Submitted:
10 December 2024
Posted:
11 December 2024
You are already at the latest version
Abstract
Cancer is a complex genetic disorder characterized by abnormalities in both coding and regulatory non-coding RNAs. microRNAs (miRNAs) are key regulatory non-coding RNAs that modulate cancer development, functioning as both tumor suppressors and oncogenes. miRNAs play critical roles in cancer progression, influencing key processes such as initiation, promotion, and metastasis. They exert their effects by targeting tumor suppressor genes, thereby facilitating cancer progression, while also inhibiting oncogenes to prevent further disease advancement. The miR-10 family, particularly miR-10a and miR-10b (miR-10a/b), is notably involved in cancer progression. Intriguingly, their functions can differ across different cancers, sometimes promoting and at other times suppressing tumor growth depending on the cancer type and molecular environment. This review explores the dual roles of miR-10a/b as tumor suppressor miRNAs (TSmiRs) or oncogenic miRNAs (oncomiRs) in various cancers by examining their molecular and cellular mechanisms and their impact on the tumor microenvironment. Furthermore, we discuss the potential of miR-10a/b as therapeutic targets, emphasizing miRNA-based strategies for cancer treatment. Insights discussed in this review aim to advance our understanding of miR-10a/b's roles in tumor biology and their application in developing innovative cancer therapies.
Keywords:
1. Introduction
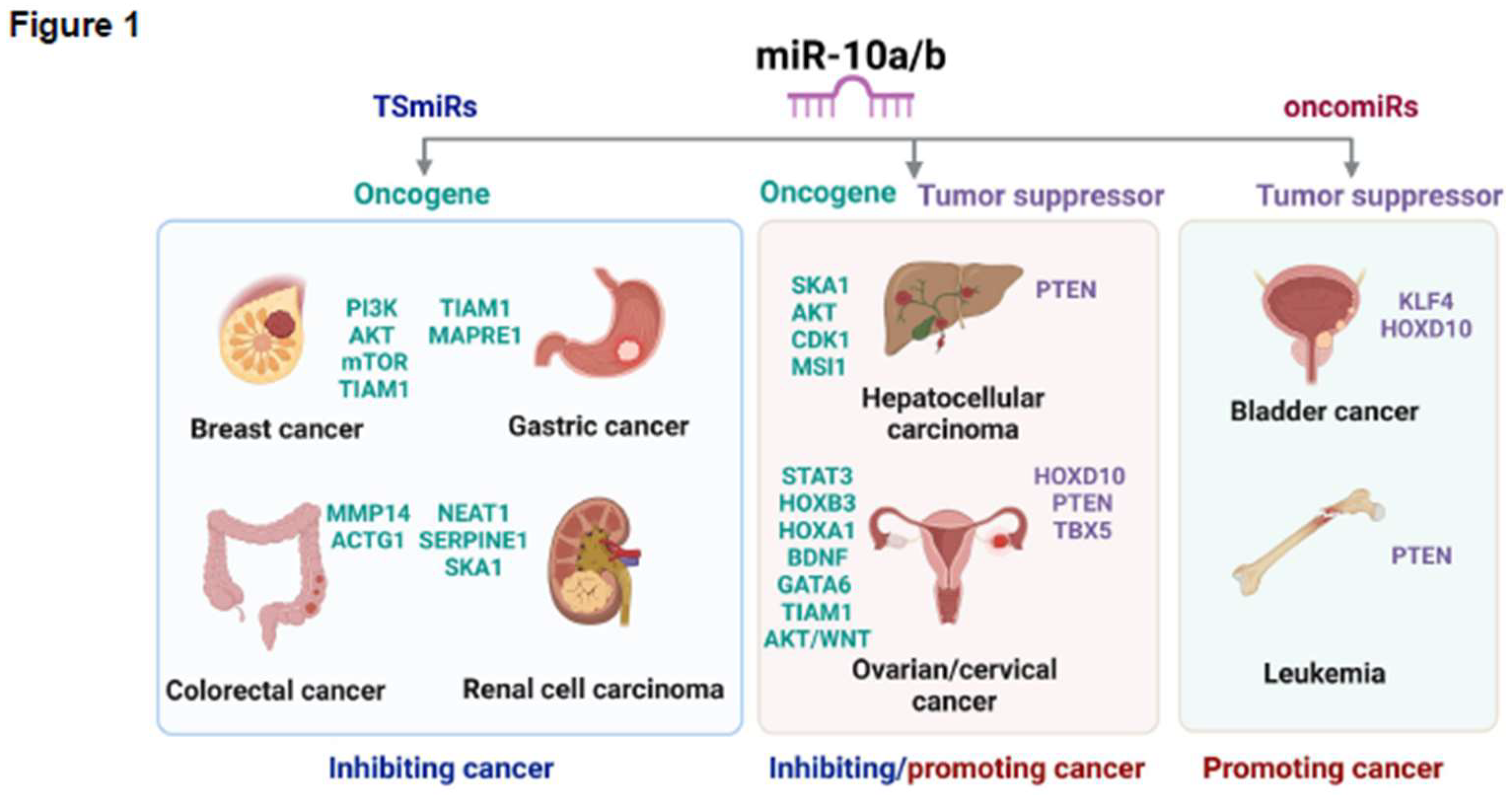
Dual Roles of miR-10a/b as Tumor Suppressors (TSmiRs) and Oncogenes (oncomiRs)
miR-10a/b as TSmiRs
Chronic Myeloid Leukemia
Esophageal Squamous Cell Carcinoma
Renal Cell Carcinoma
miR-10a/b as oncomiRs
Cholangiocarcinoma
Granulosa Cell Tumors
Acute Myeloid Leukemia
Prostate Cancer
Glioblastoma
miR-10a/b as both TSmiRs and oncomiRs
Breast Cancer
Bladder Cancer
Endometrial Cancer
Cervical Cancer
Ovarian Cancer
Gastric Cancer
Colorectal Cancer
Hepatocellular Carcinoma
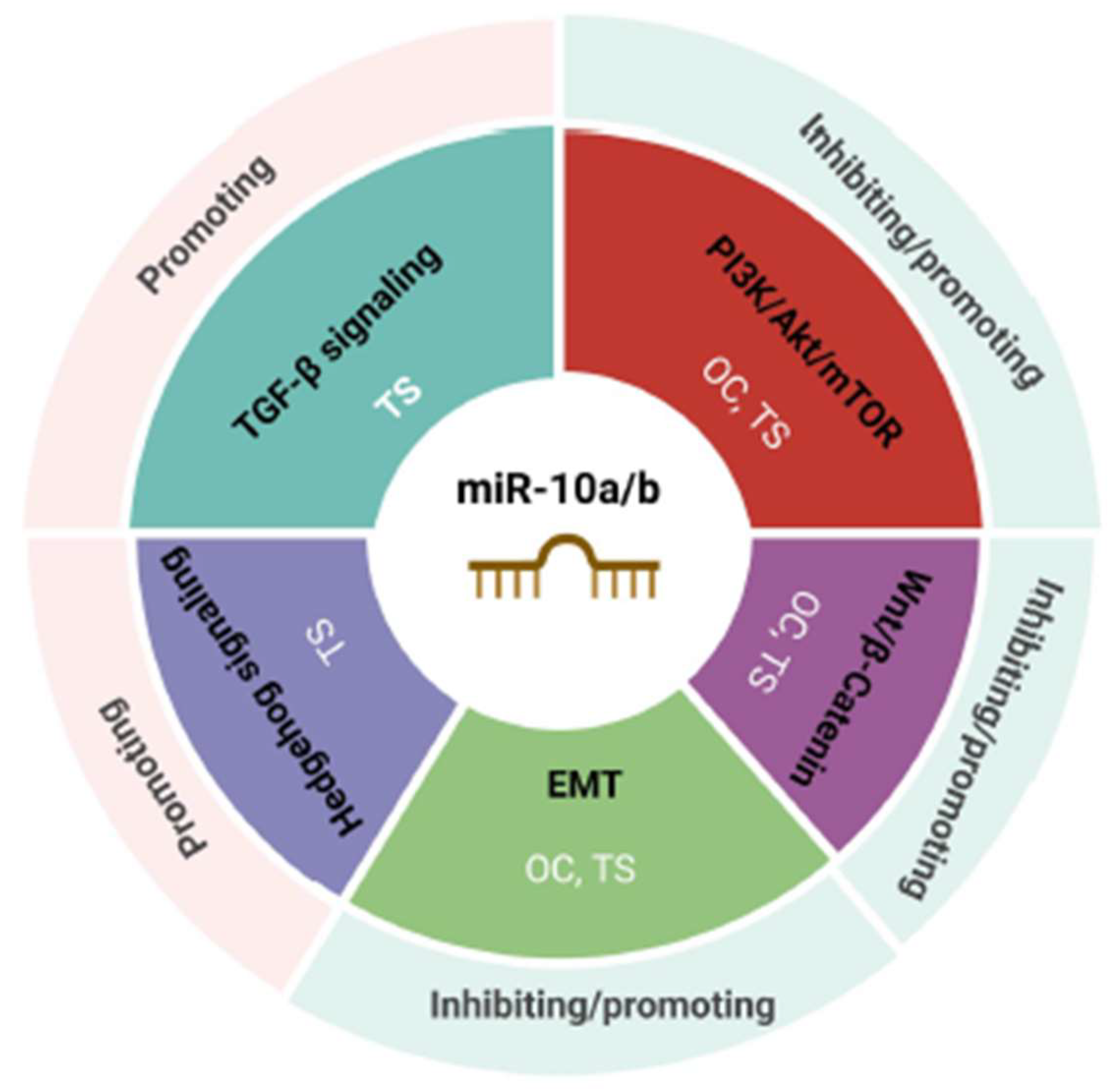
miR10a/b in the Regulation of Key Cancer Pathways
miR-10a/b and Tumor Microenvironment
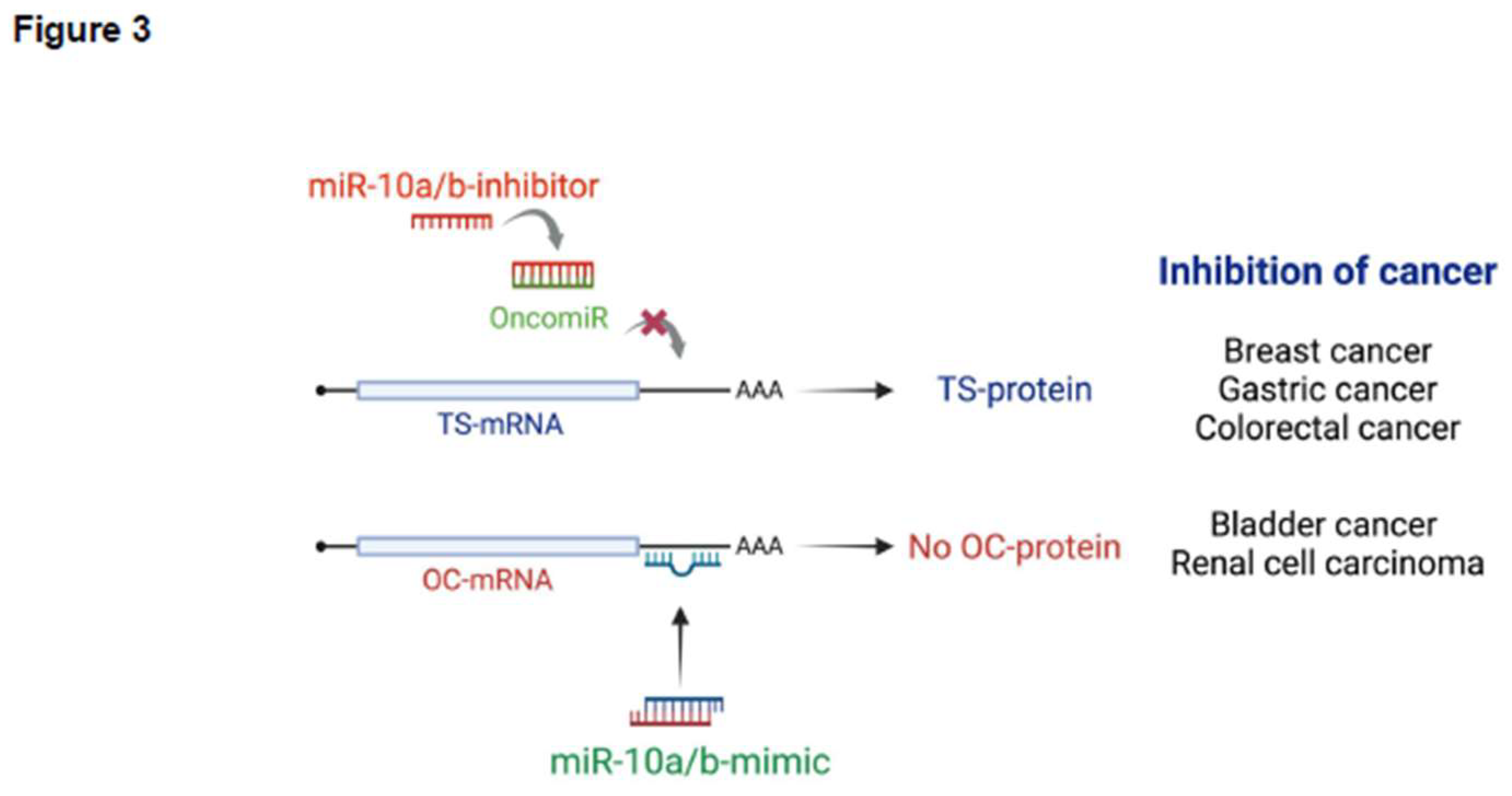
miRNA Therapeutic Targets in Cancer
Conclusion and Future Prospectives
Funding
References
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Offord, C. Duo honored for tiny RNAs key to development and disease. Science 2024, 386, 134–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A signature for cancer progression. 138, 1115; 28. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Hausser, J.; Zavolan, M. , Identification and consequences of miRNA-target interactions--beyond repression of gene expression. Nat Rev Genet 2014, 15, (9), 599–612. [Google Scholar]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ha, S.E.; Wei, L.; Jin, B.; Zogg, H.; Poudrier, S.M.; Jorgensen, B.G.; Park, C.; Ronkon, C.F.; Bartlett, A.; et al. miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology 2021, 160, 1662–1678.e18. [Google Scholar] [CrossRef] [PubMed]
- Zogg, H.; Singh, R.; Ha, S.E.; Wang, Z.; Jin, B.; Ha, M.; Dafinone, M.; Batalon, T.; Hoberg, N.; Poudrier, S.; et al. miR-10b-5p rescues leaky gut linked with gastrointestinal dysmotility and diabetes. United Eur. Gastroenterol. J. 2023, 11, 750–766. [Google Scholar] [CrossRef]
- Ha, S.E.; Singh, R.; Jin, B.; Baek, G.; Jorgensen, B.G.; Zogg, H.; Debnath, S.; Park, H.S.; Cho, H.; Watkins, C.M.; et al. miR-10a/b-5p-NCOR2 Regulates Insulin-Resistant Diabetes in Female Mice. Int. J. Mol. Sci. 2024, 25, 10147. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Ro, S. Role of microRNAs in Disorders of Gut–Brain Interactions: Clinical Insights and Therapeutic Alternatives. J. Pers. Med. 2021, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Zogg, H.; Singh, R.; Ro, S. Current Advances in RNA Therapeutics for Human Diseases. Int. J. Mol. Sci. 2022, 23, 2736. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.H. miR-10 in development and cancer. Cell Death Differ. 2009, 17, 209–214. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; Lv, X.; Han, X.; Xu, Y.; Huang, J.; Chen, X.; Yu, Z. Recent Updates on the Role of the MicroRNA-10 Family in Gynecological Malignancies. J. Oncol. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, Y.; Liu, Z.; Li, Z.; Xu, W. The emerging role of miR-10 family in gastric cancer. Cell Cycle 2021, 20, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, P.; Medarova, Z. The fundamental role of miR-10b in metastatic cancer. 2018, 8, 1674–1688.
- Singh, R.; Ha, S.E.; Park, H.S.; Debnath, S.; Cho, H.; Baek, G.; Yu, T.Y.; Ro, S. Sustained Effectiveness and Safety of Therapeutic miR-10a/b in Alleviating Diabetes and Gastrointestinal Dysmotility without Inducing Cancer or Inflammation in Murine Liver and Colon. Int. J. Mol. Sci. 2024, 25, 2266. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, X.; Zhang, H.; Yu, M.; Long, J.; Yang, T. Inhibition of miR-10a-5p suppresses cholangiocarcinoma cell growth through downregulation of Akt pathway. OncoTargets Ther. 2018, ume 11, 6981–6994. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, H.; Gu, A.; Wu, Y.; Weng, Y.; Li, S.; Song, J.; Gu, X.; Qiu, J.; Zhao, W. miRNA-10a-5p inhibits cell metastasis in hepatocellular carcinoma via targeting SKA1. Kaohsiung J. Med Sci. 2021, 37, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wang, D.; Lu, J. MicroRNA-10b inhibits proliferation, migration and invasion in cervical cancer cells via direct targeting of insulin-like growth factor-1 receptor. Oncol. Lett. 2017, 13, 5009–5015. [Google Scholar] [CrossRef] [PubMed]
- Guessous, F.; Alvarado-Velez, M.; Marcinkiewicz, L.; Zhang, Y.; Kim, J.; Heister, S.; Kefas, B.; Godlewski, J.; Schiff, D.; Purow, B.; et al. Oncogenic effects of miR-10b in glioblastoma stem cells. J. Neuro-Oncology 2013, 112, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Cheung, H.-H.; Lu, G.; Chen, Z.; Chan, W.-Y. MicroRNA-10a promotes granulosa cells tumor development via PTEN-AKT/Wnt regulatory axis. Cell Death Dis. 2018, 9, 1076. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wu, H.; Li, Y.; Zhang, Y.; Liu, M.; Li, X.; Tang, H. miR-10a suppresses colorectal cancer metastasis by modulating the epithelial-to-mesenchymal transition and anoikis. Cell Death Dis. 2017, 8, e2739–e2739. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Lou, T. MicroRNA-10a suppresses breast cancer progression via PI3K/Akt/mTOR pathway. Oncol. Lett. 2017, 14, 5994–6000. [Google Scholar] [CrossRef]
- Morgan, R.; Hunter, K.; Pandha, H.S. Downstream of the HOX genes: Explaining conflicting tumour suppressor and oncogenic functions in cancer. Int. J. Cancer 2022, 150, 1919–1932. [Google Scholar] [CrossRef]
- Lin, L.; Mahner, S.; Jeschke, U.; Hester, A. , The Distinct Roles of Transcriptional Factor KLF11 in Normal Cell Growth Regulation and Cancer as a Mediator of TGF-beta Signaling Pathway. Int J Mol Sci 2020, 21, (8). [Google Scholar] [CrossRef] [PubMed]
- Agirre, X.; Jimenez-Velasco, A.; San Jose-Eneriz, E.; Garate, L.; Bandres, E.; Cordeu, L.; Aparicio, O.; Saez, B.; Navarro, G.; Vilas-Zornoza, A.; Perez-Roger, I.; Garcia-Foncillas, J.; Torres, A.; Heiniger, A.; Calasanz, M. J.; Fortes, P.; Roman-Gomez, J.; Prosper, F. , Down-regulation of hsa-miR-10a in chronic myeloid leukemia CD34+ cells increases USF2-mediated cell growth. Mol Cancer Res 2008, 6, (12), 1830–40. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Jiang, X.; Yan, P.; Zhan, L.; Zhu, H.; Wang, T.; Wen, J. Tumor-suppressive microRNA-10a inhibits cell proliferation and metastasis by targeting Tiam1 in esophageal squamous cell carcinoma. J. Cell. Biochem. 2018, 120, 7845–7857. [Google Scholar] [CrossRef]
- Khella, H.W.Z.; Daniel, N.; Youssef, L.; Scorilas, A.; Nofech-Mozes, R.; Mirham, L.; Krylov, S.N.; Liandeau, E.; Krizova, A.; Finelli, A.; et al. miR-10b is a prognostic marker in clear cell renal cell carcinoma. J. Clin. Pathol. 2017, 70, 854–859. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Li, Y.; Jin, L.; Liu, J.; Su, Z.; Qi, Z.; Shi, M.; Jiang, Z.; NI, L.; et al. Oncogenic cAMP responsive element binding protein 1 is overexpressed upon loss of tumor suppressive miR-10b-5p and miR-363-3p in renal cancer. Oncol. Rep. 2016, 35, 1967–1978. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, W. LncRNA SNHG4 regulates miR-10a/PTEN to inhibit the proliferation of acute myeloid leukemia cells. Hematology 2020, 25, 160–164. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, Y.; Cui, Y.; Wang, Y.; Liu, C. Long non-coding RNA CHRF promotes proliferation and mesenchymal transition (EMT) in prostate cancer cell line PC3 requiring up-regulating microRNA-10b. Biol. Chem. 2018, 400, 1035–1045. [Google Scholar] [CrossRef]
- Ma, C.; Wei, F.; Xia, H.; Liu, H.; Dong, X.; Zhang, Y.; Luo, Q.; Liu, Y.; Li, Y. , MicroRNA-10b mediates TGF-beta1-regulated glioblastoma proliferation, migration and epithelial-mesenchymal transition. Int J Oncol 2017, 50, (5), 1739–1748. [Google Scholar] [CrossRef]
- Sun, L.; Yan, W.; Wang, Y.; Sun, G.; Luo, H.; Zhang, J.; Wang, X.; You, Y.; Yang, Z.; Liu, N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011, 1389, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, J.; Zhang, H.; Li, H. Long noncoding RNA-GAS5 attenuates progression of glioma by eliminating microRNA-10b and Sirtuin 1 in U251 and A172 cells. BioFactors 2019, 46, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, C. H.; Pursell, B.; Mercurio, A. M. , miR-10b targets Tiam1: implications for Rac activation and carcinoma migration. J Biol Chem 2010, 285, (27), 20541–6. [Google Scholar] [CrossRef]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R. A. , Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449, (7163), 682–8. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Zhang, H.; Lin, F.; Tan, Q.; Qin, Q.; Bao, W.; Liu, Y.; Xie, J.; Zeng, Q. , Long intergenic non-protein coding RNA 324 prevents breast cancer progression by modulating miR-10b-5p. Aging (Albany NY) 2020, 12, (8), 6680–6699. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, L.; Sun, S. LncRNA PDCD4-AS1 alleviates triple negative breast cancer by increasing expression of IQGAP2 via miR-10b-5p. Transl. Oncol. 2020, 14, 100958. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yan, S.; Weijie, Z.; Feng, W.; Liuxing, W.; Mengquan, L.; Qingxia, F. , Critical role of miR-10b in transforming growth factor-beta1-induced epithelial-mesenchymal transition in breast cancer. Cancer Gene Ther 2014, 21, (2), 60–7. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhang, P.-Y.; Zhang, Y.; Sun, S.-Y.; Yu, S.-Y.; Xi, Q.-S. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci. Monit. 2012, 18, BR299–BR308. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A.; Radojicic, J.; Lambrou, G.I.; Volanis, D.; Delakas, D.; Stathopoulos, E.N.; Spandidos, D.A. Expression of miRNAs Involved in Angiogenesis, Tumor Cell Proliferation, Tumor Suppressor Inhibition, Epithelial-Mesenchymal Transition and Activation of Metastasis in Bladder Cancer. J. Urol. 2012, 188, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, H.; Yu, G.; Xiao, W.; Hu, J.; Tang, K.; Zeng, J.; He, W.; Zeng, G.; Ye, Z.; et al. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol. Rep. 2014, 31, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, E.; Akahira, J.; Suzuki, F.; Nagase, S.; Ito, K.; Suzuki, T.; Sasano, H.; Yaegashi, N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2009, 101, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, O.; Miura, K.; Mishima, H.; Abe, S.; Kaneuchi, M.; Higashijima, A.; Miura, S.; Kinoshita, A.; Yoshiura, K.-I.; Masuzaki, H. Identification of endometrioid endometrial carcinoma-associated microRNAs in tissue and plasma. 132. [CrossRef]
- Chen, H.; Fan, Y.; Xu, W.; Chen, J.; Xu, C.; Wei, X.; Fang, D.; Feng, Y. miR-10b Inhibits Apoptosis and Promotes Proliferation and Invasion of Endometrial Cancer Cells via Targeting HOXB3. Cancer Biotherapy Radiopharm. 2016, 31, 225–231. [Google Scholar] [CrossRef]
- Zhai, L.; Li, Y.; Lan, X.; Ai, L. MicroRNA-10a-5p suppresses cancer proliferation and division in human cervical cancer by targeting BDNF. Exp. Ther. Med. 2017, 14, 6147–6151. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zhou, Q.; Wang, D.; Guan, L.; Yuan, L.; Li, S. , The Downregulation of MicroRNA-10b and its Role in Cervical Cancer. Oncol Res 2016, 24, (2), 99–108. [Google Scholar] [CrossRef]
- Yu, M.; Xu, Y.; Pan, L.; Feng, Y.; Luo, K.; Mu, Q.; Luo, G. miR-10b Downregulated by DNA Methylation Acts as a Tumor Suppressor in HPV-Positive Cervical Cancer via Targeting Tiam1. Cell. Physiol. Biochem. 2018, 51, 1763–1777. [Google Scholar] [CrossRef]
- Huang, L.; Lin, J.-X.; Yu, Y.-H.; Zhang, M.-Y.; Wang, H.-Y.; Zheng, M. Downregulation of Six MicroRNAs Is Associated with Advanced Stage, Lymph Node Metastasis and Poor Prognosis in Small Cell Carcinoma of the Cervix. PLOS ONE 2012, 7, e33762. [Google Scholar] [CrossRef]
- Sommerova, L.; Anton, M.; Bouchalova, P.; Jasickova, H.; Rak, V.; Jandakova, E.; Selingerova, I.; Bartosik, M.; Vojtesek, B.; Hrstka, R. , The role of miR-409-3p in regulation of HPV16/18-E6 mRNA in human cervical high-grade squamous intraepithelial lesions. Antiviral Res 2019, 163, 185–192. [Google Scholar] [CrossRef]
- Zeng, T.; Li, G. MicroRNA-10a enhances the metastatic potential of cervical cancer cells by targeting phosphatase and tensin homologue. Mol. Med. Rep. 2014, 10, 1377–1382. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wang, X.; Zou, B.; Mei, J.; Peng, X.; Wu, Z. Extracellular vesicles-encapsulated microRNA-10a-5p shed from cancer-associated fibroblast facilitates cervical squamous cell carcinoma cell angiogenesis and tumorigenicity via Hedgehog signaling pathway. Cancer Gene Ther. 2020, 28, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, X.; Yang, C.; Zou, X. MiR-10a-5p restrains the aggressive phenotypes of ovarian cancer cells by inhibiting HOXA1. Kaohsiung J. Med Sci. 2020, 37, 276–285. [Google Scholar] [CrossRef]
- Gao, F.; Wu, Q.; Lu, D. MicroRNA-10a-5p-mediated downregulation of GATA6 inhibits tumor progression in ovarian cancer. Hum. Cell 2023, 37, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, I.; Shibazaki, M.; Yashima-Abo, A.; Miura, F.; Sugiyama, T.; Masuda, T.; Maesawa, C. Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int. J. Oncol. 2013, 43, 63–71. [Google Scholar] [CrossRef]
- Kim, K.; Lee, H. C.; Park, J. L.; Kim, M.; Kim, S. Y.; Noh, S. M.; Song, K. S.; Kim, J. C.; Kim, Y. S. , Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics 2011, 6, (6), 740–51. [Google Scholar] [CrossRef]
- Hu, G.; Shi, Y.; Zhao, X.; Gao, D.; Qu, L.; Chen, L.; Zhao, K.; Du, J.; Xu, W. , CBFbeta/RUNX3-miR10b-TIAM1 molecular axis inhibits proliferation, migration, and invasion of gastric cancer cells. Int J Clin Exp Pathol 2019, 12, (9), 3185–3196. [Google Scholar]
- Li, Z.; Lei, H.; Luo, M.; Wang, Y.; Dong, L.; Ma, Y.; Liu, C.; Song, W.; Wang, F.; Zhang, J.; Shen, J.; Yu, J. , DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer 2015, 18, (1), 43–54. [Google Scholar] [CrossRef]
- Liu, F.; An, X.; Zhao, X.; Zhang, N.; Chen, B.; Li, Z.; Xu, W. MiR-10b-5p inhibits tumorigenesis in gastric cancer xenograft mice model through down-regulating Tiam1. Exp. Cell Res. 2021, 407, 112810. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Li, L.; Ye, Z.-Y.; Zhao, Z.-S.; Yan, Z.-L. MicroRNA-10b promotes migration and invasion through Hoxd10 in human gastric cancer. World J. Surg. Oncol. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stadthagen, G.; Tehler, D.; Høyland-Kroghsbo, N.M.; Wen, J.; Krogh, A.; Jensen, K.T.; Santoni-Rugiu, E.; Engelholm, L.H.; Lund, A.H. Loss of miR-10a Activates Lpo and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice. PLOS Genet. 2013, 9, e1003913. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Li, Z.; Zhao, X.-H.; Zuo, X.-M.; Zhang, Y.; Xiao, Y.-H.; Li, J.; Peng, Z.-H. MicroRNA-10b is upregulated and has an invasive role in colorectal cancer through enhanced Rhoc expression. Oncol. Rep. 2015, 33, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud-Dammak, R.; Chamtouri, N.; Triki, M.; Saadallah-Kallel, A.; Ayadi, W.; Charfi, S.; Khabir, A.; Ayadi, L.; Sallemi-Boudawara, T.; Mokdad-Gargouri, R. Overexpression of miR-10b in colorectal cancer patients: Correlation with TWIST-1 and E-cadherin expression. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jiang, W.; Hui, B.; Rong, D.; Fu, K.; Dong, C.; Tang, W.; Cao, H. , The circ_0021977/miR-10b-5p/P21 and P53 regulatory axis suppresses proliferation, migration, and invasion in colorectal cancer. J Cell Physiol 2020, 235, (3), 2273–2285. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.; Chen, Y.; Ma, C.; Li, B.; Hao, T. Up-regulation of mir-10b predicate advanced clinicopathological features and liver metastasis in colorectal cancer. Cancer Med. 2016, 5, 2932–2941. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Lv, Y.; Zhang, C.; Guo, S. , LncRNA meg3 suppresses hepatocellular carcinoma in vitro and vivo studies. Am J Transl Res 2019, 11, (7), 4089–4099. [Google Scholar]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. , PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol 2022, 12, 819128. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 1–37. [Google Scholar] [CrossRef]
- Makowska, M.; Smolarz, B.; Romanowicz, H. microRNAs (miRNAs) in Glioblastoma Multiforme (GBM)—Recent Literature Review. Int. J. Mol. Sci. 2023, 24, 3521. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. , Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther 2021, 6, (1), 307. [Google Scholar]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. , Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022, 7, (1), 3. [Google Scholar]
- Yan, Y.; Yan, H.; Wang, Q.; Zhang, L.; Liu, Y.; Yu, H. , MicroRNA 10a induces glioma tumorigenesis by targeting myotubularin-related protein 3 and regulating the Wnt/beta-catenin signaling pathway. FEBS J 2019, 286, (13), 2577–2592. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. 13, 1007; 73. [Google Scholar] [CrossRef]
- Jing, J.; Wu, Z.; Wang, J.; Luo, G.; Lin, H.; Fan, Y.; Zhou, C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Massague, J. , Roles of TGFbeta in metastasis. Cell Res 2009, 19, (1), 89–102. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R. R. , TGF-beta Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb Perspect Biol 2017, 9, (12). [Google Scholar] [CrossRef] [PubMed]
- I Suzuki, H.; Katsura, A.; Matsuyama, H.; Miyazono, K. MicroRNA regulons in tumor microenvironment. Oncogene 2014, 34, 3085–3094. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, P.; Wang, X.-F. Microenvironmental regulation of cancer metastasis by miRNAs. Trends Cell Biol. 2013, 24, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.H. Cells choose their words wisely. Cell 2022, 185, 1114–1116. [Google Scholar] [CrossRef]
- Nedaeinia, R.; Najafgholian, S.; Salehi, R.; Goli, M.; Ranjbar, M.; Nickho, H.; Haghjooy Javanmard, S.; G, A. F.; Manian, M. , The role of cancer-associated fibroblasts and exosomal miRNAs-mediated intercellular communication in the tumor microenvironment and the biology of carcinogenesis: a systematic review. Cell Death Discov 2024, 10, (1), 380. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. , Stromal cells in the tumor microenvironment: accomplices of tumor progression? Cell Death Dis 2023, 14, (9), 587. [Google Scholar] [CrossRef]
- Ledford, H. , MicroRNAs won the Nobel - will they ever be useful as medicines? Nature 2024. [CrossRef] [PubMed]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef] [PubMed]
- Cuciniello, R.; Filosa, S.; Crispi, S. , Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics. J Exp Clin Cancer Res 2021, 40, (1), 383. [Google Scholar]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat. Rev. Genet. 2023, 25, 211–232. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics — challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Shah, M.Y.; Ferrajoli, A.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. microRNA Therapeutics in Cancer — An Emerging Concept. 12, 42. [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Al-Qadi, N.; Kenyon, E.; Conner, K.N.; Mondal, S.K.; Medarova, Z.; Moore, A. Inhibition of miR-10b treats metastatic breast cancer by targeting stem cell-like properties. Oncotarget 2024, 15, 591–606. [Google Scholar] [CrossRef] [PubMed]
| miRNA | Taget gene | Cancer | Human | Mouse | Reference(PMID) | |||
| Name | Role | Tissue (n) | Cell (n) | Bood (n) | ||||
| miR-10a-5p | TSmiRs | PI3K/Akt/mTOR | Breast cancer (BC) | BC (2) | 29113237 | |||
| miR-10a-5p | TSmiRs | BDNF | Cervical cancer (CC) | CC (5) | 29285171 | |||
| miR-10a-5p | TSmiRs | USF2 | Chronic myeloid leukemia (CML) | CML (6) Bone marrow (85) | CML (5) | 19074828 | ||
| miR-10a-5p | TSmiRs | MMP14/ACTG1 | Colorectal cancer (CRC) | CRC (26) | CRC (2) | 28383561 | ||
| miR-10a-5p | TSmiRs | Lpo/KLF4 | Colorectal cancer (CRC) | CRC (16) | CRC (Apc) mice mir-10a KO mice | 24204315 | ||
| miR-10a-5p | TSmiRs | Tiam1 | Esophageal squamous cell carcinoma (ESCC) | ESCC (54) | ESCC (2) | ESCC xenograft mice Pulmonary metastasis mice | 30426564 | |
| miR-10a-5p | TSmiRs | SKA1 | Hepatocellular carcinoma (HCC) | HCC (30) | HCC (4) | Plasma (32) | 34002462 | |
| miR-10a-5p | TSmiRs | HOXA1 | Ovarian cancer (OC) | OC (56) | OC (4) | 33332731 | ||
| miR-10a-5p | TSmiRs | GATA6 | Ovarian cancer (OC) | OC (376) | OC (2) | OC xenograft mice | 37768544 | |
| miR-10b-5p | TSmiRs | Bladder cancer (BlC) | BlC (77) | 22704449 | ||||
| miR-10b-5p | TSmiRs | Tiam1 | Breast cancer (BC) | BC (4) | 20444703 | |||
| miR-10b-5p | TSmiRs | HOXA1 | Cervical cancer (CC) | CC (40) | CC (2) | 27296950 | ||
| miR-10b-5p | TSmiRs | IGF-1R | Cervical cancer (CC) | CC (46) | CC (5) | 28599502 | ||
| miR-10b-5p | TSmiRs | TFAP2A/Tiam1 | Cervical cancer (CC) | CC (70) | CC (3) | 30504727 | ||
| miR-10b-5p | TSmiRs | Clear cell renal cell carcinoma (ccRCC) | ccRCC (250) | 28360191 | ||||
| miR-10b-5p | TSmiRs | Endometrial serous adenocarcinoma (ENC) | ESA (21) | ESA (1) | 19891660 | |||
| miR-10b-5p | TSmiRs | Endometrioid endometrial carcinoma (EEC) | EEC (28) | Plasma (12) | 24491411 | |||
| miR-10b-5p | TSmiRs | Tiam1 | Gastric cancer (GC) | GC (12) | GC (3) | GC xenograft mice | 34487733 | |
| miR-10b-5p | TSmiRs | TIAM1 | Gastric cancer (GC) | GC (19) | GC (4) | 31934163 | ||
| miR-10b-5p | TSmiRs | Tiam1 | Gastric cancer (GC) | GC (100) | GC (4) | 24481854 | ||
| miR-10b-5p | TSmiRs | MAPRE1 | Gastric cancer (GC) | GC (32) | GC (11) | 21562367 | ||
| miR-10b-5p | TSmiRs | CREB1 | Renal cancer (RC) | RC (35) | RC (4) | 26796749 | ||
| miR-10b-5p | TSmiRs | Small cell cervical carcinoma (SCCC) | SCCC (44) | 22438992 | ||||
| miR-10b-5p | TSmiRs | Cervical cancer (CC) | CC (44) | 30711417 | ||||
| miRNA | Taget gene | Cancer | Human | Mouse | Reference(PMID) | ||
| Name | Role | Tissue (n) | Cell (n) | ||||
| miR-10a-5p | oncomiRs | Akt | Cholangiocarcinoma (CCA) | CCA (3) | CCA xenograft mice | 30410355 | |
| miR-10a-5p | oncomiRs | PTEN/Akt/Wnt | Granulosa cell tumor (GCT) | Granulosa cells (2) | mir-10a KO mice GCTxenograft mice | 30348959 | |
| miR-10a-5p | oncomiRs | PTEN | Hepatocellular carcinoma (HCC) | HCC (30) | HCC (1) | 31396320 | |
| miR-10b-5p | oncomiRs | STAT3 | Ovarian cancer (OC) | OC (6) | OC (3) | 32901049 | |
| miR-10b-5p | oncomiRs | HOXD10 | Ovarian cancer (OC) | OC (68) | OC (3) | 23670532 | |
| miR-10a-5p | oncomiRs | PTEN | Acute myeloid leukemia (AML) | AML (60) | AML (1) | 32319862 | |
| miR-10a-5p | oncomiRs | TBX5 | Cervical squamous cell carcinoma (CSCC) | CSCC (60) | CSCC (2) | GSCC xenograft mice | 33235271 |
| miR-10a-5p | oncomiRs | PTEN | Cervical cancer (CC) | CC (40) | CC (2) | 25018014 | |
| miR-10b-5p | oncomiRs | E-cadherin | Breast cancer (BC) | BC (45) | BC (2) | BC xenograft mice | 32305959 |
| miR-10b-5p | oncomiRs | IQGAP2 | Triple-negative breast cancer (TNBC) | TNBC (42) | TNBC (3) | 33248413 | |
| miR-10b-5p | oncomiRs | Twist | Breast cancer (BC) | BC (18) | BC (6) | 17898713 | |
| miR-10b-5p | oncomiRs | E-cadherin | Breast cancer (BC) | BC (44) | BC (1) | 22847191 | |
| miR-10b-5p | oncomiRs | HOXD10 | Gastric cancer (GC) | GC (436) | GC (7) | 26311318 | |
| miR-10b-5p | oncomiRs | p21 and p53 | Colorectal cancer (CRC) | CRC (63) | CRC (5) | CRC xenograft mice | 31595500 |
| miR-10b-5p | oncomiRs | FGF13 | Colorectal cancer (CRC) | CRC (28) | CRC (5 ) | 33495804 | |
| miR-10b-5p | oncomiRs | HOXD10 | Colorectal cancer (CRC) | CRC (70) | 25606801 | ||
| miR-10b-5p | oncomiRs | TWIST-1 and E-cadherin | Colorectal cancer (CRC) | CRC (50 ) | CRC (1 ) | 28345456 | |
| miR-10b-5p | oncomiRs | Colorectal cancer (CRC) | CRC (246) | 27592860 | |||
| miR-10b-5p | oncomiRs | GSK3β | Prostate cancer (PC) | PC (2) | 30844757 | ||
| miR-10b-5p | oncomiRs | TGF-β1 | Glioblastoma | GBM (15) | Glioma (2) | 28393237 | |
| miR-10b-5p | oncomiRs | HOXD10 | Glioma | Glioma (22) | Glioma (4) | 21419107 | |
| miR-10b-5p | oncomiRs | Sirt1 | Glioma | Glioma (2 | 31889362 | ||
| miR-10b-5p | oncomiRs | HOXB3 | Endometrial cancer (EC) | EC (20) | 27447302 | ||
| miR-10b-5p | oncomiRs | KLF4/HOXD10 | Bladder cancer | Bladder cancer (20) | Bladder cancer (6) | 24573354 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





