Submitted:
10 December 2024
Posted:
11 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
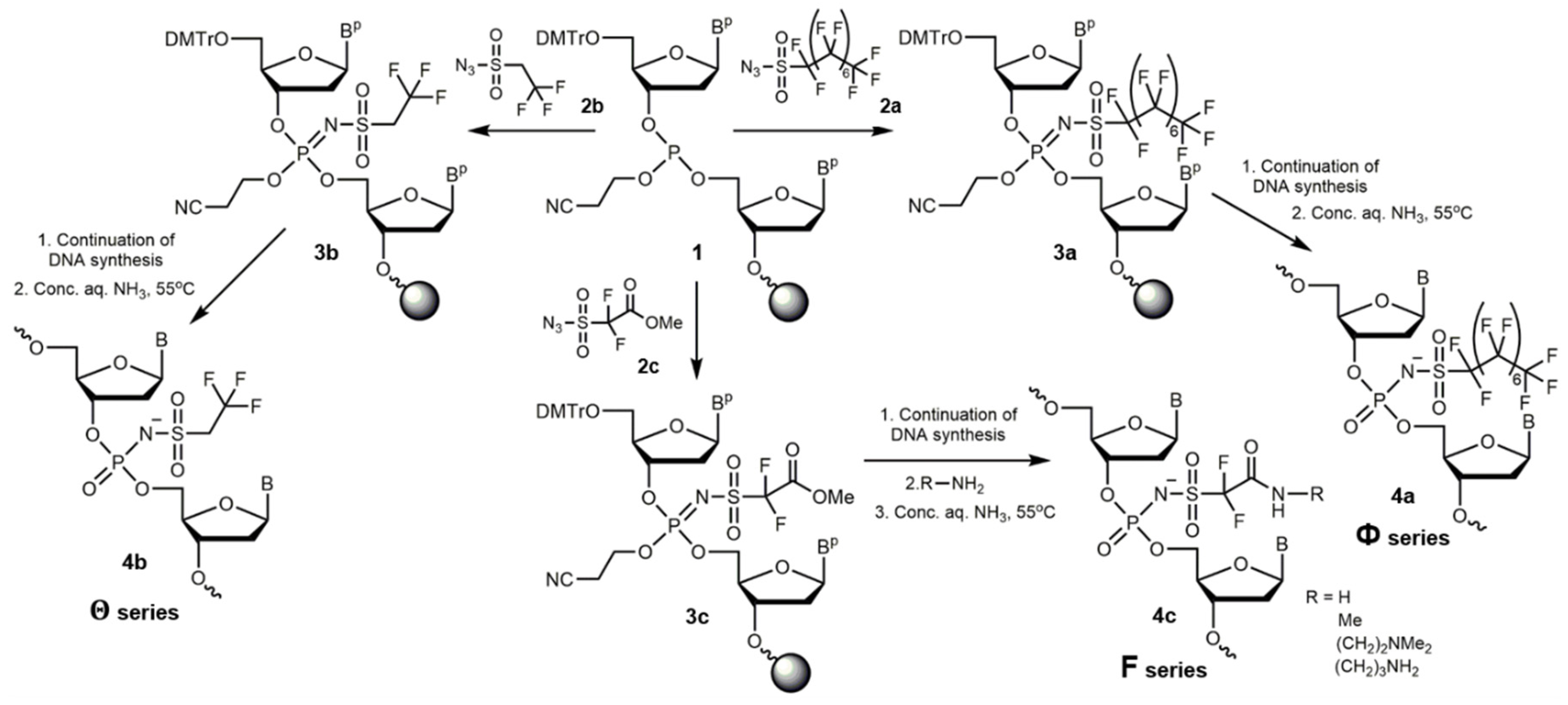
2.1. Synthesis and Characterization of New Polyfluorinated Oligonucleotide Derivatives
| Code | Oligonucleotide sequence, 5′-3′a | Amine R–NH2 |
|---|---|---|
| F1 | DMTr-TFiTTTTTb | NH3/PriOH (i) R = H |
| F2 | TFiTTTTT | |
| F3 | DMTr-TFiiTTTTT | MeNH2/EtOH (ii) R = Me |
| F4 | TFiiTTTTT | |
| F5 | TFiiiTTTTT | 1,1-Dimethylethylenediamine (iii) R = –(CH2)2NMe2 |
| F6 | TTTTTFiiiT | |
| F7 | TTTTFiiiTFiiiT | |
| F8 | TFiiiTTTTFiiiT | |
| F9 | TFiiiTFiiiTTFiiiTFiiiT | |
| F10 | TFiiiTFiiiTFiiiTFiiiTFiiiT | |
| F11 | CTCCCAGGCTCAAAFiiiT | |
| F12 | CTC CCAGGFiiiCTCAAAT | |
| F13 | CTCCCAGGCTCAAFiiiAFiiiT | |
| F14 | CFiiiTCCCAGGCTCAAAFiiiT | |
| F15 | CTCCCAGGFiiiCTCAAAFiiiT | |
| F16 | TFivTTTTT | 1,3-Diaminopropane (iv) R = –(CH2)3NH2 |
| F17 | TTTTTFivT | |
| F18 | TTTTFivTFivT | |
| F19 | TFivTTTTFivT | |
| F20 | TFivTFivTTFivTFivT | |
| F21 | TFivTFivTFivTFivTFivT |
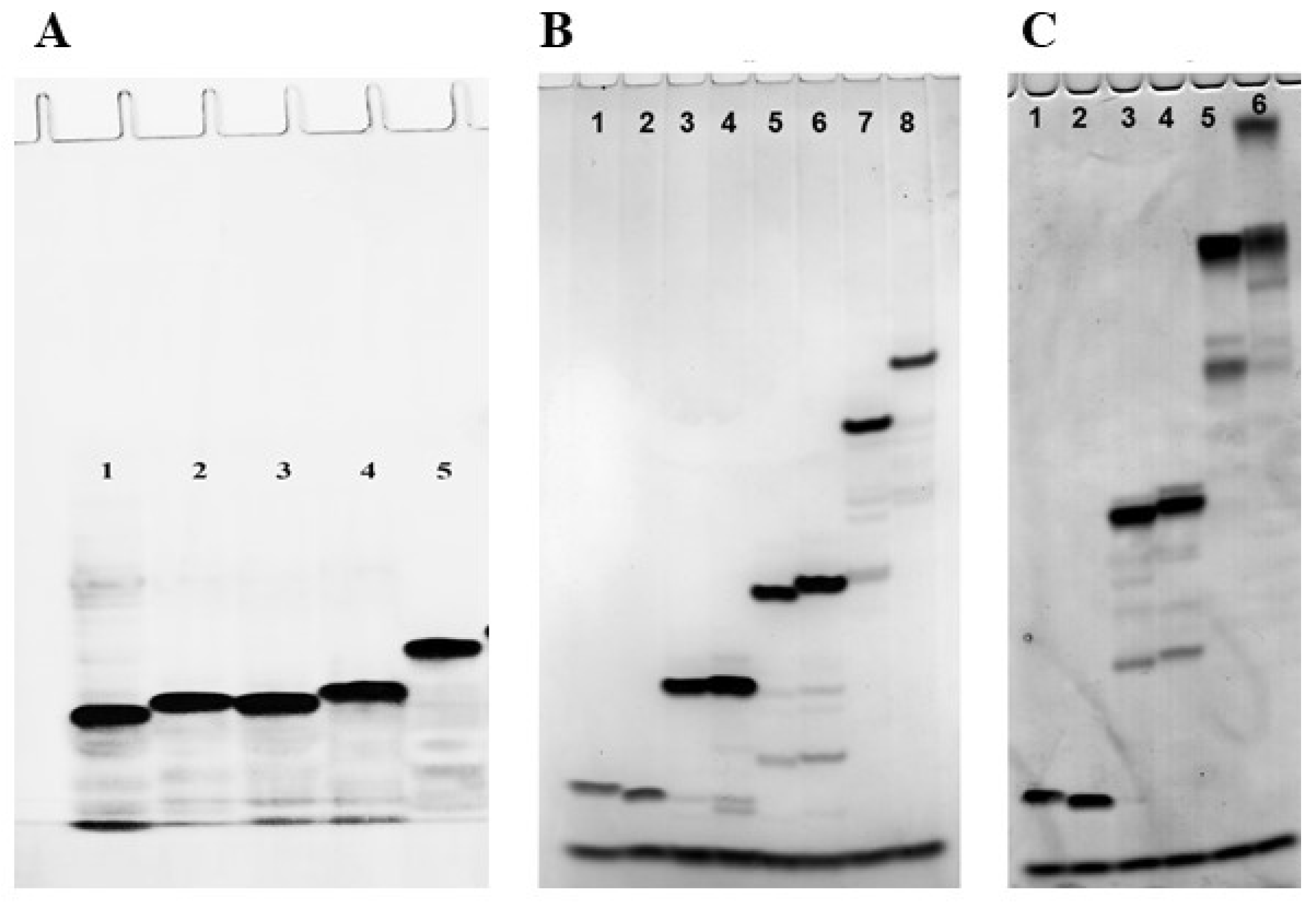
2.2. Electrophoretic Mobility of the Polyfluoro Oligonucleotides
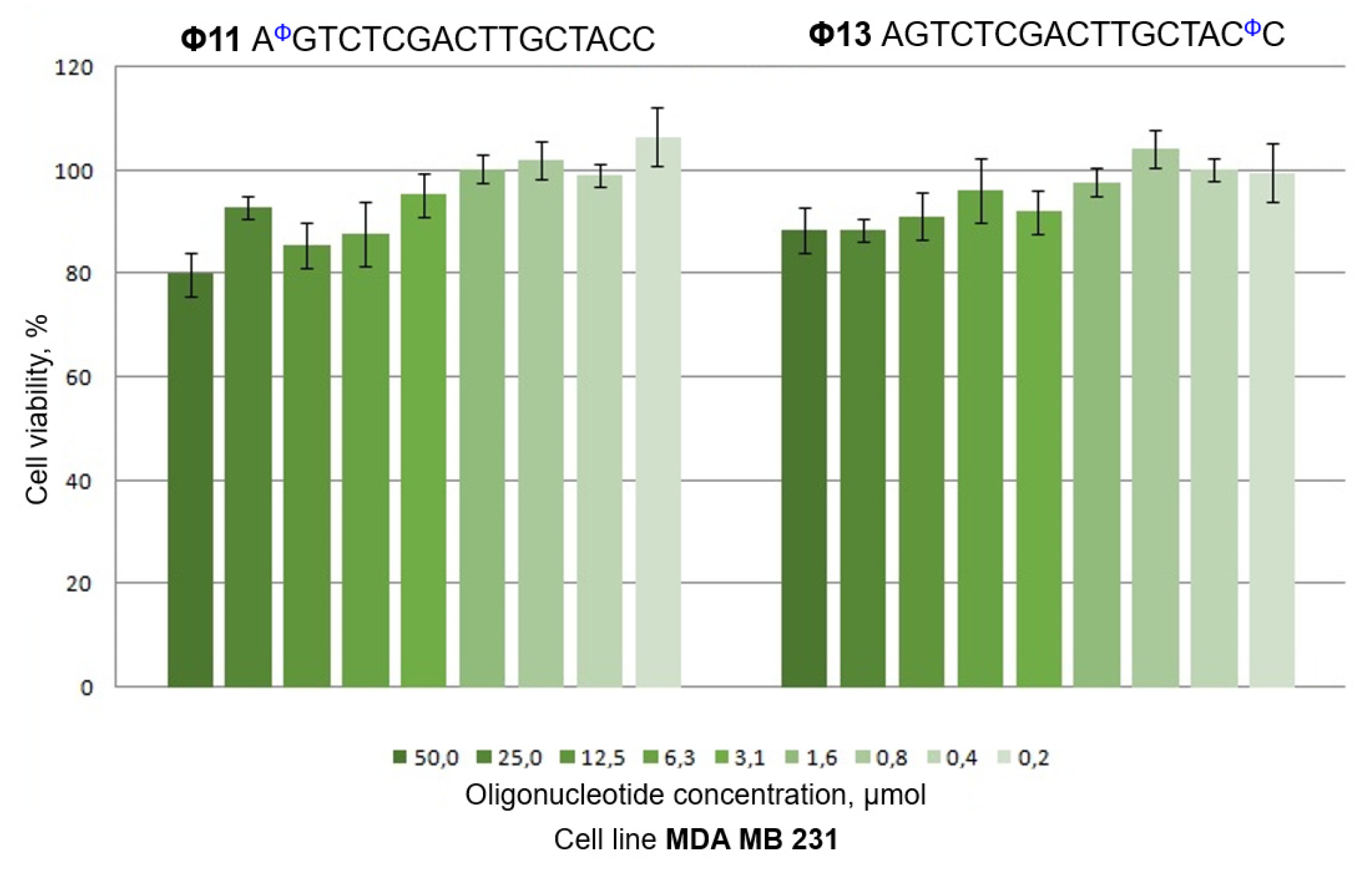
2.3. Cytotoxicity of Polyfluoro Oligonucleotides to Human Breast Cancer Cells
2.3. Thermal Denaturation Studies of Duplexes of Polyfluorinated Oligonucleotides with DNA and RNA
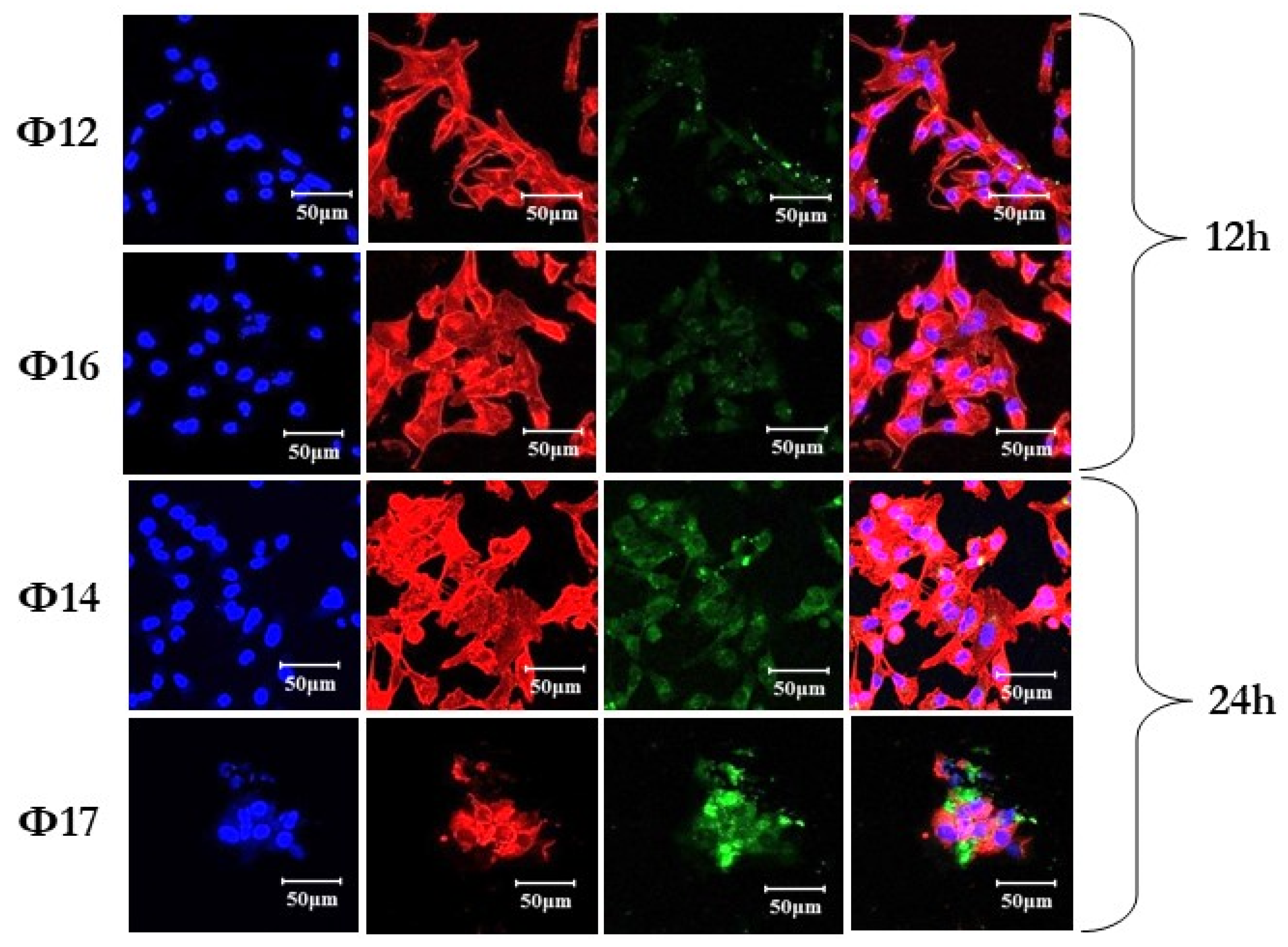
2.3. Laser Confocal Microscopy Study of the Uptake of Polyfluorinated Oligonucleotides into Human Breast Cancer Cells
3. Materials and Methods
3.1. Synthesis of Perfluoro-1-Octanesulfonyl Azide, 2,2,2-Trifluoroethanesulfonyl Azide, and 2,2-Difluoro-3-Azidosulfonylacetate
3.2. Synthesis of Oligonucleotides
3.3. Synthesis of Oligonucleotide Conjugates
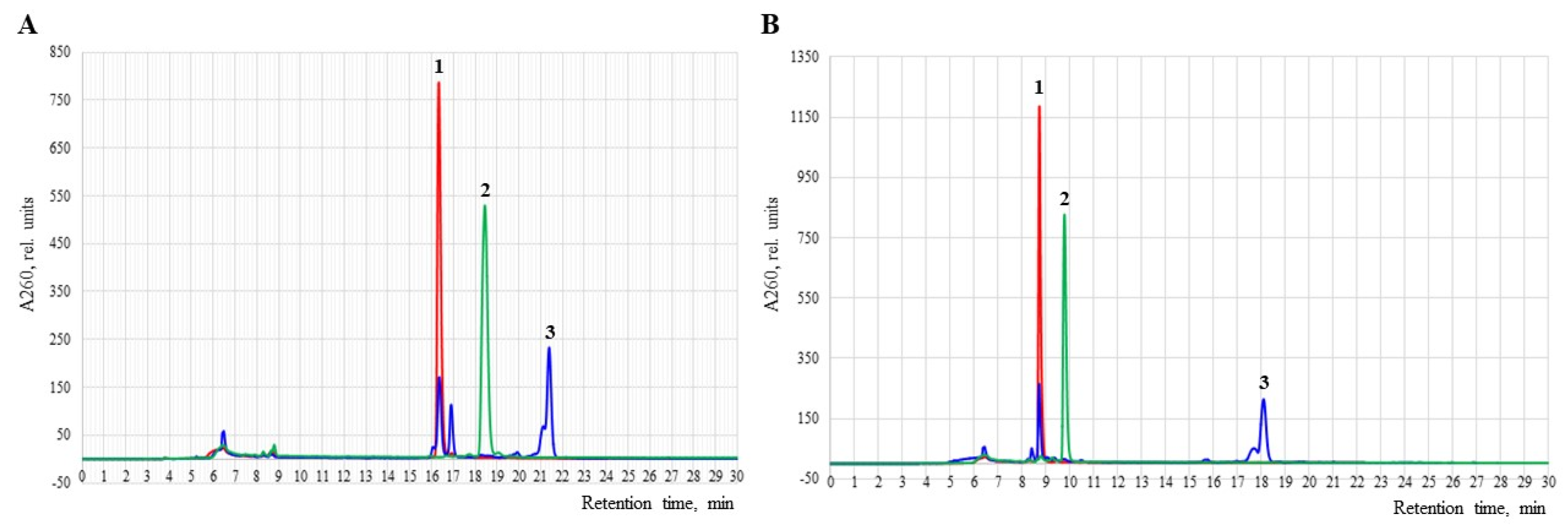
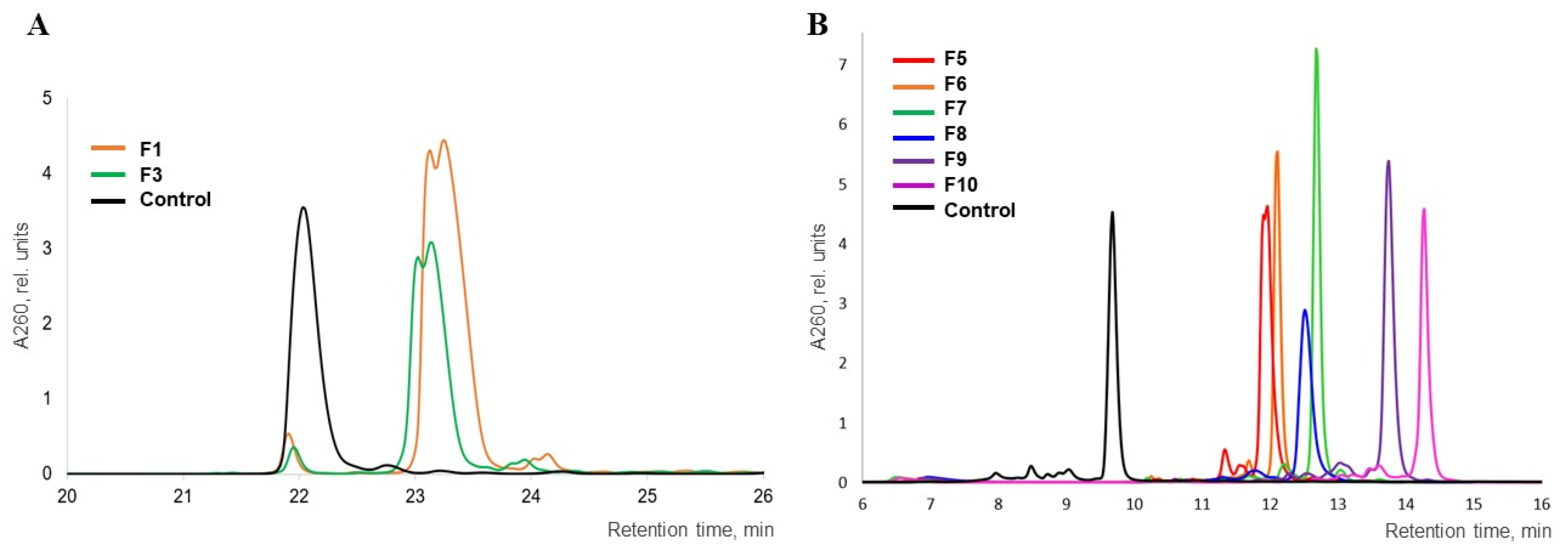
3.4. Reverse-Phase HPLC Analysis of Oligonucleotides and Conjugates
3.5. Polyacrylamide Gel Electrophoresis Under Denaturing Conditions
4.6. UV Melting Studies
4.7. Cell Line
4.8. MTT Assay of Cell Viability
4.9. Confocal Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodchild, J. Therapeutic oligonucleotides. Methods Mol. Biol. 2011, 764, 1-15. [CrossRef]
- Moumné, L.; Marie, A.C.; Crouvezier, N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics. 2022, 14, 260. [CrossRef]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238-248. [CrossRef]
- Thakur, S.; Sinhari, A.; Jain, P.; Jadhav, H.R. A perspective on oligonucleotide therapy: Approaches to patient customization. Front Pharmacol. 2022, 13, 1006304. [CrossRef]
- Egli, M.; Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 2023, 51, 2529-2573. [CrossRef]
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. U.S.A. 1978, 75, 280-284. [CrossRef]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.H. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427-453. [CrossRef]
- Aartsma-Rus, A. FDA Approval of Nusinersen for Spinal Muscular Atrophy Makes 2016 the Year of Splice Modulating Oligonucleotides. Nucleic Acid Ther. 2017, 27, 67-69. [CrossRef]
- Aartsma-Rus, A.; Krieg, A.M. FDA Approves Eteplirsen for Duchenne Muscular Dystrophy: The Next Chapter in the Eteplirsen Saga. Nucleic Acid Ther. 2017, 27, 1-3. [CrossRef]
- Heo, Y.A. Golodirsen: First Approval. Drugs 2020, 80, 329-333. [CrossRef]
- Shirley, M. Casimersen: First Approval. Drugs 2021, 81, 875-879. [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998, 391, 806-811. [CrossRef]
- Kurreck, J. RNA interference: from basic research to therapeutic applications. Angew. Chem. Int. Ed. Engl. 2009, 48, 1378-1398. [CrossRef]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625-1631. [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520-1530. [CrossRef]
- Scott, L.J. Givosiran: First Approval. Drugs 2020, 80, 335-339. [CrossRef]
- Scott, L.J.; Keam, S.J. Lumasiran: First Approval. Drugs 2021, 81, 277-282. [CrossRef]
- Keam, S.J. Vutrisiran: First Approval. Drugs 2022, 82, 1419-1425. [CrossRef]
- Lamond, A.I.; Sproat, B.S. Antisense oligonucleotides made of 2′-O-alkylRNA: their properties and applications in RNA biochemistry. FEBS Lett. 1993, 325, 123-127. [CrossRef]
- Majlessi, M.; Nelson, N.C.; Becker, M.M. Advantages of 2′-O-methyl oligoribonucleotide probes for detecting RNA targets. Nucleic Acids Res. 1998, 26, 2224-2229. [CrossRef]
- Kawasaki, A.M.; Casper, M.D.; Freier, S.M.; Lesnik, E.A.; Zounes, M.C.; Cummins, L.L.; Gonzalez, C.; Cook, P.D. Uniformly modified 2′-deoxy-2′-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J. Med. Chem. 1993, 36, 831-841, doi:1 0.1021/jm00059a007.
- El-Khoury, R.; Damha, M.J. 2′-Fluoro-arabinonucleic Acid (FANA): A Versatile Tool for Probing Biomolecular Interactions. Acc. Chem. Res. 2021, 54, 2287-2297. [CrossRef]
- Imanishi, T.; Obika, S. BNAs: novel nucleic acid analogs with a bridged sugar moiety. Chem. Commun. 2002, 1653-1659. [CrossRef]
- Kaur, H.; Babu, B.R.; Maiti, S. Perspectives on chemistry and therapeutic applications of Locked Nucleic Acid (LNA). Chem. Rev. 2007, 107, 4672-4697. [CrossRef]
- Veedu, R.N.; Wengel, J. Locked nucleic acids: promising nucleic acid analogs for therapeutic applications. Chem. Biodivers. 2010, 7, 536-542. [CrossRef]
- Altmann, K.H.; Fabbro, D.; Dean, N.M.; Geiger, T.; Monia, B.P.; Muller, M.; Nicklin, P. Second-generation antisense oligonucleotides: structure-activity relationships and the design of improved signal-transduction inhibitors. Biochem. Soc. Trans. 1996, 24, 630-637. [CrossRef]
- Altmann, K.-H.; Dean, N.M.; Fabbro, D.; Freier, S.M.; Geiger, T.; Häner, R.; Hüsken, D.; Martin, P.; Monia, B.P.; Müller, M.; et al. Second Generation of Antisense Oligonucleotides: From Nuclease Resistance to Biological Efficacy in Animals. Chimia 1996, 50, 168. [CrossRef]
- Renneberg, D.; Bouliong, E.; Reber, U.; Schumperli, D.; Leumann, C.J. Antisense properties of tricyclo-DNA. Nucleic Acids Res. 2002, 30, 2751-2757. [CrossRef]
- Eckstein, F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014, 24, 374-387. [CrossRef]
- Miroshnichenko, S.K.; Patutina, O.A.; Burakova, E.A.; Chelobanov, B.P.; Fokina, A.A.; Vlassov, V.V.; Altman, S.; Zenkova, M.A.; Stetsenko, D.A. Mesyl phosphoramidate antisense oligonucleotides as an alternative to phosphorothioates with improved biochemical and biological properties. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 1229-1234. [CrossRef]
- Patutina, O.A.; Gaponova Miroshnichenko, S.K.; Sen’kova, A.V.; Savin, I.A.; Gladkikh, D.V.; Burakova, E.A.; Fokina, A.A.; Maslov, M.A.; Shmendel, E.V.; Wood, M.J.A.; et al. Mesyl phosphoramidate backbone modified antisense oligonucleotides targeting miR-21 with enhanced in vivo therapeutic potency. Proc. Natl. Acad. Sci. U.S. A. 2020, 117, 32370-32379. [CrossRef]
- Anderson, B.A.; Freestone, G.C.; Low, A.; De-Hoyos, C.L.; Iii, W.J.D.; Ostergaard, M.E.; Migawa, M.T.; Fazio, M.; Wan, W.B.; Berdeja, A.; et al. Towards next generation antisense oligonucleotides: mesylphosphoramidate modification improves therapeutic index and duration of effect of gapmer antisense oligonucleotides. Nucleic Acids Res 2021, 49, 9026-9041. [CrossRef]
- Kupryushkin, M.S.; Pyshnyi, D.V.; Stetsenko, D.A. Phosphoryl guanidines: a new type of nucleic Acid analogues. Acta Naturae 2014, 6, 116-118.
- Kupryushkin, M.S.; Filatov, A.V.; Mironova, N.L.; Patutina, O.A.; Chernikov, I.V.; Chernolovskaya, E.L.; Zenkova, M.A.; Pyshnyi, D.V.; Stetsenko, D.A.; Altman, S.; et al. Antisense oligonucleotide gapmers containing phosphoryl guanidine groups reverse MDR1-mediated multiple drug resistance of tumor cells. Mol. Ther. Nucleic Acids 2022, 27, 211-226. [CrossRef]
- Kandasamy, P.; Liu, Y.; Aduda, V.; Akare, S.; Alam, R.; Andreucci, A.; Boulay, D.; Bowman, K.; Byrne, M.; Cannon, M.; et al. Impact of guanidine-containing backbone linkages on stereopure antisense oligonucleotides in the CNS. Nucleic Acids Res. 2022, 50, 5401-5423. [CrossRef]
- Miller, P.S. Oligonucleoside Methylphosphonates as Antisense Reagents. Bio/Technology 1991, 9, 358-362, doi:.10.1038/nbt0491-358.
- Marshall, W.S.; Caruthers, M.H. Phosphorodithioate DNA as a potential therapeutic drug. Science 1993, 259, 1564-1570, doi:.10.1126/science.7681216.
- Summers, J.S.; Shaw, B.R. Boranophosphates as mimics of natural phosphodiesters in DNA. Curr. Med. Chem. 2001, 8, 1147-1155. [CrossRef]
- Dellinger, D.J.; Sheehan, D.M.; Christensen, N.K.; Lindberg, J.G.; Caruthers, M.H. Solid-phase chemical synthesis of phosphonoacetate and thiophosphonoacetate oligodeoxynucleotides. .J Am. Chem. Soc. 2003, 125, 940-950. [CrossRef]
- Yamada, C.M.; Dellinger, D.J.; Caruthers, M.H. Synthesis and biochemical evaluation of phosphonoformate oligodeoxyribonucleotides. J. Am. Chem. Soc. 2006, 128, 5251-5261. [CrossRef]
- Nielsen, P.E. Peptide nucleic acids (PNA) in chemical biology and drug discovery. Chem. Biodivers. 2010, 7, 786-804. [CrossRef]
- Summerton, J.; Weller, D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997, 7, 187-195. [CrossRef]
- Summerton, J. History and properties of morpholino antisense oligos. J. Drug Discov. Develop. Deliv 2016, 3, 1019.
- Juliano, R.; Bauman, J.; Kang, H.; Ming, X. Biological barriers to therapy with antisense and siRNA oligonucleotides. Mol Pharm 2009, 6, 686-695. [CrossRef]
- Juliano, R.L.; Ming, X.; Nakagawa, O. The chemistry and biology of oligonucleotide conjugates. Acc Chem Res 2012, 45, 1067-1076. [CrossRef]
- Juliano, R.L.; Carver, K.; Cao, C.; Ming, X. Receptors, endocytosis, and trafficking: the biological basis of targeted delivery of antisense and siRNA oligonucleotides. J Drug Target 2013, 21, 27-43. [CrossRef]
- Juliano, R.L.; Ming, X.; Carver, K.; Laing, B. Cellular uptake and intracellular trafficking of oligonucleotides: implications for oligonucleotide pharmacology. Nucleic Acid Ther. 2014, 24, 101-113. [CrossRef]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res 2016, 44, 6518-6548. [CrossRef]
- Godfrey, C.; Desviat, L.R.; Smedsrod, B.; Pietri-Rouxel, F.; Denti, M.A.; Disterer, P.; Lorain, S.; Nogales-Gadea, G.; Sardone, V.; Anwar, R.; et al. Delivery is key: lessons learnt from developing splice-switching antisense therapies. EMBO Mol. Med. 2017, 9, 545-557. [CrossRef]
- Sabin, J.; Alatorre-Meda, M.; Minones, J., Jr.; Dominguez-Arca, V.; Prieto, G. New insights on the mechanism of polyethylenimine transfection and their implications on gene therapy and DNA vaccines. Colloids Surf. B Biointerfaces 2022, 210, 112219. [CrossRef]
- Ravina, M.; Paolicelli, P.; Seijo, B.; Sanchez, A. Knocking down gene expression with dendritic vectors. Mini Rev. Med. Chem. 2010, 10, 73-86. [CrossRef]
- Li, X.; Feng, K.; Li, L.; Yang, L.; Pan, X.; Yazd, H.S.; Cui, C.; Li, J.; Moroz, L.; Sun, Y.; et al. Lipid–oligonucleotide conjugates for bioapplications. Nat. Sci. Rev. 2020, 7, 1933-1953. [CrossRef]
- Patwa, A.; Gissot, A.; Bestel, I.; Barthélémy, P. Hybrid lipid oligonucleotide conjugates: synthesis, self-assemblies and biomedical applications. Chem. Soc. Rev. 2011, 40, 5844-5854. [CrossRef]
- Zhao, B.; Tian, Q.; Bagheri, Y.; You, M. Lipid-Oligonucleotide Conjugates for Simple and Efficient Cell Membrane Engineering and Bioanalysis. Curr Opin Biomed Eng 2020, 13, 76-83. [CrossRef]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; El Andaloussi, S. Peptides for nucleic acid delivery. Adv Drug Deliv Rev 2016, 106, 172-182. [CrossRef]
- Winkler, J.; Saadat, K.; Díaz-Gavilán, M.; Urban, E.; Noe, C.R. Oligonucleotide–polyamine conjugates: Influence of length and position of 2′-attached polyamines on duplex stability and antisense effect. European Journal of Medicinal Chemistry 2009, 44, 670-677. [CrossRef]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017, 9, 60. [CrossRef]
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; Foged, C.; Gaina, G.; Garanto, A.; Goyenvalle, A.T.; Guzowska, M.; Holodnuka, I.; Jones, D.R.; Krause, S.; Lehto, T.; Montolio, M.; Van Roon-Mom, W.; Arechavala-Gomeza, V. Delivery of oligonucleotide-based therapeutics: challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [CrossRef]
- Matsuda, S.; Keiser, K.; Nair, J.K.; Charisse, K.; Manoharan, R.M.; Kretschmer, P.; Peng, C.G.; V Kel’in A.; Kandasamy, P.; Willoughby, J.L.; Liebow, A.; Querbes, W.; Yucius, K.; Nguyen, T.; Milstein, S.; Maier, M.A.; Rajeev, K.G.; Manoharan, M. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015, 10, 1181-1187. [CrossRef]
- Corey, D.R.; Damha, M.J.; Manoharan, M. Challenges and Opportunities for Nucleic Acid Therapeutics. Nucleic Acid Ther. 2022, 32, 8-13. Epub 2021 Dec 17. PMID: 34931905; PMCID: PMC8817707. [CrossRef]
- Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Ojima, I., Ed.; Wiley-Blackwell: 2009; pp. 1-624.
- Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 2012, 41, 31-42. [CrossRef]
- Xin, J.; Lu, X.; Cao, J.; Wu, W.; Liu, Q.; Wang, D.; Zhou, X.; Ding, D. Fluorinated Organic Polymers for Cancer Drug Delivery. Adv. Mater. 2024, 36, e2404645. [CrossRef]
- Zhang, Z.; Shen, W.; Ling, J.; Yan, Y.; Hu, J.; Cheng, Y. The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat. Commun. 2018, 9, 1377. [CrossRef]
- Lv. J.; Wang, H.; Rong, G.; Cheng, Y. Fluorination Promotes the Cytosolic Delivery of Genes, Proteins, and Peptides. Acc. Chem. Res. 2022, 55, 722-733. [CrossRef]
- Wan, Y.; Yang, Y.; Wu, M.; Feng, S. Fluorinated vectors for gene delivery. Expert Opin Drug Deliv. 2022 Nov;19(11):1435-1448. [CrossRef]
- Tang, D.; Yan, Y.; Li, Y.; Li, Y.; Tian, J.; Yang, L.; Ding, H.; Bashir, G.; Zhou, H.; Ding, Q.; Tao, R.; Zhang, S.; Wang, Z.; Wu, S. Targeting DAD1 gene with CRISPR-Cas9 system transmucosally delivered by fluorinated polylysine nanoparticles for bladder cancer intravesical gene therapy. Theranostics. 2024, 14, 203-219. [CrossRef]
- Li, J.; Wu, Y.; Xiang, J.; Wang, H.; Zhuang, Q.; Wei, T.; Cao, Z.; Gu, Q.; Liu, Z.; Peng, R. Fluoroalkane modified cationic polymers for personalized mRNA cancer vaccines. Chem. Eng. J. 2023, 456, 140930. [CrossRef]
- Romani, C,; Sponchioni, M.; Volonterio, A. Fluorinated PAMAM-Arginine Carrier Prodrugs for pH-Sensitive Sustained Ibuprofen Delivery. Pharm Res. 2024, 41, 1725-1736. [CrossRef]
- Forgham, H.; Zhu, J.; Zhang, T.; Huang, X.; Li, X.; Shen, A.; Biggs, H.; Talbo, G.; Xu, C.; Davis, T.P.; Qiao, R. Fluorine-modified polymers reduce the adsorption of immune-reactive proteins to PEGylated gold nanoparticles. Nanomedicine (Lond). 2024, 19, 995-1012. [CrossRef]
- Chen, G.; Wang, K.; Wang, Y.; Wu, P.; Sun, M.; Oupický, D. Fluorination Enhances Serum Stability of Bioreducible Poly(amido amine) Polyplexes and Enables Efficient Intravenous siRNA Delivery. Adv. Healthc. Mater. 2018, 7, 1700978. [CrossRef]
- Wang, M.M.; Liu, H.M.; Li, L.; Cheng, Y.Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat. Commun. 2014, 5, 3053. [CrossRef]
- Liu, H.M.; Wang, Y.; Wang, M.M.; Xiao, J.R.; Cheng, Y.Y. Fluorinated poly(propylenimine) dendrimers as gene vectors. Biomaterials. 2014, 35, 5407-5413. [CrossRef]
- Wang, M.; Cheng, Y. The effect of fluorination on the transfection efficacy of surface-engineered dendrimers. Biomaterials. 2014, 35, 6603-6613. [CrossRef]
- Wang, M.; Cheng, Y. Structure-activity relationships of fluorinated dendrimers in DNA and siRNA delivery. Acta Biomater. 2016, 46, 204-210. [CrossRef]
- Lv, J.; Chang, H.; Wang, Y.; Wang, M.M.; Xiao, J.R.; Zhang, Q.; Cheng, Y.Y. Fluorination on polyethylenimine allows efficient 2D and 3D cell culture gene delivery. J. Mater. Chem. B. 2015, 3, 642-650. [CrossRef]
- He, B.; Wang, Y.; Shao, N.; Chang, H.; Cheng. Y. Polymers modified with double-tailed fluorous compounds for efficient DNA and siRNA delivery. Acta Biomater. 2015, 22, 111-119. [CrossRef]
- Xiao, Y.P.; Zhang, J.; Liu, Y.H.; Zhang, J.H.; Yu, Q.Y.; Huang, Z.; Yu, X.Q. Low molecular weight PEI-based fluorinated polymers for efficient gene delivery. Eur. J. Med. Chem. 2019, 162, 602-611. [CrossRef]
- Lee, G.J.; Kim, T.I. Fluorination effect to intermediate molecular weight polyethylenimine for gene delivery systems. J. Biomed. Mater. Res. A. 2019, 107, 2468-2478. [CrossRef]
- Yu, A.; Tang, S.; Ding, L.; Foley, J.; Tang, W.; Jia, H.; Panja, S.; Holbert, C.E.; Hang, Y.; Stewart, T.M.; Smith, L.M.; Sil, D.; Casero, R.A. Jr.; Oupický, D. Hyaluronate-coated perfluoroalkyl polyamine prodrugs as bioactive siRNA delivery systems for the treatment of peritoneal cancers. Biomater. Adv. 2022, 136, 212755. [CrossRef]
- Zhou, Q.; Li, K.; Wang, K.; Hong, W.; Chen, J.; Chai, J.; Yu, L.; Si, Z.; Li, P. Fluoroamphiphilic polymers exterminate multidrug-resistant Gram-negative ESKAPE pathogens while attenuating drug resistance. Sci Adv. 2024, 10, eadp6604. [CrossRef]
- Metelev, V.; Zhang, S.; Zheng, S.; Kumar, A.T.N.; Bogdanov, A., Jr. Fluorocarbons Enhance Intracellular Delivery of Short STAT3-sensors and Enable Specific Imaging. Theranostics 2017, 7, 3354-3368. [CrossRef]
- Ezzat, K.; Aoki, Y.; Koo, T.; McClorey, G.; Benner, L.; Coenen-Stass, A.; O’Donovan, L.; Lehto, T.; Garcia-Guerra, A.; Nordin, J.; et al. Self-Assembly into Nanoparticles Is Essential for Receptor Mediated Uptake of Therapeutic Antisense Oligonucleotides. Nano Lett. 2015, 15, 4364–4373. [CrossRef]
- Derzhalova A., Markov O., Fokina A., Shiohama Y., Zatsepin T., Fujii M., Zenkova M., Stetsenko D. Novel lipid-oligonucleotide conjugates containing long-chain sulfonyl phosphoramidate groups: Synthesis and biological properties. Appl. Sci. 2021, 11, 1174. [CrossRef]
- Kupryushkin, M.S.; Apukhtina, V.S.; Vasilyeva, S.V.; Pyshnyi, D.V.; Stetsenko D.A. A new simple and convenient method for preparation of oligonucleotides containing a pyrene or a cholesterol moiety. Russ. Chem. Bull. 2015, 64, 1678-1681. [CrossRef]
- Prokhorova, D.V.; Chelobanov, B.P.; Burakova, E.A.; Fokina, A.A.; Stetsenko, D.A. New oligodeoxyribonucleotide derivatives bearing internucleotide N-tosyl phosphoramidate groups: Synthesis and complementary binding to DNA and RNA. Russ. J. Bioorg. Chem. 2017, 43, 38-42. [CrossRef]
- Chelobanov, B.P.; Burakova, E.A.; Prokhorova, D.V.; Fokina, A.A.; Stetsenko, D.A. New oligodeoxynucleotide derivatives containing N-(methanesulfonyl)-phosphoramidate (mesyl phosphoramidate) internucleotide group. Russ. J. Bioorg. Chem. 2017, 43, 664-668. [CrossRef]
- Su, Y.; Bayarjargal, M.; Hale, T.K.; Filichev, V.V. DNA with zwitterionic and negatively charged phosphate modifications: Formation of DNA triplexes, duplexes and cell uptake studies. Beilstein J. Org. Chem. 2021, 17, 749-761. [CrossRef]
- Su, Y.; Raguraman, P.; Veedu, R.N.; Filichev, V.V. Phosphorothioate modification improves exon-skipping of antisense oligonucleotides based on sulfonyl phosphoramidates in mdx mouse myotubes. Org. Biomol. Chem. 2022, 20, 3790-3797. [CrossRef]
- Santorelli, A.; Gothelf, K.V. Conjugation of chemical handles and functional moieties to DNA during solid phase synthesis with sulfonyl azides. Nucleic Acids Res. 2022, 50, 7235-7246. [CrossRef]
- Letsinger, R.L.; Heavner, G.A. Synthesis of phosphoromonoamidate diester nucleotides via the phosphite-azide coupling method. Tetrahedron Lett. 1975, 16, 147-150. [CrossRef]
- Heindl, D.; Kessler, D. Polynucleotide containing a phosphate mimetic. Patent WO2007059816.
- Heindl, D.; Kessler, D.; Schube, A.; Thuer, W.; Giraut, A. Easy method for the synthesis of labeled oligonucleotides. Nucleic Acids Symp. Ser. 2008, 405-406. [CrossRef]
- Klabenkova, K.V.; Zhdanova, P.V.; Burakova, E.A.; Bizyaev, S.N.; Fokina, A.A.; Stetsenko, D.A. A Convenient Oligonucleotide Conjugation via Tandem Staudinger Reaction and Amide Bond Formation at the Internucleotidic Phosphate Position. Int J Mol Sci 2024, 25. [CrossRef]
| Oligonucleotide sequence, 5′-3′a | Code | Oligonucleotide sequence, 5′-3′ | Code |
|---|---|---|---|
| DMTr-TθbTTTTTc | Θb1 | GCGCCAφAACA | Φ4 |
| TθTTTTT | Θ2 | GCGCCAφAACφA | Φ5 |
| GCGCCAAACθA | Θ3 | TGφTTTGGCGC | Φ6 |
| GCGCCAθAACA | Θ4 | TGTTTφGGCGC | Φ7 |
| GCGCCAθAACθA | Θ5 | TGφTTTφGGCGC | Φ8 |
| GθCθGθCθCθAθAθAθCθA | Θ6 | TTTTTTTTTTφTTTTTTTTTT | Φ9 |
| TθGθTθ TθTθGθGθCθGθC | Θ7 | AAAAAAAAAAφAAAAAAAAAA | Φ10 |
| GdCGCCAAACθA | Θ8 | AφGTCTCGACTTGCTACC | Φ11 |
| GCGCCAθAACA | Θ9 | AφGTCTCGACTTGCTACC-Flu | Φ12 |
| GCGCCAθAACθA | Θ10 | AGTCTCGACTTGCTACφC | Φ13 |
| GθCθGθCθCθAθAθAθCθA | Θ11 | Flu-AGTCTCGACTTGCTACφC | Φ14 |
| DMTr-TφeTTTTT | Φe1 | AφGTCTCGACTTGCTACφC | Φ15 |
| TφTTTTT | Φ2 | AφGTCTCGACTTGCTACφC-Flu | Φ16 |
| GCGCCAAACφA | Φ3 | Flu-AφGTCTCGACTTGCTACφC | Φ17 |
| Code | Oligonucleotide sequence, 5′-3′ | Tm, °C | ΔTm, °C | Tm, °C | ΔTm, °C | ||
| DNA template 5′-d(TGTTTGGCGC) |
RNA template 5′-r(UGUUUGGCGC) |
||||||
| Control |  |
GCGCCAAACA | 54.3 ± 0.2 | – | 49.1 ± 0.2 | – | |
| Θ3 |  |
GCGCCAAACθA | 54.6 ± 0.2 | +0.3 ± 0.3 | 49.6 ± 0.2 | +0.5 ± 0.3 | |
| Θ4 |  |
GCGCCAθAACA | 54.9 ± 0.1 | +0.6 ± 0.2 | 48.9 ± 0.1 | –0.2 ± 0.3 | |
| Θ5 |  |
GCGCCAθAACθA | 53.9 ± 0.3 | –0.4 ± 0.4 | 48.5 ± 0.1 | –0.6 ± 0.3 | |
| Θ6 |  |
GθCθGθCθCθAθAθAθCθA | 50.8 ± 0.3 | –3.6 ± 0.4 | 41.4 ± 0.2 | –7.6 ± 0.3 | |
| Φ3 |  |
GCGCCAAACφA | 54.5 ± 0.5 | 0 | – | – | |
| Φ4 |  |
GCGCCAφAACA | 53.9 ± 0.5 | 0 | – | – | |
| DNA template 5′-d(ATTTGAGCCTGGGAG) |
RNA template 5′-r(AUUUGAGCCUGGGAG) |
||||||
| Control |  |
CTCCCAGGCTCAAAT | 61.0 ± 0.4 | – | 65.7 ± 0.5 | – | |
| F11 |  |
CTCCCAGGCTCAAAF3T | 59.6 ± 0.7 | –1.4 ± 0.8 | 65.0 ± 0.9 | –0.7 ± 1.0 | |
| F12 |  |
CTC CCAGGF3CTCAAAT | 59.4 ± 0.5 | –1.6 ± 0.6 | 65.7 ± 0.6 | –0.0 ± 0.8 | |
| F13 |  |
CTCCCAGGCTCAAF3AF3T | 60.7 ± 0.1 | –0.3 ± 0.4 | 65.9 ± 0.1 | –0.2 ± 0.5 | |
| F14 |  |
CF3TCCCAGGCTCAAAF3T | 59.7 ± 0.6 | –1.3 ± 0.7 | 65.7 ± 0.9 | –0.0 ± 1.0 | |
| F15 |  |
CTCCCAGGF3CTCAAAF3T | 61.8 ± 0.3 | +0.8 ± 0.5 | 64.7 ± 0.6 | –1.0 ± 0.8 | |
| Tm, °C | ΔTm, °C | ||||||
| Control |  |
5′-d(TTTTTTTTTTTTTTTTTTTT)-3′ 3′-d(AAAAAAAAAAAAAAAAAAAA) -5′ |
50.7 ± 0.2 | – | |||
|
Φ9 Control |
 |
5′-d(TTTTTTTTTTφTTTTTTTTTT)-3′ 3′-d(AAAAAAAAAA AAAAAAAAAA)-5′ |
48.4 ± 0.1 | –2.4 ± 0.3 | |||
| Φ9:Φ10 | 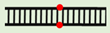 |
5′-d(TTTTTTTTTTφTTTTTTTTTT)-3′ 3′-d(AAAAAAAAAAφAAAAAAAAAA) -5′ |
44.6 ± 0.1 | –6.2 ± 0.3 | |||
| Φ3:Φ6 | 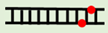 |
5′-d(GCGCCAAACφA)-3′ 3′-d(CGCGGTTTφGT)-5′ |
51.7 ± 0.1 | –2.6 ± 0.3 | |||
| Φ4:Φ7 |  |
5′-d(GCGCCAφAACA)-3′ 3′-d(CGCGGTφTTGT)-5′ |
49.5 ± 0.5 | –4.9 ± 0.3 | |||
| Θ6:Θ7 |  |
5′-d(GθCθGθCθCθAθAθAθCθA)-3′ 3′-d(CθGθCθGθGθTθTθTθGθT)-5′ |
47.7 ± 0.2 | –6.7 ± 0.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




