Submitted:
23 December 2024
Posted:
24 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Biochemical Analysis
2.3. Calculation of Ca×P/eGFR Ratio
2.4. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
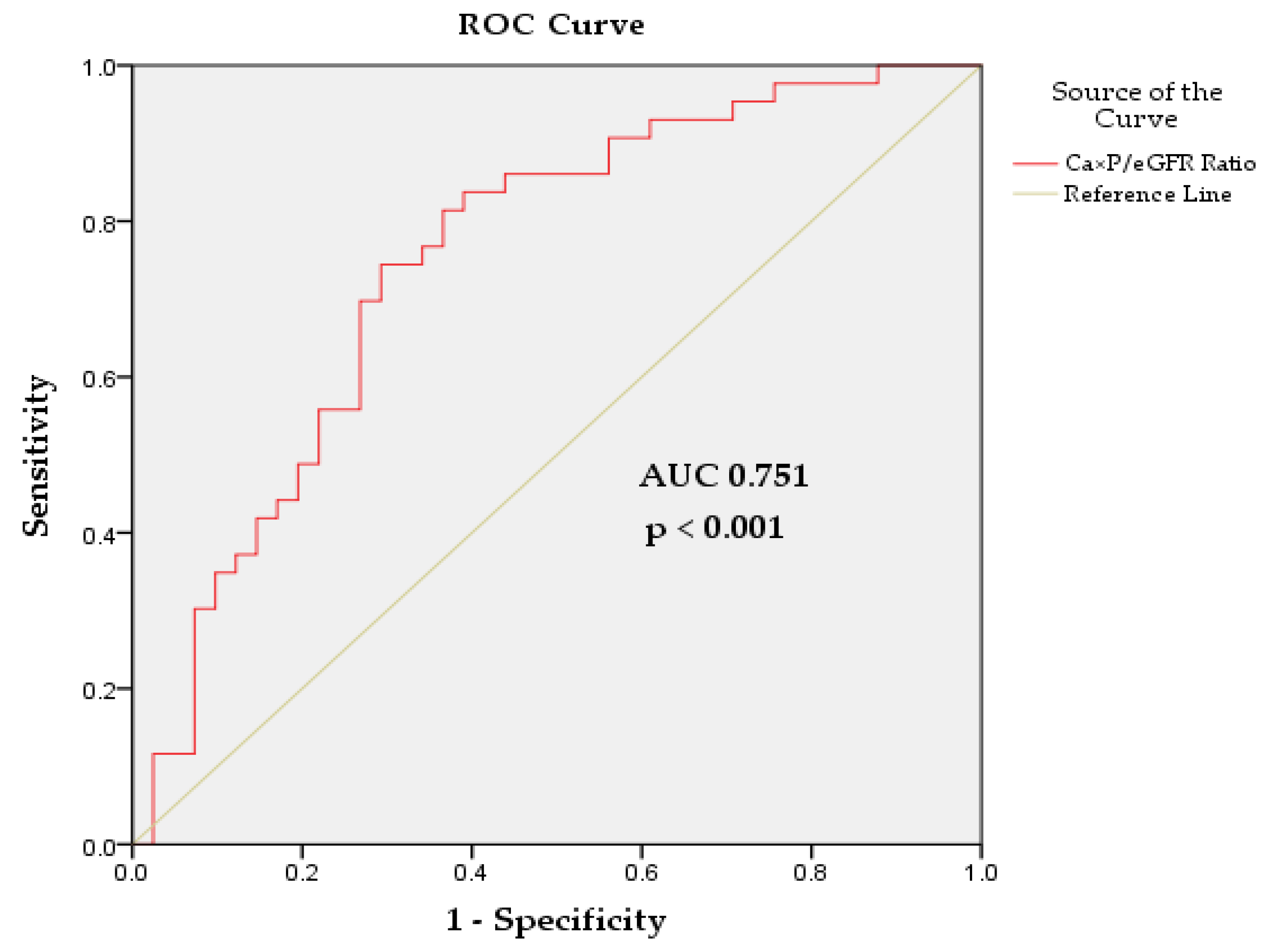
3.2. Ca×P/eGFR Ratio as a Predictor of CVD Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xie, Y., Bowe B., Mokdad, A.H., Xian, H., Yan, Y., Li, T., Maddukuri, G., Tsai, C.Y., Floyd, T., Al-Aly, Z. Analysis of the Global Burden of Disease Study Highlights the Global, Regional, and National Trends of Chronic Kidney Disease Epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567-581.
- Saeed, D., Reza, T., Shahzad, M.W., Karim Mandokhail, A., Bakht, D., Qizilbash, F.H., Silloca-Cabana, E.O., Ramadhan, A., Bokhari, S.F.H. Navigating the Crossroads: Understanding the Link Between Chronic Kidney Disease and Cardiovascular Health. Cureus 2023, 15, e51362.
- Jankowski, J., Floege, J., Fliser, D., Böhm, M., Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021,143, 1157-1172.
- Xu, C., Tsihlis, G., Chau, K., Trinh, K., Rogers, N.M., Julovi, S.M. Novel Perspectives in Chronic Kidney Disease-Specific Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 2658.
- Nakano, T. Atherosclerotic Diseases in Chronic Kidney Disease. J. Atheroscler. Thromb. 2024, RV22030.
- Ren, S.C., Mao, N., Yi, S., Ma, X., Zou, J.Q., Tang, X., Fan, J.M. Vascular Calcification in Chronic Kidney Disease: An Update and Perspective. Aging Dis. 2022, 13, 673-697.
- Ureña-Torres, P., D'Marco, L., Raggi, P., García-Moll, X., Brandenburg, V., Mazzaferro, S., Lieber, A., Guirado, L., Bover, J. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol. Dial. Transplant. 2020, 35, 2046-2053.
- Romero-González, G., González, A., López, B., Ravassa, S., Díez, J. Heart failure in chronic kidney disease: the emerging role of myocardial fibrosis. Nephrol. Dial. Transplant. 2022, 37, 817-824.
- Minciunescu, A., Genovese, L., deFilippi, C. Cardiovascular Alterations and Structural Changes in the Setting of Chronic Kidney Disease: A Review of Cardiorenal Syndrome Type 4. SN Compr. Clin. Med. 2022, 5, 15.
- Rangaswami, J., Bhalla, V., Blair, J.E.A., Chang, T.I., Costa, S., Lentine, K.L., Lerma, E.V., Mezue, K., Molitch, M., Mullens, W., Ronco, C., Tang, W.H.W., McCullough, P.A. American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation. 2019, 139, e840-e878.
- Pinheiro da Silva, A.L., Vaz da Silva, M.J. Type 4 Cardiorenal Syndrome. Rev. Port. Cardiol. 2016, 35, 601-616.
- Ronco, C., Haapio, M., House, A.A., Anavekar, N., Bellomo, R. Cardiorenal Syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527-1539.
- Mitsas, A.C., Elzawawi, M., Mavrogeni, S., Boekels, M., Khan, A., Eldawy, M., Stamatakis, I., Kouris, D., Daboul, B., Gunkel, O., Bigalke, B., van Gisteren, L., Almaghrabi, S., Noutsias, M. Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens-From a Cardiologist's View. J. Clin. Med. 2022, 11, 7041.
- Abe, S., Yoshihisa, A., Oohara, H., Sugawara, Y., Sato, Y., Misaka, T., Sato, T., Oikawa, M., Kobayashi, A., Yamaki, T., Nakazato, K., Takeishi, Y. Calcium-Phosphorus Product Is Associated with Adverse Prognosis in Hospitalized Patients with Heart Failure and Chronic Kidney Disease. Int. Heart J. 2024, 65, 84-93.
- Stevens, L.A., Coresh, J., Greene, T., Levey, A.S. Assessing Kidney Function—Measured and Estimated Glomerular Filtration Rate. N. Engl. J. Med. 2006, 354, 2473-2483.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney. Int. Suppl. 2013, 3, 1-150.
- Levey, A.S., Coresh, J., Greene, T., Marsh, J., Stevens, L.A., Kusek, J.W., Van Lente, F. Chronic Kidney Disease Epidemiology Collaboration. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 2007, 53, 766-772.
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32-35.
- Hatamizadeh, P., Fonarow, G.C., Budoff, M.J., Darabian, S., Kovesdy, C.P., Kalantar-Zadeh, K. Cardiorenal Syndrome: Pathophysiology and Potential Targets for Clinical Management. Nat. Rev. Nephrol. 2013, 9, 99-111.
- Ceravolo, G., Macchia, T., Cuppari, C., Dipasquale, V., Gambadauro, A., Casto, C., Ceravolo, M.D., Cutrupi, M., Calabrò, M.P., Borgia, P., Piccolo, G., Mancuso, A., Albiero, R., Chimenz, R. Update on the Classification and Pathophysiological Mechanisms of Pediatric Cardiorenal Syndromes. Children 2021, 8, 528.
- Sárközy, M., Kovács, Z.Z.A., Kovács, M.G., Gáspár, R., Szűcs, G., Dux, L. Mechanisms and Modulation of Oxidative/Nitrative Stress in Type 4 Cardio-Renal Syndrome and Renal Sarcopenia. Front. Physiol. 2018, 9, 1648.
- Buliga-Finis, O.N., Ouatu, A., Badescu, M.C., Dima, N., Tanase, D.M., Richter, P., Rezus, C. Beyond the Cardiorenal Syndrome: Pathophysiological Approaches and Biomarkers for Renal and Cardiac Crosstalk. Diagnostics 2022, 12, 773.
- Granata, A., Clementi, A., Virzì, G.M., Brocca, A., de Cal, M., Scarfia, V.R., Zanoli, L., Ronco, C., Corrao, S., Malatino, L. Cardiorenal Syndrome Type 4: From Chronic Kidney Disease to Cardiovascular Impairment. Eur. J. Intern. Med. 2016, 30, 1-6.
- Fu, S., Zhao, S., Ye, P., Luo, L. Biomarkers in Cardiorenal Syndromes. Biomed. Res. Int. 2018, 9617363.
- Chung, E.Y.M., Trinh, K., Li, J., Hahn, S.H., Endre, Z.H., Rogers, N.M., Alexander, S.I. Biomarkers in Cardiorenal Syndrome and Potential Insights into Novel Therapeutics. Front. Cardiovasc. Med. 2022, 9, 868658.
- Mok, Y., Wang, F., Ballew, S.H., Menez, S., Butler, K.R., Wagenknecht, L., Sedaghat, S., Lutsey, P.L., Coresh, J., Blaha, M.J., Matsushita, K. Kidney Function, Bone-Mineral Metabolism Markers, and Calcification of Coronary Arteries, Aorta, and Cardiac Valves in Older Adults. Atherosclerosis 2023, 368, 35-43.
- Kaur, R., Singh, R. Mechanistic Insights into CKD-MBD-Related Vascular Calcification and Its Clinical Implications. Life Sci. 2022, 311, 121148.
- Foley, R.N. Phosphate Levels and Cardiovascular Disease in the General Population. Clin. J. Am. Soc. Nephrol. 2009, 4, 1136-1139.
- Giachelli, C.M. The Emerging Role of Phosphate in Vascular Calcification. Kidney Int. 2009, 75, 890-897.
- Block, G., Port, F.K. Calcium Phosphate Metabolism and Cardiovascular Disease in Patients with Chronic Kidney Disease. Semin. Dial. 2003, 16, 140-147.
- Ogata, H., Sugawara, H., Yamamoto, M., Ito, H. Phosphate and Coronary Artery Disease in Patients with Chronic Kidney Disease. J. Atheroscler. Thromb. 2024, 31, 1-14.
- Lee, S.J., Lee, I.K., Jeon, J.H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685.
- Villa-Bellosta, R. Vascular Calcification: Key Roles of Phosphate and Pyrophosphate. Int. J. Mol. Sci. 2021, 22, 13536.
- Hutcheson, J.D., Goettsch, C. Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease. Circ. Res. 2023, 132, 993-1012.
- Siracusa, C., Carabetta, N., Morano, M. B., Manica, M., Strangio, A., Sabatino, J., Leo, I., Castagna, A., Cianflone, E., Torella, D., Andreucci, M., Zicarelli, M. T., Musolino, M., Bolignano, D., Coppolino, G., De Rosa, S. Understanding Vascular Calcification in Chronic Kidney Disease: Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 13096.
- Kim, J.S., Hwang, H.S. Vascular Calcification in Chronic Kidney Disease: Distinct Features of Pathogenesis and Clinical Implication. Korean Circ. J. 2021, 51, 961-982.
- Dube, P., DeRiso, A., Patel, M., Battepati, D., Khatib-Shahidi, B., Sharma, H., Gupta, R., Malhotra, D., Dworkin, L., Haller, S., Kennedy, D. Vascular Calcification in Chronic Kidney Disease: Diversity in the Vessel Wall. Biomedicines 2021, 9, 404.
- Toussaint, N.D., Kerr, P.G. Vascular Calcification and Arterial Stiffness in Chronic Kidney Disease: Implications and Management. Nephrology 2007, 12, 500-509.
- Cozzolino, M., Dusso, A.S., Slatopolsky, E. Role of Calcium-Phosphate Product and Bone-Associated Proteins on Vascular Calcification in Renal Failure. J. Am. Soc. Nephrol. 2001, 12, 2511-2516.
- Block, G.A., Hulbert-Shearon, T.E., Levin, N.W., Port, F.K. Association of Serum Phosphorus and Calcium X Phosphate Product with Mortality Risk in Chronic Hemodialysis Patients: A National Study. Am. J. Kidney Dis. 1998, 31, 607-617.
- Young, E.W., Albert, J.M., Satayathum, S., Goodkin, D.A., Pisoni, R.L., Akiba, T., Akizawa, T., Kurokawa, K., Bommer, J., Piera, L., Port, F.K. Predictors and Consequences of Altered Mineral Metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005, 67, 1179-1187.
- Thongprayoon, C., Cheungpasitporn, W., Mao, M.A., Erickson, S.B. Calcium-Phosphate Product and Its Impact on Mortality in Hospitalized Patients. Nephrology 2020, 25, 22-28.
- Cubbon, R.M., Thomas, C.H., Drozd, M., Gierula, J., Jamil, H.A., Byrom, R., Barth, J.H., Kearney, M.T., Witte, K.K. Calcium, Phosphate and Calcium Phosphate Product Are Markers of Outcome in Patients with Chronic Heart Failure. J. Nephrol. 2015, 28, 209-215.
- Menon, V., Greene, T., Pereira, A.A., Wang, X., Beck, G.J., Kusek, J.W., Collins, A.J., Levey, A.S., Sarnak, M.J. Relationship of Phosphorus and Calcium-Phosphorus Product with Mortality in CKD. Am. J. Kidney Dis. 2005, 46, 455-463.
- Cheungpasitporn, W., Thongprayoon, C., Hansrivijit, P., Medaura, J., Chewcharat, A., Bathini, T., Mao, M.A., Erickson, S.B. Impact of Admission Calcium-Phosphate Product on 1-Year Mortality among Hospitalized Patients. Adv. Biomed. Res. 2020, 9, 14.
- Matsushita, K., Ballew, S.H., Wang, A.Y., Kalyesubula, R., Schaeffner, E., Agarwal, R. Epidemiology and Risk of Cardiovascular Disease in Populations with Chronic Kidney Disease. Nat. Rev. Nephrol. 2022, 18, 696-707.
- Cabrera, C.S., Lee, A.S., Olsson, M., Schnecke, V., Westman, K., Lind, M., Greasley, P.J., Skrtic, S. Impact of CKD Progression on Cardiovascular Disease Risk in a Contemporary UK Cohort of Individuals with Diabetes. Kidney Int. Rep. 2020, 5, 1651-1660.
- Matsushita, K., Coresh, J., Sang, Y., Chalmers, J., Fox, C., Guallar, E., Jafar, T., Jassal, S.K., Landman, G.W., Muntner, P., Roderick, P., Sairenchi, T., Schöttker, B., Shankar, A., Shlipak, M., Tonelli, M., Townend, J., van Zuilen, A., Yamagishi, K., Yamashita, K., Gansevoort, R., Sarnak, M., Warnock, D.G., Woodward, M., Ärnlöv, J., CKD Prognosis Consortium. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514-525.
- van der Velde, M., Matsushita, K., Coresh, J., Astor, B.C., Woodward, M., Levey, A., de Jong, P., Gansevoort, R.T., Chronic Kidney Disease Prognosis Consortium, van der Velde, M., Matsushita, K., Coresh, J., Astor, B.C., Woodward, M., Levey, A.S., de Jong, P.E., Gansevoort, R.T., Levey, A., El-Nahas, M., Eckardt, K.U., Kasiske, B.L., Ninomiya, T., Chalmers, J., Macmahon, S., Tonelli, M., Hemmelgarn, B., Sacks, F., Curhan, G., Collins, A.J., Li, S., Chen, S.C., Hawaii Cohort, K.P., Lee, B.J., Ishani, A., Neaton, J., Svendsen, K., Mann, J.F., Yusuf, S., Teo, K.K., Gao, P., Nelson, R.G., Knowler, W.C., Bilo, H.J., Joosten, H., Kleefstra, N., Groenier, K.H., Auguste, P., Veldhuis, K., Wang, Y., Camarata, L., Thomas, B., Manley, T. Lower Estimated Glomerular Filtration Rate and Higher Albuminuria Are Associated with All-Cause and Cardiovascular Mortality. A Collaborative Meta-Analysis of High-Risk Population Cohorts. Kidney Int. 2011, 79, 1341-1352.
- Bello, A.K., Hemmelgarn, B., Lloyd, A., James, M.T., Manns, B.J., Klarenbach, S., Tonelli, M., Alberta Kidney Disease Network. Associations among Estimated Glomerular Filtration Rate, Proteinuria, and Adverse Cardiovascular Outcomes. Clin. J. Am. Soc Nephrol. 2011, 6, 1418-1426.
- Fox, C.S., Matsushita, K., Woodward, M., Bilo, H.J., Chalmers, J., Heerspink, H.J., Lee, B.J., Perkins, R.M., Rossing, P., Sairenchi, T., Tonelli, M., Vassalotti, J.A., Yamagishi, K., Coresh, J., de Jong, P.E., Wen, C.P., Nelson, R.G., Chronic Kidney Disease Prognosis Consortium. Associations of Kidney Disease Measures with Mortality and End-Stage Renal Disease in Individuals with and without Diabetes: A Meta-Analysis. Lancet 2012, 380, 1662-1673.
- Kottgen, A., Russell, S.D., Loehr, L.R., Crainiceanu, C.M., Rosamond, W.D., Chang, P.P., Chambless, L.E., Coresh, J. Reduced Kidney Function as a Risk Factor for Incident Heart Failure: The Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Soc. Nephrol. 2007, 18, 1307-1315.
- Ku, E., Lee, B.J., Wei, J., Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 120-131.
- Nagata, D., Hishida, E., Masuda, T. Practical Strategy for Treating Chronic Kidney Disease (CKD)-Associated with Hypertension. Int. J. Nephrol. Renovasc. Dis. 2020, 13, 171-178.
- Burnier, M., Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050-1063.
| Variables | All Patients (n=84) |
Non-CVD Group (n=41) |
CVD Group (n=43) | p-Value (ANOVA) |
|---|---|---|---|---|
| Age, years 1 | 64.71±12.95 | 64.70±13.82 | 64.72±12.22 | 0.996 |
| Male/Female, n/n | 32/52 | 11/30 | 21/22 | - |
| SCr, µmol/L 1 | 114.65±52.92 | 93.73±52.03 | 134.60±46.06 | <0.001 |
| BUN, mmol/L 1 | 8.27±3.78 | 6.79±2.79 | 9.69±4.08 | <0.001 |
| UA, µmol/L 1 | 313.22±97.23 | 283.43±87.15 | 332.25±99.68 | 0.059 |
| eGFR, mL/min/1.73 m2 1 | 61.60±27.79 | 74.09±27.42 | 49.69±22.63 | <0.001 |
| CKD 1, n (%) 2 | 15 (18) | 12 (29) | 3 (7) | - |
| CKD 2, n (%) 2 | 25 (30) | 16 (39) | 9 (21) | - |
| CKD 3, n (%) 2 | 32 (38) | 10 (25) | 22 (51) | - |
| CKD 4, n (%) 2 | 12 (14) | 3 (7) | 9 (21) | - |
| HTN, n (%) | 43 (51) | - | 43 (100) | - |
| CAD, n (%) | 19 (23) | - | 19 (44) | - |
| HF, n (%) | 13 (15) | - | 13 (30) | - |
| CVC, n (%) | 18 (21) | - | 18 (42) | - |
| PAD, n (%) | 8 (10) | - | 8 (19) | - |
| Arrhythmias, n (%) | 4 (5) | - | 4 (9) | - |
| Ca, mmol/L 1 | 2.49±0.21 | 2.51±0.29 | 2.48±0.10 | 0.444 |
| Pi, mmol/L 1 | 1.16±0.17 | 1.13±0.15 | 1.18±0.18 | 0.202 |
| Ca×P, mmol 2 /L2 1 | 2.90±0.51 | 2.86±0.53 | 2.94±0.50 | 0.480 |
| PTH, mmol/L 1 | 84.39±50.39 | 89.07±67.41 | 82.13±41.02 | 0.677 |
| ALP, mmol/L 1 | 72.44±19.54 | 70.53±18.98 | 74.25±20.11 | 0.387 |
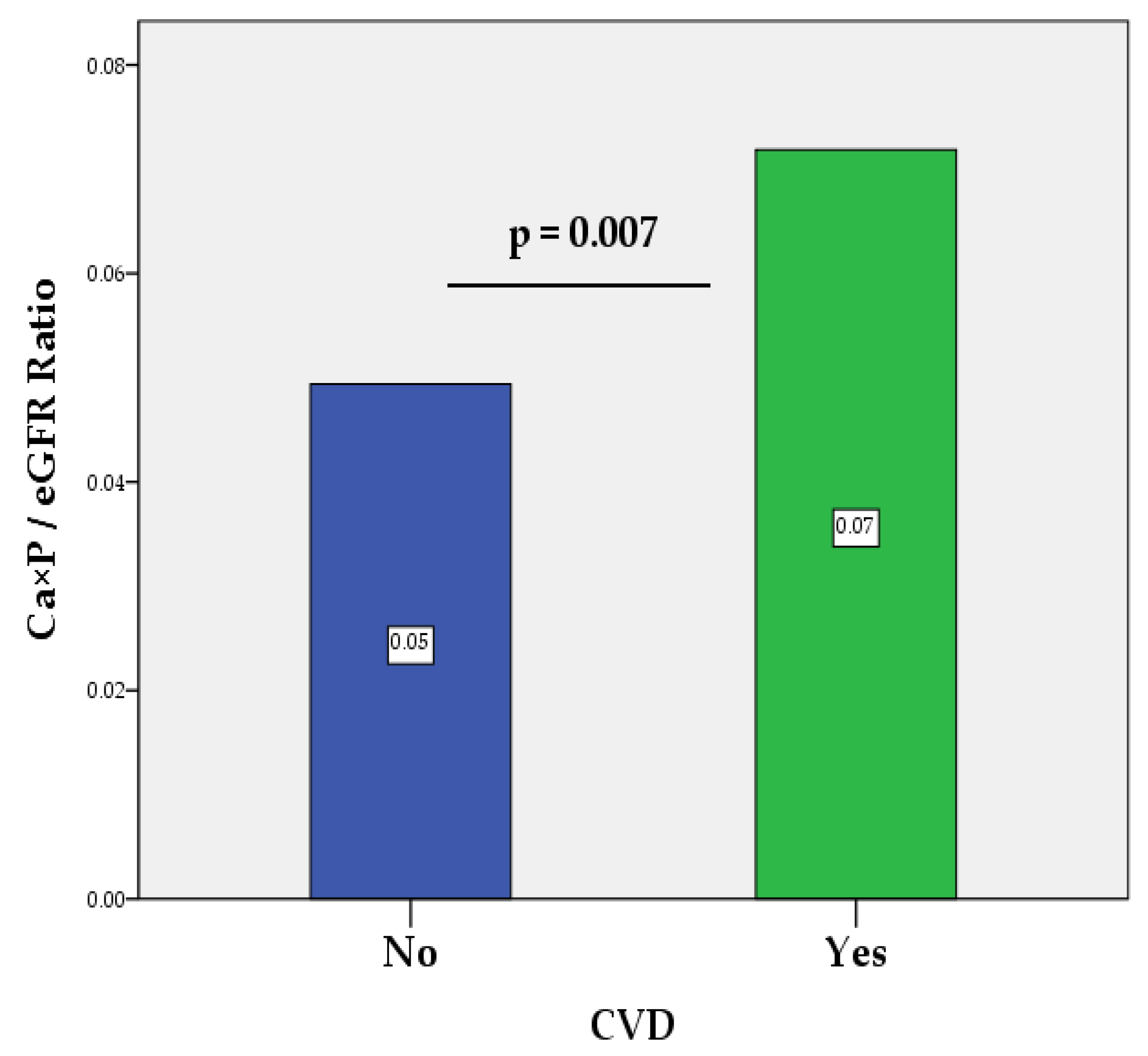
| Ca×P/eGFR Ratio, (mmol/L)²/(mL/min/1.73 m²) 1 | 0.06±0.04 | 0.05±0.04 | 0.07±0.04 | 0.007 |
| Variables | Univariate Analysis * (Single Predictors) |
Multivariate Analysis ** (Predictors in Model) |
||||
|---|---|---|---|---|---|---|
| Coefficient (B) | OR (95% CI) | p-Value | Coefficient (B) | OR (95% CI) | p-Value | |
| BUN, mmol/L | 0.275 | 1.317 (1.109-1.563) | 0.002 | - | - | - |
| SCr, µmol/L | 0.018 | 1.018 (1.007-1.029) | 0.001 | - | - | - |
| eGFR, mL/min/1.73 m2 | -0.038 | 0.963 (0.944-0.982) | <0.001 | -0.038 | 0.963 (0.944-0.982) | <0.001 |
| Ca×P/eGFR Ratio, (mmol/L)²/(mL/min/1.73 m²) | 0.187 | 1.206 (1.042-1.395) | 0.012 | - | - | - |
| Correlations | Pearson Correlation Coefficient (r) |
R-Squared (R²) | p-Value |
|---|---|---|---|
| Ca×P/eGFR and SCr | 0.841** | 0.707 | <0.001 |
| Ca×P/eGFR and eGFR | -0.796** | 0.633 | <0.001 |
| Ca×P/eGFR and BUN | 0.723** | 0.523 | <0.001 |
| Ca×P/eGFR and UA | 0.264* | 0.070 | 0.043 |
| Ca×P/eGFR and Ca | 0.491** | 0.241 | <0.001 |
| Ca×P/eGFR and Pi | 0.458** | 0.210 | <0.001 |
| Ca×P/eGFR and PTH | 0.366* | 0.134 | 0.016 |
| Ca×P/eGFR and ALP | 0.399** | 0.159 | <0.001 |
| Risk Classification |
Ca×P/eGFR Ratio * | Ca×P/eGFR Ratio (%) * | Risk Description |
| Very Low Risk | < 0.03 | < 3 | Very low likelihood of CV complications |
| Low Risk | 0.03 - 0.5 | 3 - 5 | Low likelihood of CV complications |
| Moderate Risk | 0.05 - 0.10 | 5 - 10 | Moderate likelihood of CV complications |
| High Risk | 0.10 - 0.15 | 10 - 15 | Increased likelihood of CV complications |
| Very High Risk | 0.15 - 0.20 | 15 - 20 | Significant likelihood of serious CV events |
| Extremely High Risk | > 0.20 | > 20 | Critical likelihood of severe CV events |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





