Submitted:
17 December 2024
Posted:
18 December 2024
You are already at the latest version
Abstract

Keywords:
Introduction
Methods
Study Type
Techniques and Instruments
Literature Search
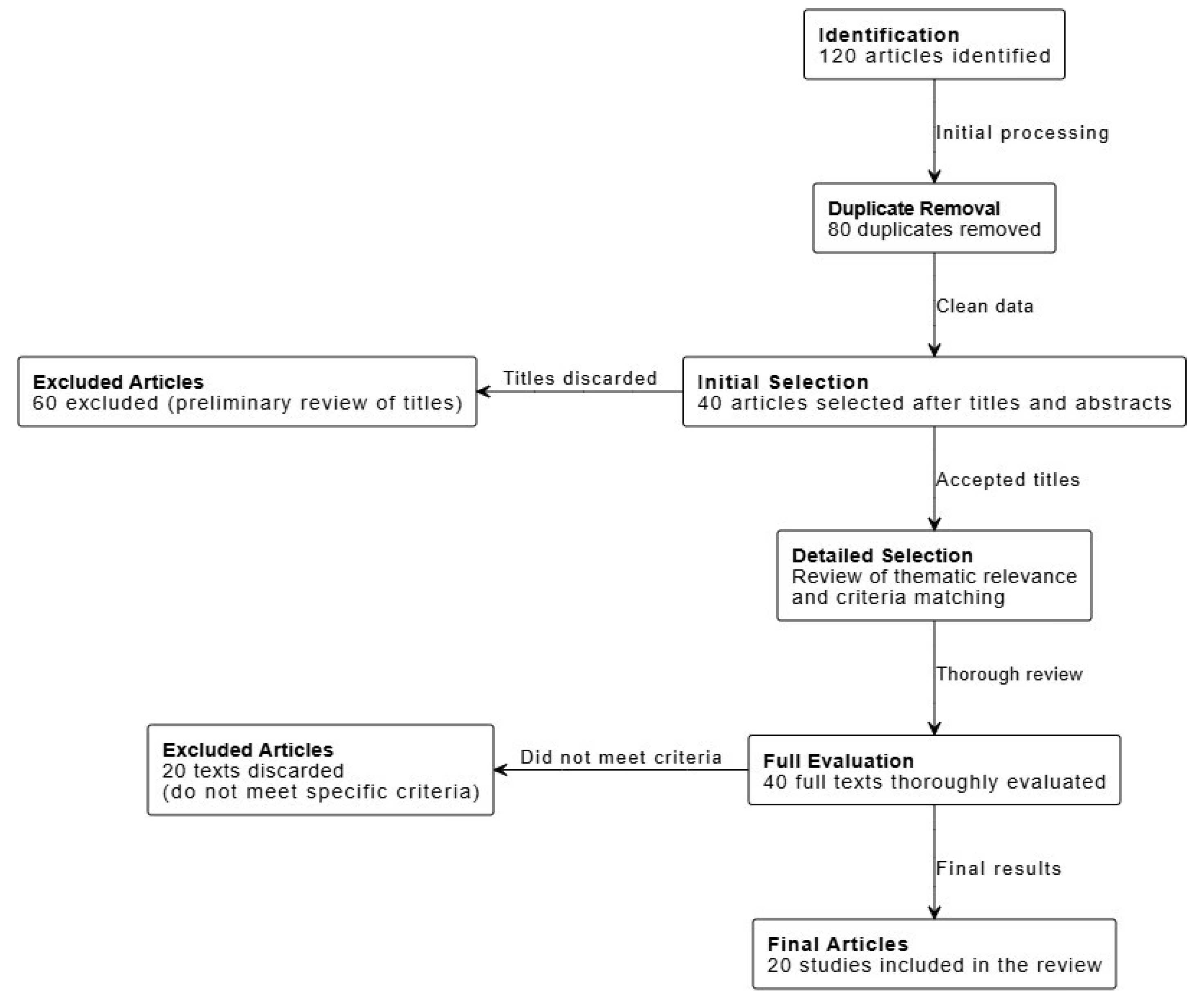
Study Analysis
Results
| No. | Author | Year | Sample | Context | Metrics | Algorithm | Results |
|---|---|---|---|---|---|---|---|
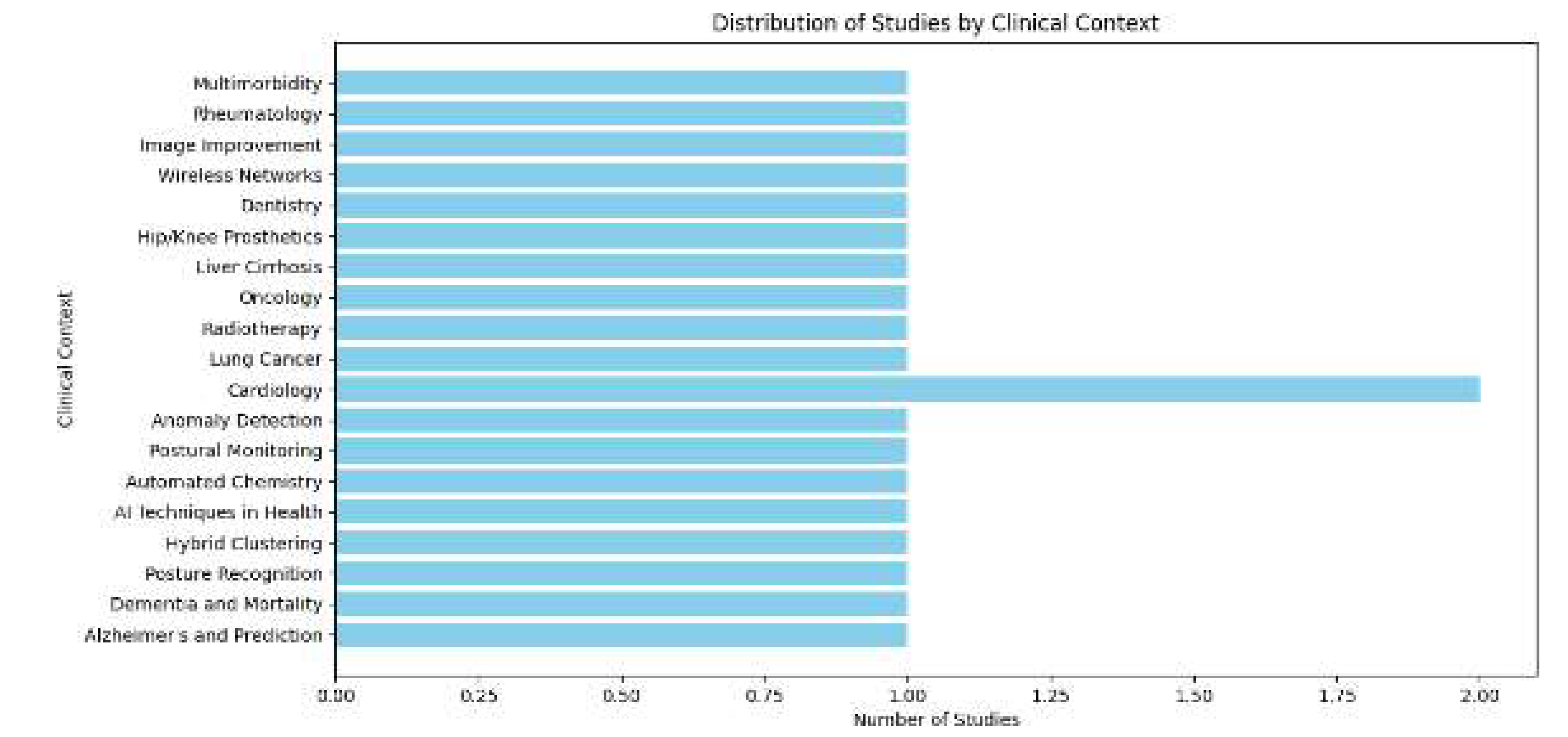
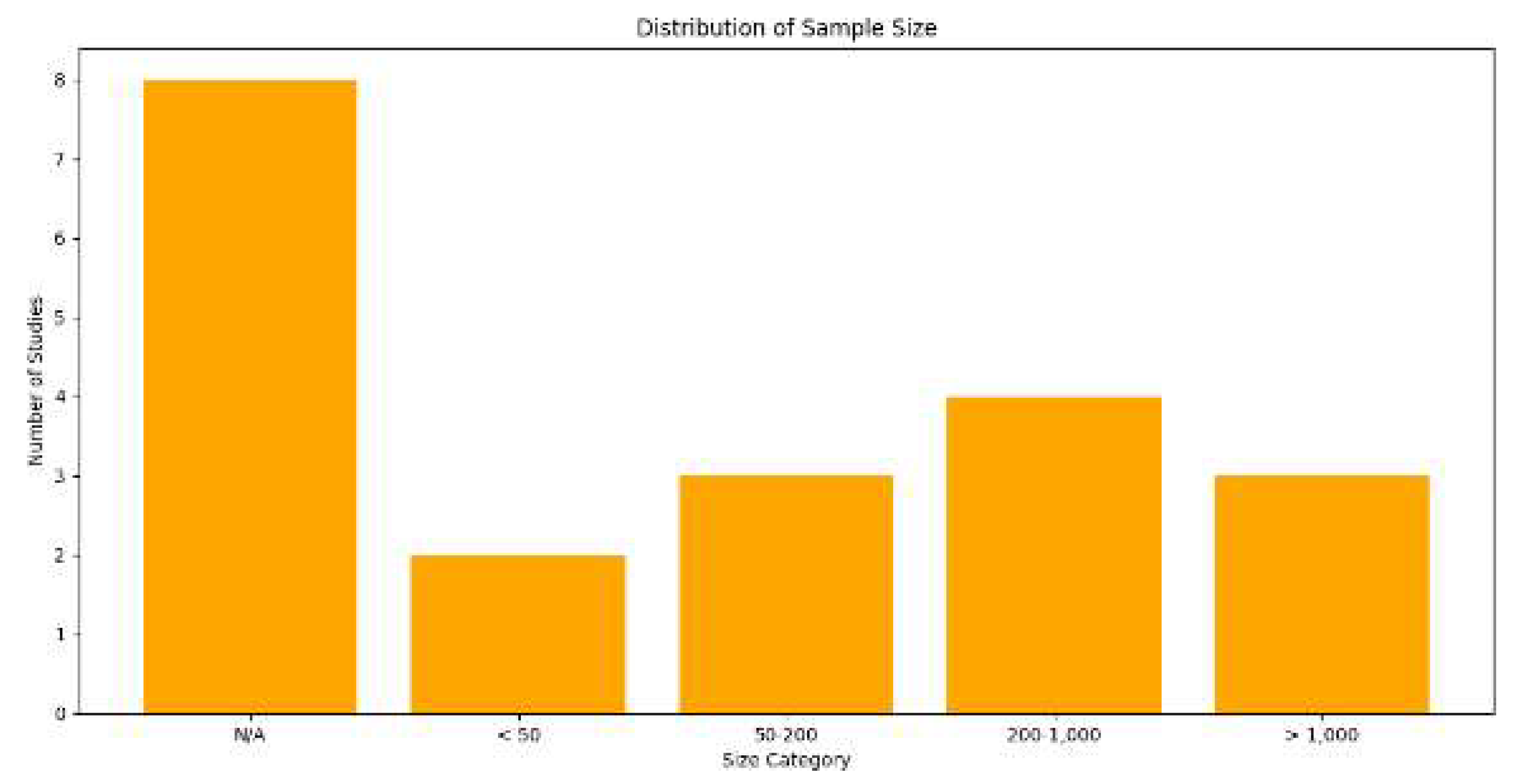
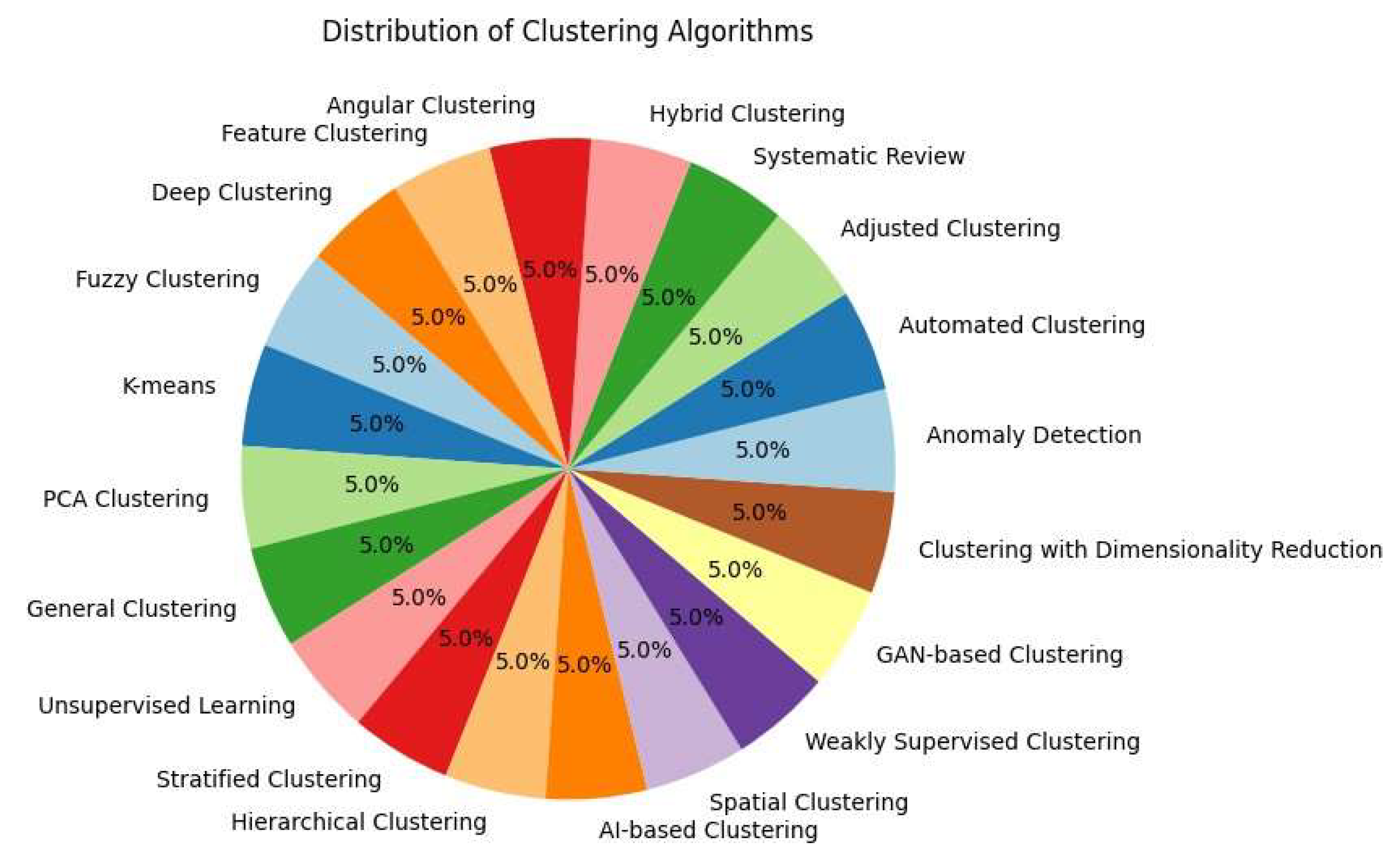
| 1 | Marina Lleal et al. | 2022 | 740 patients | Multimorbidity | Medication Quality | Fuzzy Clustering | 4 clusters linked to PIP and PIM |
| 2 | Yiming Shi et al. | 2024 | N/A | Rheumatology | Prediction Accuracy | K-means | Improved diagnostic accuracy with K-means and ML |
| 3 | Habte Tadesse Likassa et al. | 2021 | N/A | Image Enhancement | Image Quality Improvement | PCA Clustering | Improved images with PCA |
| 4 | Sakib Iqram Hamim et al. | 2023 | N/A | Wireless Networks | Clustering Efficiency | General Clustering | Energy optimization using hybrid clustering |
| 5 | Min Joo Kim et al. | 2023 | 200 images | Dentistry | Unsupervised Diagnosis | Unsupervised Learning | Periodontal diagnosis with unsupervised clustering |
| 6 | Irene Salvi et al. | 2024 | 1,000 patients | Hip/Knee Prosthesis | Results Interpretation | Stratified Clustering | Stratification with EQ-5D-3L |
| 7 | Sara Palomino-Echeverria et al. | 2024 | 50 patients | Liver Cirrhosis | Clinical Subgroup Identification | Hierarchical Clustering | Identification of subgroups in cirrhosis |
| 8 | Ryuji Hamamoto et al. | 2022 | N/A | Oncology | Clinical Decision Support | AI-based Clustering | Personalized oncology with AI and clustering |
| 9 | Andrew Wentzel et al. | 2024 | 200 patients | Radiotherapy | Long-term Risk Prediction | Spatial Clustering | 3D stratification in radiotherapy |
| 10 | Xueting Ren et al. | 2021 | N/A | Lung Cancer | Subtype Classification | Weakly Supervised Clustering | Lung cancer classification with weak clustering |
| 11 | Ying Fu et al. | 2024 | 1,000 images | Cardiology | Data Augmentation | GAN-based Clustering | Data augmentation with GANs |
| 12 | Michael Selle et al. | 2023 | N/A | Anomaly Detection | Outlier Detection | Dimensionality Reduction Clustering | Dimensionality reduction and outlier detection in CT |
| 13 | Patrick Vermander et al. | 2021 | 50 individuals | Postural Monitoring | Anomaly Prevention | Anomaly Detection | Postural anomaly detection with clustering |
| 14 | Jos´e T. Moreira Filho et al. | 2024 | 200 chemicals | Automated Chemistry | Automated Chemical Analysis | Automated Clustering | Chemical grouping with KNIME |
| 15 | Victorine P. Muse et al. | 2022 | 1,000 patients | Cardiology | Reference Intervals | Adjusted Clustering | Seasonal clustering in cardiovascular diseases |
| 16 | Lucas Greif et al. | 2024 | N/A | AI Techniques in Healthcare | Systematic Review of AI | Systematic Review | Clustering review in AI applied to SDGs |
| 17 | Daisy Nkele Molokomme et al. | 2023 | N/A | Hybrid Clustering | Clustering Optimization | Hybrid Clustering | Optimization with hybrid clustering |
| 18 | Debanjan Borthakur et al. | 2021 | 100 images | Posture Recognition | Pose Recognition Accuracy | Angular Clustering | Pose estimation with angular clustering |
| 19 | Jimmy Zhang et al. | 2024 | 1,500 patients | Dementia and Mortality | Mortality Prediction | Feature Clustering | Predictive clustering for mortality in dementia |
| 20 | Yu Chen et al. | 2024 | N/A | Alzheimer’s Prediction | Polygenic Analysis | Deep Clustering | Risk analysis in Alzheimer’s using deep learning |
Discussion
References
- Lleal, M. , Bar´e, M., Ortonobes, S., et al. Comprehensive Multimorbidity Patterns in Older Patients Are Associated with Quality Indicators of Medication—MoPIM Cohort Study.
- Shi, Y. , Zhou, M., Chang, C., et al. Ad- vancing precision rheumatology: applica- tions of machine learning for rheumatoid arthritis management.
- Hamamoto, R. , Koyama, T., Kouno, N., et al. Introducing AI to the molecular tu- mor board: one direction toward the es- tablishment of precision medicine using large-scale cancer clinical and biological information.
- Fu, Y. , Gong, M., Yang, G., et al. Evolu- tionary GAN–Based Data Augmentation for Cardiac Magnetic Resonance Image.
- Kim, M. J. , Chae, S. G., Bae, S. J., et al. Unsupervised few shot learning architec- ture for diagnosis of periodontal disease in dental panoramic radiographs.
- Ren, X. , Jia, L., Zhao, Z., et al. Weakly su- pervised label propagation algorithm clas- sifies lung cancer imaging subtypes.
- Wentzel, A. , Mohamed, A. S. R., Naser, M. A., et al. Multi-organ spatial stratifi- cation of 3-D dose distributions improves risk prediction of long-term self-reported severe symptoms in oropharyngeal cancer patients receiving radiotherapy.
- Palomino-Echeverria, S. , Huergo, E., Ortega-Legarreta, A., et al. A robust clus- tering strategy for stratification unveils unique patient subgroups in acutely de- compensated cirrhosis.
- Selle, M. , Kircher, M., Schwennen, C., et al. Dimension reduction and outlier de- tection of 3-D shapes derived from multi- organ CT images.
- Molokomme, D. N. , Onumanyi, A. J., Abu-Mahfouz, A. M. Hybrid metaheuris- tic schemes with different configurations and feedback mechanisms for optimal clus- tering applications.
- Salvi, I. , Ehlig, D., Vogel, J., et al. How to interpret patient-reported out- comes? Stratified adjusted minimal im- portant changes for the EQ-5D-3L in hip and knee replacement patients.
- Vermander, P. , Mancisidor, A., Cabanes, I., et al. Intelligent systems for sitting pos- ture monitoring and anomaly detection: an overview.
- Moreira Filho, J. T. , Ranganath, D., Con- way, M., et al. Democratizing cheminfor- matics: interpretable chemical grouping using an automated KNIME workflow.
- Muse, V. P. , Placido, D., Haue, A. D., et al. Seasonally adjusted laboratory reference intervals to improve the performance of machine learning models for classification of cardiovascular diseases.
- Greif, L. , R¨ockel, F., Kimmig, A., Ovtcharova, J. A systematic review of cur- rent AI techniques used in the context of the SDGs.
- Likassa, H. T. , Chen, D.-G., Wang, Y., Zhu, W., et al. Robust PCA with Lw and L2 Norms: A Novel Method for Low- Quality Retinal Image Enhancement.
- Hamim, S. I. , Ab Rahman, A. B. Optimiz- ing Wireless Sensor Networks: A Survey of Clustering Strategies and Algorithms.
- Borthakur, D. , Paul, A., Kapil, D., et al. Yoga Pose Estimation Using Angle-Based Feature Extraction.
- Zhang, J. , Song, L., Miller, Z., et al. Ma- chine learning models identify predictive features of patient mortality across demen- tia types.
- Chen, Y. , Ip, F. C. F., Jiang, Y., et al. Deep learning-based polygenic risk analy- sis for Alzheimer’s disease prediction.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




