Submitted:
09 December 2024
Posted:
10 December 2024
You are already at the latest version
Abstract
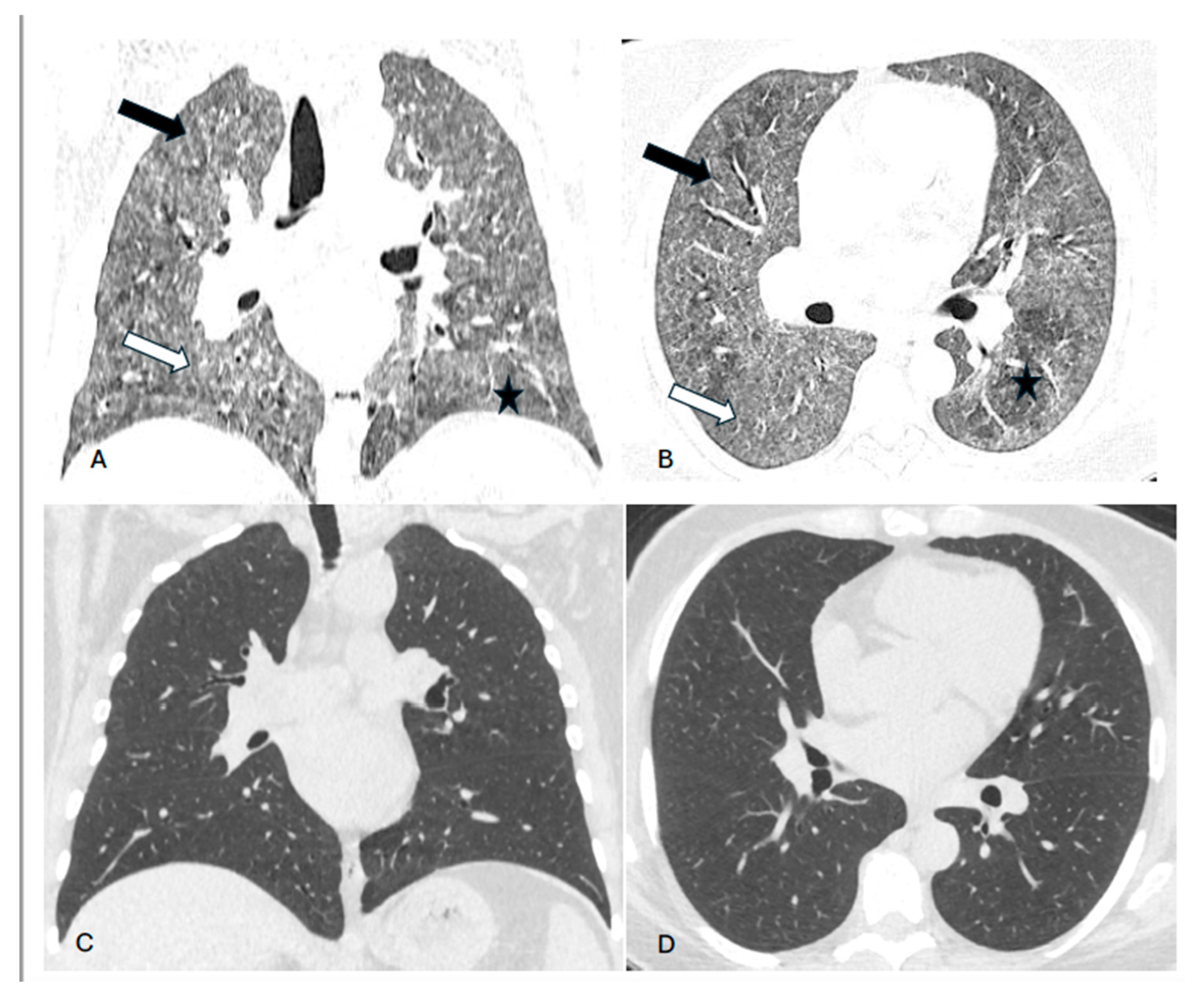
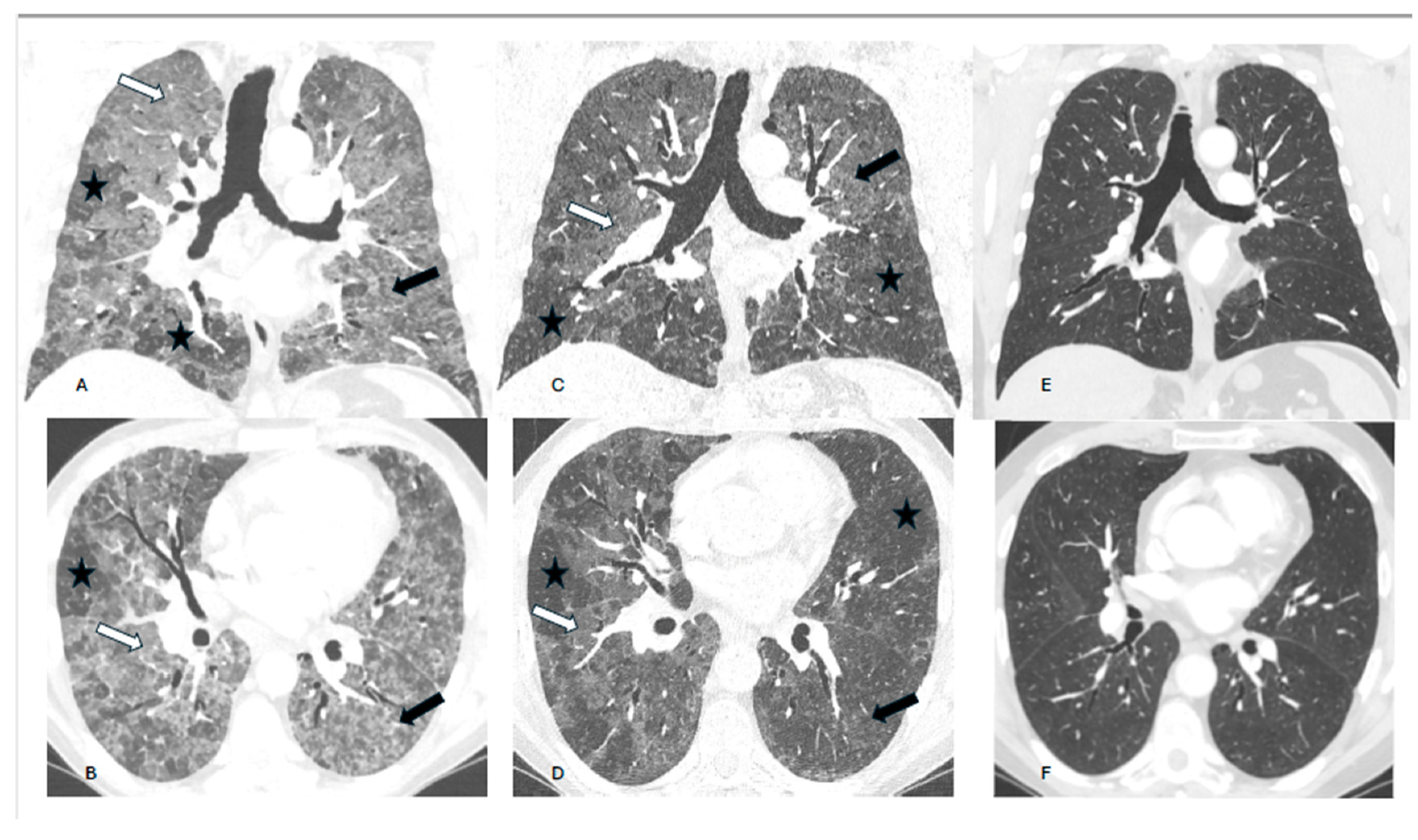
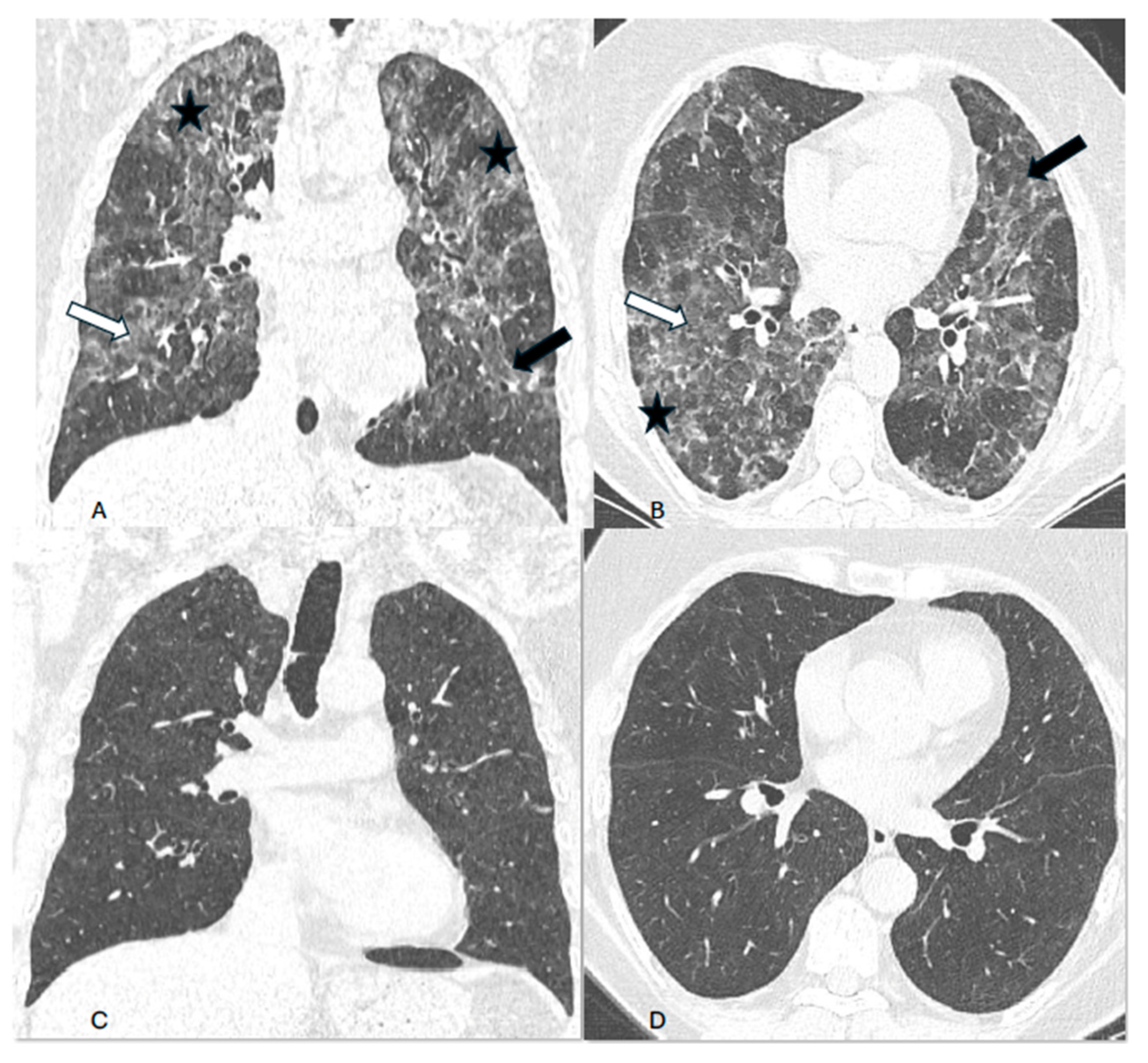
Background. Hypersensitivity pneumonitis (HP) is an increasingly recognized interstitial lung disease, developing as a result of exposition to inhaled, mostly organic, antigens. Two types of the disease are presently distinguished based on HRCT pattern and/or lung biopsy: fibrotic and non-fibrotic (non-fHP). Complete antigen avoidance is the principle of non-fHP treatment. The indications for steroids use in non-fHP depend on the clinical course of the disease. Case presentations. We present three patients in whom acute respiratory failure was diagnosed as the first sign of non-fHP. Intravenous prednisolone, followed by oral therapy with prednisone in diminishing doses, resulted in marked clinical improvement. Respiratory failure subsided within a few days, but regression of lung opacities lasted from 3 to 7 months. In one patient, the discrete reticular opacities, suggestive of the early phase of lung fibrosis, were present on HRCT, but complete regression of lung disease was achieved in the course of treatment. The patients were instructed to avoid antigens exposure; nevertheless, in one of them, a relapse of the disease, requiring a temporal increase of prednisone dose, was observed. Conclusions. Non-fHP may present as sudden-onset hypoxemic respiratory failure. In such patients, the diagnosis is based on medical history concerning the exposition to inciting antigens and characteristic HRCT pattern. Intravenous therapy with prednisolone results in quick resolution of respiratory failure, and BAL performed with a few days of delay may still be of diagnostic value. Ground glass opacities and air trapping may persist for months from exposure.
Keywords:
1. Introduction
2. Case presentations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pedraza-Serrano F, Jimenez-Garcia R, Lopez-de-Andres A et al. Characteristics and outcomes of patients hospitalized with interstitial lung diseases in Spain, 2014 to 2015. Medicine 2019; 98:21 (e15779).
- Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: Insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012; 186: 314-324.
- Karakatsani A, Papacsta D, Rapti A et al. Epidemiology of interstitial lung diseases in Greece. Respiratory Medicine 2009; 103: 1122-1129. [CrossRef]
- Fernandez-Perez ER, Kong AM, Raimundo K et al. Epidemiology of hypersensitivity pneumonitis among an insured population in United States: a claims-based cohort analysis. Ann Am Thorac Soc. 2018; 15: 460-469. [CrossRef]
- Rittig AH, Hilberg O, Ibsen R, Lokke A. Incidence, comorbidity and survival rate of hypersensitivity pneumonitis: a national population-based study. ERJ Open Res. 2019; 5: 00259-2018. [CrossRef]
- Raghu G, Remy-Jardin M, Ryerson CJ et al. Diagnosis of hypersensitivity pneumonitis in adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2020; 202: e36-e69.
- Hambly N, Farooqi MM, Dvorkin-Gheva A et al. Prevalence and characteristics of progressive fibrosing interstitial lung disease in a prospective registry. Eur Respir J 2022; 60: 2102571. [CrossRef]
- Cano-Jimenez E, Villar Gomez A, Velez Segovia E et al. Prognostic factors of progressive fibrotic hypersensitivity pneumonitis: a large, retrospective, multicentre, observational cohort study. ERJ Open Res. 2024; 10: 00405-2023. [CrossRef]
- Flaherty KR, Wells AU, Cottin V et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019; 381: 1718-1727.
- Dixon G, Hague S, Mulholland S et al. Real-world experience of nintedanib for progressive fibrosing interstitial lung disease in the UK. ERJ Open Res. 2024; 10: 00529-2023. [CrossRef]
- Fernandez Perez ER, Crooks JL, Lynch DA et al. Pirfenidone in fibrotic hypersensitivity pneumonitis: a double-blind, randomised clinical trial of efficacy and safety. Thorax 2023; 78: 1097-1104.
- De Sadeleer LJ, Hermans F, De Dycker E et al. Effect of corticosteroid treatment and antigen avoidance in a large hypersensitivity pneumonitis cohort: a single-centre cohort study. J Clin Med. 2019; 8: 14.
- Tomioka H, Miyazaki Y, Inoue Y et al. Japanese clinical practice guide for hypersensitivity pneumonitis. Respir Invest. 2024; 62: 16-43.
- Vasakova M, Selman M, Morell F et al. Hypersensitivity pneumonitis: current concepts of pathogenesis and potential targets for treatment. Am J Respir Crit Care Med. 2019; 200: 301-308.
- Petnak T, Moua T. Exposure assessment in hypersensitivity pneumonitis: a comprehensive review and proposed screening questionnaire. ERJ Open Res 2020; 6: 00230-2020.
- Mullin ML, Fernandez G, Marinescu D-C et al. Impact of antigen exposure on outcomes and treatment response in fibrotic hypersensitivity pneumonitis. Chest 2024; 165: 1435-1443. [CrossRef]
- Walters GI, Mokhlis JM, Moore VC et al. Characteristics of hypersensitivity pneumonitis diagnosed by interstitial and occupational lung disease multi-disciplinary team consensus. Respir Med. 2019; 155: 19-25. [CrossRef]
- Onishi Y, Kawamura T, Higashino T et al. Clinical features of chronic summer-type hypersensitivity pneumonitis and proposition of diagnostic criteria. Respir Invest. 2020; 58: 59-67.
- Sakamoto S, masuoka M, Usui Y et al. ATS/JRS/ALAT Hypersensitivity pneumonits guidelines for diagnosis of humidifier lung and summer-type hypersensitivity pneumonitis. Respir Invest. 2023; 61: 660-667.
- Vasakova M, Morell F, Walsh S et al. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med. 2017; 196: 680-689.
- Ryu K, Fukutomi Y, Sekiya K et al. Identification of fungi causing humidifier lung: 2 rare cases and a review of the literature. Asia Pac Allergy 2022; 12: e43. [CrossRef]
- Brambilla A, Sangiorgio A. Mould growth in energy efficient buildings: causes, health implications and strategies to mitigate the risk. Renewable and Sustainable Energy Reviews 2020; 132: 110093.
- Moran-Mendoza O, Aldhaheri S, Connor JA et al. Mold in foam pillows and mattresses. A novel cause of hypersensitivity pneumonitis. Chest 2021; 160: e259-e263.
- Morisset J, Johannson KA, Jones KD et al. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis. Am J Respir Crit care Med. 2018; 197: 1036-1044.
- Fernandez Perez ER, Travis WD, Lynch DA et al. Executive summary. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST Guideline and Expert Panel Report. Chest 2021; 160: 595-615. [CrossRef]
- Szturmowicz M, Barańska I, Jędrych ME, Demkow U. Correlation of bronchoalveolar lavage lymphocyte count with the extent of lung fibrosis and with plethysmographic lung volumes in patients with newly recognized hypersensitivity pneumonitis. Cent Eur J Immunol 2020; 45: 276-282. [CrossRef]
- Aderley N, Humphreys CJ, Barnes H et al. Bronchoalveolar lavage fluid lymphocytosis in chronic hypersensitivity pneumonitis: a systematic review and meta-analysis. Eur Respir J. 2020; 56: 2000206.
- Fernandez Perez ER, Swigris JJ, Forssen AV, Tourin O. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest 2013; 144: 1644-1651. [CrossRef]
- Jenkins AR, Chua A, Chami H et al. uestionnaires or serum immunoglobulin G testing in the diagnosis of hypersensitivity pneumonitis among patients with interstitial lung disease. Ann Am Thorac Soc. 2021; 18: 130-147.
- Iijima Y, Ejima M, Yamana T et al. Assessment of clinical relevance of antigen improves diagnostic accuracy of hypersensitivity pneumonitis. BMC Pulm Med. 2024; 24: 84.
- Calaras D, David A, Vasarmidi E, Antoniou K, Corlateanu A. Hypersensitivity pneumonitis: challenges of a complex disease. Canadian Respir J. 2024 doi:10.1155/2024/4919951.
- Rea G, Bocchino M, Lieto R et al. The unveiled triad: clinical, radiological and pathological insights into hypersensitivity pneumonitis. J Clin Med. 2024; 13: 797. [CrossRef]
- Nishida T, Kawate E, Ishiguro T et al. Antigen avoidance and outcome of nonfibrotic and fibrotic hypersensitivity pneumonitis. ERJ Open Res 2022; 8: 00474-2021. [CrossRef]
| WBC (x109/L) N: 4.00-8.00 |
Neutrophils (%) N: 34 - 71 |
TSH (mIU/mL) N: 0.27 – 4.2 |
IgE (IU/mL) N: <100 |
NT-proBNP (pg/mL) N: <125 |
ANA (screen) | CRP (mg/l) N:<5 | |
|---|---|---|---|---|---|---|---|
| Case 1 | 4.5 | 11 | 67 | 0.41 | 10 | 154 | negative |
| Case 2 | 130 | 14 | 77 | 0.42 | - | 683 | - |
| Case 3 | 44 | 11 | 75 | 0.45 | - | 383 | negative |
| FVC (%pred) | TLC (%pred) | RV/TLC (%pred) | TL,co (%pred) | 6MWD (m) | 6MWT SpO2-1 | 6MWT SpO2-2 | |
|---|---|---|---|---|---|---|---|
| Case 1 | 60 | 83 | 140 | 44 | 480 | 95 (+O2) | 92 (+O2) |
| Case 2 | 80 | 92 | 116 | 60 | 640 | 97 (-O2) | 87 (-O2) |
| Case 3 | 74 | 87 | 113 | 28 | 388 | 95(+O2) | 94(+O2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





