Submitted:
23 December 2024
Posted:
24 December 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Biomimetics in Tissue Engineering
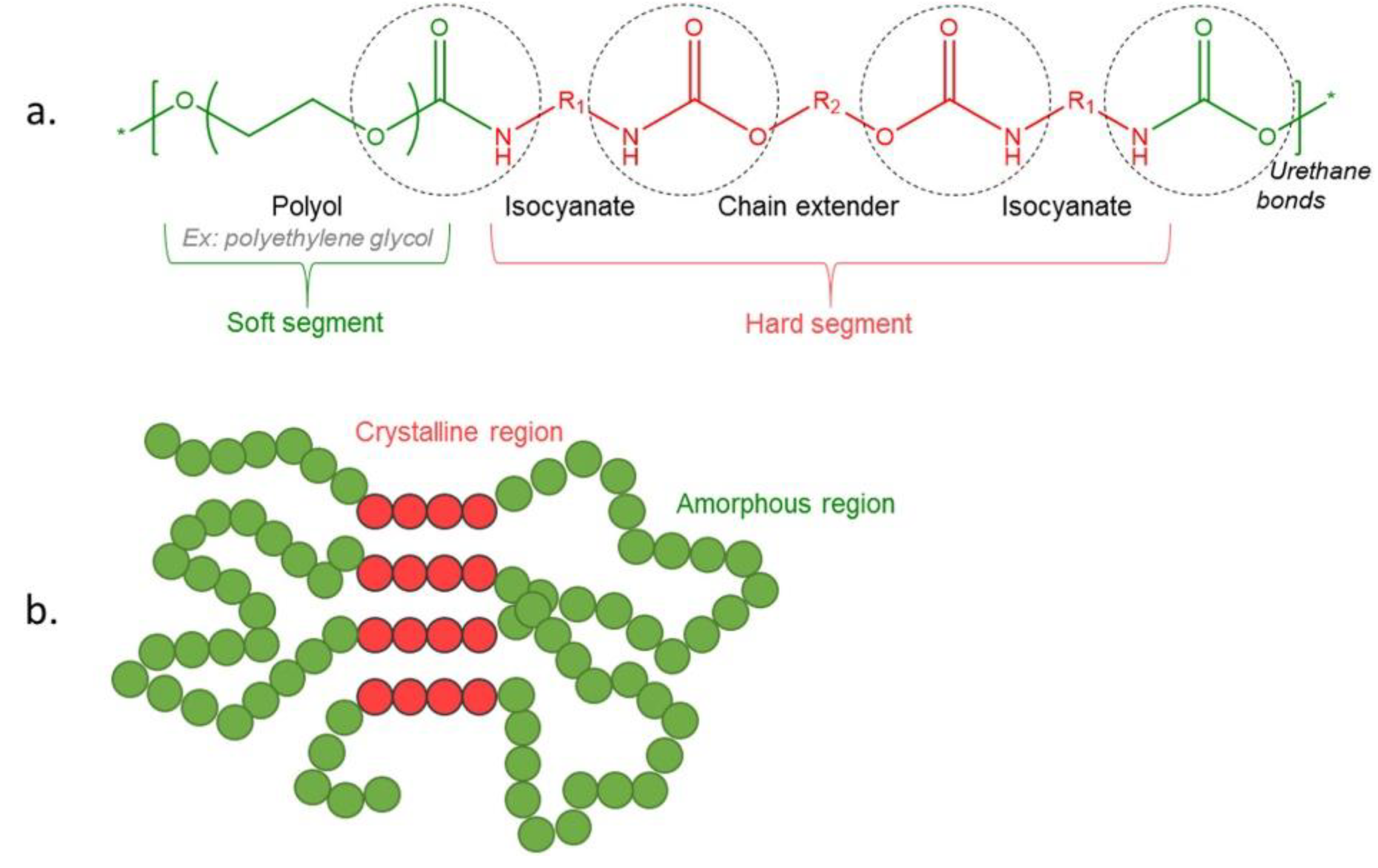
Polyurethane Chemistry
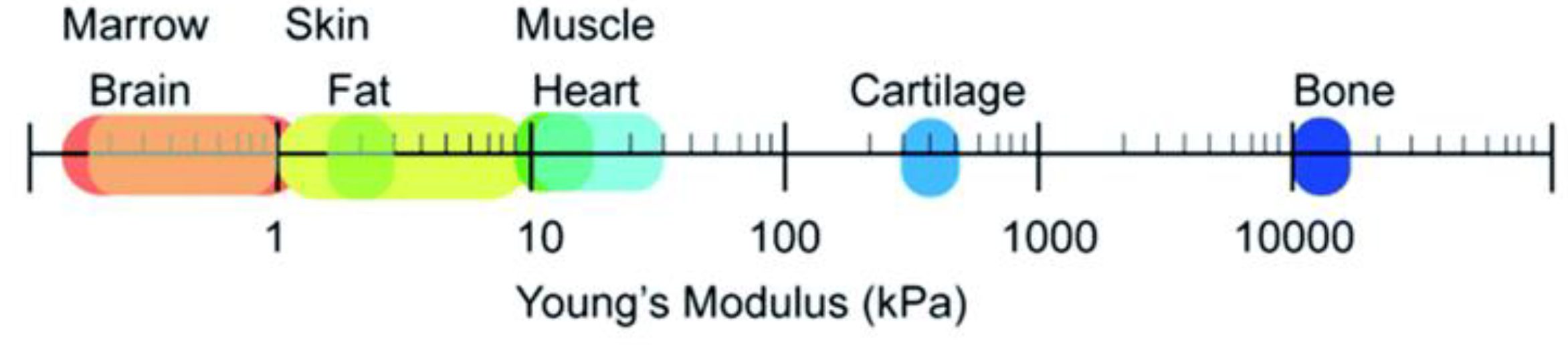
Soft Tissues, Hard Tissue
Soft-Tissue Engineering
5.Skeletal Muscle Tissue Engineering
5.1.Polyurethane Scaffolds for Skeletal Muscle Engineering
5.Cardiovascular Tissue Engineering
- - Blood vessels: Creating bio-engineered blood vessels that can replace damaged or blocked vessels is crucial to restoring proper blood circulation. The use of biodegradable scaffolds and hydrogels allows for the development of a structure that not only provides physical support but also promotes integration with existing tissue.
- - Heart muscle: Regeneration or replacement of damaged heart muscle after heart attacks or other diseases is an important goal. Research into stem cells and various biomaterials for tissue engineering could lead to new therapies that speed healing and improve heart function.
5.2.Polyurethane Scaffolds for Cardiac Muscle Engineering
5.Cardiac Tissue Engineering
5.3.Polyurethane Scaffolds for Cardic Valve
5.Vascular Tissue Engineering
5.4.Polyurethane Scaffolds for Vascular Engineering.
Polyurethanes for Hard-Tissue Engineering
6.Bone Tissue Engineering
6.1. Polyurethane Scaffolds for Bone Engineering
Conclusions and Future Trends
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bar-Cohen, Y. Biomimetics – Biologically Inspired Technologies, 1st ed.; Taylor & Francis Group, Boca Raton, 2005, 18-40.
- Bar-Cohen, Y. Biomimetics – using nature to inspire human innovations. Bioinsp. Biomim. 2006, 1, 1–12. [Google Scholar] [CrossRef]
- Dąbrowska, A. Biomimetism and nanotechnology – materials and methods inspired by nature. Przegląd mechaniczny 2017, 1. [Google Scholar]
- Bhushan, B. Biomimetics: lessons from nature – an overview. Phil. Trans. R. Soc. A 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Vincent., J.F.V. Biomimetics – a review. Proceedings of the Institution of Mechanical Engineers Part H Journal of Engineering in Medicine 2009, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Bonser, R.H.C.; Vincent, J.F.V. Technology trajectories, innovation, and the growth of biomimetics. Proc. IMechE, Part C: J. Mechanical Engineering Science 2007, 221, 1177–1180. [Google Scholar] [CrossRef]
- Peppas, N.A.; Langer, R.L. New challenges in biomaterials. Science 1994, 263, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Tathe, A.; Ghodke, M.; Nikalje, A.P. A brief review: biomaterials and their application. Int..J Pharm Pharm. Sci. 2010, 2, 19–23. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S. Biomaterials science: an introduction to materials in medicine, 1st ed.; Academic Press: 1996.
- Badylak, S. The extracellular matrix as a scaffold for tissue reconstruction. Cell and Developmental Biology 2002, 13. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, M.L. Current concepts of extracellular matrix. J. Orthop. Sci. 2006, 11, 326–331. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Leong, B. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. European Spine Journal 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Kima, K.; Parkb, I.; Hoshibac, T.; Jiangd, H. Design of artificial extracellular matrices for tissue engineering. Progress in Polymer Science, 2011, 36, 238–268. [Google Scholar] [CrossRef]
- Kim, T.; Shin, H.; Lim, D. Biomimetic scaffolds for tissue engineering. Advanced Functional Materials, 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Kim, B.; Mooney, D. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends in Biotechnology 1998, 16, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Rosso, F.; Giordano, A.; Barbarisi, M.; Barbarisi, A. From cell–ECM interactions to tissue engineering. Journal of Cellular Physiology. 2004, 199, 174–180. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.; Szostak, D.; Malejczyk, J. Tissue engineering in oral surgery – review of new methodology. Nowa Stomatologia 2002, 1. [Google Scholar]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-Polyvinyl Alcohol Film for Tissue Engineering: A Concise Review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef]
- Akter, F. Tissue engineering made easy. Academic Press, London 2016.
- Grayson, W.L.; Martens, T.P.; Eng, G.M.; Radisic, M.; Vunjak-Novakovic, G. Biomimetic approach to tissue engineering. Seminars in Cell & Developmental Biology 2009, 20, 665–673. [Google Scholar]
- Jakab, K.; Norotte, C.; Marga, F.; Murphy, K.; Vunjak-Novakovic, G.; Forgacs, G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010, 2. [Google Scholar] [CrossRef]
- Tabata, Y. Significance of release technology in tissue engineering. Drug Discovery Today 2005, 10, 1639–1646. [Google Scholar] [CrossRef]
- Chan, B.; Leong, B. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. European Spine Journal 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Chan, C.; Ramakrishna, S. Stem cells and biomimetic materials strategies for tissue engineering. Materials Science and Engineering C 2008, 28, 1189–1202. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J. Design and development of three-dimensional scaffolds for tissue engineering. Chemical Engineering Research and Design 2008, 85, 1051–1064. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.; Du, Z.; Chua, C. The design of scaffolds for use in tissue engineering part I. Traditional factors. Tissue Engineering 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.; Shoichet, M. Design of three-dimensional biomimetic scaffolds. Journal of Biomedical Materials Research A 2010, 94, 1321–1331. [Google Scholar] [CrossRef]
- Seidi, A.; Ramalingam, M.; Elloumi-Hannachi, I.; Ostrovidov, S. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomaterialia 2011, 7, 1441–1451. [Google Scholar] [CrossRef]
- Ma, P. Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Chan, G.; Mooney, D. New materials for tissue engineering: towards greater control over the biological response. Trends in Biotechnology 2008, 26, 382–392. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polymer Degradation and Stability 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. Journal of Materials Science: Materials in Medicine 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Koria, P. Delivery of Growth Factors for Tissue Regeneration and Wound Healing. BioDrugs 2012, 26, 163–175. [Google Scholar] [CrossRef]
- Fortunato, T.; De Bank, P.; Pula, G. Vascular Regenerative Surgery: Promised Land for Tissue Engineers? Int. J. Stem. Cell. Res. Transplant. 2017, 5, 268–276. [Google Scholar]
- Williams, D.F. To engineer is to create: the link between engineering and regeneration. Trends Biotechnol. 2006, 24, 4–8. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hashimoto, Y.; Negishi, J. Fabrication of a cell culture scaffold that mimics the composition and structure of bone marrow extracellular matrix. Journal of Bioscience and Bioengineering 2024, 138, 83–88. [Google Scholar] [CrossRef]
- Butler, D.L.; Goldstein, S.A.; Guilak, F. Functional tissue engineering: the role of biomechanics. J. Biomech. Eng. 2000, 122, 570–575. [Google Scholar] [CrossRef]
- Adachia, T.; Osakoa, Y.; Tanakaa, M.; Hojoa, M.; Hollister, S.J. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 2006, 27, 3964–3972. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Advance Drug Delivery Reviews 2008, 60, 243–262. [Google Scholar] [CrossRef]
- Błażewicz, S.; Stoch, L. Biocybernetyka i Inżynieria Biomechaniczna. Akademicka Oficyna Wydawnicza EXIT, Warszawa, 1999.
- Bronzino, J.D. Tissue Engineering and Artificial Organs. CRC Press, Boca Raton, Florida, 2006.
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone microparticles and their biodegradation. Polymer Degradation and Stability 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Wyleżoł, M.; Ostrowska, B.; Wróbel, E.; Muzalewska, M.; Grabowski, M.; Wyszyński, D.; Zubrzycki, J.; Piech, P.; Klepka, T. Inżynieria biomedyczna. Metody przyrostowe w technice medycznej. Politechnika Lubelska, Agencja TOP, Lublin, 2016.
- Nair, L.; Laurencin, C. Biodegradable polymers as biomaterials. Progress in Polymer Science 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P. Polymeric scaffolds for bone tissue engineering. Annals of Biomedical Engineering 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Piozzi, A.; Francolini, I. Chapter 9: Biomimetic Polyurethanes. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications. Muñoz-Bonilla, A.; Cerrada, M.; Fernández-García, M. Royal Society of Chemistry, 2013, 224 – 278.
- Wu, Y.; Simonovsky, F.I.; Ratner, B.D.; Horbett, T.A. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: A comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J. Biomed. Mater. Res. 2005, 74A, 722–738. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. The AAPS Journal 2010, 12, 188–196. [Google Scholar] [CrossRef]
- Florjańczyk, Z.; Penczek, S. Chemia polimerów, v.II, Oficyna Wydawnicza Politechniki Warszawskiej, Warszawa, 1998.
- Wirpsza, Z. Poliuretany: chemia, technologia, zastosowanie, Wydawnictwo Naukowo-Techniczne, Warszawa, 1991.
- Randall, D.; Lee, S. The polyurethane book, Wiley Ltd., New York, 2002.
- Szycher, M. Szycher’s handbook of polyurethane, CRC Press, Taylor &Francis Group, 2003.
- Magnina, A.; Polleta, E.; Phalipb, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnology Advances 2020, 39, 107457–107481. [Google Scholar] [CrossRef] [PubMed]
- Oertel, G.; Abele, L. Polyurethane handbook : chemistry, raw materials, processing, application, properties. Hanser, 1994.
- Prisacariu, C. Polyurethane Elastomers – from morphology to mechanical aspects, Springer Wien, New York, 2011.
- Rueda-Larraz, L.; Fernandez d’Arlas, B.; Tercjak, A.; Ribes, A.; Mondragon, I.; Eceiza, A. Synthesis and microstructure–mechanical property relationships of segmented polyurethanes based on a PCL–PTHF–PCL block copolymer as soft segment. European Polymer Journal 2009, 45, 2096–2109. [Google Scholar] [CrossRef]
- Sanchez-Adsuar, M.S.; Pastor-Blas, M.M.; Martín-Martínez, J.M.; Villenave, J.J. Properties of elastomeric polyurethanes obtained with ϵ-caprolactone macroglycol. International Journal of Adhesion and Adhesives 1997, 17, 155–161. [Google Scholar] [CrossRef]
- Petrovic, Z.S.; Javni, I.; Divjakovic, V. Structure and physical properties of segmented polyurethane elastomers containing chemical crosslinks in the hard segment. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 221–235. [Google Scholar] [CrossRef]
- Fernández-D’Arlas, B.; Alonso-Varona, A.; Palomares, T.; Corcuera, M.A.; Eceiza, A. Studies on the morphology, properties and biocompatibility of aliphatic diisocyanate-polycarbonate polyurethanes. Polym. Degrad. Stab. 2015, 122, 153–160. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Tien, Y.; Wei, K. Hydrogen bonding and mechanical properties in segmented montmorillonite/polyurethane nanocomposites of different hard segment ratios. Polymer 2001, 42, 3213–3221. [Google Scholar] [CrossRef]
- Coleman, M.M.; Lee, K.H.; Skrovanek, D.J.; Painter, P.C. Hydrogen Bonding in Polymers. Infrared Temperature Studies of a Simple Polyurethane. Macromolecules 1986, 19, 2149–2157. [Google Scholar] [CrossRef]
- Seymour, R.W.; Estes, G.X. Infrared Studies of Segmented Polyurethan Elastomers. Hydrogen Bonding. Polyurethan Elastomers 1970, 3, 579–583. [Google Scholar] [CrossRef]
- Seymour, R.W.; Cooper, S.L. Thermal Analysis of Polyurethane Block Polymers. Macromolecules 1973, 6, 48–53. [Google Scholar] [CrossRef]
- Xu, C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioactive Materials 2022, 15, 250–271. [Google Scholar] [CrossRef]
- Ciardelli, G.; Sartori, S.; Silvestri, A.; Serafini, P.; Caporale, A.; Boccafoschi, F. Multiblock polyurethanes in biomedical applications: fine tuning of degradation and biomimetic properties. AIP Conf. Proc. 2010, 1255, 8–16. [Google Scholar]
- Niu, Y.Q.; Chen, K.V.C.; He, T.; Yu, W.Y.; Huang, S.W.; Xu, K.T. Scaffolds from block polyurethanes based on poly(e-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials 2014, 35, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Hedrick, M.; Pareek, G.; Renzulli, J.; Haleblian, G.; Webster, T.J. Nanostructured polyurethane-poly-lactic-co-glycolic acid scaffolds increase bladder tissue regeneration: an in vivo study. Int. J. Nanomed. 2013, 8, 3285–3296. [Google Scholar]
- Xu, Y.; Guan, J. Interaction of cells with polyurethane scaffolds. In Advances in Polyurethane Biomaterials. Cooper, S.L.; Guan, J. Elsevier Ltd., 2016, 523-542.
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xu, M.; Lu, F.; Chang, Q. Adipogenesis or osteogenesis: destiny decision made by mechanical properties of biomaterials. RSC Adv. 2022, 12, 24501–24510. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, T.C.; Woodhouse, K.A.; Hauschka, S.D.; Murry, C. E.; Stayton, P.S. Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. J. Biomed. Mater. Res. A 2003, 66, 586–595. [Google Scholar] [CrossRef]
- Guan, J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, V.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef]
- Sharifpoor, S.; Labow, R.S.; Santerre, J.P. Synthesis and Characterization of Degradable Polar Hydrophobic Ionic Polyurethane Scaffolds for Vascular Tissue Engineering Applications. Biomacromolecules 2009, 10, 2729–2739. [Google Scholar] [CrossRef]
- Riboldi, S.A.; Sampaolesi, M.; Neuenschwanderc, P.; Cossu, G.; Mantero, S. Biomaterials 2005, 26, 4606–4615. [CrossRef] [PubMed]
- Grad, S.; Kupcsik, L.; Gorna, K.; Gogolewski, S.; Alini, M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials 2003, 24, 5163–5171. [Google Scholar] [CrossRef]
- Yıldırım, A.M.; Sanli, A.; Türkoglu, N.; Denktaş, C. Fabrication of electrospun nanofibrous clinoptilolite doped thermoplastic polyurethane scaffolds for skeletal muscle tissue engineering. J Appl Polym Sci. 2023, 140, 54233–54245. [Google Scholar] [CrossRef]
- Warren, G.L.; Hulderman, T.; Jensen, N.; McKinstry, M.; Mishra, M.; Luster, M.I.; Simeonova, P.P. Physiological role of tumor necrosis factor α in traumatic muscle injury. FASEB Journal 2002, 16, 1630–1632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, G.; Liu, Z.; Guo, M.; Jiang, X.; Taskin, M.B.; Zhang, Z.; Liu, J.; Tang, J.; Bai, R.; Besenbacher, F.; Chen, M.; Chen, C. Mussel inspired polynorepinephrine functionalized electrospun Polycaprolactone microfibers for muscle regeneration. Sci. Rep. 2017, 7, 8197–81107. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, I.; Seol, Y.-. .J.; Ko, I.K.; Yoo, J.J.; Atala, A.; Lee, S.J. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020, 11, 1–12. [Google Scholar]
- Gotti, C.; Sensini, A.; Fornaia, G.; Gualandi, C.; Zucchelli, A.; Focarete, M.L. Biomimetic hierarchically arranged nanofibrous structures resembling the architecture and the passive mechanical properties of skeletal muscles: a step forward toward artificial muscle. Front. Bioeng. Biotechnol. 2020, 8, 767–789. [Google Scholar] [CrossRef]
- Chen, J.; Dong, R.; Ge, J.; Guo, B.; Ma, P.X. Biocompatible, Biodegradable, and Electroactive Polyurethane-Urea Elastomers with Tunable Hydrophilicity for Skeletal Muscle Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 28273–28285. [Google Scholar] [CrossRef]
- Chen, J.; Ge, J.; Guo, B.; Gao, K.; Ma, P.X. Nanofibrous polylactide composite scaffolds with electroactivity and sustained release capacity for tissue engineering. J. Mater. Chem. B 2016, 4, 2477–2485. [Google Scholar] [CrossRef]
- Xu, C.; Yepez, G.; Wei, Z.; Liu, F.; Bugarin, A.; Hong, Y. Synthesis and characterization of conductive, biodegradable, elastomeric polyurethanes for biomedical applications. Journal of Biomedical Materials Research A. 2016, 104, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhu, Y.; Gao, R.; Yu, W.; Li, L.; Xu, K. Synthesis, Characterizations and Biocompatibility of Novel Block Polyurethanes Based on Poly(lactic acid) (PLA) and Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB). J. Inorg. Organomet. Polym. 2015, 25, 81–90. [Google Scholar] [CrossRef]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. ; Effects of synthetic micro- and nano-structured surfaces on cell behawior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Advanced Drug Delivery Reviews 2015, 84, 208–221. [Google Scholar] [CrossRef]
- Meng, JX.; Hou, CY.; Zhang, QH.; Li, YG.; Wang, HZ. Light-driven artificial muscles based on electrospun microfiber yarns. Sci. China Tech. Sci. 2019, 62, 965–970. [Google Scholar] [CrossRef]
- Łopianiak, I.; Kawecka, A.; Civelek, M.; Wojasinski, ́M.; Cicha, I.; Ciach, T.; Butruk-Raszeja, B.A. Characterization of Blow-Spun Polyurethane Scaffolds−Influence of Fiber Alignment and Fiber Diameter on Pericyte Growth. ACS Biomater. Sci. Eng. 2024, 10, 4388–4399. [Google Scholar] [CrossRef] [PubMed]
- Łopianiak, I.; Wojasinski, ́ M.; Butruk-Raszeja, B. Properties of Polyurethane Fibrous Materials Produced by Solution Blow Spinning. Chem. Process Eng. 2020, 41, 267–276. [Google Scholar]
- Blake, C.; Massey, O.; Boyd-Moss, M.; Firipis, K.; Rifai, A.; Franks, S.; Quigley, A.; Kapsa, R.; Nisbet, D.R.; Williams, R.J. Replace and repair: Biomimetic bioprinting for effective muscle engineering. APL Bioeng. 2021, 5, 031502-1–031502-16. [Google Scholar] [CrossRef]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef] [PubMed]
- Azarmgin, S.; Torabinejad, B.; Kalantarzadeh, R.; Garcia, H.; Velazquez, C.A.; Lopez, G.; Vazquez, M.; Rosales, G.; Heidari, B.S.; Davachi, S.M. Polyurethanes and Their Biomedical Applications. ACS Biomater. Sci. Eng. 2024, 10, 6828–6859. [Google Scholar] [CrossRef] [PubMed]
- Gokyer, S.; Yilgor, E.; Yilgor, I.; Berber, E.; Vrana, E.; Orhan, K.; Monsef, Y.A.; Guvener, O.; Zinnuroglu, M.; Oto, C.; Huri, P.Y. 3D Printed Biodegradable Polyurethaneurea Elastomer Recapitulates Skeletal Muscle Structure and Function. ACS Biomater. Sci. Eng. 2021, 7, 5189–5205. [Google Scholar] [CrossRef] [PubMed]
- Ketabat, F.; Alcorn, J.; Kelly, M.E.; Badea, I.; Chen, X. Cardiac Tissue Engineering: A Journey from Scaffold Fabrication to In Vitro Characterization. Small Sci. 2024, 4, 2400079–2400096. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering: state of the art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, S.; Asadpour, S.; Akbarzadeh, A.; Faridi-Majidi, R.; Ghanbari, H. Heart valve tissue engineering: an overview of heart valve decellularization processes. Regener. Med. 2018, 13, 41–54. [Google Scholar] [CrossRef]
- Akter, F.; Hamid, H. Chapter 6 - Cardiovascular Tissue Engineering. In Tissue Engineering Made Easy. Akter, F. Elsevier Inc., 2016, 55-65.
- Sartori, S.; Chiono, V.; Tonda-Turo, C.; Mattu, C.; Gianluca, C. Biomimetic polyurethanes in nano and regenerative medicine. J. Mater. Chem. B 2014, 2, 5128–5144. [Google Scholar] [CrossRef] [PubMed]
- Jawad, H.; Lyon, A.R.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Myocardial tissue engineering. Br. Med. Bull. 2008, 87, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Shah, G.; Wu, Y.; Torre-Amione, G.; King, N.M.; Lahmers, S.; Witt, C.C.; Becker, K.; Labeit, S.; Granzier, H.L. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 2004, 110, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Udriste, A.S.; Niculescu, A.G.; Iliută, L.; Bajeu, T.; Georgescu, A.; Grumezescu, A.M.; Bădilă, E. Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration. Polymers 2023, 15, 1177–1199. [Google Scholar] [CrossRef] [PubMed]
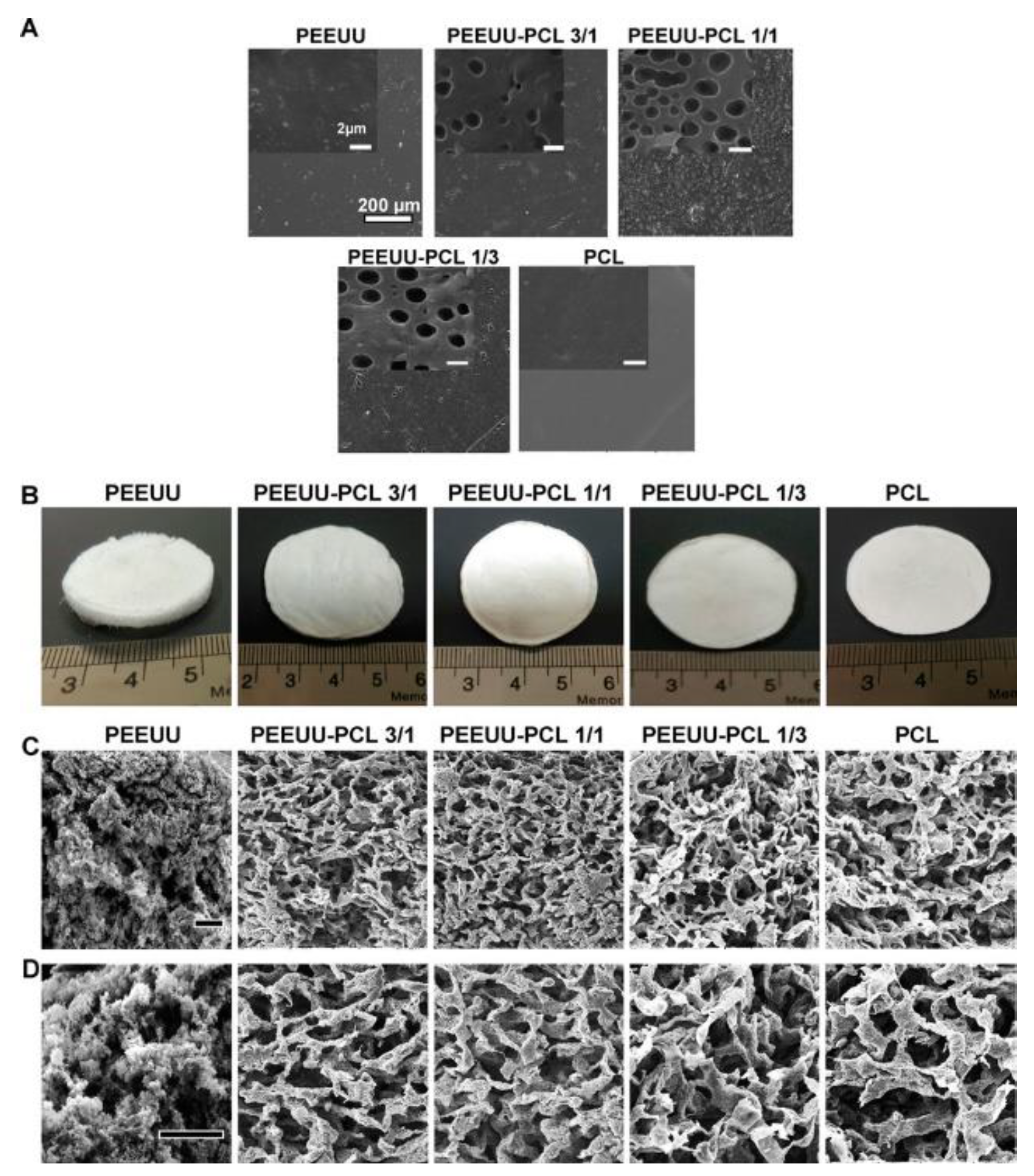
- Asadpour, S.; Yeganeh, H.; Ai, J.; Kargozar, S.; Rashtbar, M.; Alexander Seifalian, A.; Ghanbari, H. Polyurethane-Polycaprolactone Blend Patches: Scaffold Characterization and Cardiomyoblast Adhesion, Proliferation, and Function. ACS Biomaterials Science & Engineering 2018, 4, 4299–4310. [Google Scholar]
- Baheiraei, N.; Gharibi, R.; Yeganeh, H.; Miragoli, M.; Salvarani, N.; Di Pasquale, E.; Condorelli, G. ; Electroactive polyurethane/siloxane derived from castor oil as a versatile cardiac patch, part I: Synthesis, characterization, and myoblast proliferation and differentiation. J. Biomed. Mater. Res. Part A 2015, 104, 775–787. [Google Scholar] [CrossRef]
- Baheiraei, N.; Gharibi, R.; Yeganeh, H.; Miragoli, M.; Salvarani, N.; Di Pasquale, E.; Condorelli, G. ; Electroactive polyurethane/siloxane derived from castor oil as a versatile cardiac patch, part II: HL-1 cytocompatibility and electrical characterizations. J. Biomed. Mater. Res. Part A 2016, 104, 1398–1407. [Google Scholar] [CrossRef]
- Bahrami, S.; Solouk, A.; Mirzadeh, H.; Seifalian, A.M. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Composites Part B 2019, 168, 421–431. [Google Scholar] [CrossRef]
- Mani, M.P.; Jaganathan, S.K.; Ahmad Athif Faudzi, A.A.; Sunar, M.S. Engineered Electrospun Polyurethane Composite Patch Combined with Bi-functional Components Rendering High Strength for Cardiac Tissue Engineering. Polymers 2019, 11, 705–721. [Google Scholar] [CrossRef]
- Tao, Z.W.; Wu, S.; Cosgriff-Hernandez, E.M.; Jacot, J.G. Evaluation of a polyurethane-reinforced hydrogel patch in a rat right ventricle wall replacement model. Acta Biomaterialia 2019, 101, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Liao, H.C.; Hsu, S.H.; Chen, R.S.; Wu, M.C.; Yang, Y.F.; Wu, C.C.; Chen, M.H.; Su, W.F. A novel polyurethane/cellulose fibrous scaffold for cardiac tissue engineering. RSC Adv. 2015, 5, 6932–6939. [Google Scholar] [CrossRef]
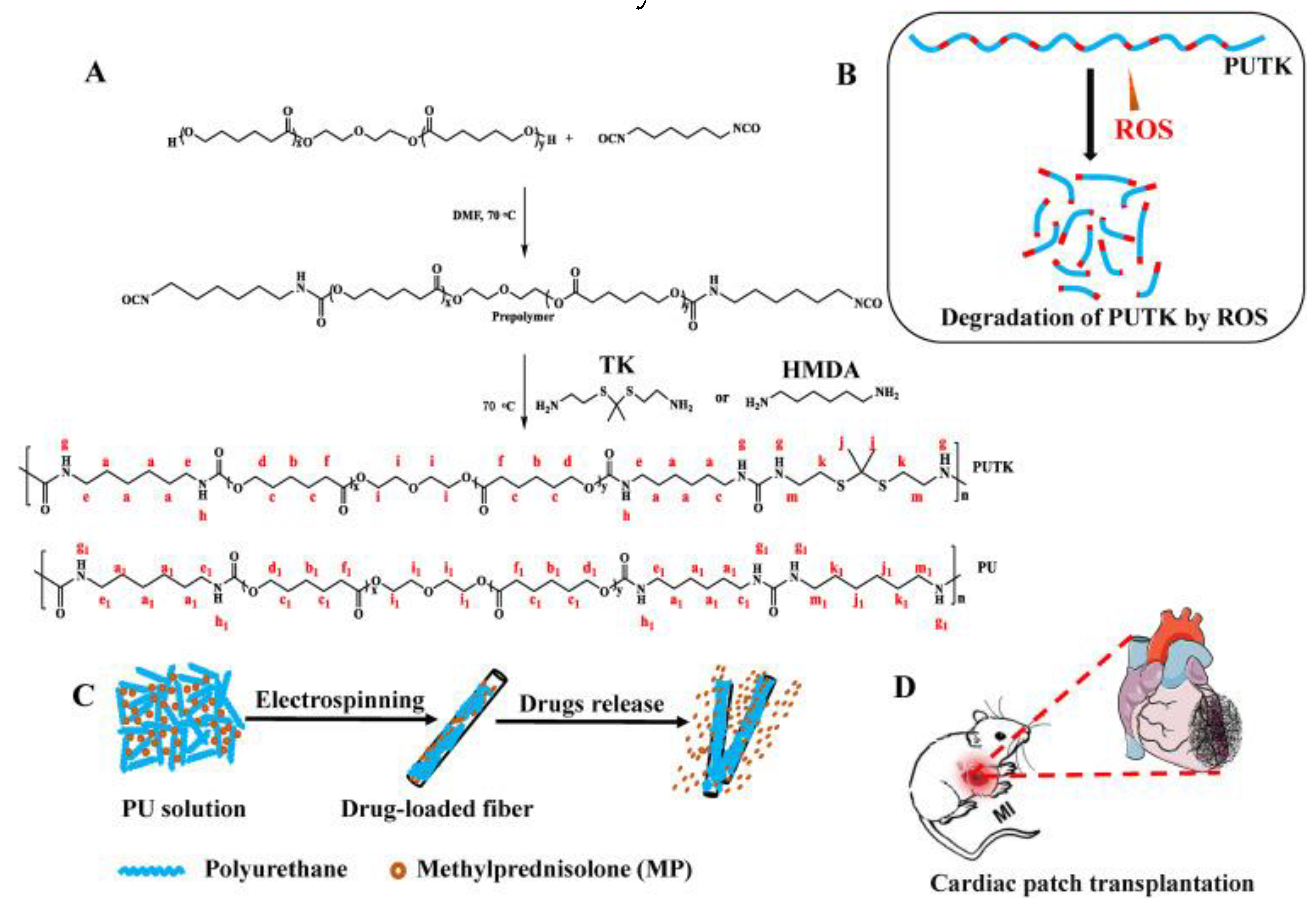
- Yao, Y.; Ding, J.; Wang, Z.; Zhang, H.; Xie, J.; Wang, Y.; Hong, L.; Mao, Z.; Gao, J.; Gao, C. ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials 2020, 232, 119726–119740. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of Polyurethane Fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Boffito, M.; Sartori, S.; Ciardelli, G. Polymeric scaffolds for cardiac tissue engineering: requirements and fabrication technologies. Polym. Int. 2013, 63, 2–11. [Google Scholar] [CrossRef]
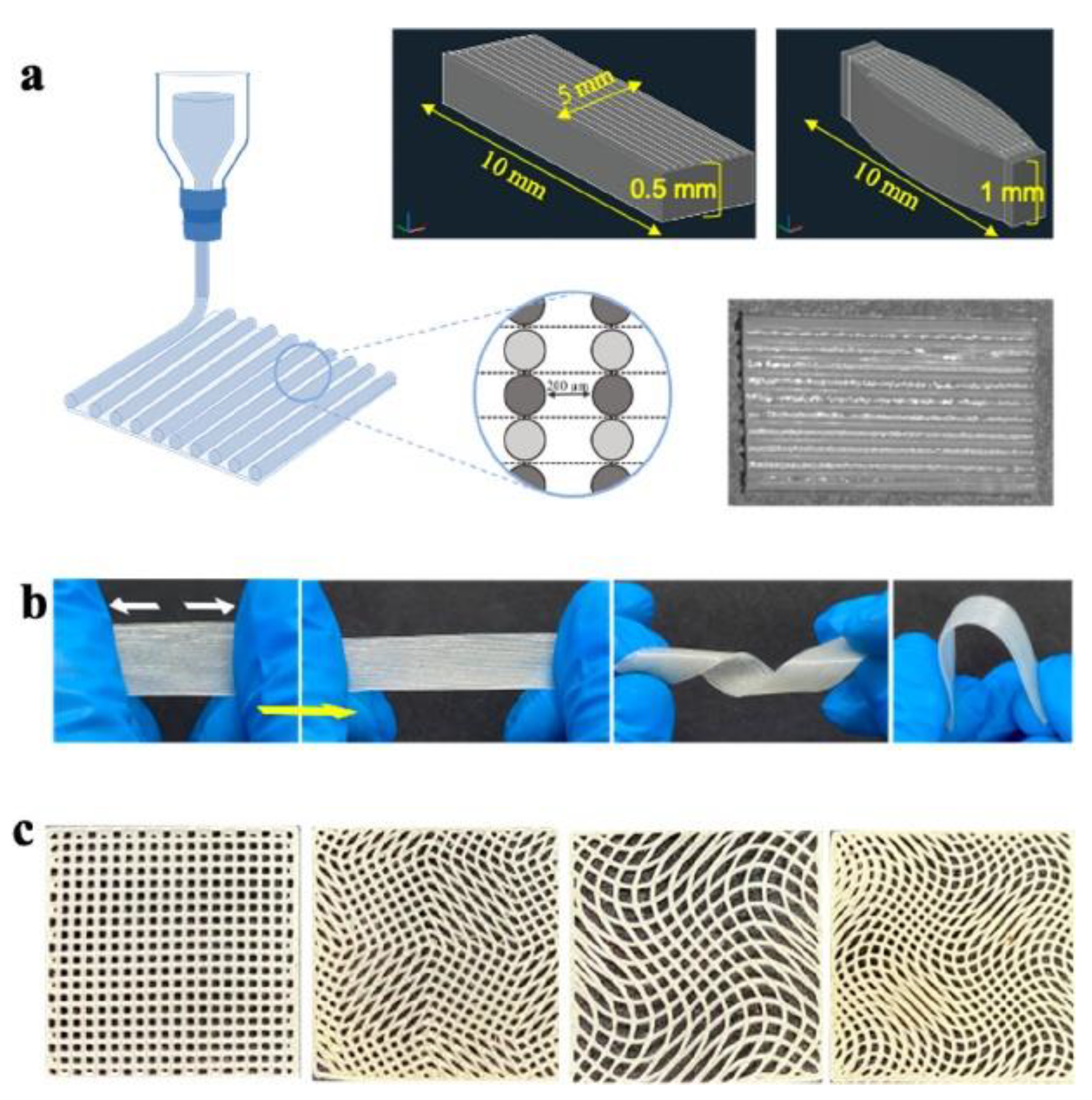
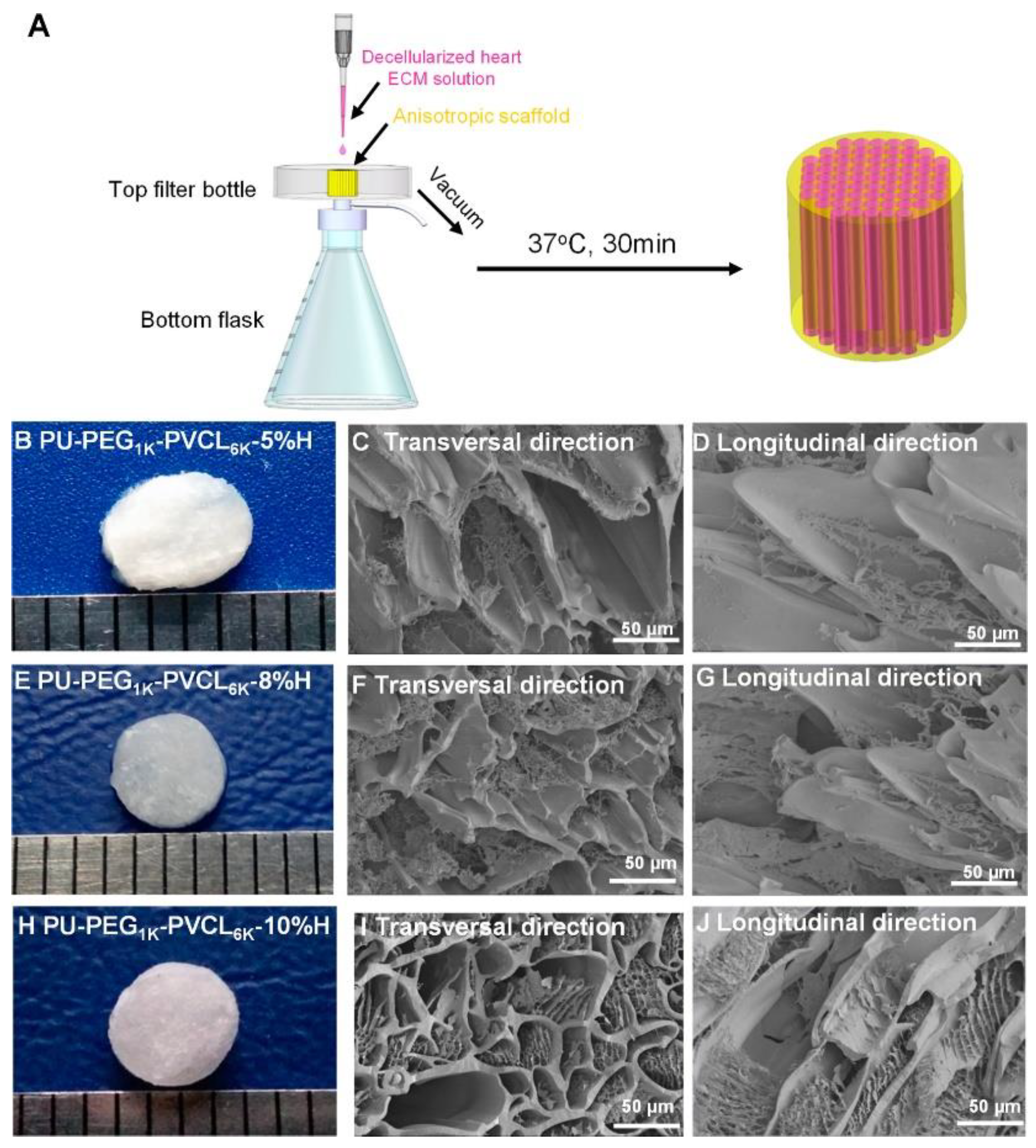
- Xu, C.; Okpokwasili, C.; Huang, Y.; Shi, X.; Wu, J.; Liao, J.; Tang, L.; Hong, Y. Optimizing Anisotropic Polyurethane Scaffolds to Mechanically Match with Native Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 2757–2769. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Tao, T.; Xu, H.; Cen Chen, C.; Lee, I.S.; Chung, S.; Dong, Z.; Li, W.; Ma, L.; Bai, H. Qianming Chen Recent progress in biomaterials for heart valve replacement: Structure, function, and biomimetic design. VIEW 2021, 2, 20200142–20200159. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Answini, G.A.; Yakubov, S.J.; Rai, B.; Smith, J.M.; Duff, S.; Shannon, F.L.; Sakwa, M.; Beith, J.; Heimansohn, D. Preliminary evaluation of a novel polymeric valve following surgical implantation for symptomatic aortic valve disease. J. Am. Coll. Cardiol. Intv. 2021, 14, 2754–2756. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y. A new nanocomposite copolymer based on functionalised Graphene oxide for development of heart valves. Sci. Rep. 2020, 10, 5271–5285. [Google Scholar] [CrossRef]
- Rezvova, M.A.; Klyshnikov, K.Y.; Gritskevich, A.A.; Ovcharenko, E.A. Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices. Int. J. Mol. Sci. 2023, 24, 3963–3993. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate risk patients. The New England journal of medicine. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Kodali, S.; Makkar, R.R.; Herrmann, H.C.; Williams, M.; Babaliaros, V.; Leon, M.B. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. The Lancet 2016, 387, 2218–2225. [Google Scholar] [CrossRef]
- Rahmani, B.; Tzamtzis, S.; Sheridan, R.; Mullen, M.J.; John Yap, J.; Seifalian, A.M.; Burriesci, G. In Vitro Hydrodynamic Assessment of a New Transcatheter Heart Valve Concept (the TRISKELE). J. of Cardiovasc. Trans. Res. 2016, 10, 104–115. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; . Answini, G.A.; Yakubov, S.J.; Rai, B.; Smith, J.M.; Duff, S.; Shannon, F.L.; Sakwa, M.; Beith, J.; Heimansohn, D. Preliminary Evaluation of a Novel Polymeric Valve Following Surgical Implantation for Symptomatic Aortic Valve Disease. JACC Cardiovascular Interventions 2021, 14, 2754–2756. [Google Scholar] [CrossRef]
- Evgeny, A.O.; Amelia Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikano, E.A.; Chernonosova, V.S.; Shevelev, G.Y.; Shishkova, D.K.; Krivkin, E.O.; Kudryavceva, Y.A.; Seifalian, A.M.; Barbarash, L.S. A New Nanocomposite Copolym Based On Functionalised Graphen Oxide for Development of Heart Valves. Scientific Reports 2020, 10, 527–541. [Google Scholar]
- Hasan, A.; et al. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 47, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.F.; Nondonfaz, A.; Aqil, A.; Pierrard, A.; Hulin, A.; Delierneux, C.; Ditkowski, B.; Gustin, M.; Legrand, M.; Tullemans, B.M.E.; Brouns, S.L.N.; Nchimi, A.; Carrus, R.; Dejosé, A.; Heemskerk, J.W.M.; Kuijpers, M.J.E.; Jan Ritter, J.; Steinseifer, U.; Clauser, J.C.; Jérôme, C.; Lancellotti, P.; Oury, C. Design, manufacturing and testing of a green non-isocyanate polyurethane prosthetic heart valve. Biomater. Sci. 2024, 12, 2149–2164. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, E.; Gaudio, C.D.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2013, 9, 861–888. [Google Scholar] [CrossRef]
- Singh, C.; Cynthia, S.; Wong, C.S.; Wang, X. Medical textiles as vascular implants and their success to mimic natural arteries. Journal of Functional Biomaterials 2015, 6, 500–525. [Google Scholar] [CrossRef] [PubMed]
- Liber-Kneć, A.; Łagan, S. Metody badań biomateriałów i tkanek-wstęp do ćwiczeń labarotoryjnych. Politechnika Krakowska, Kraków, 2020.
- Haruguchi, H.; Teraoka, S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J. Artif. Organs. 2003, 6, 227–235. [Google Scholar] [CrossRef]
- Jirofti, N.; Mohebbi-Kalhori, D.; Samimi, A.; Hadjizadeh, A.; Kazemzadeh, G.G. Small-diameter Vascular Graft Using Co-Electrospun of Composite PCL/PU Nanofibers. Biomed. Mater. 2018, 13, 055014–005020. [Google Scholar] [CrossRef]
- Zhen, L.; Creason, S.A.; Simonovsky, F.I.; Snyder, J.M.; Lindhartsen, S.L.; Mecwan, M.M.; Johnson, B.W.; Himmelfarb, J.; Ratner, B.D. Precision-porous polyurethane elastomers engineered for application in pro-healing vascular grafts: Synthesis, fabrication and detailed biocompatibility assessment. Biomaterials 2021, 279, 121174–121191. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, A.; Daemi, H.; Rajabi, S.; Baharvand, H. Highly tough and ultrafast self-healable dual physically crosslinked sulfated alginate-based polyurethane elastomers for vascular tissue engineering. Carbohydrate Polymers 2021, 257, 117632–117641. [Google Scholar] [CrossRef] [PubMed]
- Kianpour, G.; Bagheri, R.; Pourjavadi, A.; Ghanbari, H. Synergy of titanium dioxide nanotubes and polyurethane properties for bypass graft application: Excellent flexibility and biocompatibility. Materials & Design 2022, 215, 11523–11533. [Google Scholar]
- Yu, E.; Mi, H.Y.; Zhang, J.; Thomson, J. A.; Turng, L.S. Development of Biomimetic Thermoplastic Polyurethane/Fibroin Small-Diameter Vascular Grafts via a Novel Electrospinning Approach. J. Biomed. Mater. Res. A 2018, 106, 985–996. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Ferrone, M.L.; Raut, C.P. Modern surgical therapy: limb salvage and the role of amputation for extremity soft-tissue sarcomas. Surg. Oncol. Clin. N. Am. 2012, 21, 201–213. [Google Scholar] [CrossRef] [PubMed]
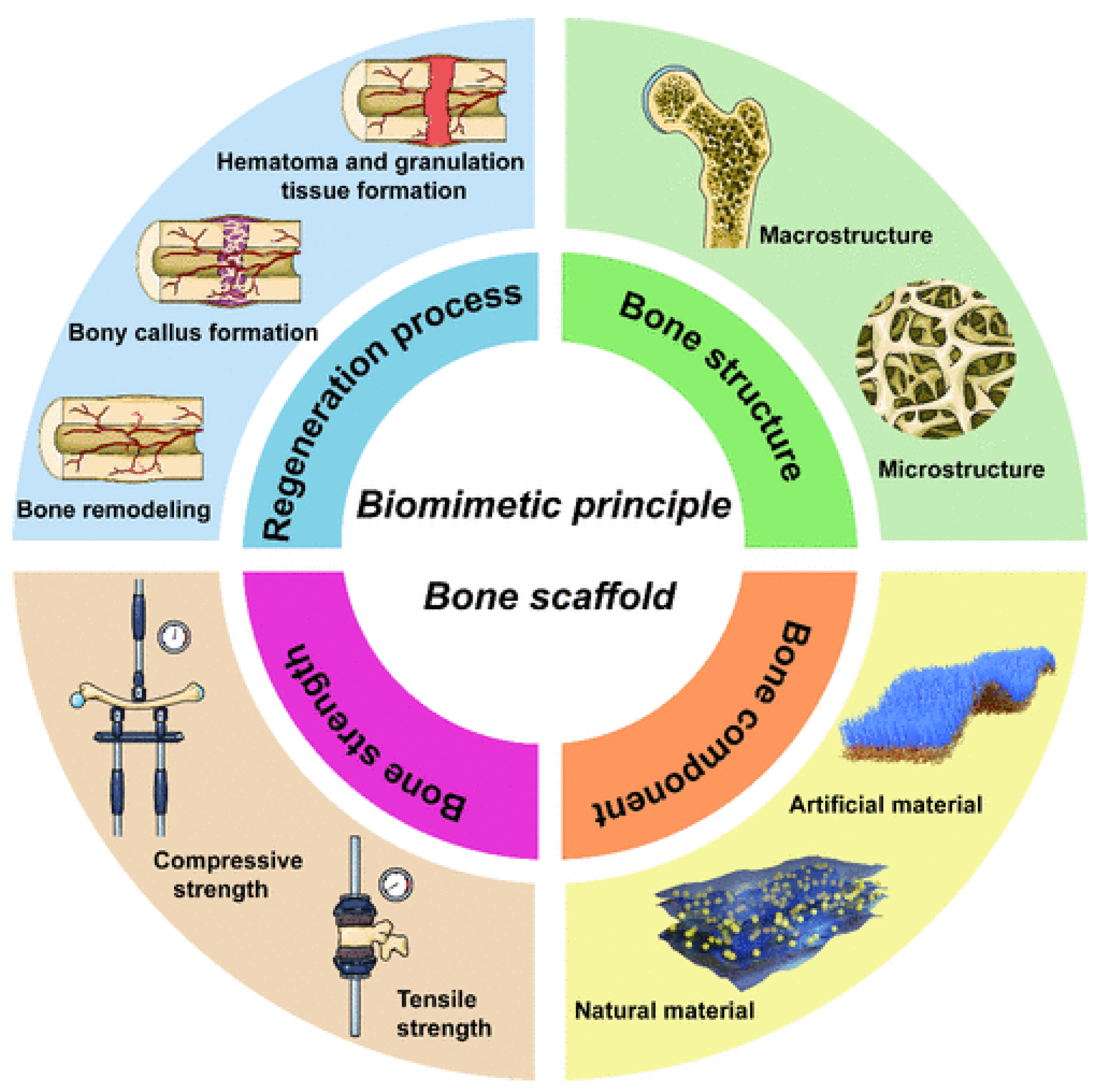
- Xu, H.; Hui Li, H.; Liu, R.; Liu, Y.; Tian, F.; Gao, X.; Wang, Z.; Lu, B.; Wu, W.; Hui, H.; Chen, X.; Hu, C.; Jia, S.; Hao, D.; Zhu, L. Four-Dimensional Perspective on Biomimetic Design and Fabrication of Bone Scaffolds for Comprehensive Bone Regeneration. CS Materials Lett. 2024, 6, 4262–4281. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ethier, C.R.; Simmons, C.A. Skeletal biomechanics. In Introductory Biomechanics From Cells to Organisms. Ethier, C.R.; Simmons, C.A. Cambridge University Press, 2007, 379–443.
- Guo, X.E.; Goldstein, S.A. Is trabecular bone tissue different from cortical bone tissue? Forma 1997, 12, 85–196. [Google Scholar]
- Becker, S.T.; et al. Biocompatibility of individually designed scaffolds with human periosteum for use in tissue engineering. Journal of Materials Science: Materials in Medicine 2010, 21, 1255–1262. [Google Scholar] [CrossRef]
- Brunello, G.; et al. Powder-based 3D printing for bone tissue engineering. Biotechnology Advances 2016, 34, 740–753. [Google Scholar] [CrossRef]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. The International Journal of Advanced Manufacturing Technology 2016, 82, 559–571. [Google Scholar] [CrossRef]
- Warnke, P.H.; et al. Ceramic scaffolds produced by computer-assisted 3D printing and sintering: characterization and biocompatibility investigations. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 212–217. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Materials Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Torres, F.G.; et al. Mechanical properties and bioactivity of porous PLGA/TiO2 nanoparticle-filled composites for tissue engineering scaffolds. Composites Science and Technology 2007, 67, 1139–1147. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Industrial & Engineering Chemistry Research 2019, 58, 6163–6194. [Google Scholar]
- McEnery, M.A.P.; Lu, S.; Gupta, M.K.; Zienkiewicz, K.J.; Wenke, J.C.; Kalpakci, K.N.; Shimko, D.A.; Duvalla, C.L.; Guelcher, S.A. Oxidatively degradable poly(thioketal urethane)/ceramic composite bone cements with bone-like strength. RSC Adv. 2016, 6, 109414–109424. [Google Scholar] [CrossRef] [PubMed]
- McGough, M.A.P.; Shiels, S.M.; Boller, L.A.; Zienkiewicz, K.J.; Duvall, C.L.; Wenke, J.C.; Guelcher, S.A. Poly(Thioketal Urethane) Autograft Extenders in an Intertransverse Process Model of Bone Formation. Tissue Eng. Part A. 2019, 25, 949–963. [Google Scholar] [CrossRef]
- McGough, M.A.P.; Boller, L.A.; Groff, D.M.; Schoenecker, J.G.; Nyman, J.S.; Wenke, J.C.; Rhodes, C.; Shimko, D.; Duvall, C.L.; Guelcher, S.A. Nanocrystalline Hydroxyapatite−Poly(thioketal urethane) Nanocomposites Stimulate a Combined Intramembranous and Endochondral Ossification Response in Rabbits. ACS Biomater. Sci. Eng. 2020, 6, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Boller, L.A.; Jones, A.A.; Cochran, D.L.; Guelcher, S.A. Compression-resistant polymer/ceramic composite scaffolds augmented with rhBMP-2 promote new bone formation in a nonhuman primate mandibular ridge augmentation model. Int. J. Oral Maxillofac. Implants. 2020, 35, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Talley, A.D.; McEnery, M.A.; Kalpakci, K.N.; Zienkiewicz, K.J.; Shimko, D.A.; Guelcher, S.A. Remodeling of injectable, low-viscosity polymer/ceramic bone grafts in a sheep femoral defect model. J. Biomed. Mater. Res. Part B 2017, 105, 2333–2343. [Google Scholar] [CrossRef]
- Shiels, S.M.; Anne, D.; Talley, A.D.; McGough, M.A.P.; Zienkiewicz, K.J.; Kalpakci, K.; Shimko, D.; Guelcher, S.A.; Wenke, J.C. Injectable and compression-resistant low viscosity polymer/ceramic composite carriers for rhBMP-2 in a rabbit model of posterolateral fusion: a pilot study. Journal of Orthopaedic Surgery and Research 2017, 12, 107–116. [Google Scholar] [CrossRef]
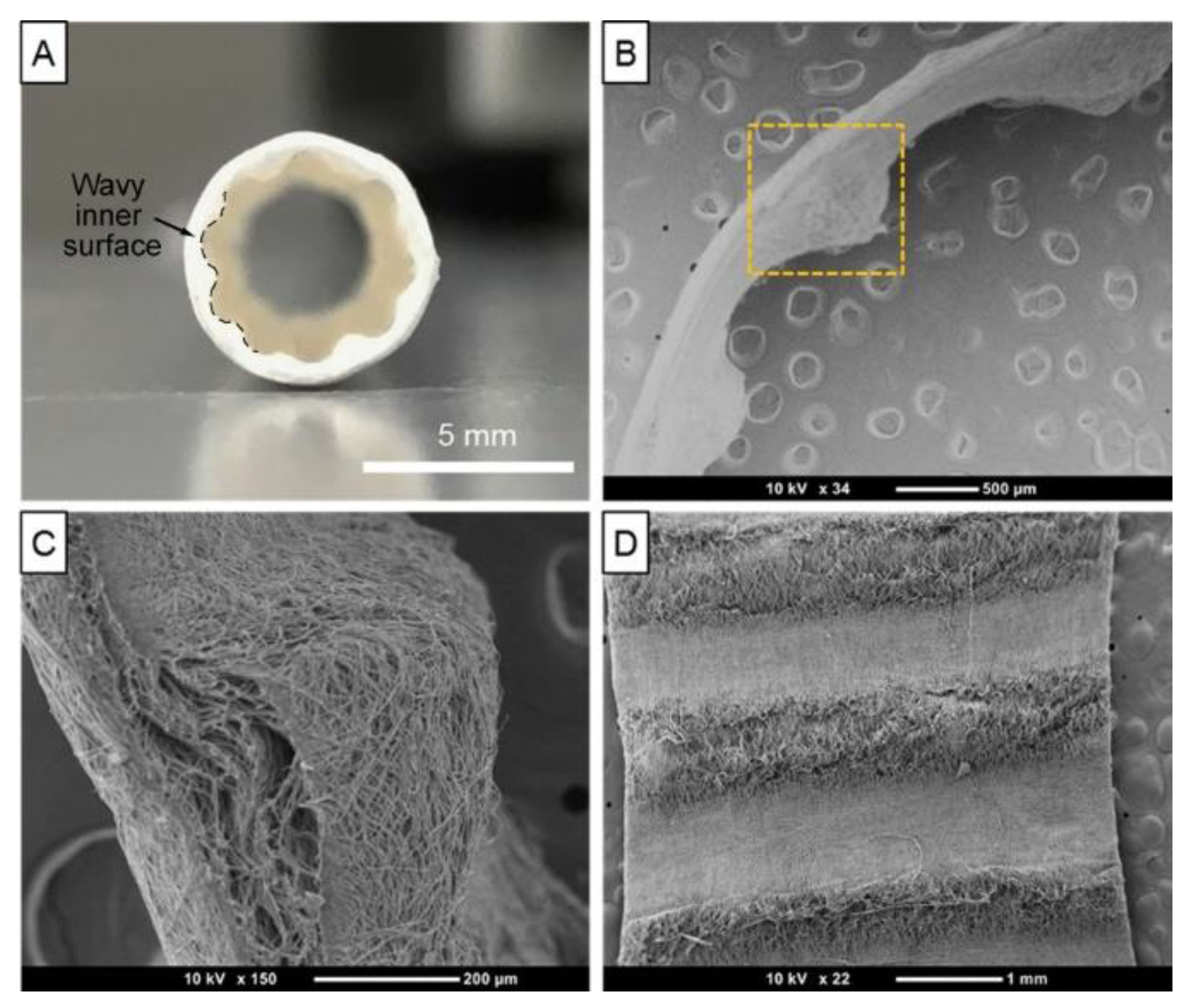
- Lavrador, C.; Mascarenhas, R.; Coelho, P.; Brites, C.; Pereira, A.; Gogolewski, S. Elastomeric enriched biodegradable polyurethane sponges for critical bone defects: a successful case study reducing donor site morbidity. J. Mater. Sci: Mater. Med. 2016, 27, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Zhu, Q.; Wang, X.; Xiao, H.; Zheng, Z. In Vitro and in Vivo Characterization of a Foam-Like Polyurethane Bone Adhesive for Promoting Bone Tissue Growth. ACS Biomater. Sci. Eng. 2019, 5, 5489–5497. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, X.; Fan, Z.; Xu, Y.; Niu, H.; Lia, C.; Dang, Y.; Huang, Z.; Wang, Y.; Guan, J. Biomimetic polyurethane/TiO2 nanocomposite scaffolds capable of promoting biomineralization and mesenchymal stem cell proliferation. Materials Science & Engineering C 2018, 85, 79–87. [Google Scholar]
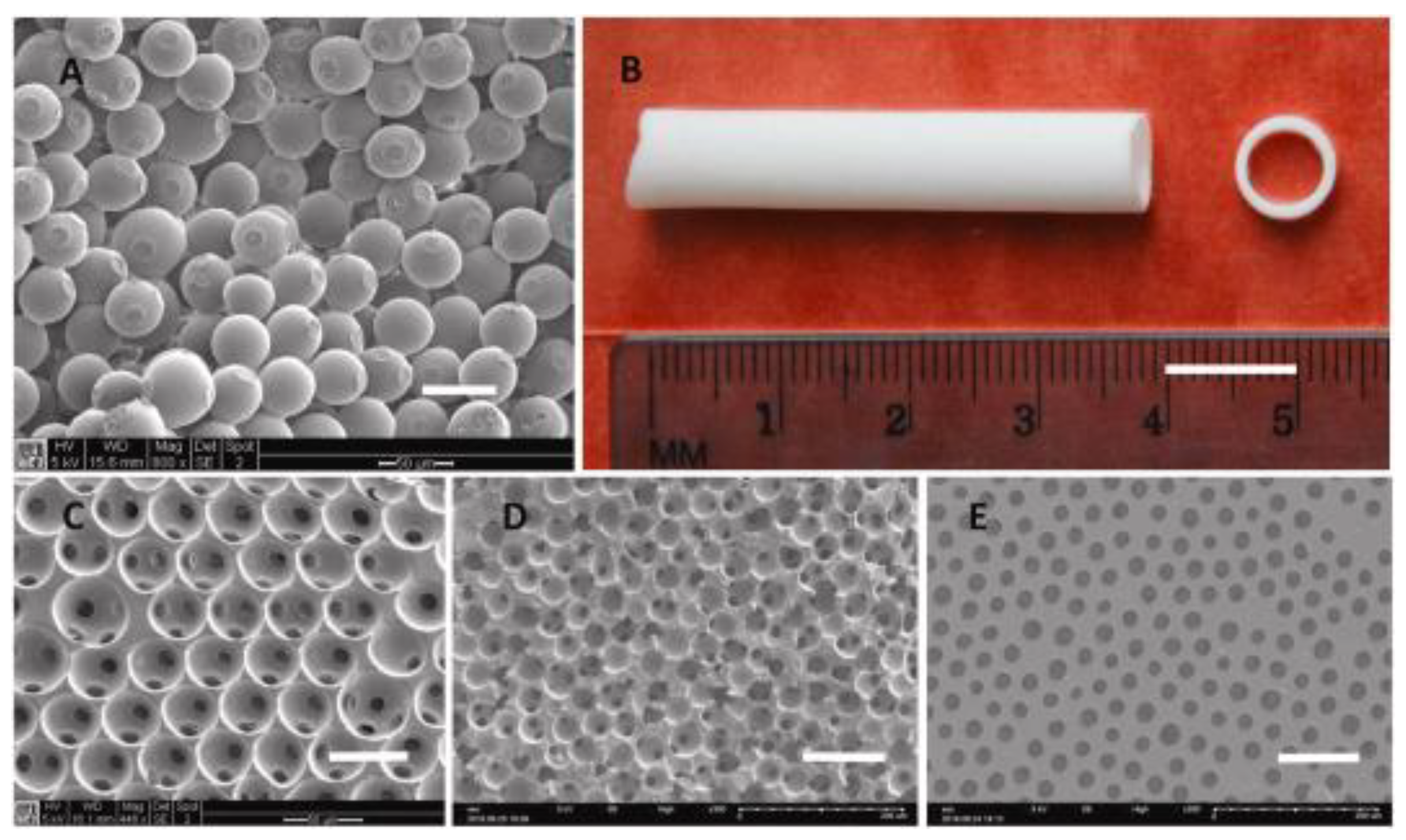
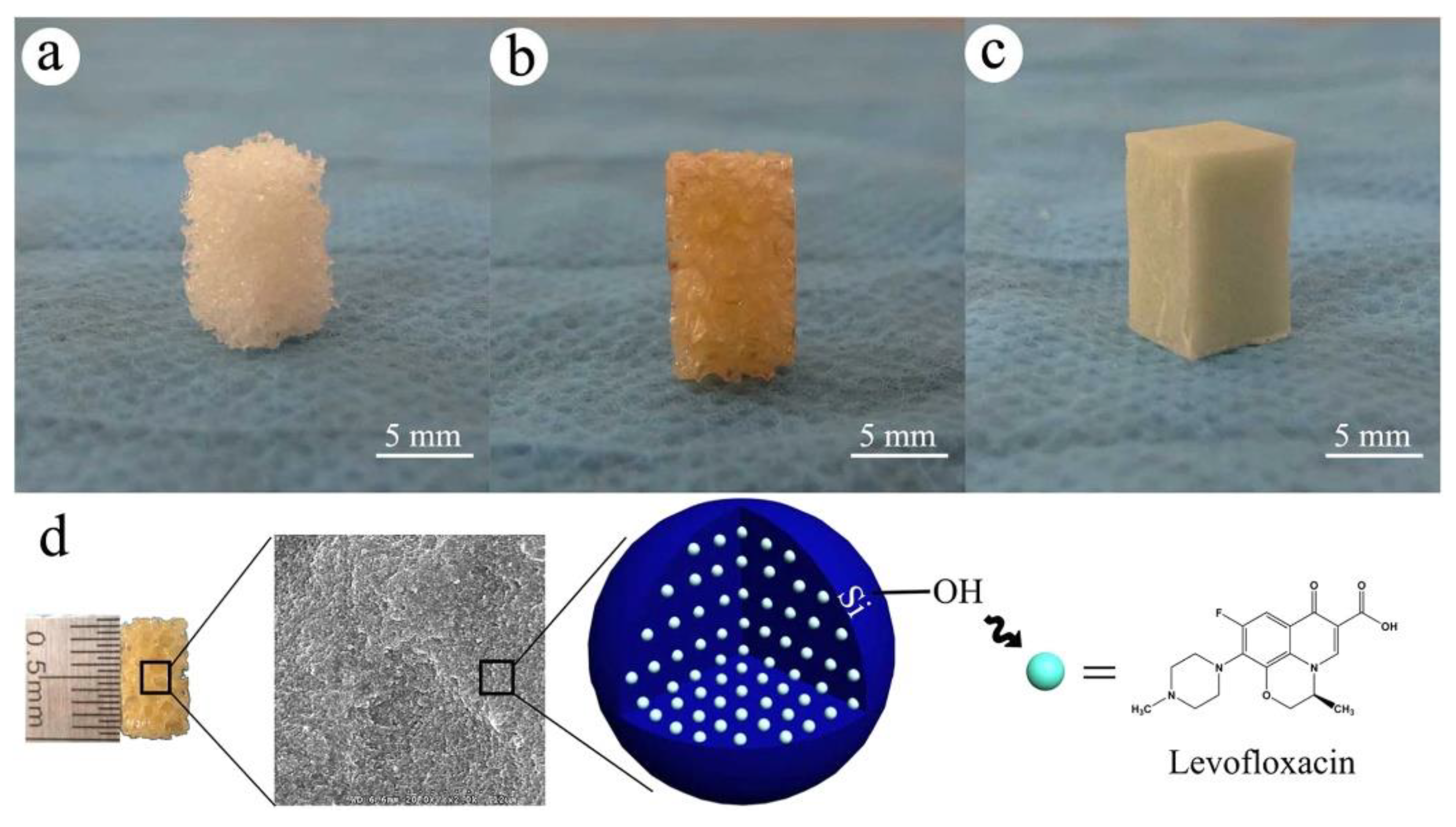
- Wang, Q.; Chen, C.; Liu, W.; He, X.; Zhou, N.; Zhang, D.; Gu, H.; Li, J.; Jiang, J.; Huang, W. Levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Scientific Reports 2017, 7, 41808–41821. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Wu, X.D.; He, X.; Lin, X.; Wang, H.; Li, J.; Jiang, J.; Huang, W. Efficacy of novel nano-hydroxyapatite/polyurethane composite scaffolds with silver phosphate particles in chronic osteomyelitis. Journal of Materials Science: Materials in Medicine 2019, 30–59. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D. , de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends in Biotechnology 2012, 30, 499–511. [Google Scholar] [CrossRef]
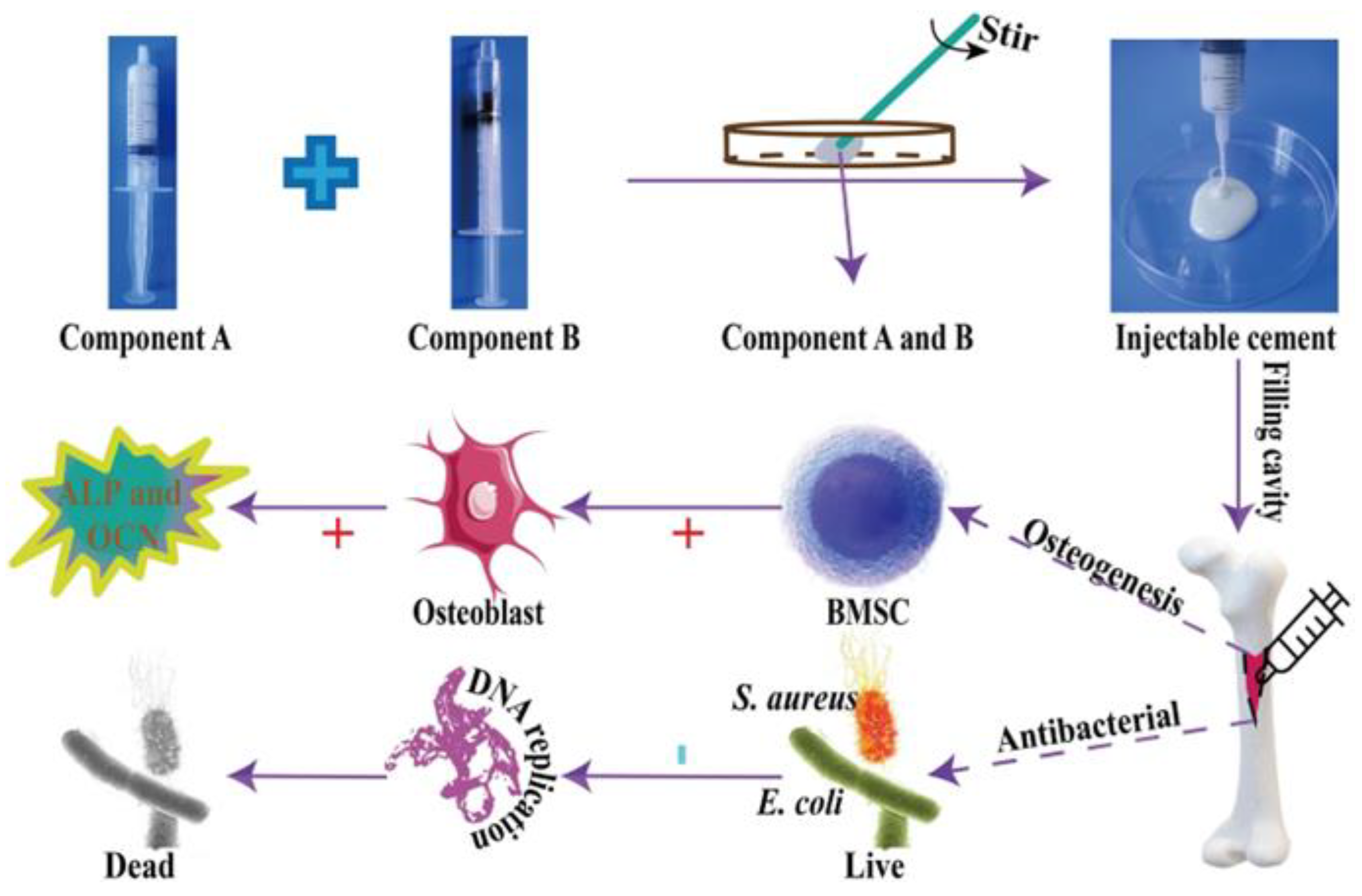
- Zhang, J.; Lei, X.; Tang, J.; Chen, J.; Zhao, Q.; Fang, W.; Zhang, Y.; Li, Y.; Zuo, Y. Efect of Antibacterial Enoxacin on the Properties of Injectable Nano-hydroxyapatite/Polyurethane Cement for Bone Repairing. Journal of Bionic Engineering 2022, 19, 483–496. [Google Scholar] [CrossRef]
- Anderson, J.M.; Marchant, R.E. Biomaterials: Factors favoring colonization and infection. In F. A. Waldvogel & A. L. Bisno (Eds.), Infections associated with indwelling medical devices. 2000, 89–109, ASM Press.
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Materials Science and Engineering C: Materials for Biological Applications, 2021, 118, 111382–111399. [Google Scholar] [CrossRef]
- Wanga, C.; Huang, W.; Zhoue, Y.; Hea, L.; Hea, Z.; Chena, Z.; Hea, X.; Tian, S.; Liao, J.; Lua, B.; Weid, Y.; Wang, M. 3D printing of bone tissue engineering scaffolds. Bioactive Materials 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; K, S.S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; Camci-Unal, G.; Ramalingam, M. 3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives. International Journal of Nanomedicine 2021, 16, 4289–4319. [Google Scholar] [CrossRef] [PubMed]
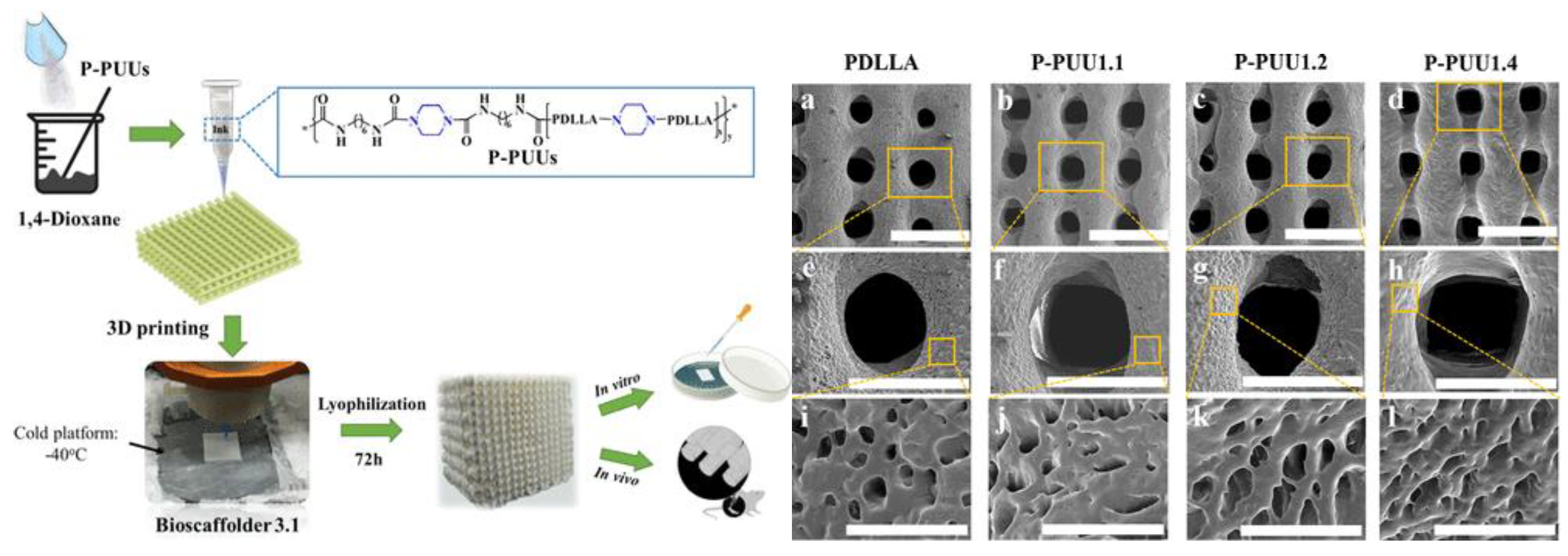
- Ma, Y.; Hu, N.; Liu, J.; Zhai, X.; Wu, M.; Hu, C.; Li, L.; Lai, Y.; Pan, H.; Lu, W.W.; Zhang, X.; Luo, Y.; Ruan, C. Three-Dimensional Printing of Biodegradable Piperazine-Based Polyurethane-Urea Scaffolds with Enhanced Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 9415–9424. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




