1. Introduction
High-Intensity Focused Ultrasound (HIFU) is a non-invasive treatment that deposits energy inside the body without causing harm. The first preclinical publication dates back to 1942 when Lynn et al. [
1] tested a “focused supersonic beam” in the animal brain. Since then, and for over eighty years, it has been gradually used as a reliable and effective technology for various medical applications in humans.
The primary effects of HIFU on the tissue include thermal heating or mechanical forces. Magnetic Resonance-guided Focused Ultrasound (MRgFUS) is an innovative and non-invasive technique employing HIFU alongside Magnetic Resonance Imaging (MRI) system guidance to target and deliver energy inside the lesion, resulting in considerable tissue heating, leading to necrosis in the target focal zone (
Figure 1).
The development of MRgFUS devices continues to be an active area of research and clinical trials. This combined system offers two principal advantages: first, it provides highly accurate and comprehensive information regarding tumor localization, thereby facilitating three-dimensional treatment planning; second, it offers continuous thermal monitoring of the treatment area, with the help of MR thermography. Implementing MRgFUS ablation enables exact tumor targeting, resulting in immediate temperature elevation within the specified zone and subsequent induction of cell death. Other non-thermal effects contribute to tissue destruction.
Compared to other ablation technologies, this approach is characterized by its less invasive nature and the elimination of the need for complex image-guided interventional skills [
2,
3].
MRgFUS is an alternative treatment for various oncological, neurological, and musculoskeletal diseases. In oncology, it is used to treat primary and secondary bone lesions, as well as some solid tumors [
4].
2. Physical Principles, Biological Effect and Technique Notes
Medical therapeutic ultrasound is classified into two types based on intensity: High-Intensity Focused Ultrasound (HIFU) and Low-Intensity Focused Ultrasound (LIFU). Both use a concave transducer, lens, or phased array to focus ultrasonic waves into a precise tissue volume. Intensity is measured as the total power delivered per unit area in the focal region (W/cm²). Considering a train of sonic waves propagating in an absorbing medium having attenuation coefficient μ, the intensity (I) of the ultrasound at depth x follows the following exponential law:
where I
0 is the intensity of the ultrasound beam at the point of generation (x=0), while μ is the attenuation coefficient of the medium per unit path. Specifically, the attenuation coefficient of the incident beam intensity depends on the biological tissue and the ultrasound frequency according to a power law having the following form:
where f is the frequency and the coefficients a and
b are tissue-specific constants. It is important to underline that it is precisely this dependence of the attenuation coefficient, and therefore of each specific biological tissue, on the frequency that makes ultrasound particularly suitable for non-invasive and targeted therapy, but it is also the cause of some significant challenges in the optimization of target destruction induced by HIFU. According to a power law, increasing the ultrasound frequency determines an increase in the attenuation coefficient. This implies that a greater heat deposition and a decrease in the penetration depth are obtained. Therefore, in a surgical procedure, a correct and optimal choice of ultrasound frequency is specific for each application and represents a compromise between the treatment depth and the desired heating rate at the target.
Frequencies between 0.5 and 1 MHz have proven to be more useful for heat deposition for deep treatments, whereas frequencies as high as 8 MHz are convenient for more superficial treatments [
7,
8].
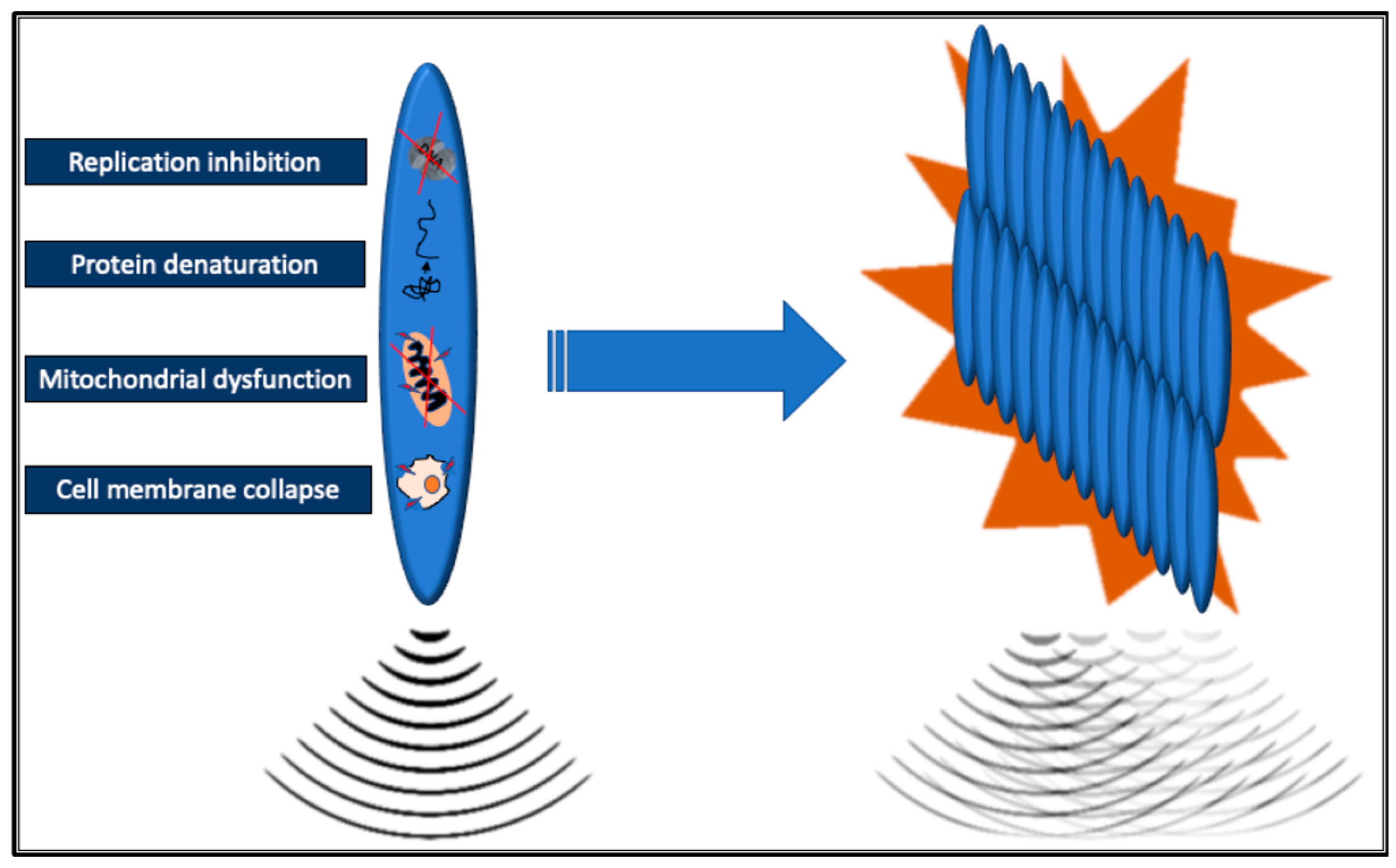
Current commercially available MRgFUS systems use multi-element phase array ultrasonic transducers composed of a variable number (several hundred to thousands) of individual piezoelectric ultrasonic elements. There are transducers integrated into the MRI table or dedicated relocatable. As ultrasound waves travel through tissue, they are absorbed and can cause both thermal and non-thermal (mechanical) effects. The ultrasound waves are focused into a very small volume, the focal zone, which significantly increases the intensity of the sound waves inducing energy release in the target tissue, defined as sonication. Within the focal zone, this high energy density raises the temperature to over 60°C within seconds, leading to the denaturation of tissue proteins. Extensive denaturation of proteins has an immediate cytotoxic effect leading to coagulative necrosis of tissue in the focal zone; other lethal effects are related to the loss of cell membrane integrity, mitochondrial dysfunction, and inhibition of DNA replication [
9]. The resulting necrotic lesions are typically small and elliptical, measuring 50-300 mm³ in volume [
10]. Combining multiple single lesions makes treating larger target volumes, such as solid tumors, possible without leaving gaps (
Figure 2).
Adequate pauses between individual sonications are crucial to avoid tissue boiling and bubbles forming. These bubbles can reflect and distort the ultrasound field, potentially resulting in unpredictable lesion growth, insufficient treatment of the target volume, and unintended lesion formation in surrounding areas [
10]. Another phenomenon to take into account when using HIFU is the mechanical effect of ultrasonic energy. FUS (Focused Ultrasound) can cause the oscillation of small gas bubbles trapped in the tissues, a process called
cavitation. These bubbles undergo repeated cycles of rarefaction and compression. If the mechanical pressure continues to increase, the bubbles reach a threshold size where they collapse violently during the compression part of the cycle. This phenomenon is called inertial cavitation [
11]. Cavitation occurs only with high-intensity ultrasound waves, generating high pressures and temperatures, significant shear stress, and microflow jets of liquid that can damage cell walls. In a predominantly liquid, freely moving medium, the movement of the liquid creates microscopic flows contributing to cell apoptosis. The nuclei of the apoptotic cells ultimately self-destruct through deoxyribonucleic acid degradation by endonucleases [
12].
The effectiveness of HIFU ablation largely stems from its ability to induce these mechanical effects, particularly cavitation, which is crucial for the technique’s usefulness.
MRI offers detailed 3D planning in targeting specific tissues, demonstrating high tumor detection sensitivity and excellent anatomical resolution. Additionally, it allows for the pre-definition of sonication's position, size, and physical characteristics (such as energy, frequency, and duration) [
13].
LIFU uses ultrasonic sound waves often pulsed in a sinusoidal waveform to propagate through bone and tissue and for the human skull the range used is often 250 kHz to 650 kHz. Unlike HIFU, LIFU results in low intensities (0.125—3 W/cm
2) focused ultrasound and causes low-temperature mechanical agitation [
14,
15].
Real-time imaging during therapeutic procedures is crucial for ensuring both safety and treatment effectiveness. Moreover, MR-thermometry allows for calculating thermal doses and provides a superimposed representation of the anatomical area where temperatures reach cytotoxic levels. During treatment, it controls energy deposition with a temperature accuracy of ±1°C, a spatial resolution of 1 mm, and a temporal resolution of 1 second. This precision is achievable because many MRI parameters, including T1 and T2 relaxation times, proton resonance frequency, and magnetization transfer coefficients, exhibit temperature dependencies that can be utilized. In proton-resonance frequency shift thermometry, temperature-dependent phase changes in gradient-recalled echo pulse sequences are employed to determine temperature variations [
3,
16,
17].
At the end of the procedure, the treatment's success is evaluated by obtaining T1-weighted MR images enhanced with gadolinium-based contrast material, where non-enhancing regions indicate the Non-Perfused Volume (NPV), an MR biomarker of clinical efficacy [
18].
The MRI, used for disease detection and diagnosis, in this field can play also a feasible role in the real-time assessment of precise energy delivering inside tumor lesions and, consequently, in the assessment of response to therapy, allowing the evaluation of the completeness of the ablation also during follow-up [
19].
3. An Overview of Current and Prospective Applications in Oncology
MRgFUS is currently a valuable incision-less option in the multidisciplinary management of primary and secondary malignant tumors or painful bone metastases.
3.1. Bone Metastases
Bone metastases play a significant role in causing cancer-related pain, which can greatly impact an individual's quality of life. Radiotherapy (RT) and analgesics represent the standard of care for the management of localized metastatic pain; however, approximately two-thirds of patients are known to continue to experience residual pain after treatment [
20]. MRgFUS is a non-invasive treatment that utilizes focused ultrasound waves to destroy the pain-transmitting periosteal nerves at the bone surface near the tumor. This approach delivers rapid and lasting relief for patients suffering from
painful bone metastases.
HIFU is believed to alleviate pain through thermal denervation of the bone and periosteum; however, the volumetric reduction of metastases may also play a role [
21].
The acoustic absorption and low thermal conductivity of cortical bone limit the diffusion of focused ultrasound energy to its surface. Focusing acoustic energy on the intact surface of cortical bone rapidly raises the temperature, causing critical thermal damage to the adjacent periosteum for pain relief. In the ablation of bone lesions, there are two scenarios. With intact cortical bone, the equipment set includes lower-frequency modulated protocols that reach the medullary and subcortical lesion; when the cortex is destroyed, as there are no absorption barriers, a high-frequency protocol with multiple ablative spots inside the lesion will be used [
22].
MRgFUS effectively delivers complete or partial pain relief in approximately 79% (95% CI: 73% - 83%) of patients. Furthermore, it maintains low-grade and high-grade treatment-related adverse event rates below 6% and 1%, respectively [
23,
24].
Patients typically experience pain relief within 3 days to 2 weeks after the treatment, many reducing or stopping their pain medication. Bony targets are localized (painful) non-spinal lesions (apart from the posterior elements below the level of the conus medullaris) or noncranial lesions, which can be identified by radiological imaging. The safety distance from the skin and the main nerve bundles is ≥ 1 cm; bone injuries should not require surgical stabilization, with low fracture risk (Mirel score ≤ 7). There should be no interposition of non-target bone, hollow viscera with airborne contents, or extensive scars along the trajectory of the ultrasound beam [
25]. MRgFUS is a valuable opportunity in painful bone metastasis management, particularly in patients who have previously not responded to RT [
26].
Additionally, MRgFUS can be recommended as an alternative to external beam radiation therapy or after it for patients with skeletal oligometastases (1-5 metastatic bone lesions)
devices accessible, representing a highly effective therapy able to provide local tumor control (LTC) in 84% (95% CI: 66% - 97%) of lesions treated, superior to other percutaneous ablation procedures that reached 65% (95% CI: 51% - 78%) of LTC [
2].
Compared with RT, MRgFUS has the advantage of not having dose limits and allowing for more treatment sessions, making it an effective option, especially for non-radiosensitive tumors or when a dose limit for adjacent radiosensitive organs is conceivable. Furthermore, a significant pain-relieving effect can usually be achieved already in a single treatment session [
27].
3.2. Prostate Cancer
MRgFUS can effectively treat patients with intermediate-risk
prostate cancer who wish to avoid radical whole-gland treatment, with a low rate of genitourinary adverse effects [
28]. Two types of devices have been developed and tested: transrectal and transurethral MRgFUS systems.
In a prospective phase II trial of Ghai et al. [
29], 41 of 44 men (93%; 95% CI: 82% - 98%) had no residual disease at the treatment site with a transrectal MRgFUS system and only one man (2%; 95% CI: 0.4% - 11.8%) had severe pelvic pain that persisted and reported a grade 3 adverse event at 5 months following treatment. This therapy is suitable for individuals with unilateral, MRI-visible, primary, intermediate-risk, previously untreated prostate adenocarcinoma, characterized by a prostate-specific antigen level of ≤20 ng/mL, Grade Group 2 or 3, and tumor classification of ≤T2, all confirmed via fusion biopsy. After 2 years, 39 of the 43 participants (91%) exhibited no evidence of clinically significant prostate cancer at the treatment site, and 36 of the 43 participants (84%) were found to have no cancer present in the entire gland, results-based with multiparametric MRI as well as targeted and systematic biopsies follow-up [
30]. In a Phase I trial by Chin et al. [
31], the transurethral MRgFUS system (MRI-TUSLA) showed that men with biopsy-proven low-risk (80%) and intermediate-risk (20%) prostate cancer had a median PSA reduction of 87% at one month, stabilizing at 0.8 ng/ml by twelve months. There was also a 61% decrease in total cancer length, with clinically significant disease in 9 of 29 patients (31%; 95% CI: 15% - 51%) and any disease in 16 of 29 patients (55%; 95% CI: 36% - 74%).
In this trial the MRgFUS treatment included a minimum margin of 3-5mm in the target area and in some cases up to 10 mm, beyond the tumor visible on the magnetic resonance imaging was included in the treatment plan. This procedural aspect increases the likelihood of treating the entire volume of the histological tumor during focal ablative therapy. Furthermore, using thermographic maps overlaid on anatomical MR images during treatment allowed for monitoring of the effective ablation, and the effects on urinary control, erectile function, pain, and morbidity were minimal.
3.3. Breast Cancer
MRgFUS seems to be a non-invasive treatment for localized, clinically non-palpable
early-stage breast cancer, potentially replacing lumpectomy [
32]. HIFU breast cancer therapy offers, among other benefits, also the preservation of tissue integrity, no scars, and slight changes in breast shape with good cosmetic results.
Merckel et al. [
33] report their initial experiences with MRgFUS ablation for breast cancer using a dedicated breast platform, finding it safe and resulting in confirmed tumor necrosis. The dedicated MRgFUS platform for the breast is a working plan with the breast targeted in the cup of the breast filled with water surrounded by transducers distributed on a circular arc of 270°; during treatment, patients are positioned prone on the table with the targeted breast inside the cup [
34]. In a prospective study of Furusawa et al. [
35], thirty women with invasive breast cancer (T1–2, N0–2, M0) were treated using MRgFUS, followed by excision or mastectomy. Tumor histology revealed that the average necrosis of the targeted breast tumors was 96.9% of the tumor volume. Treatment was generally well tolerated, with few adverse effects while only one patient suffered a third-degree burn due to the user error. Pathology from a lumpectomy indicated that residual tumors in two patients were at the margins of the treatment area, underscoring the need for a 5-mm safety margin in the prescribed treatment region.
Patients with large T2 tumors, however, are generally not appropriate candidates for treatment with minimally invasive ablation techniques such as the HIFU. Furthermore, it is important to consider how the location of the tumor within the breast can impact treatment options and outcomes. If the tumor is close to the skin, there is a risk of burns or leaving tumor cells if the overlying tissue is not treated properly.
3.4. Abdominal Cancers
MRgFUS is a feasible and repeatable ablative technique for patients with unresectable and device-accessible
hepatic and pancreatic lesions [
36]. Preliminary results of Anzidei et al. [
37] in one patient with unresectable hepatocellular carcinoma distant from intrahepatic vessels and two patients with pancreatic body adenocarcinoma are encouraging, especially regarding the pain relief response and the palliation of local tumor growth. MRgFUS effectively controls tumors and alleviates symptoms in pancreatic adenocarcinoma by damaging the celiac plexus. In hepatocellular carcinoma, lesion ablation is confirmed through imaging and histopathology. Once fast organ tracking technology is available, it has the potential to serve as an alternative to surgical resection for
malignant renal tumors [
38].
3.5. Targeting Drugs Delivery
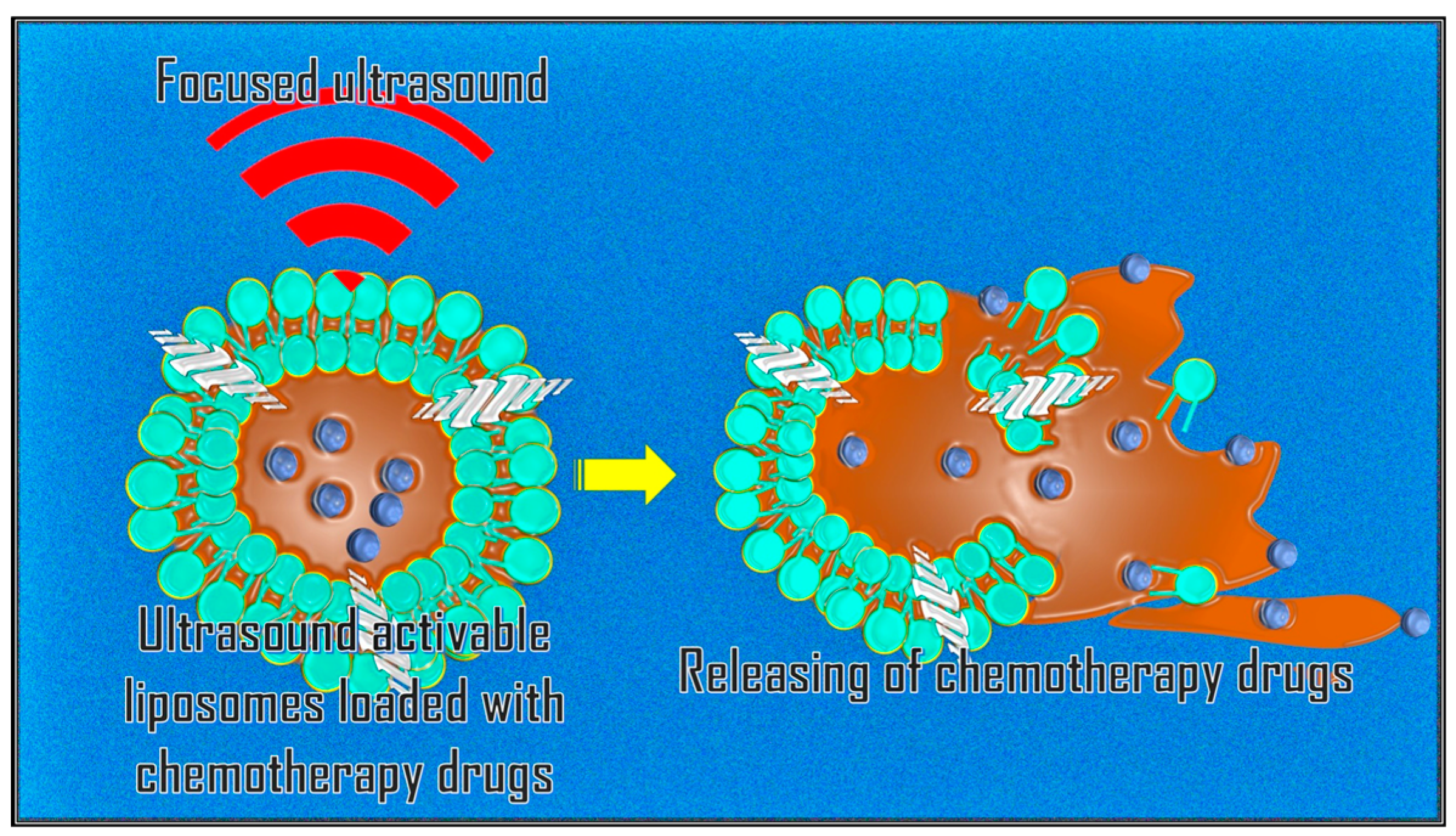
A promising application of MRgFUS is targeted drug release through systemic administration of nanocarriers sensitive to mechanical forces and/or sensitive to temperature [
39]. Anticancer drugs (e.g., Doxorubicin, etc.) can be encapsulated in gas-filled bubbles or thermosensitive liposomes attached to nanoparticles. These are released locally by rupturing the bubbles during sonication of the target area (
Figure 3).
3.6. Immunological Effect
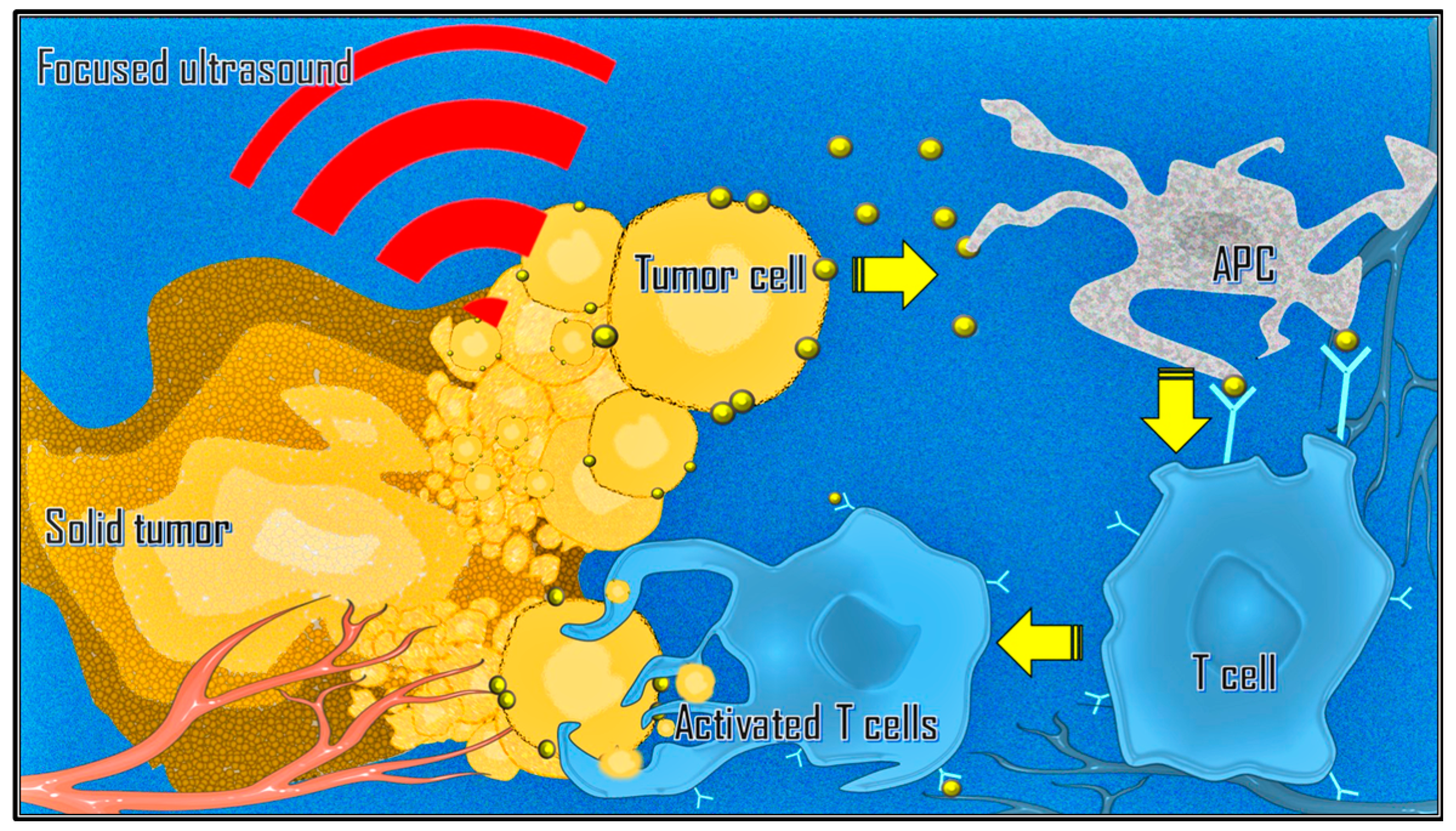
There is also growing interest in combining immunotherapy with ablative strategies such as MRgFUS due to mechanical changes in the tumor microenvironment and inflammatory-mediated changes in immune phenotypes [
40,
41]. Focused ultrasound can stimulate immune responses in tumors by releasing tumor-associated antigens. This process can lead to T cell-specific responses, an increase in tumor-infiltrating lymphocytes, and antigen-presenting cells within the tumor microenvironment (
Figure 4). Additionally, it can alter the immune context of the tumor, induce the abscopal effect, and reverse T cell anergy and tolerance [
42,
43,
44].
3.7. Neuro-Oncology
Trans cranial MRgFUS (tcMRgFUS) has mainly been used for movement disorders [
45]. Since the FDA approved unilateral thalamotomy for drug-refractory essential tremor in 2016, its applications have expanded also into neurosurgical adult and pediatric oncology. The first successful treatment using HIFU thermal ablation in a Glioblastoma Multiforme was conducted by Coluccia et al. in 2014 [
46]. Currently, phase I trials are exploring complete lesion ablation [
47,
48]. Recent studies have examined low-intensity pulsatile focused ultrasound (LIFU) for temporarily enhancing the blood-brain barrier (BBB) permeability [
49]. Unlike HIFU, which can cause permanent brain damage, LIFU safely disrupts the tight junctions between endothelial cells. This allows the barrier to open briefly for several hours. Combining this technique with the administration of exogenous microbubbles (i.e., ultrasound contrast) could improve chemotherapy trans-BBB delivery for the targeted treatment of primary and secondary brain tumors, maximizing local concentration and reducing systemic toxicity [
50,
51]. Several studies have indicated that tcMRgFUS can effectively stimulate both innate and acquired immune responses [
52].
4. Conclusions
The effectiveness of the MRgFUS technique is closely tied to identifying lesions accessible by the device and the absence of contraindications to MRI. MRgFUS has several advantages, such as the repeatability of the procedure if necessary and a favorable safety profile, with no dose limit reported for focused ultrasound energy. Given the clinical circumstances of patients, MRgFUS can be considered a viable treatment option, either by itself or in combination with other therapies to cure the disease. At present, the term “Evolving Nonsurgical Precision Ablation” appears to be an appropriate descriptor for specific applications of MRgFUS in the field of oncology. Combining this approach with other interventions and drug delivery may enhance their effectiveness and therapeutic outcomes. The advancements in platforms and devices will enable the expansion of anti-cancer MRgFUS applications soon.
Author Contributions
“Conceptualization, M.L.; investigation, and resources, M.L., F.C., and R.S.; writing—original draft preparation, M.L., and F.C.; validation, and supervision, D.F., C.C., and R.I.; all authors have read and agreed to the published version of the manuscript.”.
Funding
“This research received no external funding”.
Institutional Review Board Statement
“Not applicable”.
Informed Consent Statement
“Not applicable”.
Conflicts of Interest
“The authors declare no conflicts of interest.”.
References
- Lynn, J.; Zwemer, R.; Chick A.; Miller A. A new method for the generation and use of focused ultrasound in experimental biology." J Gen Physiol. 1942,26,179-193. [CrossRef]
- Leporace, M.; Lancellotta, v.; Baccolini, V.; Calabria, F.; Castrovillari, F.; Filippiadis, D.; Tagliaferri, L.; Iezzi, R. Magnetic resonance-guided focused ultrasound versus percutaneous thermal ablation in local control of bone oligometastases: a systematic review and meta-analysis. Radiol Med, 2024, 129, 291-306.
- Pediconi, F.; Napoli, A.; Di Mare, L.; Vasselli F.; Catalano, C. MRgFUS: from diagnosis to therapy. Eur J Radiol, 2012, 81, S118-120.
- Lei, S.; He, J.; Gao, P.; Wang, Y.; Hui, H.; An aY.; Tian, J. Correction to: Magnetic Particle Imaging-Guided Hyperthermia for Precise Treatment of Cancer: Review, Challenges, and Prospects. Mol Imaging Biol, 2023, 25, 1151.
- Haar. G.; Coussios, C. High intensity focused ultrasound: physical principles and devices". Int J Hyperthermia, 2007, 23, 89-104.
- Bamber, J.; ter Haar, G.; Hill, C. Physical Principles of medical Ultrasound.Wiley, London, United Kingtom, 2004.
- Hill, C. Optimum acoustic frequency for focused ultrasound surgery. Ultrasound Med Biol, 1994, 20, 271-277. [CrossRef]
- Hynynen, K.; Watmough, D.; Mallard, J. The effects of some physical factors on the production of hyperthermia by ultrasound in neoplastic tissues. Radiat Environ Biophys, 1981, 19, 215-226. [CrossRef]
- Chu K.; Dupuy, D. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer, 2014, 14, 199-208. [CrossRef]
- Jenne, J.; Preusser, T.; Günther, M. High-intensity focused ultrasound: principles, therapy guidance, simulations and applications. Z Med Phys, 2012, 22, 311-322. [CrossRef]
- Coussios, C.; Farny, C.; Haar G.; Roy, R. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int J Hyperthermia, 2007, 23, 105-120. [CrossRef]
- Zhou, Y. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol, 2011, 10, 2, 8-27.
- Cline, H.; Schenck, J.; Hynynen, K.; Watkins, R.; Souza, S.; Jolesz, F. MR-guided focused ultrasound surgery. J Comput Assist Tomogr, 1992, 16, 956-965. [CrossRef]
- Cox, S.; Connolly, D.; Peng, X.; Badran, B. A Comprehensive Review of Low-Intensity Focused Ultrasound Parameters and Applications in Neurologic and Psychiatric Disorders. Neuromodulation, 2024, 3, S1094-7159. [CrossRef]
- Mungur, R.; Zheng, J.; Wang, B.; Chen, X.; Zhan, R.; Tong, Y. Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment. Front Oncol, 2022, 12, 903059. [CrossRef]
- Napoli, A.; Anzidei, M.; Ciolina, F.; Marotta, E.; Cavallo, M.B.; Brachetti, G.; Di Mare, L.; Cartocci, G.; Boni, F.; Noce, V.; et al. MR-guided high-intensity focused ultrasound: current status of an emerging technology. Cardiovasc Intervent Radiol, 2013, 36, 1190-1203. [CrossRef]
- Haar, G.; Coussios, C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia, 2007, 23, 99-104.
- Hectors, S.; Jacobs, I.; Moonen, C.; Strijkers, G.; Nicolay, K. MRI methods for the evaluation of high intensity focused ultrasound tumor treatment: Current status and future needs. Magn Reson Med, 2016, 75, 302-317. [CrossRef]
- Wimper, Y.; Fütterer, J.; Bomers, J. MR Imaging in Real Time Guiding of Therapies in Prostate Cancer. Life (Basel), 2022, 12, 302. [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol, 2007, 25, 1423–1436.
- Brown, M.; Farquhar-Smith, P.; Williams, J.; ter Haar, G.; deSouza, N. The use of high-intensity focused ultrasound as a novel treatment for painful conditions-a description and narrative review of the literature. Br J Anaesth, 2015, 115, 520-530.
- Napoli, A.; Anzidei, M.; Marincola, B.; Brachetti, G.; Noce, V.; Boni, F.; Bertaccini, L.; Passariello, R.; Catalano, C. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics, 2013, 33, 1555-1568. [CrossRef]
- Baal, J.; Chen, W.; Baal, U.; Wagle, S.; Baal, J.; Link, T.; Bucknor, M. Efficacy and safety of magnetic resonance-guided focused ultrasound for the treatment of painful bone metastases: a systematic review and meta-analysis. Skeletal Radiol, 2021, 50, 2459-2469.
- McGill, K.; Baal, J.; Bucknor, M. Update on musculoskeletal applications of magnetic resonance-guided focused ultrasound. Skeletal Radiol, 2014, 53, 1869-1877. [CrossRef]
- Yeo, S.; Bratke, G.; Grüll, H. High Intensity Focused Ultrasound for Treatment of Bone Malignancies-20 Years of History. Cancers (Basel), 2022, 15, 108. [CrossRef]
- Hurwitz, M.; Ghanouni, P.; Kanaev, S.; Iozeffi, D.; Gianfelice, D.; Fennessy, F.; Kuten, A.; Meyer, E.; LeBlang, S.; Roberts, A.; et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst, 2014, 106, 082. [CrossRef]
- Huisman, M.; ter Haar, G.; Napoli, A.; Hananel, A.; Ghanouni, P.; Lövey, G.; Nijenhuis, R.; van den Bosch, M.; Rieke, V.; Majumdar, S.; et al. International consensus on use of focused ultrasound for painful bone metastases: Current status and future directions. Int J Hyperthermia, 2015, 31, 251-259. [CrossRef]
- Yuh, B.; Liu, A.; Beatty, R.; Jung, A.; Wong, J.Y.C. Focal therapy using magnetic resonance image-guided focused ultrasound in patients with localized prostate cancer. J Ther Ultrasound, 2016, 11, 4-8. [CrossRef]
- Ghai, S.; Finelli, A.; Corr, K.; Chan, R.; Jokhu, S.; Li, X.; McCluskey, S.; Konukhova, A.; Hlasny, E.; van der Kwast, T.; et al. MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial. Radiology, 2021, 298, 695-703. [CrossRef]
- Ghai, S.; Finelli, A.; Corr, K.; Lajkosz, K.; McCluskey, S.; Chan, R.; Gertner, M.; van der Kwast, T.; Incze, P.; Zlotta, A.; et al. MRI-guided Focused Ultrasound Focal Therapy for Intermediate-Risk Prostate Cancer: Final Results from a 2-year Phase II Clinical Trial. Radiology, 2024, 310, e231473. [CrossRef]
- Chin, J.; Billia, M.; Relle, J.; Roethke, M.; Popeneciu, I.; Kuru, T.; Hatiboglu, G.; Mueller-Wolf, M.; Motsch, J.; Romagnoli, C.; et al. Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Tissue in Patients with Localized Prostate Cancer: A Prospective Phase 1 Clinical Trial. Eur Urol, 2016, 70, 447-455.
- Matsutani, A.; Ide, Y.; Miura, S.; Takimoto, M.; Amano, S.; Nakamura, S. Innovative use of magnetic resonance imaging-guided focused ultrasound surgery for non-invasive breast cancer: a report of two cases. Surg Case Rep, 2020, 6, 294. [CrossRef]
- Merckel, L.; Knuttel, F.; Deckers, R.; van Dalen, T.; Schubert, G.; Peters, N.; Weits, T.; van Diest, P.; Mali, W.; Vaessen, P.; et al. First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation. Eur Radiol, 2016, 26, 4037-4046. [CrossRef]
- Merckel, L.; Bartels, L.; Köhler, M.; van den Bongard, H.; Deckers, R.; Mali, W.; Binkert, C.; Moonen, C.; Gilhuijs, K.; van den Bosch, M. MR-guided high-intensity focused ultrasound ablation of breast cancer with a dedicated breast platform. Cardiovasc Intervent Radiol, 2013, 36, 292-301. [CrossRef]
- Furusawa, H.; Namba, K.; Thomsen, S.; Akiyama, F.; Bendet, A.; Tanaka, C.; Yasuda, Y.; Nakahara, H. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg, 2006, 203, 54-63. [CrossRef]
- Orsi, F.; Arnone, P.; Chen, W.; Zhang, L. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther, 2010, 6, 414-420.
- Anzidei, M.; Napoli, A.; Sandolo, F.; Marincola, B.; Di Martino, M.; Berloco, P.; Bosco, S.; Bezzi, M.; Catalano, C. Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: a feasibility study in selected cases of pancreatic and liver cancer. Cardiovasc Intervent Radiol, 2014, 37, 1611-1617. [CrossRef]
- Saeed, M.; Krug, R.; L. Do, Hetts, S.; Wilson, M. "Renal ablation using magnetic resonance-guided high intensity focused ultrasound: Magnetic resonance imaging and histopathology assessment.," World J Radiol, 2016, 8, 298-307.
- Thanou, M.; Gedroyc, W. MRI-Guided Focused Ultrasound as a New Method of Drug Delivery. J Drug Deliv, 2013, 616197.
- Silvestrini, M.; Ingham, E.; Mahakian, L.; Kheirolomoom, A.; Liu, Y.; Fite, B.; Tam, S.; Tucci, S.; Watson, K.; Wong, A.; et al. Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight, 2017, 2, e90521. [CrossRef]
- Joiner, J.; Pylayeva-Gupta Y.; Dayton, P. Focused Ultrasound for Immunomodulation of the Tumor Microenvironment. J Immunol, 2020, 205, 2327-2341.
- Lu, P.; Zhu, X.; Xu, Z.; Zhou, Q.; Zhang, J.; Wu, F. Increased infiltration of activated tumor-infiltrating lymphocyte safter high intensity focused ultrasound ablation of human breast cancer. Surgery, 2009, 145,286–293.
- Xu, Z.; Zhu, X.; Lu, P.; Zhou, Q.; Zhang, J.; Wu, F. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer, Ultrasound Med Biol, 2009, 35, 50–57.
- Xia, J.; Xie, F.; Ran, L.; Xie, X.; Fan, Y.; Wu, F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol, 2012, 38, 1363–1371. [CrossRef]
- Deuschl, G.; Antonini, A.; Costa, J.; Śmiłowska, K.; Berg, D.; Corvol, J.; Fabbrini, G.; Ferreira, J.; Foltynie, T.; Mir, P.; et al. European Academy of Neurology/Movement Disorder Society-European Section Guideline on the Treatment of Parkinson's Disease: I. Invasive Therapies. Mov Disord, 2022, 37, 1360-1374. [CrossRef]
- Coluccia, D.; Fandino, J.; Schwyzer, L.; O'Gorman, R.; Remonda, L.; Anon, J.; Martin, E.; Werner, B. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound, 2014, 2, 17.
- A Study to Evaluate the Safety and Feasibility of Transcranial MRI-Guided Focused Ultrasound Surgery in the Treatment of Brain Tumors.- [ClinicalTrials.gov ID NCT00147056].
- Magnetic Resonance (MR) Guided Focused Ultrasound in the Treatment of Brain Tumors (FUS-Tumor) - [ClinicalTrials.gov ID NCT01698437].
- McMahon, D.; Poon, C.; Hynynen, K. Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability. Expert Opin Drug Deliv, 2019, 16, 129-142.
- Beccaria, K.; Canney, M.; Bouchoux, G.; Puget, S.; Grill, J.; Carpentier, A. Blood-brain barrier disruption with low-intensity pulsed ultrasound for the treatment of pediatric brain tumors: a review and perspectives. Neurosurg Focus., 2020, 48, E10.
- Grasso, G.; Torregrossa, F.; Noto, M.; Bruno, E.; Feraco, P.; Buscemi, F.; Bartolotta, T.; Gagliardo, C. MR-guided focused ultrasound-induced blood-brain barrier opening for brain metastasis: a review. Neurosurg Focus, 2023, 55, E11.
- Liu, H.; Hsieh, H.; Lu, L.; Kang, C.; Wu, M.; Lin, C. Low-pressure pulsed focused ultrasound with microbubbles promotes an anticancer immunological response. J Transl Med, 2012, 10, 221.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).