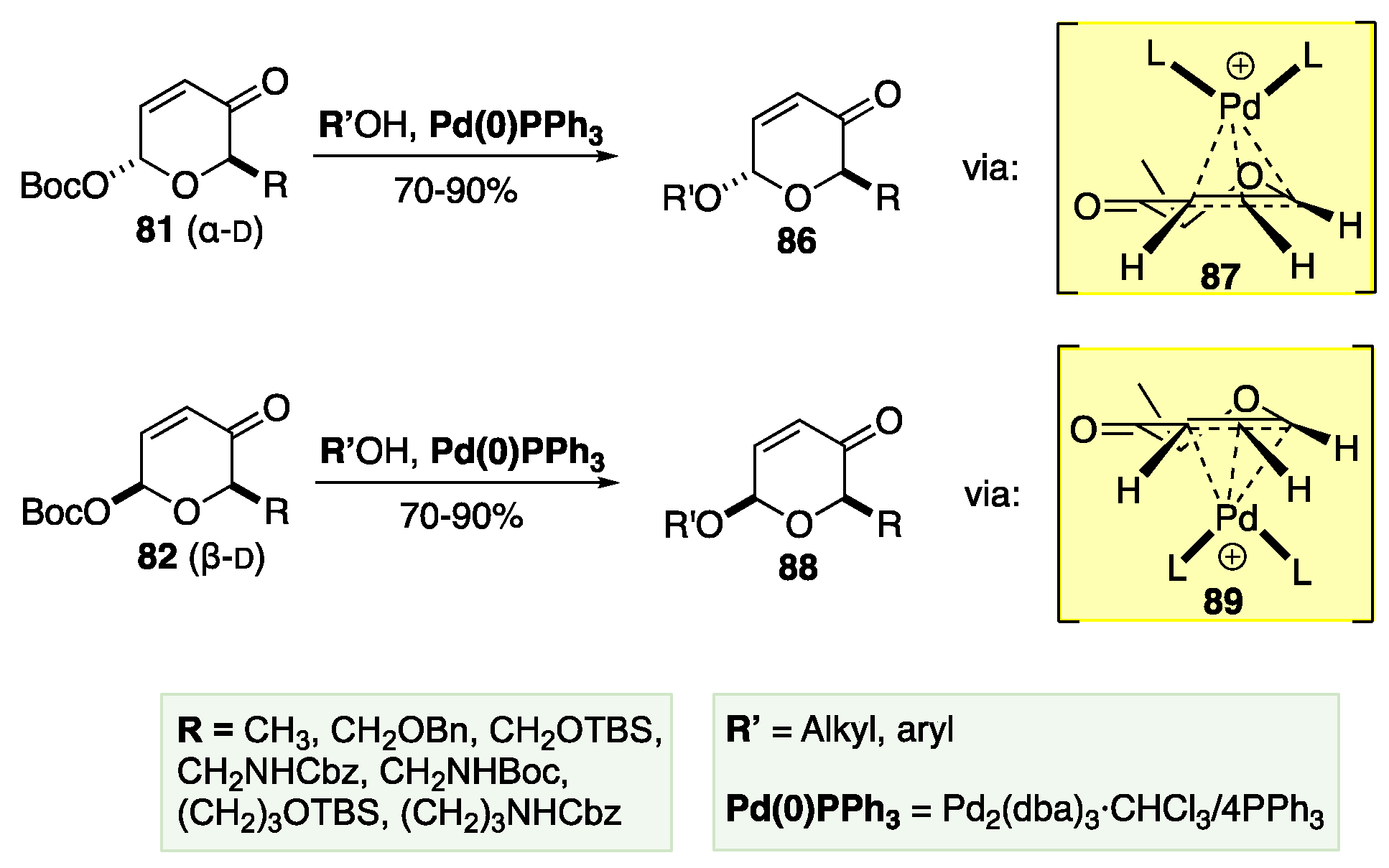
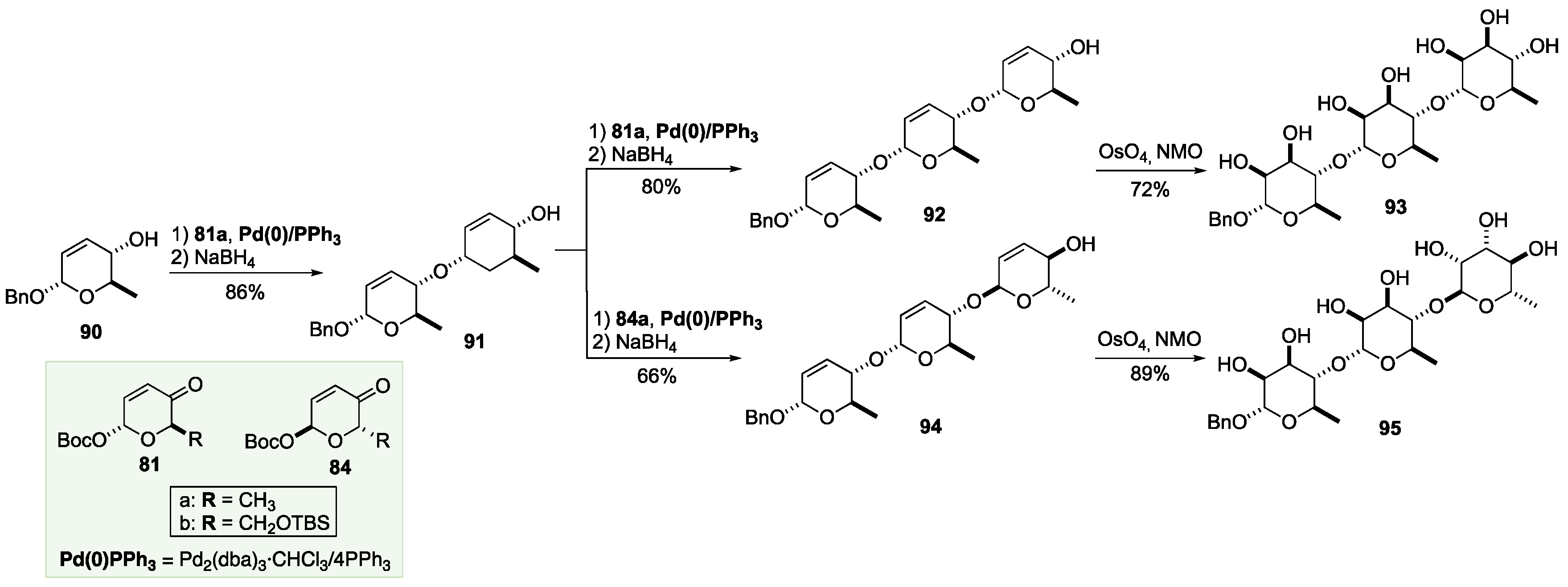
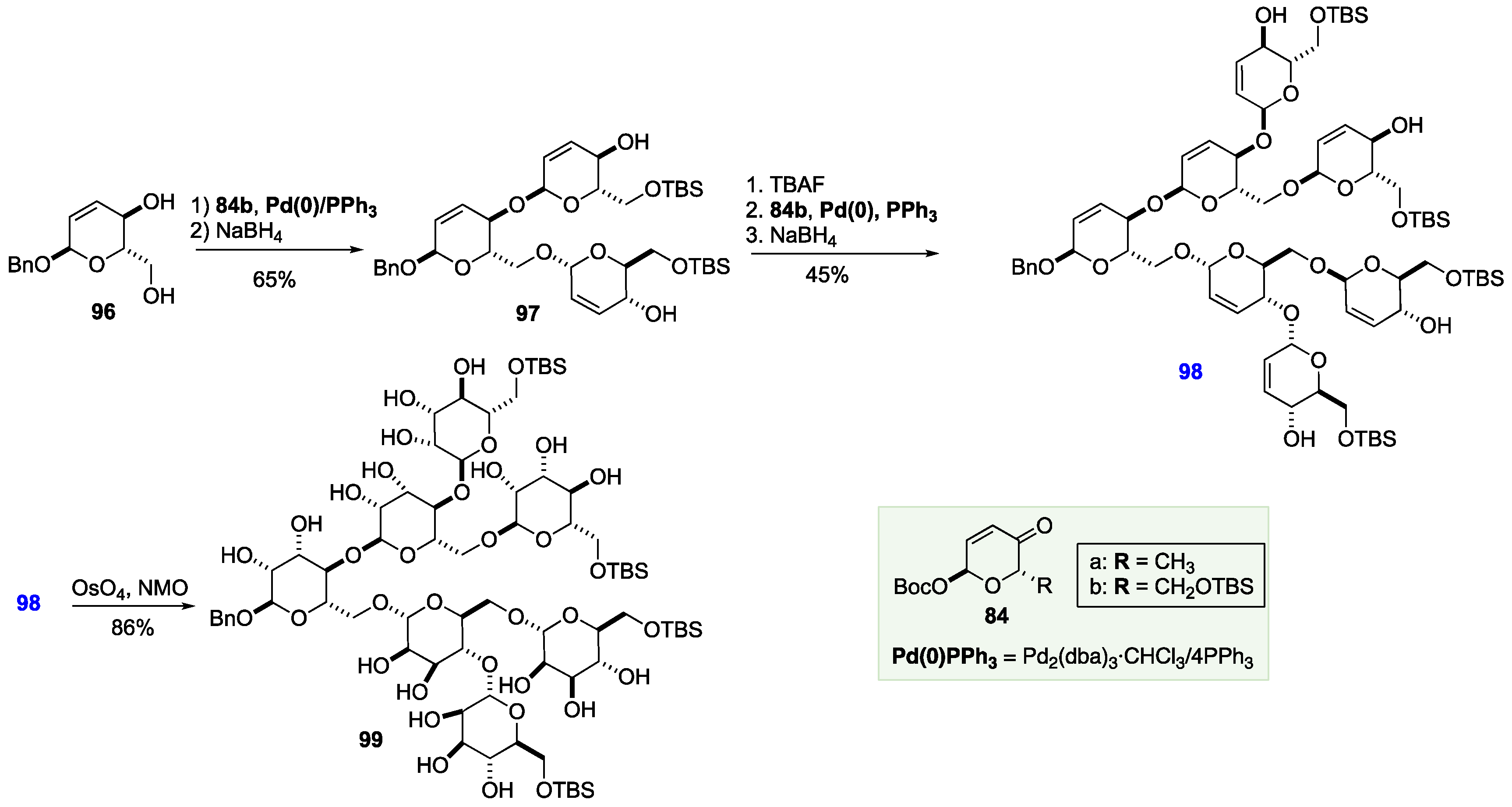
6. De Novo Asymmetric Achmatowicz Approach to Carbohydrates
6. Rhee Synthesis of Carbohydrates
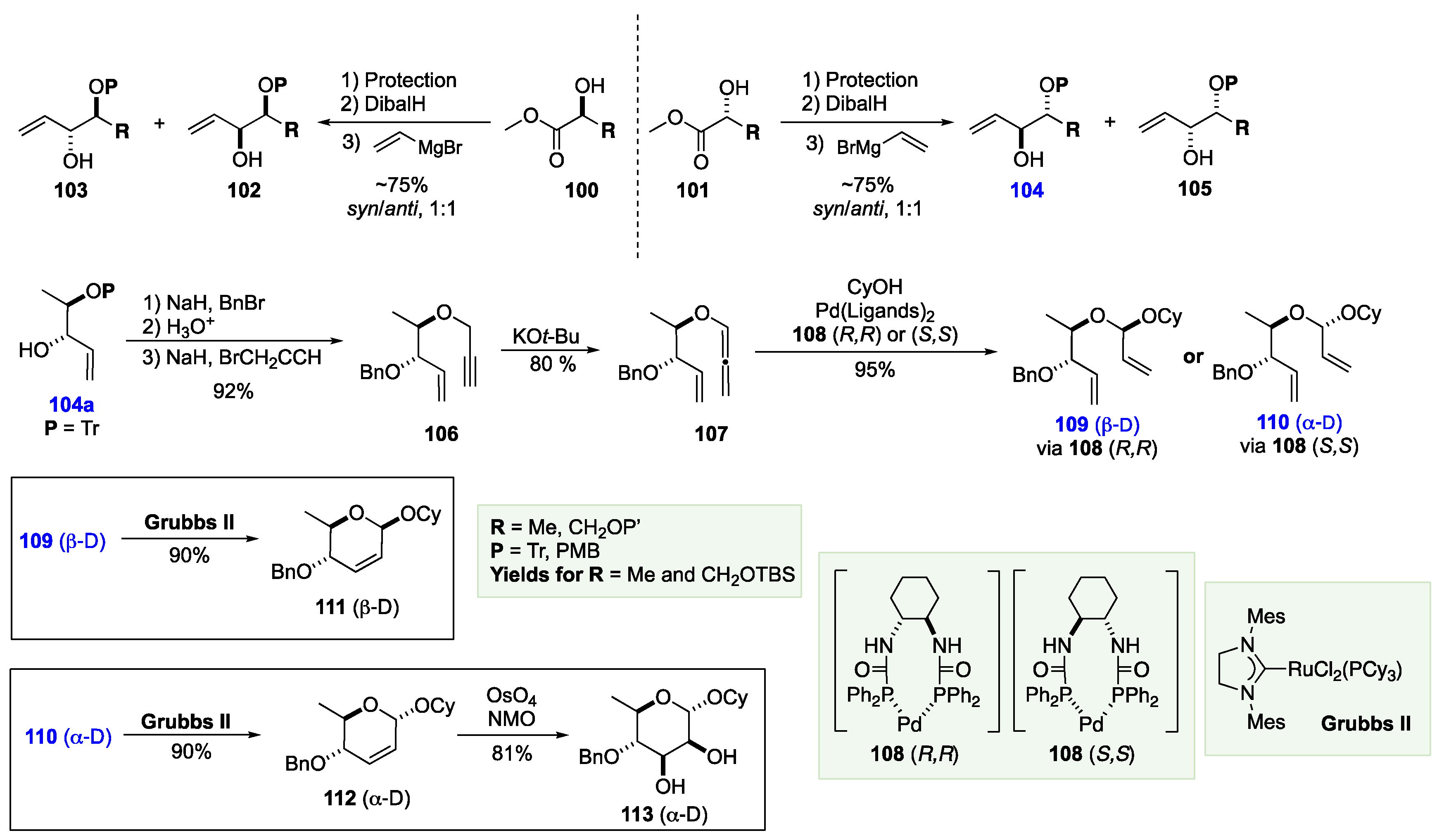
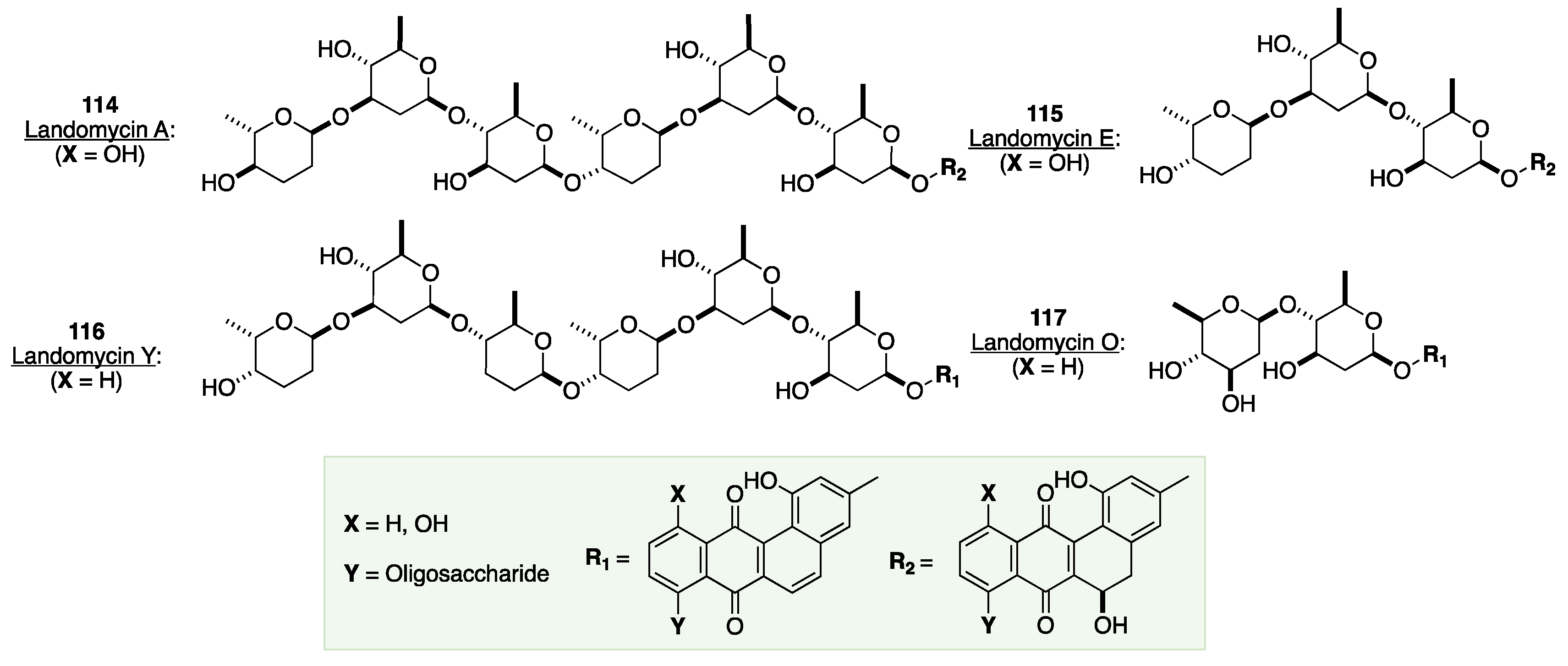
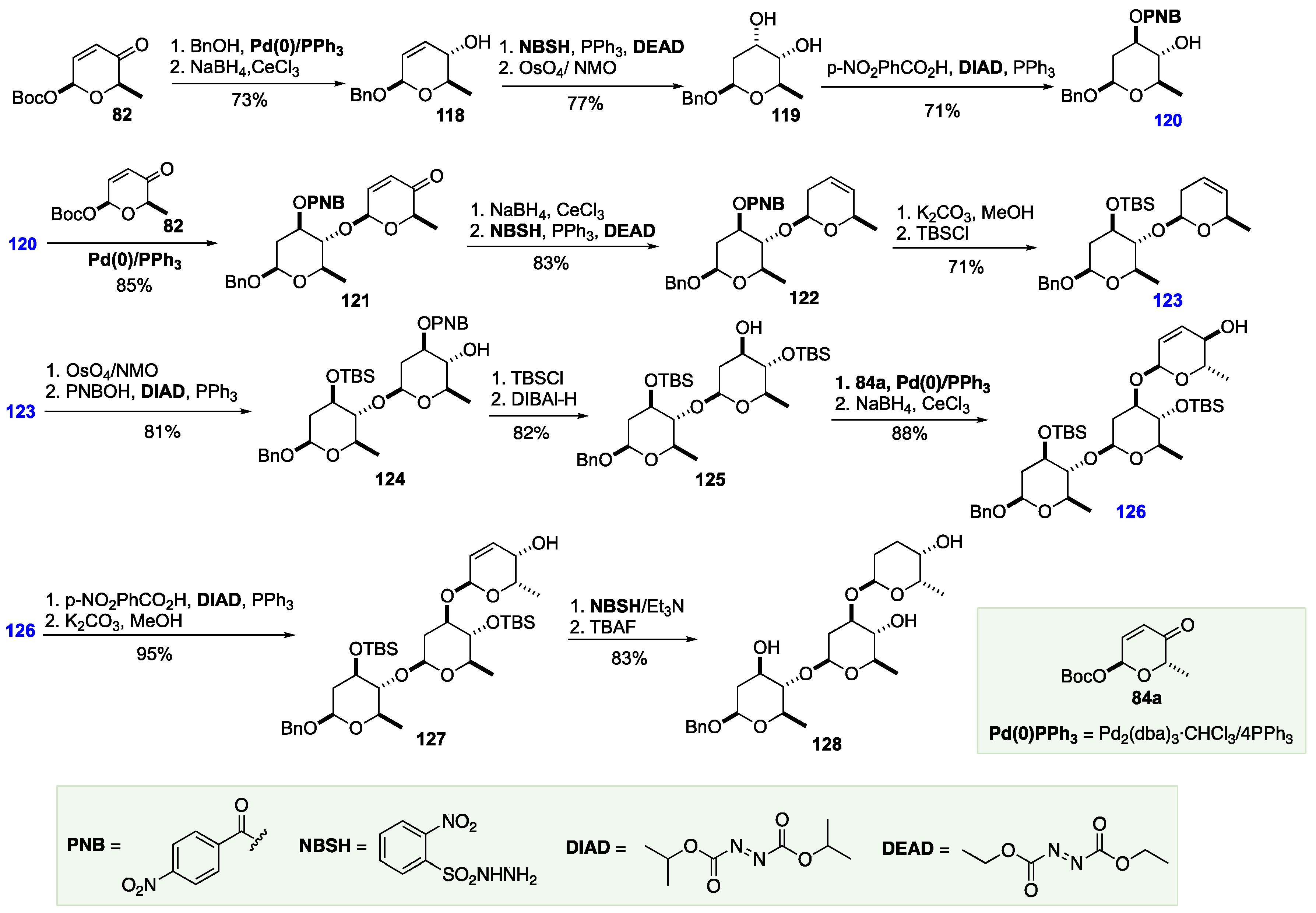
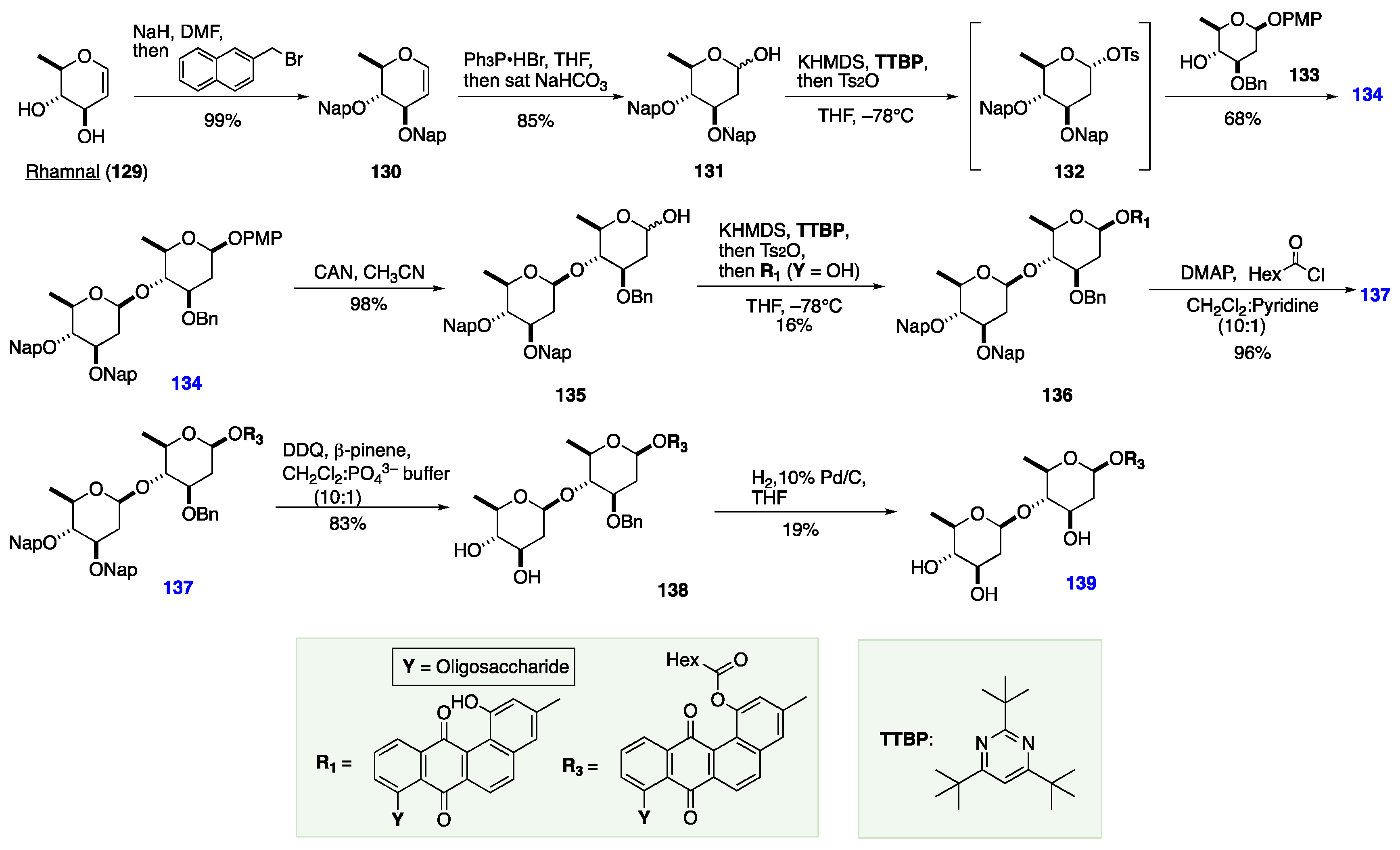
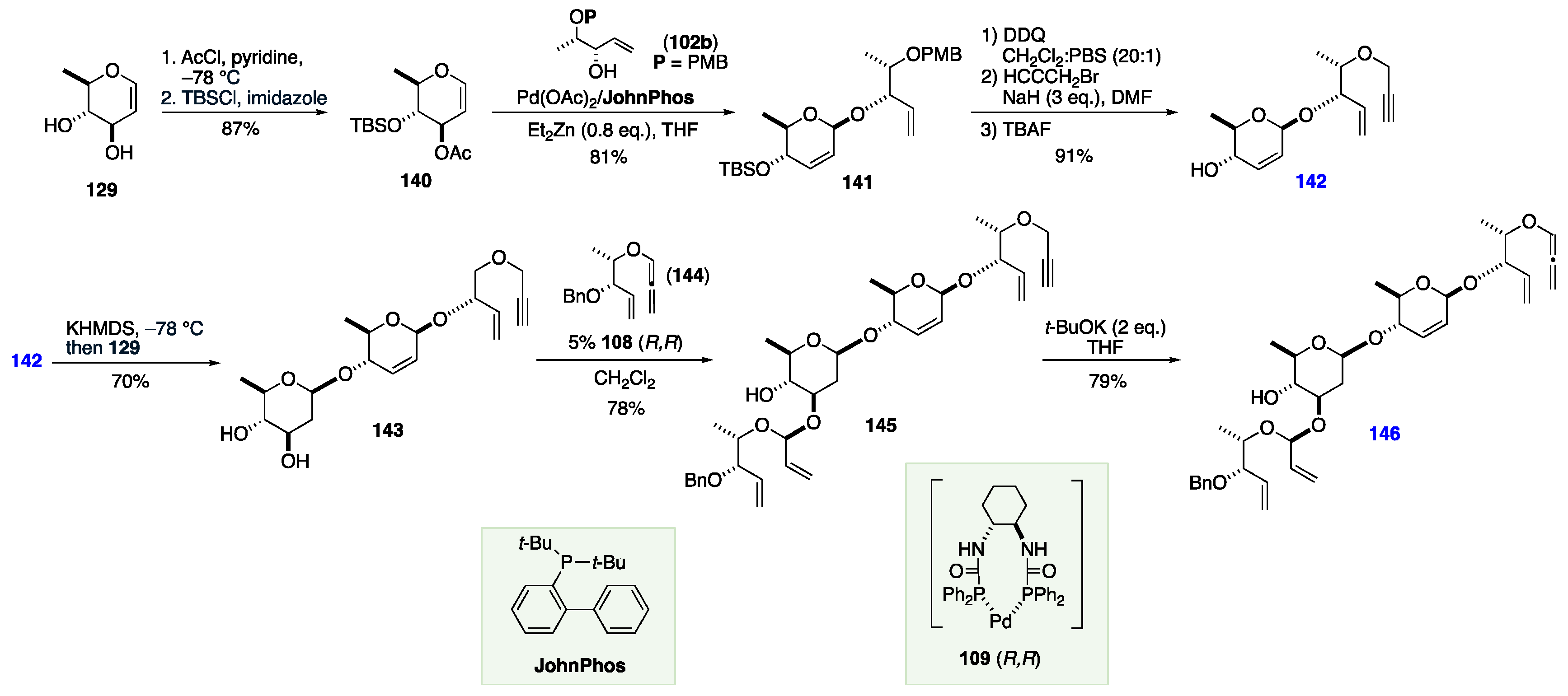
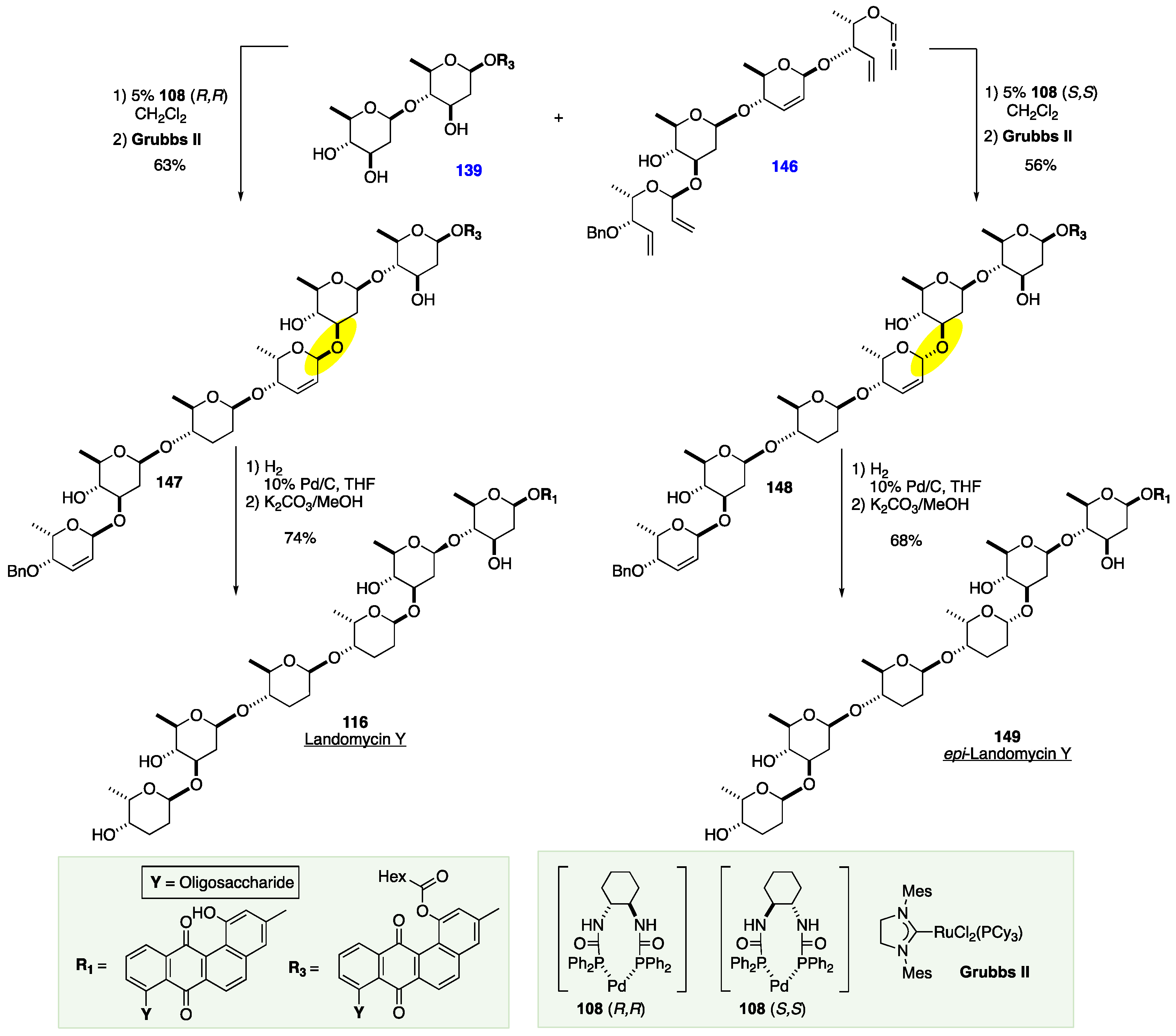
7. The De Novo Asymmetric Synthesis of the Landomycins
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- “When Did We Become So Obsessed With Being Symmetrical,” Rhonda Garelick, Published NY Times, 23 August 2022.
- Leigh, W. Simmons, Gillian Rhodes, Marianne Peters, Nicole Koehler, Are human preferences for facial symmetry focused on signals of developmental instability? Behavioral Ecology, Volume 15, Issue 5, 04, Pages 864–871. 20 September. [CrossRef]
- Misselhorn, C. "Empathy with inanimate objects and the uncanny valley". Minds and Machines. 2009, 19, 345–359. [Google Scholar] [CrossRef]
- McManus, I.C. "Symmetry and asymmetry in aesthetics and the arts". Eur. Rev. 2005, 13, 157–180. [Google Scholar] [CrossRef]
- Eliel, E.; Willen, S.H.; Mander, L.N. "Stereochemistry of Organic Compounds". John Wiley & Sons, Inc., New York, NY. 1994. 0167. [Google Scholar]
- Mizuno, T.; Weiss, A.H. Synthesis and utilization of formose sugars. Adv. Carbohydr. Chem. Biochem. 1974, 29, 173–227. [Google Scholar]
- Aljahdali, A.Z.; Shi, P.; Zhong, Y.; O’Doherty, G.A. , De Novo Asymmetric Synthesis of the Pyranoses: From Monosaccharides To Oligosaccharides. Advances in Carbohydrate Chemistry and Biochemistry, 2013; 69. [Google Scholar]
- Kim, S.; Oiler, J.; Xing, Y.; O’Doherty, G.A. Asymmetric Achmatowicz Approach to Oligosaccharides. Chem. Commun. 2022, 58, 12913–12926. [Google Scholar] [CrossRef]
- Kim, S.; O’Doherty, G.A. De novo asymmetric synthesis of the pyranoses—from monosaccharides to oligosaccharides: An update. Advances in Carbohydrate Chemistry and Biochemistry David Baker Ed. 2024; 85. [Google Scholar]
- Zheng, J.; O’Doherty, G.A. De Novo Synthesis of Oligosaccharides Via Metal Catalysis in Comprehensive Glycoscience, 2nd Edition, Joseph Barchi Ed., Elsevier: Oxford 2021; Volume 2., pp. 435-463.
- Adams, R.; C. S. Marvel. Benzoin. Org. Synth. 1921, 1, 33. [Google Scholar]
- Matsumoto, T.; Yamamoto, H.; Inoue, S. Selective Formation of Triose from Formaldehyde Catalyzed by Thiazolium Salt. J. Am. Chem. Soc. 1984, 106, 4829–4832. [Google Scholar] [CrossRef]
- Stetter, H. Catalyzed addition of aldehydes to activated double bonds-a new synthetic approach. Angew. Chem. Int. Ed. 1976, 15, 639. [Google Scholar] [CrossRef]
- de Alaniz, J.R.; Kerr, M.S.; Moore, J.L.; Rovis, T. Scope of the asymmetric intramolecular Stetter reaction catalyzed by chiral nucleophilic triazolinylidene carbenes. J. Org. Chem. 2008, 73, 2033. [Google Scholar] [CrossRef]
- Breslow, R. On the Mechanism of Thiamine Action. IV. Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719. [Google Scholar] [CrossRef]
- Dondoni, A.; Perrone, D. Thiazole-based routes to amino hydroxyl aldehydes, and their use of the synthesis of biologically active compounds. Aldrichimica Acta 1997, 30, 35–46. [Google Scholar]
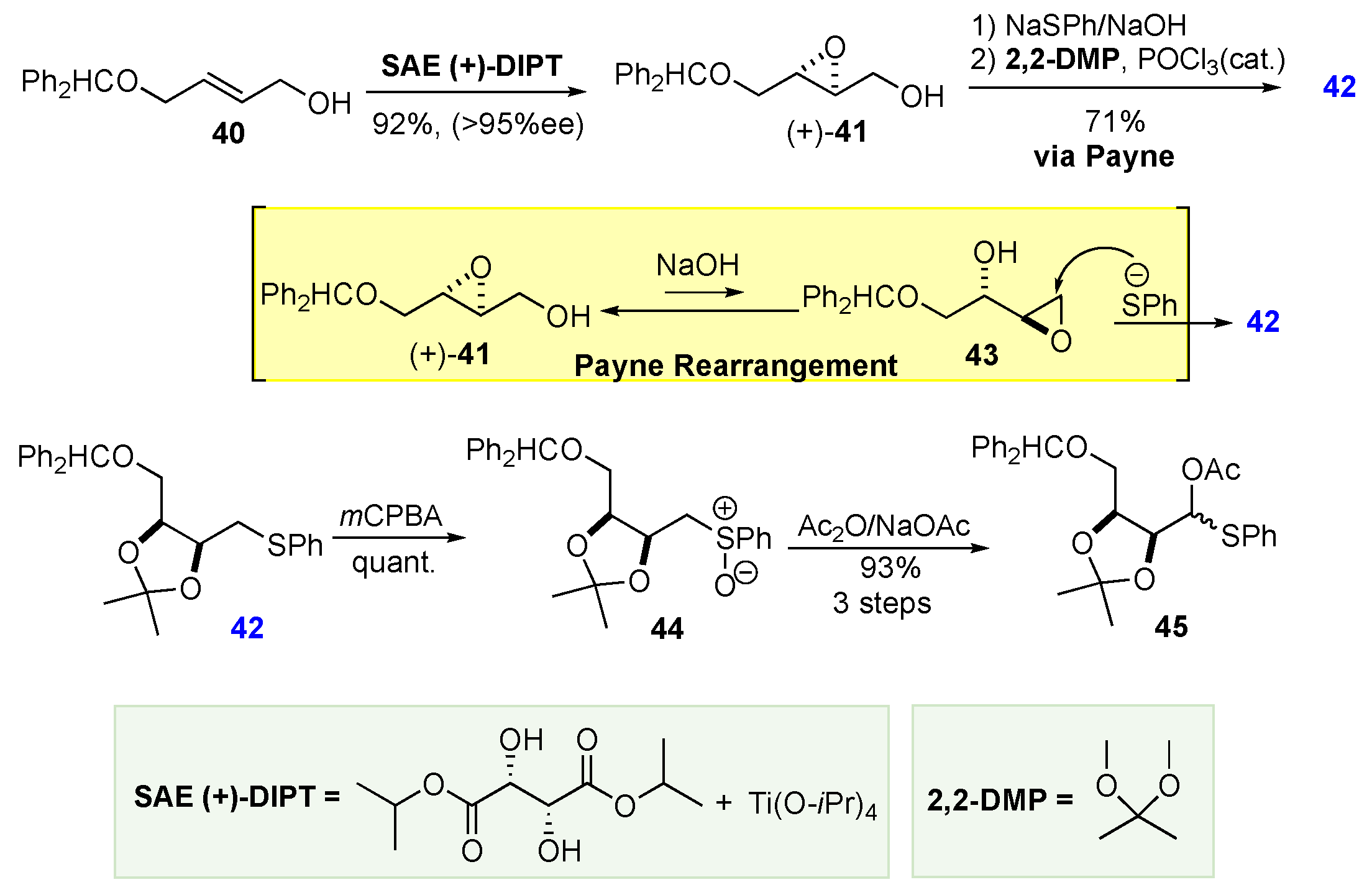
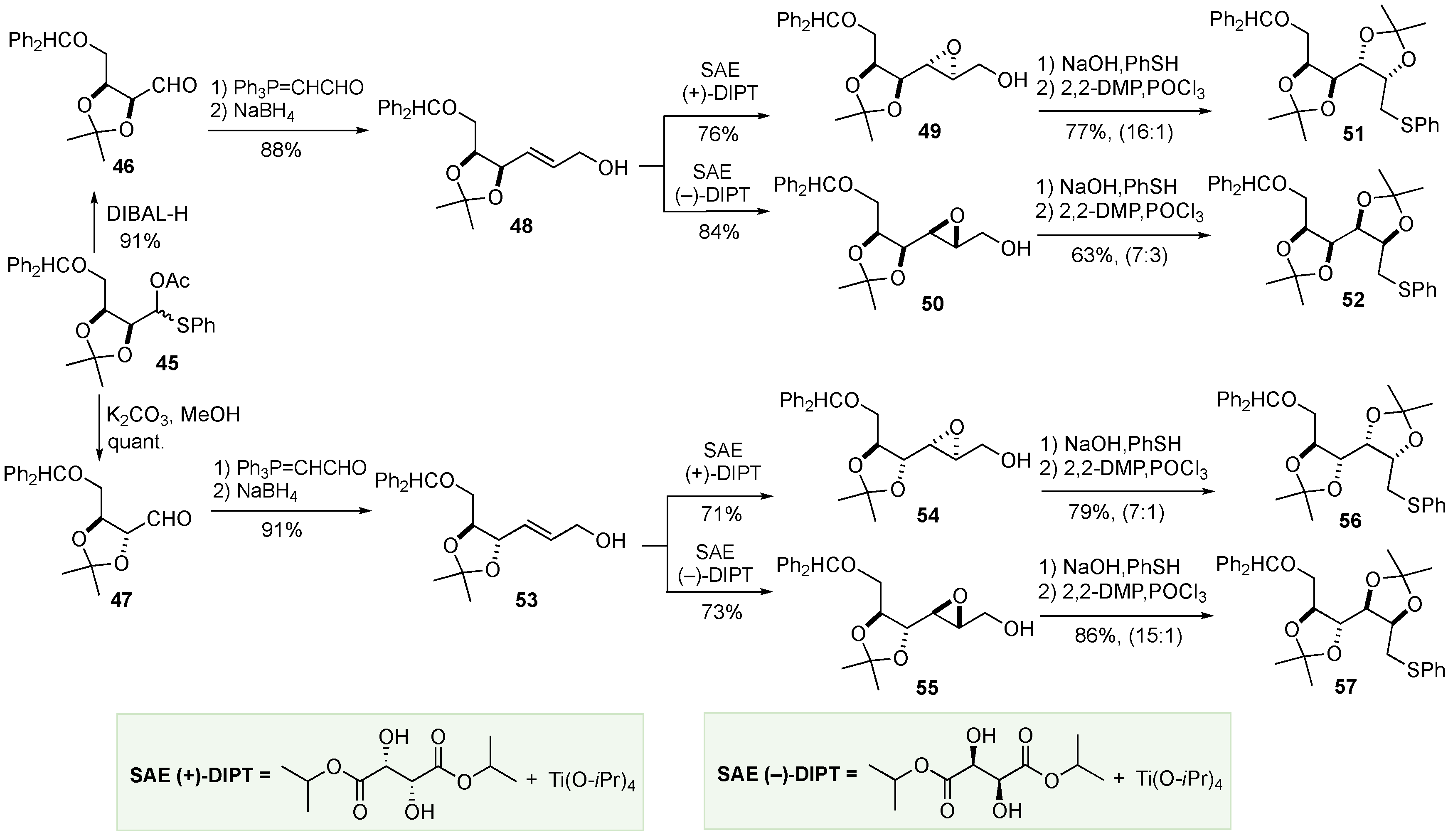
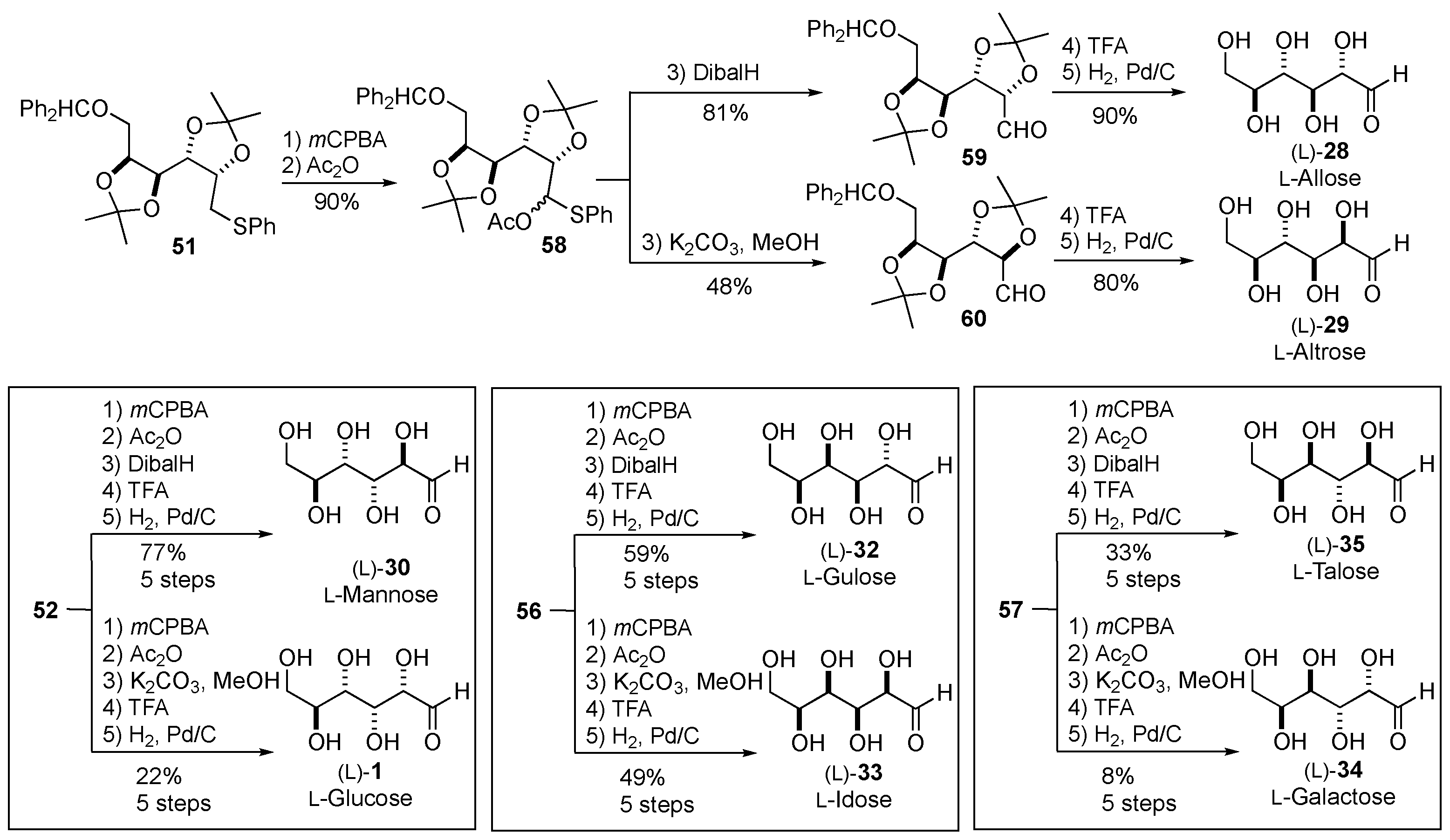
- Ko, S.Y.; Lee, A.W. M.; Masamune, S.; Reed III, L.A.; Sharpless, K.B.; Walker, F.J. Total Synthesis of the L-Hexoses. Science 1983, 220, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Lee, A.W. M.; Masamune, S.; Reed III, L.A.; Sharpless, K.B.; Walker, F.J. Total synthesis of the L-hexoses. Tetrahedron 1990, 46, 245–264. [Google Scholar] [CrossRef]
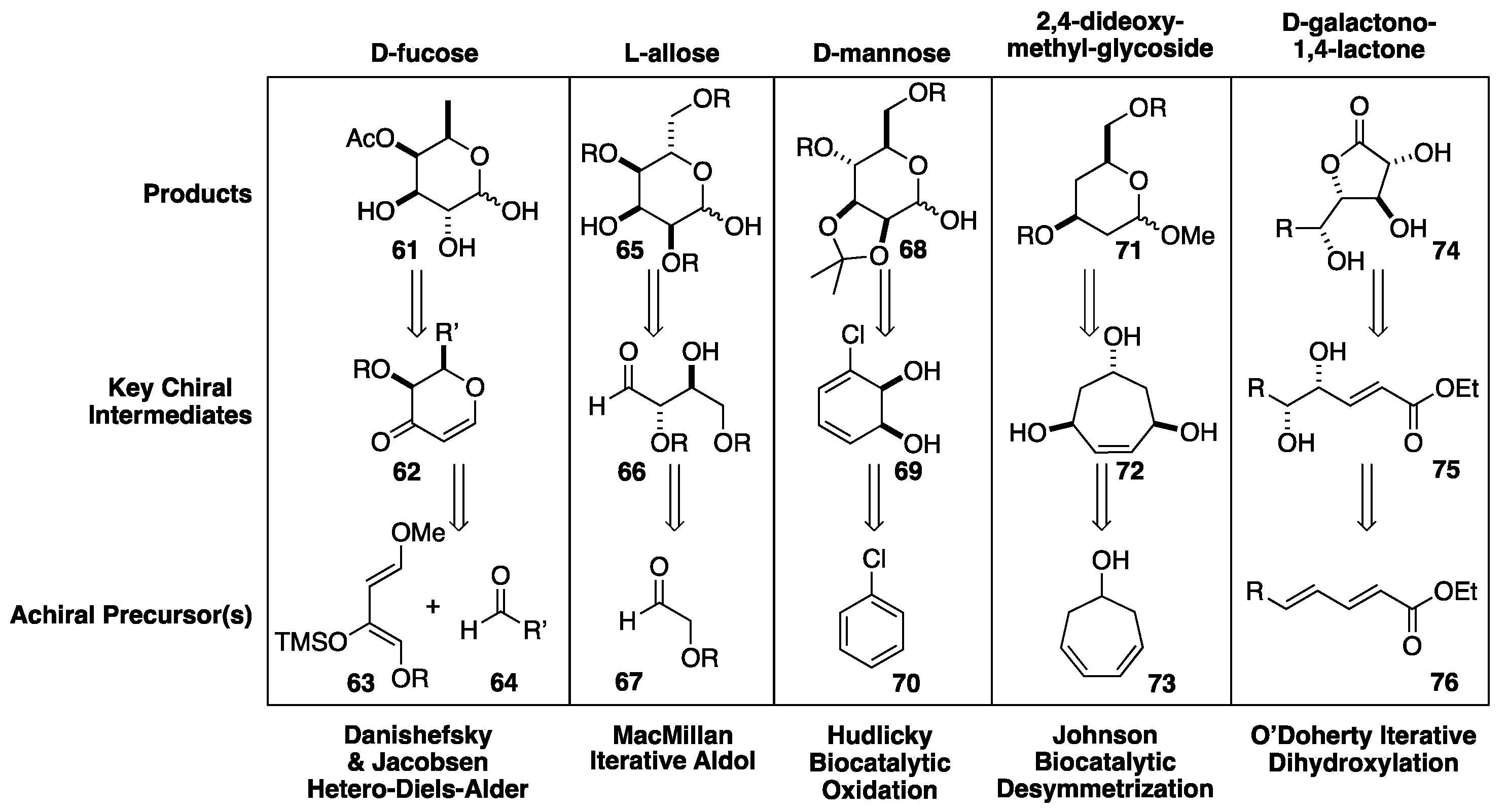
- Danishefsky, S.J. Cycloaddition and cyclocondensation reactions of highly functionalized dienes: Applications to organic synthesis. Chemtracts 1989, 273–297. [Google Scholar] [CrossRef]
- Schaus, S.E.; Branalt, J.; Jacobsen, E.N. Asymmetric Hetero-Diels-Alder Reactions Catalyzed by Chiral (Salen)Chromium(III) Complexes. J. Org. Chem. 1998, 63, 403–405. [Google Scholar] [CrossRef]
- Northrup, B.; Macmillan, D.W.C. Two-step synthesis of carbohydrates by selective aldol reactions, Science 2004, 305 1752--1755.
- B. Northhrup, I.K. Mangion, F. Hettche, and D. W. C MacMillan, Enantioselective organocatalytic direct aldol reactions of α-oxyaldehydes: Step one in a two-Step synthesis of carbohydrates, Angew. Chem., Int. Ed. 2004, 43, 2152–2154.
- Hudlicky, T.; Pitzer, K.K.; Stabile, M.R.; Thorpe, A.J.; Whited, G.M. , Biocatalytic Syntheses of Protected D-Mannose-d(5), D-Mannose-d(7), D-Mannitol-2,3,4,5,6-d(5), and D-Mannitol-1,1,2,3,4,5,6,6-d(8) J. Org. Chem. 1996, 61, 4151–4153. [Google Scholar] [CrossRef]
- Gibson, D.T.; Koch, J.R.; Kallio, R.E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry 1968, 7, 2653-–2662. [Google Scholar] [CrossRef]
- Johnson, C.R.; Golebiowski, A.; Steensma, D.H.; Scialdone, M.A. Enantio- and diastereoselective transformations of cycloheptatriene to sugars and related products. J. Org. Chem. 1993, 58, 7185–7194. [Google Scholar] [CrossRef]
- JBackvall, K.; Bystrom, S.E.; Nordberg, R.E. Stereo- and regioselective palladium-catalyzed 1,4-diacetoxylation of 1,3-dienes. J.Org. Chem. 1984, 49, 4619–4631. [Google Scholar]
- Henderson, I.; Sharpless, K.B.; Wong, C.-H. Synthesis of Carbohydrates via Tandem Use of the Osmium-Catalyzed Asymmetric Dihydroxylation and Enzyme-Catalyzed Aldol Addition Reactions. J. Am. Chem. Soc. 1994, 116, 558–561. [Google Scholar] [CrossRef]
- Kolb, H.C.; Van Nieuwenhze, M.S.; Sharpless, K.B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547. [Google Scholar] [CrossRef]
- Kolb, H.C.; Anderson, P.G.; Sharpless, K.B. Toward an Understanding of the High Enantioselectivity in the Osmium-Catalyzed Asymmetric Dihydroxylation (AD). 1. Kinetics. J. Am. Chem. Soc. 1994, 116, 1278. [Google Scholar] [CrossRef]
- Zhang, Y.; O’Doherty, G.A. Remote steric effect on the regioselectivity of Sharpless asymmetric dihydroxylation, Tetrahedron 2005, 61 6337-6351. 61.
- Ahmed, M.M.; O'Doherty, G.A. De Novo Asymmetric Syntheses of D- and L-Talose via an Iterative Dihydroxylation of Dienoates. J. Org. Chem. 2005, 70, 10576–10578. [Google Scholar] [CrossRef]
- Ahmed, M.M.; O'Doherty, G.A. De novo synthesis of a galacto-papulacandin moiety via an iterative dihydroxylation strategy. Tetrahedron Lett. 2005, 46, 4151–4155. [Google Scholar] [CrossRef]
- Gao, D.; O'Doherty, G.A. Enantioselective Synthesis of 10-epi-Anamarine via an Iterative Dihydroxylation Sequence. Org. Lett. 2005, 7, 1069–1072. [Google Scholar] [CrossRef]
- Ahmed, M.M.; O'Doherty, G.A. De novo synthesis of galacto-sugar δ-lactones via a catalytic osmium/palladium/osmium reaction sequence. Tetrahedron Lett. 3019; 46, 3015–3019. [Google Scholar]
- Ahmed, M.M.; Berry, B.P.; Hunter, T.J.; Tomcik, D.J.; O'Doherty, G.A. De Novo Enantioselective Syntheses of Galacto-Sugars and Deoxy Sugars via the Iterative Dihydroxylation of Dienoate. Org. Lett. 2005, 7, 745–748. [Google Scholar] [CrossRef]
- Harris, J.M.; Keranen, M.D.; O’Doherty, G.A. Syntheses of d- and l-mannose, gulose, and talose via diastereoselective and enantioselective dihydroxylation reactions, J. Org. Chem. 1999, 64, 2982-–2983. [Google Scholar] [CrossRef]
- Harris, J.M.; Keranen, M.D.; Nguyen, H.; Young, V.G.; O’Doherty, G.A. Syntheses of four d- and l-hexoses via diastereoselective and enantioselective dihydroxylation reactions, Carbohydr. Res. 2000, 328, 17–36. [Google Scholar]
- Bushey, M.L.; Haukaas, M.H.; O’Doherty, G.A. Asymmetric aminohydroxylation of vinylfuran, J. Org. Chem. 1999, 64, 2984–2985. [Google Scholar] [CrossRef]
- Cuccarese, M.F.; Li, J.J.; O’Doherty, G.A. De Novo Approaches to Monosaccharides and Complex Glycans in Modern Synthetic Methods in Carbohydrate Chemistry, Sebastien Vidal and Daniel B. Werz Eds., Wiley-VCH Verlag GmbH & Co. KG, Weinheim 2014, pp. 1-28.
- Ashmus, R.; Jayasuriya, A.; Lim, Y.-J.; O’Doherty, G.A.; Lowary, T.L. De novo asymmetric synthesis of a 6-O-methyl-D-glycero-L-gluco-heptopyranose-derived thioglycoside for the preparation of Campylobacter jejuni NCTC11168 capsular polysaccharide fragments. J. Org. Chem. 2016, 81, 3058–3063. [Google Scholar] [CrossRef]
- Cunha, V.L.S.; O'Doherty, G.A.; Lowary, T.L. Exploring a de novo route to bradyrhizose and its diastereomers: synthesis and isomeric equilibrium of reducing bicyclic carbohydrates. Chem. Eur. J. 2024, 30. [Google Scholar] [CrossRef] [PubMed]
- Balachari, D.; O’Doherty, G.A. Sharpless Asymmetric Dihydroxylation of 5-Aryl-2-vinylfurans: Application to the Synthesis of the Spiroketal Moiety of Papulacandin D. Org. Lett. 2000, 2, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Balachari, D.; O’Doherty, G.A. Enantioselective Synthesis of the Papulacandin Ring System: Conversion of the Mannose Diastereoisomer into a Glucose Stereoisomer. Org. Lett. 2000, 2, 4033–4036. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; O'Doherty, G.A. De Novo Asymmetric Synthesis of a Galacto-Papulacandin Moiety Via an Iterative Dihydroxylation Strategy. Tetrahedron Lett. 4155; 46, 4151–4155. [Google Scholar]
- Haukaas, M.H.; O’Doherty, G.A. Enantioselective synthesis of N-Cbz-protected 6-amino-6-deoxy-mannose, gulose and talose. Org. Lett. 3899; 3, 3899–3992. [Google Scholar]
- Haukaas, M.H.; O’Doherty, G.A. Enantioselective Synthesis of 2-Deoxy and 2,3-Dideoxy-hexoses. Org. Lett. 2002, 4, 1771–1774. [Google Scholar] [CrossRef]
- Guo, H.; O’Doherty, G.A. De Novo Asymmetric Synthesis of Daumone via a Palladium Catalyzed Glycosylation Org. Lett. 2005, 7, 3921–3924. [Google Scholar]
- Xing, Y. , O’Doherty, G. A. De Novo Asymmetric Approach to Aspergillide-C. ChemistrySelect 2022, 7, e202200266. [Google Scholar]
- Guppi, S.R.; Zhou, M.; O’Doherty, G.A. De Novo Asymmetric Synthesis of Homo-Adenosine via a Palladium Catalyzed N-Glycosylation Org. Lett. 2006, 8, 293–296. [Google Scholar]
- Noyri, R.; Ohkuma, T. Asymmetric catalysis by architectural and functional molecular engineering: practical chemo-and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 2001, 40, 40. [Google Scholar] [CrossRef]
- Noyori, R.; Yamakawa, M.; Hashiguchi, S. Metal- ligand bifunctional catalysis: a nonclassical mechanism for asymmetric hydrogen transfer between alcohols and carbonyl compounds. J. Org. Chem. 2001, 66. [Google Scholar] [CrossRef]
- Li, M.; O’Doherty, G.A. An enantioselective synthesis of phomopsolide D. Tetrahedron Lett. 2004, 45, 6407–6411. [Google Scholar] [CrossRef]
- Li, M.; Scott, J.G.; O’Doherty, G.A. Synthesis of 7-oxa-phomopsolide E and its C-4 epimer. Tetrahedron Lett. 2004, 45, 1005–1009. [Google Scholar] [CrossRef]
- Babu, R.S.; O’Doherty, G.A. A Palladium-Catalyzed Glycosylation Reaction: The De Novo Synthesis of Natural and Unnatural Glycosides. J. Am. Chem. Soc. 2003, 125, 12406–12407. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.S.; O’Doherty, G.A. Palladium Catalyzed Glycosylation Reaction: De-Novo Synthesis of Trehalose Analogues. J. Carb. Chem. 2005, 24, 169–177. [Google Scholar] [CrossRef]
- Sharif, E.U.; Wang, H.-Y. L.; Akhmedov, N.G.; O’Doherty G., A. Merremoside D: De novo synthesis of its purported structure, NMR analysis and comparison of spectral data. Org. Lett. 2014, 16, 492–495. [Google Scholar] [CrossRef]
- Babu, R.S.; Guppi, S.R.; O’Doherty, G.A. Synthetic Studies Towards Mannopeptimycin-E: Synthesis of a O-Linked Tyrosine 1,4-,-Manno,Manno-Pyanosyl-Pyranoside. Org. Lett. 2006, 8, 1605–1608. [Google Scholar] [CrossRef]
- Feringa, L.; Comely, A.C.; Eelkema, R.; Minnaard, A.J.; Feringa, B.L. De novo asymmetric bio- and chemocatalytic synthesis of saccharides - stereoselective formal O-glycoside bond formation using palladium catalysis. J. Am. Chem. Soc. 2003, 125, 8714–8715. [Google Scholar]
- Kim, H.; Men, H.; Lee, C. Stereoselective palladium-catalyzed O-glycosylation using glycals. J. Am. Chem. Soc. 2004, 126, 1336–1337. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C. A mild and efficient method for the stereoselective formation of C-O bonds: palladium-catalyzed allylic etherification using zinc(II) alkoxides. Org. Lett. 2002, 4, 4369–4372. [Google Scholar] [CrossRef]
- Wu, B.; Li, M.; O’Doherty, G.A. Synthesis of several cleistrioside and cleistetroside natural products via a divergent de novo asymmetric approach, Org. Lett. 2010, 12, 5466–5469. [Google Scholar]
- Guo, H.; O’Doherty, G.A. De novo asymmetric synthesis of the anthrax tetrasaccharide by a palladium-catalyzed glycosylation reaction. Angew. Chem. Int. Ed. 2007, 46, 5206–5208. [Google Scholar] [CrossRef]
- Guo, H.; O’Doherty, G.A. De novo asymmetric synthesis of anthrax tetrasaccharide and related tetrasaccharide, J. Org. Chem. 2008, 73, 5211–5220. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y. L.; Guo, H.; O’Doherty, G.A. De novo asymmetric synthesis of rhamno di- and tri-saccharides related to the anthrax tetrasaccharide, Tetrahedron 2013, 69 3432-3436. 69.
- Bajaj, S.O.; Sharif, E.U.; Akhmedov, N.G.; O’Doherty, G.A. De novo asymmetric synthesis of the mezzettiaside family of natural products via the iterative use of a dual B-/Pd-catalyzed glycosylation. Chem. Sci. 2014, 5, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Sharif, E.U.; Wang, H.-Y. L.; Akhmedov, N.G.; O’Doherty G., A. Merremoside D: De novo synthesis of its purported structure, NMR analysis and comparison of spectral data. Org. Lett. 2014, 16, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Ray, D. and O’Doherty, G.A. De Novo Asymmetric Synthesis of Oligosaccharides Using Atom-Less Protecting Group in Protecting Groups: Strategies and Applications in Carbohydrate Chemistry, Sebastien Vidal Ed., Wiley-VCH Verlag GmbH Co. KG, Weinheim, 2019, pp. 327-351Weinheim, 2019, pp. 327-351.
- Babu, R.S.; Zhou, M.; O’Doherty, G.A. De-Novo Synthesis of Oligosaccharides Using a Palladium Catalyzed Glycosylation Reaction. J. Am. Chem. Soc. 2004, 126, 3428–3429. [Google Scholar] [CrossRef]
- Babu, R.S.; Chen, Q.; Kang, S.-W.; Zhou, M.; O’Doherty, G.A. De Novo Synthesis of Oligosaccharides Using Green Chemistry Principles. J. Am. Chem. Soc. 2012, 134, 11952–11955. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, H.W.; Jeong, W.; Moon, D.; Rhee, Y.H. Palladium-catalyzed asymmetric nitrogen-selective addition reaction of indoles to alkoxyallenes. Org. Lett. 2018, 20, 1248–1251. [Google Scholar] [CrossRef]
- Lim, W.; Kim, J.; Rhee, Y.H. Pd-catalyzed asymmetric intermolecular hydroalkoxylation of allene: an entry to cyclic acetals with activating group-free and flexible anomeric control. J. Am. Chem. Soc. 2014, 136, 13618–13621. [Google Scholar] [CrossRef]
- Trost, M.; Fandrick, D.R.; Dinh, D.C. . Dynamic kinetic asymmetric allylic alkylations of allenes. J. Am. Chem. Soc. 2005, 127, 14186–14187. [Google Scholar] [CrossRef]
- Trost, M.; Lee, C.B. . Geminal dicarboxylates as carbonyl surrogates for asymmetric synthesis. Part I. Asymmetric addition of malonate nucleophiles. J. Am. Chem. Soc. 2001, 123, 3671–3686. [Google Scholar] [CrossRef]
- Kang, J.; Rhee, Y.H. . Convergent Synthesis of Tetrasaccharide Fragment of Cervimycin K. Org. Lett. 2021, 23, 4468–4472. [Google Scholar] [CrossRef]
- Barpuzary, B.; Kim, M.; Rhee, Y.H. . Synthetic Study toward Saccharomicin Based upon Asymmetric Metal Catalysis. Org. Lett. 2021, 23, 5969–5972. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, J.; Lee, S.; Rhee, Y.H. . Flexible Total Synthesis of 11-Deoxylandomycins and Their Non-Natural Analogues by Way of Asymmetric Metal Catalysis. Angew. Chem. 2020, 132, 2369–2373. [Google Scholar]
- Yang, X.; Fu, B.; Yu, B. Total Synthesis of Landomycin A, a Potent Antitumor Angucycline Antibiotic. J. Am. Chem. Soc. 2011, 133, 12433–12435. [Google Scholar] [CrossRef]
- Yang, X.; Wang, P.; Yu, B. Tackling the Challenges in the Total Synthesis of Landomycin A. Chem. Rec. 2013, 13, 70–84. [Google Scholar] [CrossRef]
- Lai, Y-H. ; Mondal, S.; Su, H-T.; Huang, S-C.; Wu, M-H.; Huang, I-W.; Lauderdale, T-S. Y.; Song, J-S.; Shia, K-S.; Mong, K-K. T. Total synthesis of landomycins Q and R and related core structures for exploration of the cytotoxicity and antibacterial properties. RSC Adv. 2021, 11, 9426–9432. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S.; Kim, J.; Moon, D.; Rhee, Y.H. A Convergent Synthetic Strategy towards Oligosaccharides containing 2,3,6-Trideoxypyranoglycosides. Angew. Chem. Int. Ed. 2018, 58, 628–631. [Google Scholar] [CrossRef]
- Yalamanchili, S.; Lloyd, D.; Bennet, C.S. Synthesis of the Hexasaccharide Fragment of Landomycin A Using a Mild, Reagent-Controlled Approach. Org. Lett. 2019, 21, 3684–3677. [Google Scholar] [CrossRef]
- Guo, Y.; Sulikowski, G.A. Synthesis of the Hexasaccharide Fragment of Landomycin A: Application of Glycosyl Tetrazoles and Phosphites in the Synthesis of Deoxyoligosaccharide. J. Am. Chem. Soc. 1998, 120, 1392–1397. [Google Scholar] [CrossRef]
- Roush, W.R.; Bennett, C.E. A Highly Stereoselective Synthesis of the Landomycin A Hexasaccharide Unit. J. Am. Chem. Soc. 2000, 122, 6124–6125. [Google Scholar] [CrossRef]
- Yu, B.; Wang, P. Efficient Synthesis of the Hexasaccharide Fragment of Landomycin A: Using Phenyl 2,3-O-Thionocarbonyl-1-thioglycosides as 2-Deoxy-β-glycoside Precursors. Org. Lett. 2002; 4, 1919–1922. [Google Scholar]
- Tanaka, H.; Yamaguchi, S.; Yoshizawa, A.; Takagi, M.; Shin-ya, K.; Takahashi, T. Combinatorial Synthesis of Deoxyhexasaccharides Related to the Landomycin A Sugar Moiety, Based on an Orthogonal Deprotection Strategy. Chem. Asian. J. 2010, 5, 1407–1424. [Google Scholar] [CrossRef]
- Zhou, M.; O’Doherty, G.A. De Novo Synthesis of the Trisaccharide Subunit of Landomycins A and E. Org. Lett. 2008, 10, 2283–2286. [Google Scholar] [CrossRef] [PubMed]
- Ruei, J-H. ; Venukumar, P.; Ingle, A.B.; Mong, K-K. T. C6 picoloyl protection: a remote stereodirecting group for 2-deoxy-β-glycoside formation. Chem. Commun. 2015, 51, 5394–5397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Baryal, K.N.; Adhikari, S.; Zhu, J. Direct Synthesis of 2-Deoxy-β-glycosides via Anomeric O-Alkylation with Secondary Electrophiles. J. Am. Chem. Soc. 2014, 136, 3172–3175. [Google Scholar] [CrossRef] [PubMed]
- McDonald, F.E.; Reddy, K.S. Discovery of the tungsten carbonyl-catalyaed endo-selective alkynyl alcohol cylcoisomerization reaction: applications to stereoselective syntheses of D-olivose, D-olivose disaccharide substructures of landomycin and mithramycin. J. Organomet. Chem. 2001, 617, 444–452. [Google Scholar] [CrossRef]
- Issa, J.P.; Bennett, C.S. A Reagent-Controlled SN2-Glycosylation for the Direct Synthesis of β-Linked 2-Deoxy-Sugars. J. Am. Chem. Soc. 2014, 136, 5740–5744. [Google Scholar] [CrossRef]
- Lloyd, D.; Bylsma, M.; Bright, D.K.; Chen, X.; Bennett, C.S. Mild method for 2-naphthylmethyl ether protecting group removal using a combination of 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone (DDQ) and β-pinene. J. Org. Chem. 2017, 82, 3926–3934. [Google Scholar] [CrossRef]
- Lloyd, D.; Bennett, C.S. An Improved Approach to the Direct Construction of 2-Deoxy-β-Linked Sugars: Applications to Oligosaccharide Synthesis. Chem. Eur. J. 2018, 24, 7610–7614. [Google Scholar] [CrossRef]
- Yu, X.; O’Doherty, G.A. De Novo Asymmetric Synthesis and Biological Evaluation of the Trisaccharide Portion of PI-080 and Vineomycin B2. Org. Lett. 2008, 10, 4529–4532. [Google Scholar] [CrossRef]
- Yu, X.; Li, M.; O’Doherty, G.A. De Novo Asymmetric Synthesis of the D-/L-Disaccharide Portion of Sch 47555. Heterocycles 2011, 82, 1577–1584. [Google Scholar]
- Shi, P.; Silva, M.; Wu, B. ; Wang, H-Y. L.; Akhmedov, N.G.; Li, M.; Beuning, P.; O’Doherty, G.A. Structure activity relationship study of the cleistrioside/cleistetroside natural products for antibacterial/anticancer activity, ACS Med. Chem. Lett. 2012, 3, 1086–1090. [Google Scholar]
- Bajaj, S.O.; Shi, P.; Beuning, P.J.; O’Doherty, G.A. Structure activity relationship study of Mezzettiasides natural products and its four new disaccharide analogues for anticancer/antibacterial activity. ChemMedComm. 2014, 5, 1138–1142. [Google Scholar]
- Zhou, M.; O’Doherty, G.A. De novo approach to 2-deoxy-O-glycosides: asymmetric syntheses of digioxose and digitoxin. J. Org. Chem. 2007, 72, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; O’Doherty, G.A. De novo asymmetric synthesis of digitoxin via a palladium catalyzed glycosylation reaction. Org. Lett. 2006, 8, 4339–4342. [Google Scholar] [CrossRef]
- Iyer, A.; Zhou, M.; Azad, N.; Elbaz, H.; Wang, L.; Rogalsky, D.K.; Rojanasakul, Y.; O’Doherty, G.A.; Langenhan, J.M. A direct comparison of the anticancer activities of digitoxin MeON-neoglycosides and O-glycosides: oligosaccharide chain length-dependant induction of caspase-9-mediated apoptosis. ACS Med. Chem. Lett. 2010, 1, 326–330. [Google Scholar] [CrossRef]
- Zhou, M.; O’Doherty, G.A. The de novo synthesis of oligosaccharides: application to the medicinal chemical study of digitoxin. Curr. Top. Med. Chem 2008, 8, 114–125. [Google Scholar]
- Hinds, J.W.; McKenna, S.B.; Sharif, E.U.; Wang, H-Y. L.; Akhmedov N. G.; O’Doherty, G.A. C3'/C4'-stereochemical effects of digitoxigenin -L-/-D-glycoside in cancer cytotoxicity. ChemMedChem. 2013, 8, 63–69. [Google Scholar] [CrossRef]
- Wang, H.-Y. L.; Xin, W.; Zhou, M.; Stueckle, T.A.; Rojanasakul, Y.; O’Doherty, G.A. Stereochemical survey of digitoxin monosaccharides, ACS Med. Chem. Lett. 2011, 2, 73–78. [Google Scholar]
- Wang, H.-Y.L.; Wu, B.; Zhang, Q.; Kang, S.-W.; Rojanasakul, Y.; O’Doherty, G.A. C5’-Alkyl Substitution Effects on Digitoxigenin -L-Glycoside Epithelial Human Lung Cancer Cells Cytotoxicity, ACS Med. Chem. Lett. 2011, 2, 259–263. [Google Scholar]
- Wang, H.-Y.L.; Rojanasakul, Y.; O’Doherty, G.A. Synthesis and Evaluation of the α-D-/α-L-Rhamnosyl and Amicetosyl Digitoxigenin Oligomers as Anti-tumor Agents, ACS Med. Chem. Lett. 2011, 2, 264–259. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




