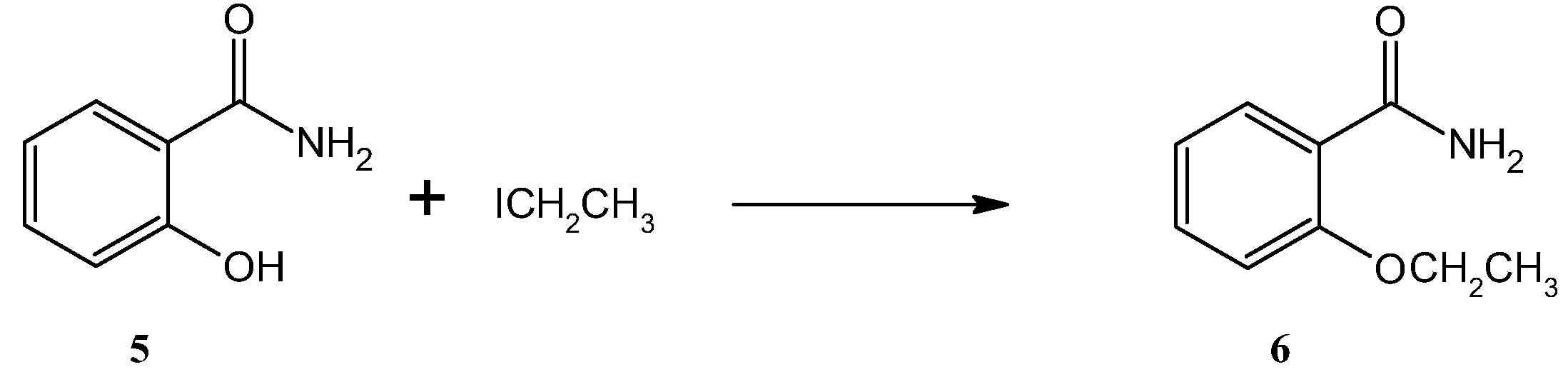1. Introduction
Salicylic acid (
1) derivatives are one of the oldest active substances used in pharmacy. Initially isolated mainly from willow bark (
Salix alba). The first records of the therapeutic analgesic properties of willow bark extracts were described by Hippocrates in the 5th century BC, but it is known that the anti-inflammatory properties of this plant were also known in ancient Egypt and China [
1,
2,
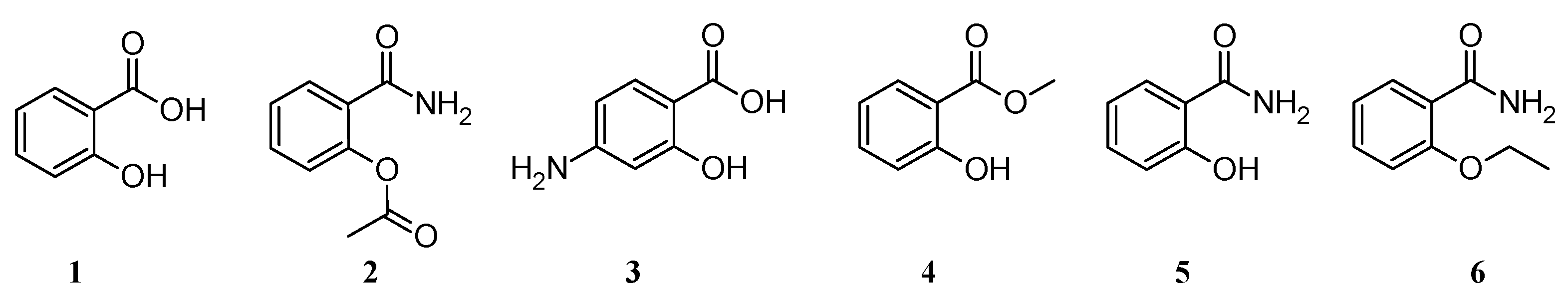
3] . Currently, both salicylic acid and its derivatives are obtained mainly chemically, although herbal medicines based on salicylates are still very popular. Salicylic acid was first obtained in the Kolbe reaction from phenol and carbon dioxide, and then there was a development in the synthesis of other derivatives of this acid such as acetylsalicylic acid (
2),
p-aminosalicylic acid (
3), methyl salicylate (
4), salicylamide (
5) or ethenzamide (
6) (
Figure 1.).
Most derivatives of salicylic acid possess analgesic, anti-inflammatory and antipyretic effects and are classified as non-steroidal anti-inflammatory drugs (NSAIDs). This group includes salicylamide (
5), which, even though this compound is not as effective as the popular acetylsalicylic acid (
2), is still used as an analgesic, antipyretic, antirheumatic and as a fungicide [
4]. Interestingly,
O-derivatives of salicylic acid are a common building block of bioactive compounds tested for their potential use as new anticancer drugs [
5,
6,
7,
8,
9] antihistamines [
10], and also being explored for the treatment of schizophrenia and depression [
11,
12,
13,
14,
15], tuberculosis [
16] and cardiovascular diseases [
17,
18,
19].
Ethenzamide (
6) is one of the pharmaceutically interesting derivatives of salicylamide, which has analgesic, anti-inflammatory effects and reduces skeletal muscle tension [
20,
21,
22,
23,
24,
25]. Most often, in combination with paracetamol or acetylsalicylic acid and caffeine, it is recommended for colds, headaches, toothaches, menstrual cramps or fever. Ethenzamide, like other
O-alkyl derivatives of salicylamide, is most often obtained by the Williamson synthesis in the presence of sodium, sodium or potassium hydroxide or potassium carbonate in solvents such as acetone, ethanol, dimethylformamide, and the process usually takes at least several hours [
26]. Considering that ethenzamide is a widely used active pharmaceutical ingredient (API) worldwide, we decided to develop a new ecological-friendly and efficient method for the synthesis of this compound.
2. Materials and Methods
Reagents from Sigma Aldrich and TCI CHEMICALS, organic solvents from POCH. The reaction progress was analyzed by thin-layer chromatography (TLC) on silica gel 60 F254 plates on aluminum sheets (Merck, Darmstadt, Germany). UPLC was performed on a Waters Acquity system - Waters TQD (ESI-tandem quadrupole), PDA detector, Acquity UPLC BEH C18, 1.7, 2.1 × 100 mm column (Waters Corporation, Milford, MA, USA). Mobile phase: methanol:water + formic acid (4:6 + 0.1%, v/v). Melting points were determined on a Boetius apparatus and are uncorrected. FTS-165 spectrometer (FTIR Biorad) was used for IR spectra. 1H NMR and 13C NMR spectra were recorded on Bruker Avance 400 MHz and Varian Unity Plus 300 MHz spectrometers using CDCl3 with TMS as internal standard. Chemical shifts are expressed in δ (ppm), and the coupling constants J in hertz (Hz).
Reactions in the presence of microwave radiation were carried out in glass reactors in a CEM Discovery device (50 W, 2 min, max. temperature 120 °C), preliminary tests were carried out using SAMSUNG Model M182DN device, with adjustable microwave output power of 100W-850W. Reactions in the presence of ultrasound were carried out in a round bottom flask cooled with an ice bath in a Vibra-Cell™ VCX 130 (A = 70%, 5-6 W, interval/break = 60 s/10 s, Temp. 40-45 °C). The mechanochemical reactions were carried out in glass reactors in a ball mill (zirconium oxide balls with a size of Ø20 mm, Planetary Ball Mill Pulverisette 7 premium line, Fritsch GmbH).
3. Results
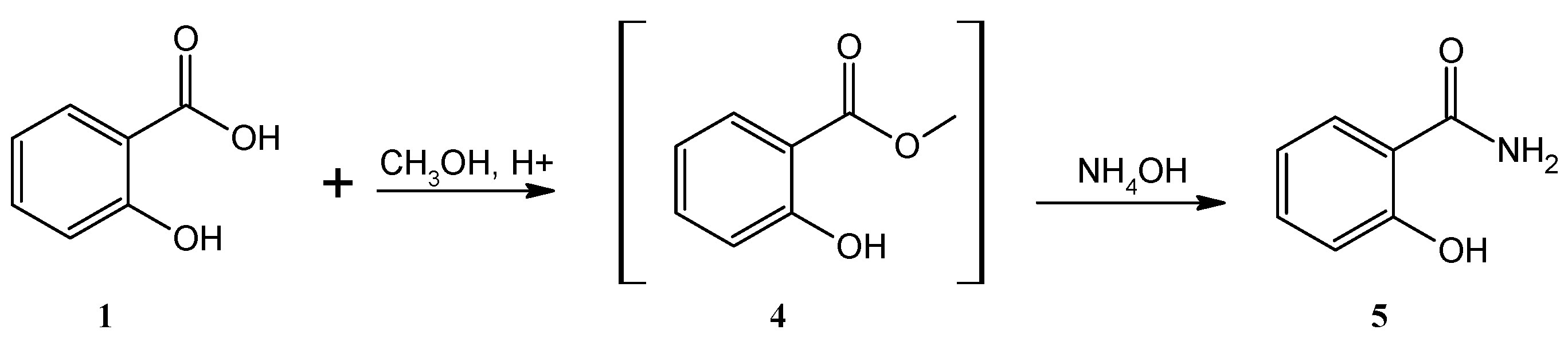
The key intermediate for the synthesis of ethenzamide, salicylamide (
5), was obtained through a two-step process. First, an acid-catalyzed esterification reaction of salicylic acid (
1) with methanol was performed to obtain methyl salicylaten (
4) without further isolation; then ammonium hydroxide (NH
4OH)-based aminolysis of (
4) was carried out in the second step to yield the desired salicylamide (
5), which was subsequently crystallized from water (Yield: 44%, m.p. 143-145°C) (
Scheme 1).
Given that
O-derivatives of salicylamide are commonly obtained through the Williamson reaction, we decided to use this reaction for the synthesis of ethenzamide as well. This synthesis consists of the reaction of an alkoxide or phenolate with a haloalkane, which takes place in an alkaline environment according to the SN
2 mechanism. It should be noted that primary haloalkanes should be used in Williamson reactions to avoid competitive elimination to the alkene. In the synthesis reactions of
O-derivatives of salicylamide described in the literature, organic solvents such as acetone, ethanol, dimethylformamide, 2-ethoxyethanol or acetonitrile are often used. The reaction is carried out in an alkaline conditions in the presence of potassium carbonate, sodium sodium/potassium hydroxide or, and the process usually lasts from a few to several hours [
9,
12,
13,
14,
26,
28].
Scheme 2.
Synthesis of ethenzamide (6) via the O-alkylation reaction of salicylamide (5).
Scheme 2.
Synthesis of ethenzamide (6) via the O-alkylation reaction of salicylamide (5).
3.1. Conventional Methods of Ethenzamide Synthesis
The research was started with the synthesis by classical methods of obtaining ethenzamide, i.e. in the presence of sodium hydroxide in ethanol. The reactions were carried out under reflux, with progress monitored by TLC (Thin Layer Chromatography) until salicylamide was no longer detected. The expected ethenzamide was obtained after 3 hours with a yield of 43%, following crystallization from methanol (
Table 1, no. 1). In the next stage of the study,
O-alkylation of salicylamide (
5) was carried out in the presence of K
2CO
3 and KI, in dimethylformamide (DMF) as a solvent, which allowed obtaining ethenzamide (
6) after 4 hours with a yield of 60% (
Table 1, no. 2).
Considering that from an ecological point of view it is necessary to "minimize the use of toxic organic solvents, we also decided to carry out the synthesis in solvent-free conditions. The lack of a solvent in the reaction environment brings many benefits, for example it reduces the risk of explosion, allows to eliminate toxic waste, often also has a positive effect on shortening the reaction time, improves the ease of isolating the product and has a positive effect on the product efficiency. By eliminating expensive and toxic solvents, the synthesis process is much cheaper and more ecological, which allows such reactions to be classified as "green chemistry" processes. This concept was first used by Paul Anastas in 1991, who defined it as: "Searching for, designing and implementing chemical products and processes that enable the reduction or elimination of the use and production of hazardous substances"[
29,
30,
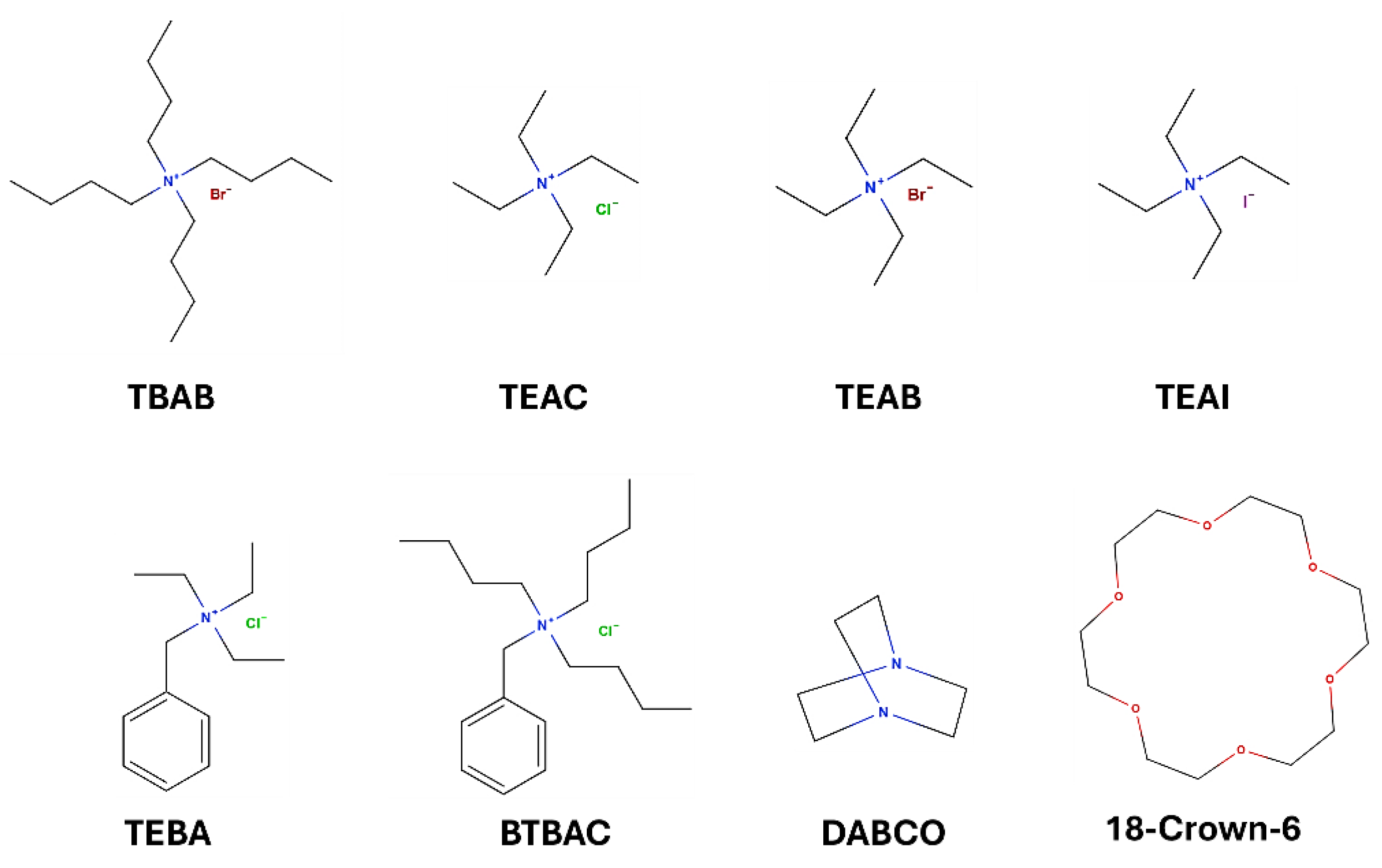
31]. We began our research by evaluating the effect of temperature on the process, conducting the reaction at temperatures ranging from 20 °C to 80 °C. In these reactions, TBAB (tetrabutylammonium bromide) was used as a catalyst, which is one of the most commonly used PTC (Phase Transfer Catalyst) in the synthesis of bioactive compounds due to its non-toxicity, easy availability and favorable low price. Due to the lack of reaction progress at lower temperatures, i.e. up to 60 °C, the reaction was stopped, despite the presence of salicylamide in the reaction mixture. The products isolated in these reactions were clearly contaminated with unreacted salicylamide (
Table 1, no. 3-5). In the next stage of the research, the effectiveness of the catalysts on the rate and efficiency of the process was evaluated. The following PTC catalysts were used in the studies: TEBA (triethylbenzylammonium chloride), TEAC (tetraethylammonium chloride), TEAI (tetraethylammonium iodide), TEAB (tetraethylammonium bromide) and other catalysts: DABCO (1,4-diazobicyclo [2.2.2]octane) or 18-Crown-6 (
Table 1, no. 7-12).The molecular structures of the PTCs were presented in
Scheme 3.
It is worth noting here that the reactions conducted in solvent-free conditions significantly exceed both the yield and the short duration of the process the reactions known so far in the literature. Another significant advantage of the reactions is that the obtained products are highly pure, UPLC-MS (Ultra Performance Liquid Chromatography - Mass Spectrometer) evaluation has proven that in the case of these reactions, ethenzamide (6) is obtained with a purity of 98-99% before crystallization and 100% after crystallization from methanol. Higher reaction temperatures (>60°C) and the addition of PTCs (TBAB, TEBA, and TEAB) normally resulted in higher yields. Notably, under solvent-free conditions, the optimized reaction result with the shortest reaction time (15 min, 79% yield) was found with the TBAB at 80°C. Moreover, the different catalysts used, the yields of ethenazamide (6) are rather similar and range from 69 to 86% during 15 minutes of synthesis.
3.2. Synthesis of Ethenzamide in the Presence of Microwave Radiation, Ultrasound and Mechanochemically
An important advantage of reactions conducted under PTC conditions is the possibility of carrying out the process both in the case of classical methods and in the presence of microwave radiation, ultrasound or in mechanochemical reactions.
The research was continued using microwave radiation (MW) first, because numerous literature data and our previous experience in synthesis show that in the case of these reactions the reaction time can be shortened several times by minimizing the share of organic solvents or replacing them with a more ecological alternative such as glycerin or water. Interestingly, in MW processes, it is beneficial to use small amounts of dimethylformamide, which, in addition to the ability to transfer microwave energy, also acts as a catalyst. As shown in
Table 2, microwave-assisted reactions were conducted to synthesize ethenzamide (
6) with various catalysts, solvents, and reaction conditions. TBAB-catalyzed reaction (with K₂CO₃) under solvent-free conditions achieved the highest yield (92%) in just 90 sec, indicating the very high yield using direct microwave-assisted reaction. Using DMF as a solvent, the reaction yield was slightly dropped to 85%; the glycerin-DIPEA system has a lower reaction yield (69%). Water as a solvent with TBAB and K₂CO₃ produces a substantial yield (80%). Besides, BTBAC and TEAC-catalyzed reaction also have high yields (~90%). Under PTC-free condition in water, the yield dropped to 78%. Without base-catalysts, no reaction was occurred.
We continued our research into the possibility of using ultrasound as a source of energy for the reaction system. Under ultrasound (US)-assisted PTC of ethenzamide (with K₂CO₃), TBAB in water could result in the reaction yield of 84% in 10 min. No reaction occurs in the absence of K₂CO₃. BTBAC and TEBA could result in higher yields (88% and 89%, respectively) compared to that of TBAB. TEAC performs less effectively (74%). In the absence of PTC, the reaction yield drops significantly to 30%. These results showed that US-assisted PTC synthesis of ethenzamide could achieve high efficiency and yields within short period.
Table 3.
Synthesis of ethenzamide in the presence of ultrasound (US) conditions.
Table 3.
Synthesis of ethenzamide in the presence of ultrasound (US) conditions.
| No |
Catalyst PTC |
Solvent |
Conditions |
Time
[min]
|
Yield [%] |
| 1 |
TBAB |
H2O |
K2CO3
|
10 |
84 |
| 2 |
TBAB |
H2O |
- |
10 |
0 |
| 3 |
BTBAC |
H2O |
K2CO3
|
10 |
88 |
| 4 |
TEBA |
H2O |
K2CO3
|
10 |
89 |
| 5 |
TEAC |
H2O |
K2CO3
|
10 |
74 |
| 6 |
- |
H2O |
K2CO3
|
10 |
30 |
To further investigate the PTCs under solvent-free conditions, mechanochemical synthesis of ethenzamide were conducted under TBAB, TEAC, BTBAB and TEBA. Compared to the above MW and US conditions, the mechanochemical synthesis exhibits low efficiency due to the lack of solvents to disperse the reactants and PTCs, which reduced the catalytic efficacy of the PTCs and not be able to lower activation energy (E
a) for efficient
O-alkylation. With the increased grinding time from 20 min to 60 min for TEBA-catalyzed reaction, the yield was improved from 52% to 60%. The relatively low yields may be further improved by optimizing the reaction conditions (
Table 4).
4. Synthesis
4.1. Synthesis of Salicylamide (5)
A round-bottomed flask was filled with 0.105 mol (15 g) of salicylic acid (1), 60 cm3 of methanol and 5 cm3 of concentrated sulfuric acid. The reaction mixture was refluxed for 8 hours. After cooling, 100 cm3 of concentrated ammonia water was added, heated on a water bath for 20 min and stirred on a magnetic stirrer to obtain a homogeneous system. The contents of the flask were transferred to a beaker cooled with ice water and acidified with 20% hydrochloric acid solution to pH approx. 2. The precipitate was filtered on a Buchner funnel and washed with 50 cm3 of cold water. The crude amide was mixed with 100 cm3 of saturated NaHCO3 solution (to remove unreacted salicylic acid), filtered, washed with 25 cm3 of cold water and dried. 7.3 g (51%) of salicylamide (5) was obtained, which was crystallized from water to give 6.3 g (44%), mp 143-145 °C, Rf = 0.3, MS: m/z 138 (M+), Rt = 4.22 min.
4.2. Synthesis of Ethenzamide (6)
4.2.1. Synthesis of Ethenzamide (6) in Conventional Conditions in Ethanol
In a round-bottomed flask, 0.020 mol (0.8 g) of sodium hydroxide was dissolved in 15 cm3 of water, then 0.02 mol (2.7 g) of salicylamide (5), 10 cm3 of methanol and 0.026 mol of ethyl iodide were added. The reaction mixture was reflux for 3 hours until the alkaline reaction disappeared, then the residue was evaporated to dryness. After purification by crystallization from methanol, 1.42 g (43%) of ethenzamide was obtained, melting point 125-128 °C [mp lit. 129-133 °C]. 1H NMR (400 MHz, CDCl3) δ 8.24 (dd, J = 7.8, 1.8 Hz, 1H), 7.91 (brs, 1H), 7.48 (ddd, J = 8.4, 7.4, 1.9 Hz, 1H), 7.11 – 7.06 (m, 1H), 6.99 (d, J = 8.3 Hz, 1H), 6.03 (brs, 1H), 4.23 (q, J = 7.0 Hz, 2H), 1.54 (t, J = 7.0 Hz, 3H), 13C NMR (101 MHz, CDCl3) δ 167.21, 157.29, 133.37, 132.58, 121.13, 112.30, 64.72, 14.86; Rf = 0.58; IR 1643.19 (C=O), (NH2) 3367.74 cm-1; MS: m/z 166 (M+), Rt = 3.79 min.
4.2.2. Synthesis of Ethenzamide (6) in Conventional Conditions in DMF
A suspension consisting of 0.01 mol (1.37g) of salicylamide (5), 0.012 mol of ethyl iodide, 0.01 mol (1.38g) of potassium carbonate and catalytic amounts of potassium iodide in 15 cm3 of DMF was placed in a round-bottomed flask. Then the reaction mixture was reflux at a temperature of about 70 °C for 4 hours, monitoring the reaction time on TLC plates. After the process was completed, 40 cm3 of water was added to the mixture and after a while the resulting precipitate was filtered off on a Buchner funnel, which was then dried. After crystallization from methanol, ethenzamide (6) with a melting point of 126-128 °C was obtained in the amount of 0.96 g (60%).
4.2.3. Synthesis of Ethenzamide (6) in Conventional and Solvent-Free Conditions
A mixture consisting of 0.01 mol (1.37 g) of salicylamide (5), 0.03 mol (4.14 g) of potassium carbonate and 0.001 mol of PTC catalyst was ground in a mortar until a powdery consistency was obtained. Then the mixture was placed in a flask, 0.012 mol of ethyl iodide was added and heated on an oil bath at 20-80 °C, monitoring the reaction progress using the TLC method. After the process was completed, 40 cm
3 of water was added and the resulting precipitate was filtered on a Buchner funnel. The crude product was crystallized from methanol. The obtained results are summarized in
Table 1.
4.2.4. Synthesis of Salicylamide (5) in MW Conditions
The reaction mixture was prepared exactly as in the conventional and solvent-free procedure (4.2.3) and then placed in a glass pressure reactor and placed in a microwave reactor. The results are summarized in
Table 2.
4.2.5. Synthesis of Salicylamide (5) in US Conditions
The reaction mixture was prepared exactly as in the case of the procedure in conventional and solvent-free conditions (4.2.3) and then placed in a glass pressure reactor and 1 cm
3 of water was added, and then placed in an ultrasonic reactor. The results are summarized in
Table 3.
4.2.6. Synthesis of Salicylamide (5) in in Mechanochemical Conditions
The reaction mixture was prepared exactly as in the procedure under conventional and solvent-free conditions (4.2.3) and then placed in a ball mill. The results are summarized in
Table 4.
5. Discussion
The research was aimed at determining the optimal conditions for the O-alkylation reaction, based on the example of the synthesis of ethenzamide (6) by the reaction of salicylamide (5) with ethyl iodide. The synthesis was carried out under conventional conditions in the presence of organic solvents such as ethanol or dimethylformamide as well as in solvent-free conditions. The reactions were supported by phase transfer and other catalysts such as DABCO and crown ether. The results were very interesting because they proved that after just 15 minutes, the expected product can be obtained in solvent-free conditions with a yield of 69-86%.
Then we continued our research in a microwave reactor, which brought amazing results because it was observed that after just 2 minutes, an efficiency of up to 90% could be achieved. Interestingly, in MW-assisted reactions, even in the absence of a catalyst, an efficiency of 78% is achieved. It is worth noting, however, that the lack of potassium carbonate in the reaction medium makes it impossible to obtain ethenzamide (6). We observed similar dependencies in the reactions in the presence of ultrasound, i.e. the non-catalytic process occurred but with lower efficiency, while the lack of potassium carbonate was a critical parameter for obtaining the product. In US-assisted reactions, yields of 74-89% were achieved within 10 minutes, which is a very satisfactory result considering that the process took place in very mild conditions (atmospheric pressure, temperature 40 °C).
The last stage of the research included attempts to carry out the process in mechanochemical conditions, which allowed obtaining the expected ethenzamide (
6) within 20 min with yields even above 50% also in the non-catalytic process. Despite lower yields than in other methods, this one is certainly equally interesting but undoubtedly requires optimization. Undoubtedly, the stage of product isolation from the post-reaction mixture should also be refined, e.g. by adding zeolites or sand, which we have already tested in our previous studies in the synthesis of aripiprazole [
32].
Based on the latest literature data and patent databases regarding the synthesis of organic compounds, a trend of searching for new environmentally friendly technologies is clearly observed. This phenomenon is also present in the pharmaceutical industry, where at the stage of searching for new drugs, and especially in the area of synthesis, methods from the area of green chemistry are promoted. Undoubtedly, an important aspect in the case of environmentally friendly processes is the use of catalysts that are non-toxic and significantly affect the selectivity and efficiency of the process. All these aspects are met when the process is carried out under phase transfer catalysis (PTC) conditions.
Bearing the above in mind, the O-alkylation methods we have developed are consistent with current trends in organic synthesis. The conditions used allowed to minimize toxic conditions and significantly improve the product isolation process, which was based only on adding water and filtering the product. This means that not only the synthesis process itself but also the product isolation is more ecological than in the case of other conventional synthesis methods, where not only the reaction but also the product isolation is carried out using organic solvents.
Our latest research proves that the developed synthesis methods are universal. We are currently conducting research on the application of the developed method of ethenzamide synthesis in the presence of microwave radiation and ultrasound for the synthesis of new O-derivatives of salicylamide with anticancer properties that can be used in the treatment of breast cancer and for the synthesis of new acetylcholinesterase inhibitors.
Author Contributions
Conceptualization and methodology J.J., P.M., R.S.,; investigation, J.J., P.N.-Ć., P.P., W.B., R.S., K.Z.-T.; writing—original draft preparation, J.J., R.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thun, M. Beyond willow bark: Aspirin the prevention of chronic disease. Epidemiology 2000, 11, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Hedner, T.; Everts, B. Clinical history of salicylates in rheumatology and pain. Clin. Rheumatol. 1998, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.L.; Scheidt, S. History of drugs for thrombotic disease. Discovery, development, and directions for the future. Circulation 1994, 89, 432–439. [Google Scholar] [CrossRef]
- Velcheva, E.A.; Stamboliyska, B.A. Structural changes caused by the conversion of 2-hydroxybenzamide (salicylamide) into the oxyanion, J. Mol. Struct. 2008, 875, 264–271. [Google Scholar] [CrossRef]
- Javaid, M.H.; Murray Smith, G.C.; Morrison Barr Martin, N.; Gomez, S.; Ming Lai Loh, V. Jr.; Fan Cockcroft, X.L.; Menear, K.A. PARP inhibitors, Panent WO-2006067472-A1 2024.
- Javaid, M.H.; Morrison Barr Martin, N.; Gomez, S.; Fan Cockcroft, X.L.; Menear, K.A. 2-oxybenzamide derivatives as PARP inhibitors, Patent WO-2007144639-A1 2006.
- Javaid, M.H.; Morrison Barr Martin, N.; Gomez, S.; Fan Cockcroft, X.L.; Menear, K.A. PARP inhibitors, Patent WO-2007144652-A2 2006.
- Lin, C.F.; Yang, J.S.; Chang, C.Y.; Kuo, S.C.; Lee, M.R.; Huang, L.J. ; Synthesis and anticancer activity of benzyloxybenzaldehyde derivatives against HL-60 cells, Bioorg. Med. Chem. 2005, 13, 1537–1544. [Google Scholar] [CrossRef]
- Lee, S.; Yi, Y.K.; Lee, B.H.; Oh, K.S. Studies on Benzofuran-7-carboxamides as Poly(ADP-ribose) Polymerase-1 (PARP-1) Inhibitors. Bulletin of the Korean Chemical Society 2012, 33, 1147–1153. [Google Scholar] [CrossRef]
- Broughton, B.J.; Chaplen, P.; Knowles, P.; Lunt, E.; Marshall, S.M.; Pain, D.L.; Wooldrige, K.R. H. Antiallergic Activity of 2-Phenyl-8-azapurin-6-ones, J. Med. Chem. 1975, 18, 1117–1122. [Google Scholar] [CrossRef]
- Kamei, K.; Maeda, N.; Nomura, K.; Shibata, M.; Katsuragi-Ogino, R.; Koyama, M.; Nakajima, M.; Inoue, T.; Ohno, T.; Tatsuoka, T. ; Synthesis, SAR studies and evaluation of 1,4-benzoxazepine derivatives as selective 5-HT1A receptor agonists with neuroprotective effect: Discovery of Piclozotan, Bioorg. Med. Chem. 2006, 14, 1978–1992. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wahi, A.K.; Singh, R. Synthesis, computational studies and preliminary pharmacological evaluation of new arylpiperazines as potential antipsychotics, Med. Chem. Res. 2012, 21, 1218–1225. [Google Scholar] [CrossRef]
- Kowalski, P.; Jaśkowska, J.; Bojarski, A.J.; Duszyńska, B.; Bucki, A.; Kołaczkowski, M. ; Evaluation of 1-Arylpiperazine Derivative of Hydroxybenzamides as 5-HT1A and 5-HT7 Serotonin Receptor Ligands: An Experimental and Molecular Modeling Approach, J. Heterocycl. Chem. 2011, 48, 192–198. [Google Scholar] [CrossRef]
- Kowalski, P.; Jaśkowska, J.; Bojarski, A.J.; Duszyńska, B. ; M. ; The Synthesis of Cyclic and Acyclic Long-chain Arylpiperazine Derivatives of Salicylamide as Serotonin Receptor Ligands, J. Heterocycl. Chem. 2008, 45, 209–214. [Google Scholar] [CrossRef]
- Jaśkowska, J.; Drabczyk, A.K.; Śliwa, P.; Jodłowski, P.; Pindelska, E.; Kułaga, D.; Zaręba, P.; Majka, Z.; Siwek, A.; Wolak, M.; Kołaczkowski, M. Ultrasound assisted one-pot synthesis and preliminary in vitro studies of salicylamide arylpiperazines as dual 5-HT1A/5-HT7 ligands J. Mol. Str. 2023, 1275, 134585. [Google Scholar] [CrossRef]
- Qiao, C.; Gupte, A.; Boshoff, H.I.; Wilson, D.J.; Benett, E.M.; Somu, R.V.; Barry, C.E.; Aldrich, C.C. 5’-O-[(N-Acyl)sulfamoyl]adenosines as Antitubercular Agents that Inhibit MbtA: An Adenylation Enzyme Required for Siderophore Biosynthesis of the Mycobactins, J. Med. Chem. 2007, 50, 6080–6094. [Google Scholar] [CrossRef] [PubMed]
- Żmudzka, E.; Lustyk, K.; Siwek, A.; Wolak, M.; Gałuszka, A.; Jaśkowska, J.; Kołaczkowski, M.; Sapa, J.; Pytka, K. Novel Arylpiperazine Derivatives of Salicylamide with α1-Adrenolytic Properties Showed Antiarrhythmic and Hypotensive Properties in Rats. Int. J. Mol. Sci. 2023, 24, 293. [Google Scholar] [CrossRef]
- Żmudzka, E.; Lustyk, K.; Głuch-Lutwin, M.; Wolak, M.; Jaśkowska, J.; Kołaczkowski, M.; Sapa, J.; Pytka, K. Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice. Pharmaceuticals 2023, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Żmudzka, E.; Lustyk, K.; Sałaciak, K.; Siwek, A.; Jaśkowska, J.; Kołaczkowski, M.; Sapa, J.; Pytka, K. Potential Anti-Amnesic Activity of a Novel Multimodal Derivative of Salicylamide, JJGW08, in Mice. Pharmaceuticals 2023, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- García-March, F.J.; García-Domenech, R.; Gálvez, J.; Antón-Fos, G.M.; Julián-Ortiz, J.V.; Giner-Pons, R.; Recio-Iglesias, M.C. Pharmacological Studies of 1-(p-Chlorophenyl)propanol and 2-(1-Hydroxy-3-butenyl)phenol: Two New Non-narcotic Analgesics Designed by Molecular Connectivity. J Pharm Pharmacol. 1997, 1997. 49, 10–15. [Google Scholar] [CrossRef]
- Nikaido, T.; Maruyama, C.; Hamanaka, M.; Yamaguchi, C.; Fujimaru, Y.; Nakanishi, Y.; Asano, T.; Takaoka, A. Ethenzamide Exerts Analgesic Effect at the Spinal Cord via Multiple Mechanisms of Action Including the 5HT2B Receptor Blockade in the Rat Formalin Test. Biol Pharm Bull. 2020, 43, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Darias, V.; Bravo, L.; Abdallah, S.S.; Sánchez Mateo, C.C.; Expósito-orta M., A.; Lissavetsky, J.; Manzanares, J. Synthesis and Preliminary Pharmacological Study of Thiophene Analogues of the Antipyretic and Analgesic Agent Ethenzamide. Archiv der Pharmazie, 1992, 325, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Guo, H.R. ; Frequent analgesics consumption in migraineurs: Comparison between chronic and episodic migraineurs. J. Headache Pain 2004, 5, 30–35. [Google Scholar] [CrossRef]
- Bertholdt, H.; Michalczyk, D.; Hartl, R.; Lieb, H. Medicament with an analgesic, antipyretic and/or antiphlogistic action and use of 2-ethoxybenzoic acid. Patent US5419915 A 1995.
- Mathur, K.C.; Gupta, S.; Khadikar, P.V. Topological modelling of analgesia. Bioorg. Med. Chem. 2003, 11, 1915–1928. [Google Scholar] [CrossRef]
- Fahmy, H.H.; El-Eraky, W. Synthesis and evaluation of the analgesic and antiinflammatory activities of O-substituted salicylamides. Arch Pharm Res. 2001, 24, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ohnacker, G.; Kottler, A. ; Process for the production of benzoic acid derivatives, USA Patent 2895992, 1959. [Google Scholar]
- Li, S.; Cao, X.; Chen, C.; Ke, S. Novel salicylic acid-oriented thiourea-type receptors as colorimetric chemosensor: Synthesis, characterizations and selective naked-eye recognition properties. Spectrochim Acta A Mol Biomol Spectrosc 2012, 96, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Allais, F.; Brogi, S.; Castro, G.R.; Vidali, V.P. Editorial: Advances in green synthesis for drug discovery. Front. Chem 2023, 11, 1166887. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998. [Google Scholar]
- Sanderson, K. ; Chemistry: It’s not easy being green. Nature. 2011, 469, 18–20. [Google Scholar] [CrossRef]
- Jaśkowska, J.; Drabczyk, A.K.; Michorczyk, P.; Kułaga, D.; Zaręba, P.; Jodłowski, P.; Majka, Z.; Jakubski, J.; Pindelska, E. Mechanochemical Synthesis Method for Drugs Used in the Treatment of CNS Diseases under PTC Conditions. Catalysts 2022, 12, 464. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).