Introduction
Sp hybridizations, in carbon atoms and, to a lesser extent, silicon, are very versatile; hosting a large number of allotropic forms, with sp, sp
2 and sp
3 hybridized bonds, which acquire physical properties, such as hardness and electronics, different from each other. Among the most common are graphite and diamond. Subsequently, nanotubes, fullerenes, and nanosheets, such as graphene and its allotropes, have been synthesized or manufactured. It has also been possible, applying modern computational methods, to theorize new forms of carbon, such as the already mentioned, bct-C4 [
1], the forms: C, F, M, O, P, T12, W and Z
2.
In this work, new variants of the bct - C4 phase are proposed, composed of several fused Si4 rings; as well as others that combine Si4 rings with other Si8 cubes[ 5, also fused. Forming new bct, silicon variants.
1. Structures
To define the possible bct - C4 variants, we have based ourselves on the results of some works [ 3, 4, which predict graphene allotropes, of sp2 hybridization, based on the Demsity Function Theory (DFT). Specifically, the structures C4 -10 -I, C4 - 8 - R and C4 - 12- These results have been translated into a three-dimensional sp3 hybridization, corresponding to a bct-type structure, composed of flat square rings, C4 or Si4.
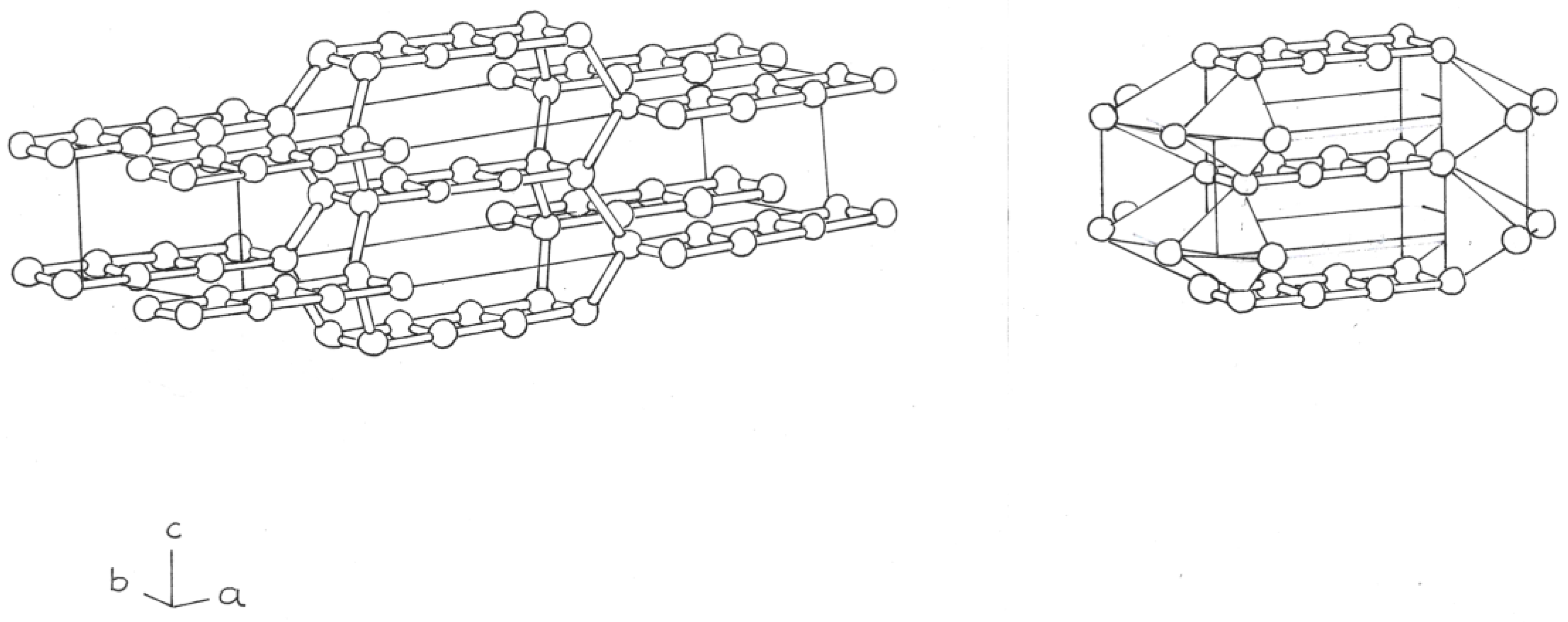
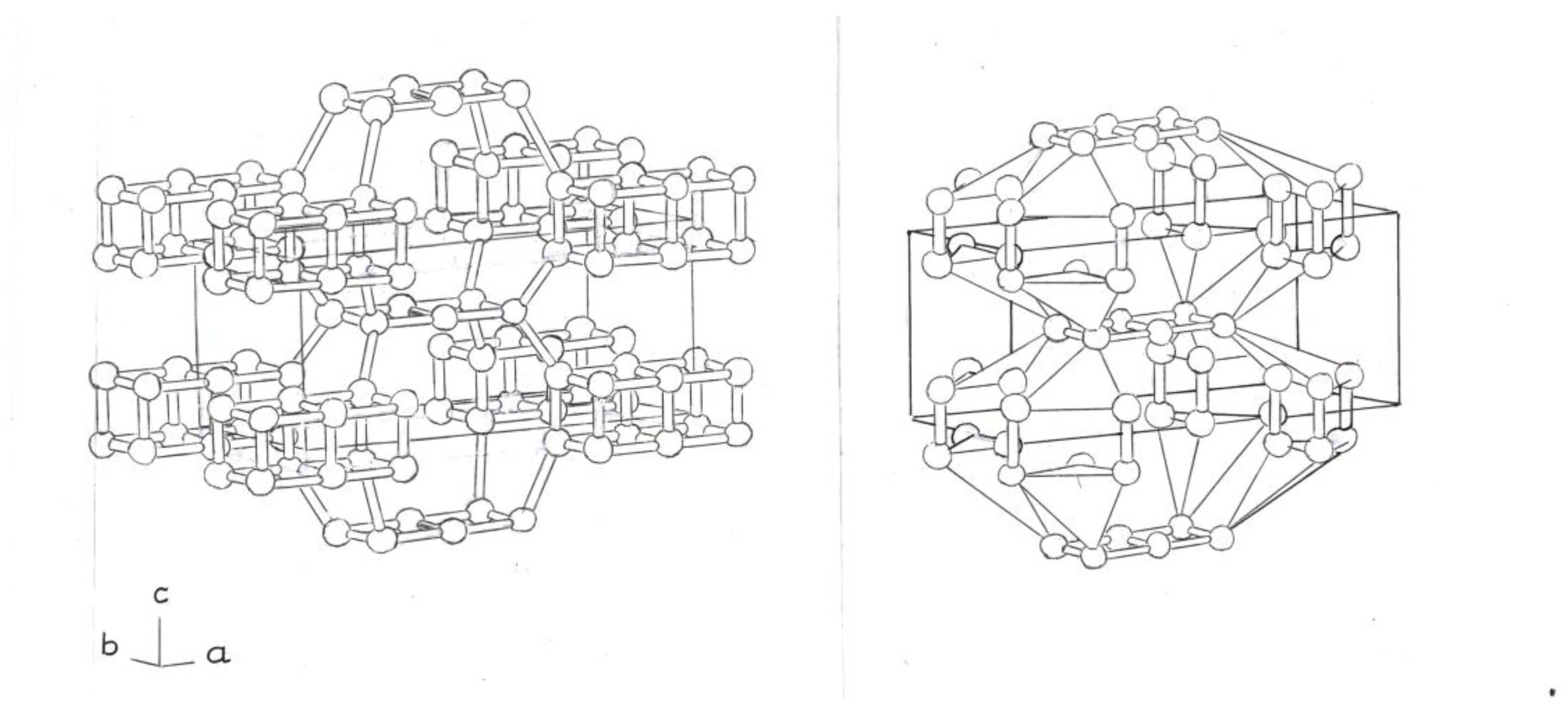
The first of these new structures, derived from the simpler bct - Si
4 phase, is formed by two fused Si
4 rings, with single and double bonds; in the two atoms of the two rings, (
Figure 1)
Another variant phase would be composed of three flat square rings, Si
4. The ends of the central ring would be formed by two simple links, at the top and bottom of it; and two double bonds, on the sides of the central ring. (
Figure 2)
Both phases form 8 tetrahedra, 5 silicon atoms, adjacent, at the ends (
Figure 1 and
Figure 2, right).
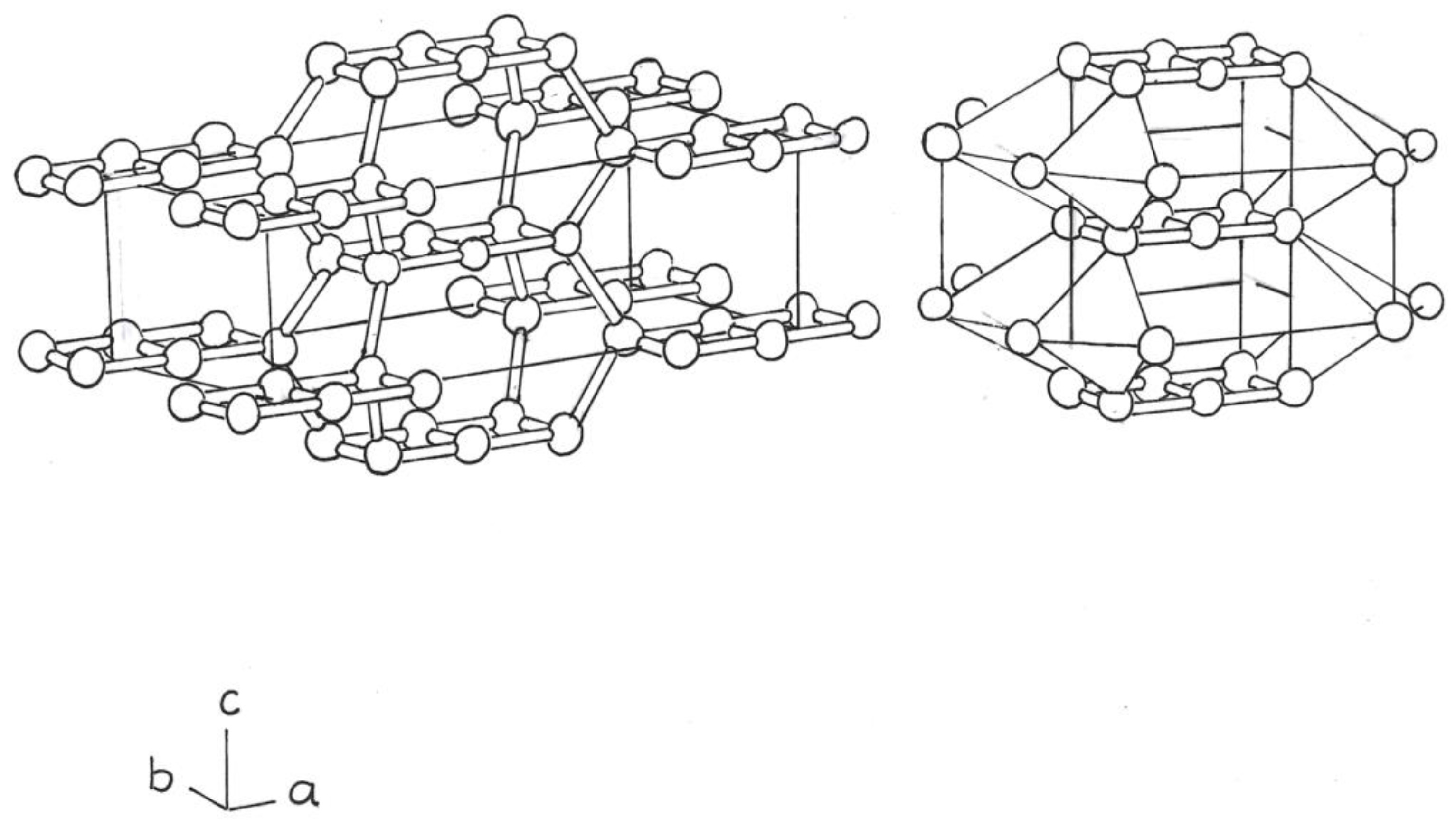
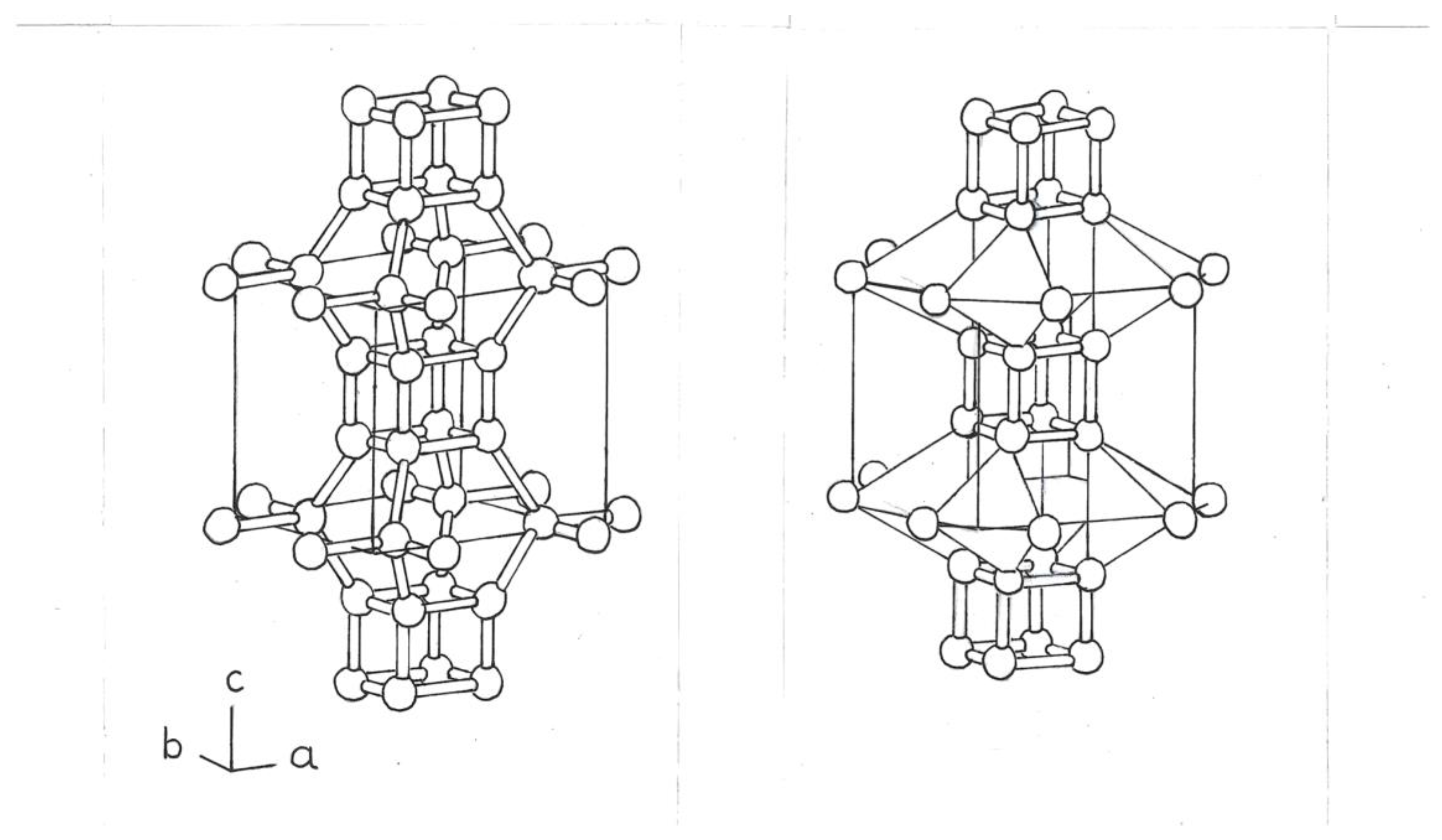
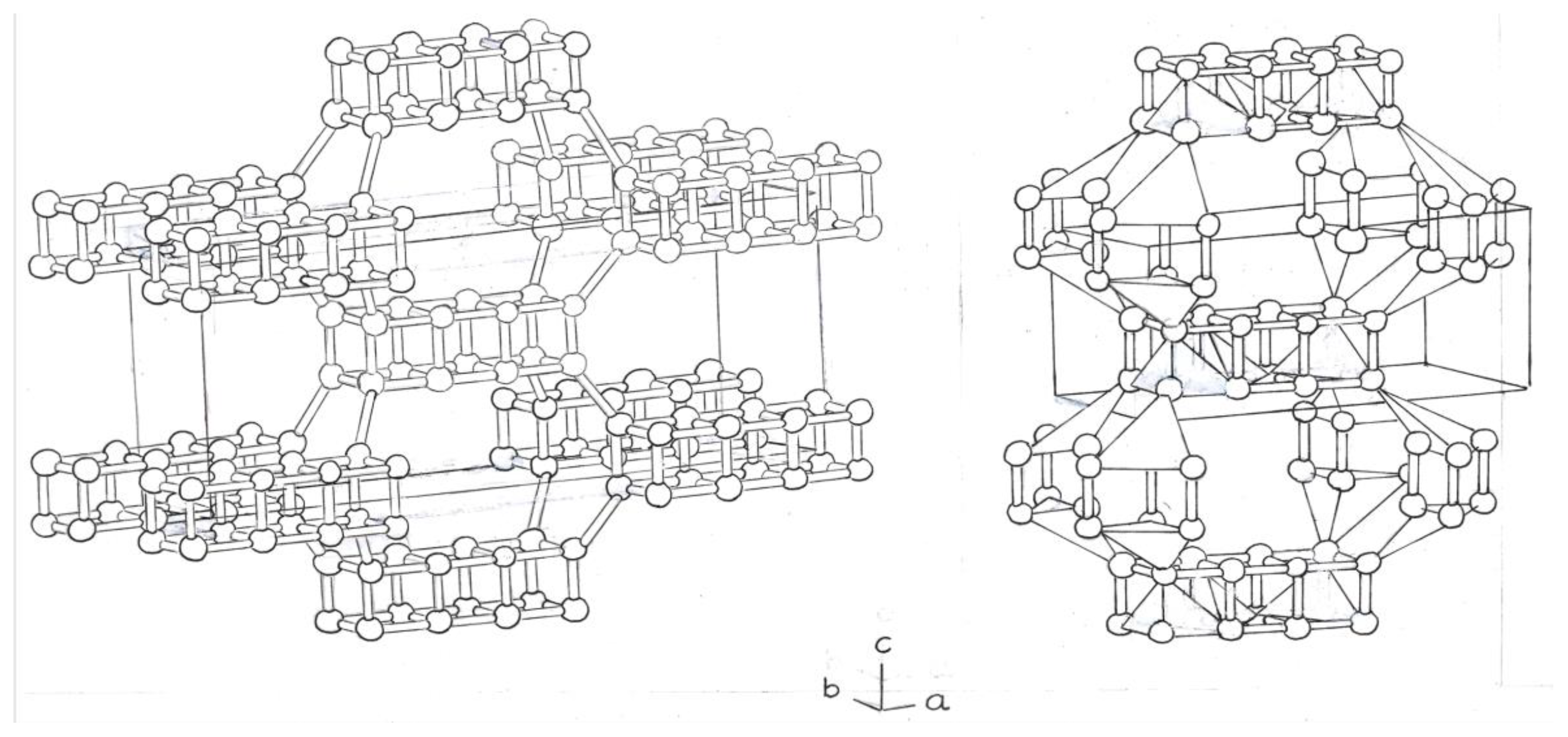
The next phase would be composed of a Si
8 cube, centered, and eight Si
4 rings on the edges of a tetrahedron (
Figure 3).
The tetrahedra, at the top, are separated from those at the bottom, by dense bonds of the Si atoms, of the central cbo, Si
8 (
Figure 3b).
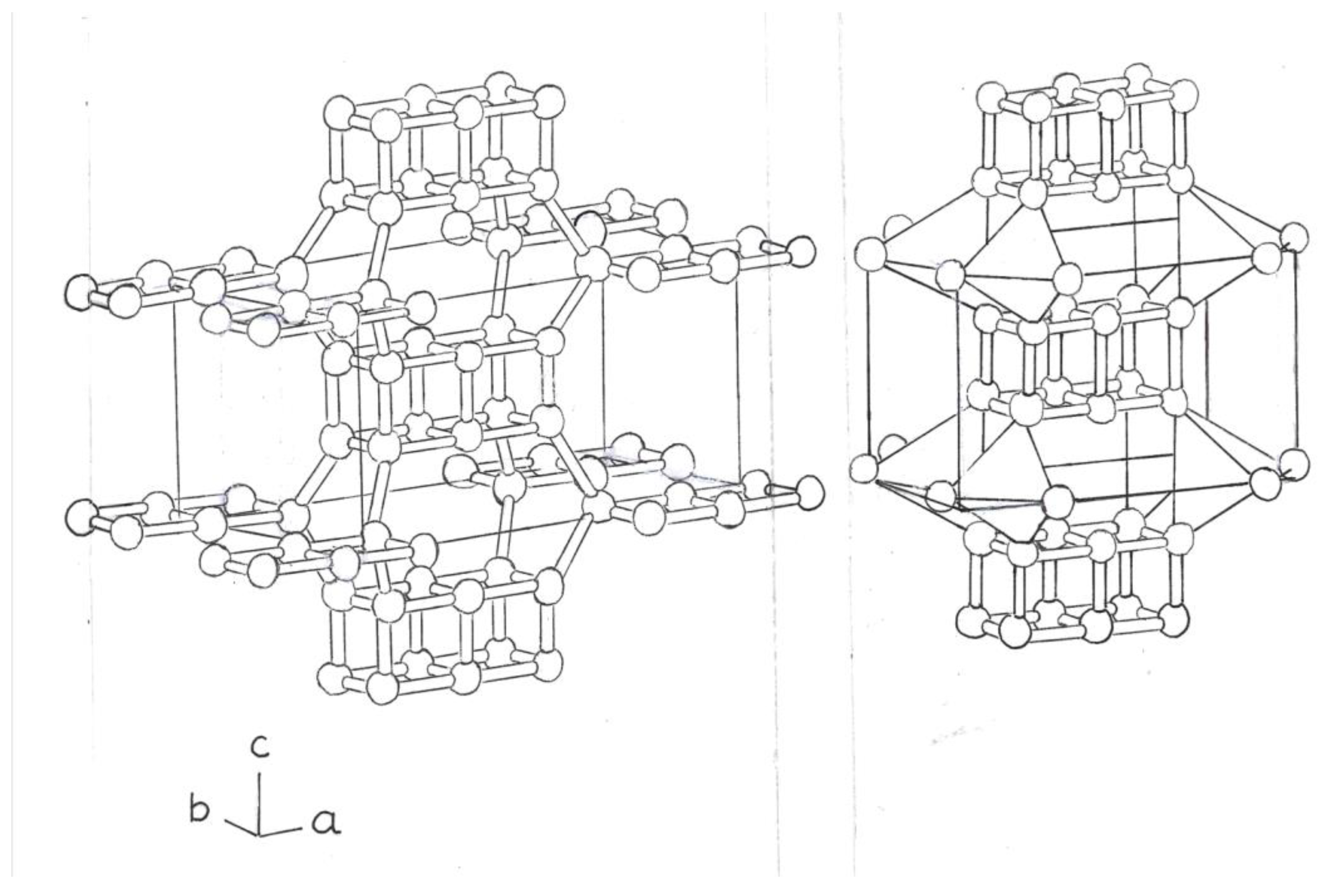
The following variants would be formed by two cubes of Si
8, and two rings, Si
4, fused together. The 2 central atoms, Si, form double and single bonds. (
Figure 4)
Another variant, of the previous one, would be formed by three flat rings, Si
4, fused; and three cubes, also composed of silicon atoms, Si
8, fused, in this case, in the form of a double prism. (
Figure 5).
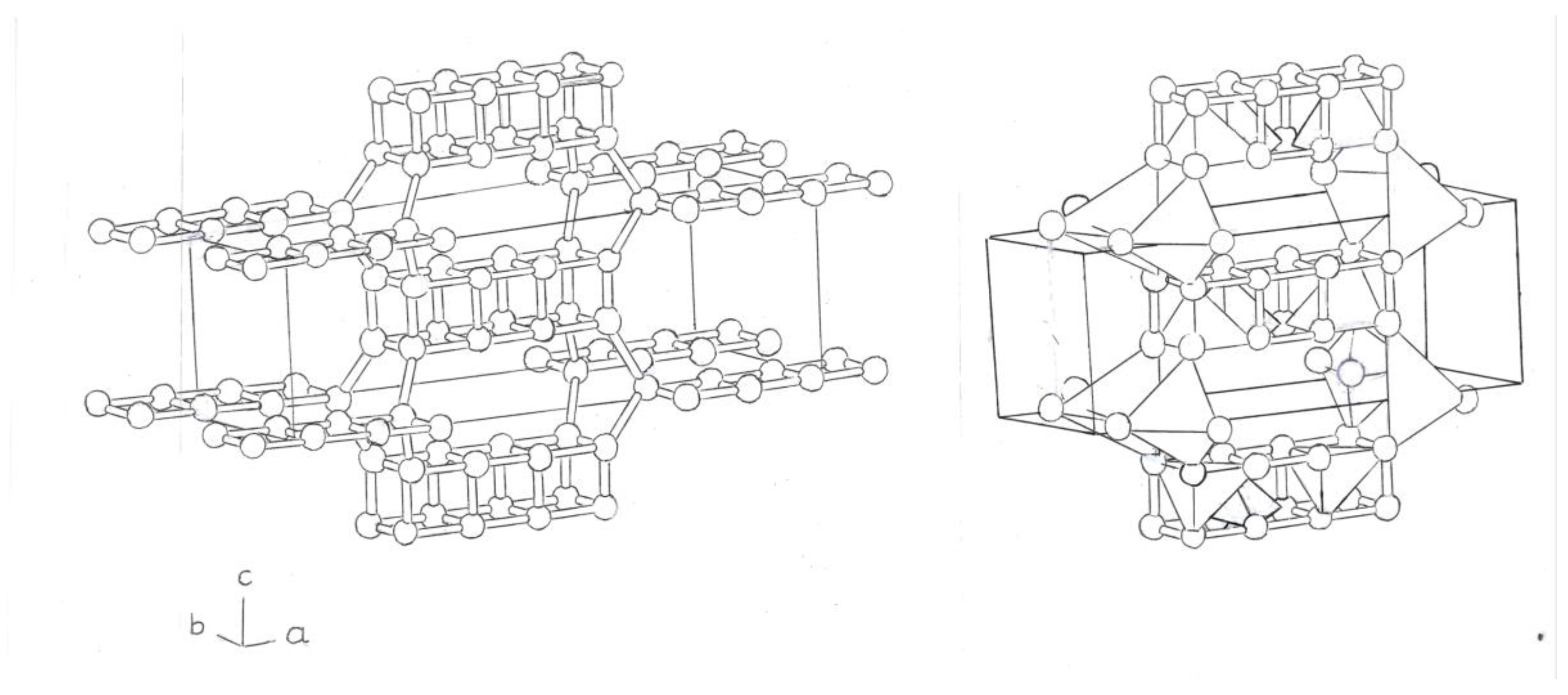
It is also possible to form a Bct phase, composed of two fused Si
8 cubes, at the edges of a tetrahedron; and two flat rings, centered on the body (
Figure 6). The central atoms, of the double rings, fused, form single and double bonds.
The tetrahedrons that form between the planes and cubes of Si4 and Si8, fused, will be separated by single bonds, which form the silicon cubes, Si8.
A new Bct phase, a variant of the previous one, would be composed of fused prisms, with three cubes, Si
8; located at the edges and in the center of the tetrahedron (
Figure 7 ) . All the atoms of Si, , form simple bonds in it.
All these new Bct phases form 8 octahedrons, with other Si atoms adjacent; located on the edges of the tetrahedron. The difference between them would lie in the direct contact between them, in the case of being composed of fused flat rings (
Figure 1 and
Figure 2). Or because they are separated by a simple link, as in
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7.
2. Calculation Model
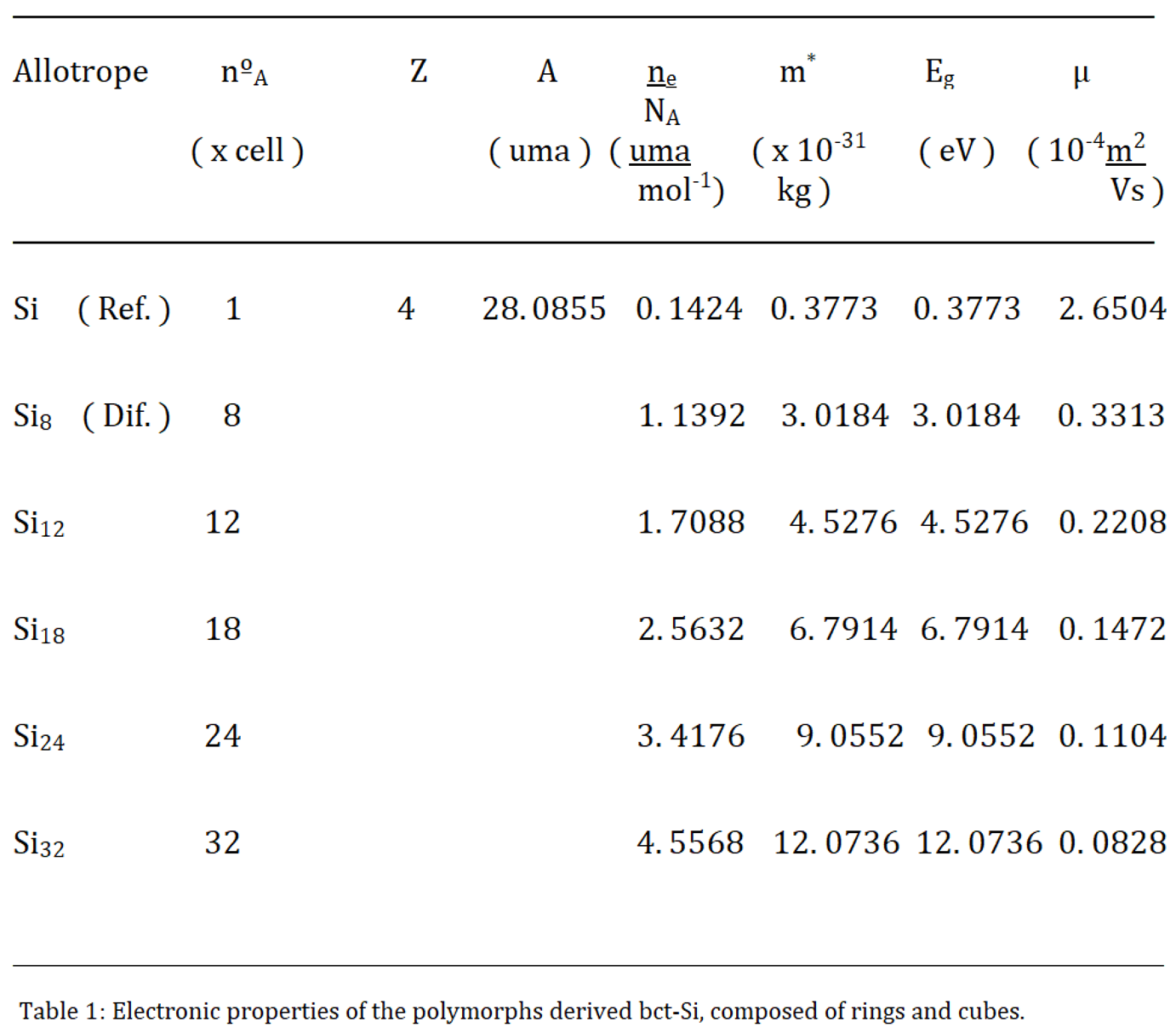
2.1. Electronic Properties
The electrons that move freely through the solid, from the electrons of density, were established by the free electron gas model of Drude and Sommerfeld [
4,
5].
NA, is Avogadro's number or constant, Z indicates the electronic valence number, A is the atomic mass of the chemical element (in g/mol), ρm, is the mass density (mass, divided by volume) and ne, They are the conduction electrons, or number of free electrons. This is the basic equation to calculate its concentration.
For a unit cell, we can simplify the equation to the form:
In this equation, we replace the mass density, ρm, with nºA, which is the number of atoms that make up the unit cell [
6].
The effective or dynamic mass of the electron is defined by the formula:
where, m*, is the effective mass of the electrons, σ, is the electronic conductivity, e, is the charge of an electron, and τ, is the Relaxation Time; which is the average time between consecutive collisions of an electron. It is an average value, since not all collisions occur at regular intervals.
There is a relationship between this effective or dynamic mass of free electrons in motion, and the forbidden energy gap or band gap [
7].
And between these and electronic mobility [
7], which measures the efficiency of semiconductors.
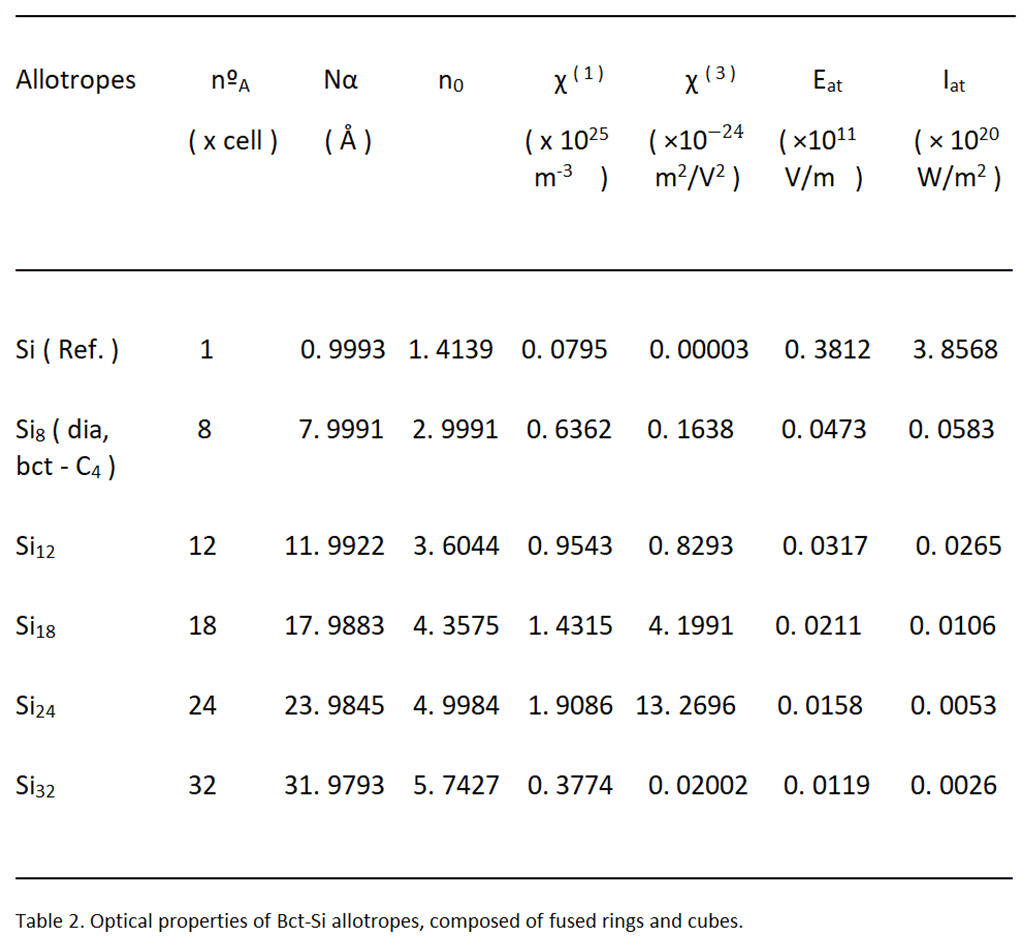
2.2. Optical Properties
To begin to study the optical properties of these allotropes, variants of the bct - Si
4, we must begin to calculate the atomic or electron polarity [
10]. of the silicon atoms, which compose them.
where, a
R, the Bohr radius of the components [
11] and 4πε
0, the inverse of Coulomb's constant, 1/k.
From this, we can define the linear refractive index, n
0 [
12], as:
In this equation, N is the number of atoms per unit volume in the material or unit cell.
Under these circumstances, we can already calculate the linear, or first-order, susceptibility χ
(1) of the solid medium [
13].
And, from this, we can derive third-order susceptibility [
13].
The strength of the electric field, atomic (E
at), is determined by the charge of the electron [
14], and the Bohr radius of silicon [
11], in the form:
The charge of the electron (e = 1.602 × 10−19 C), comes in coulombs.
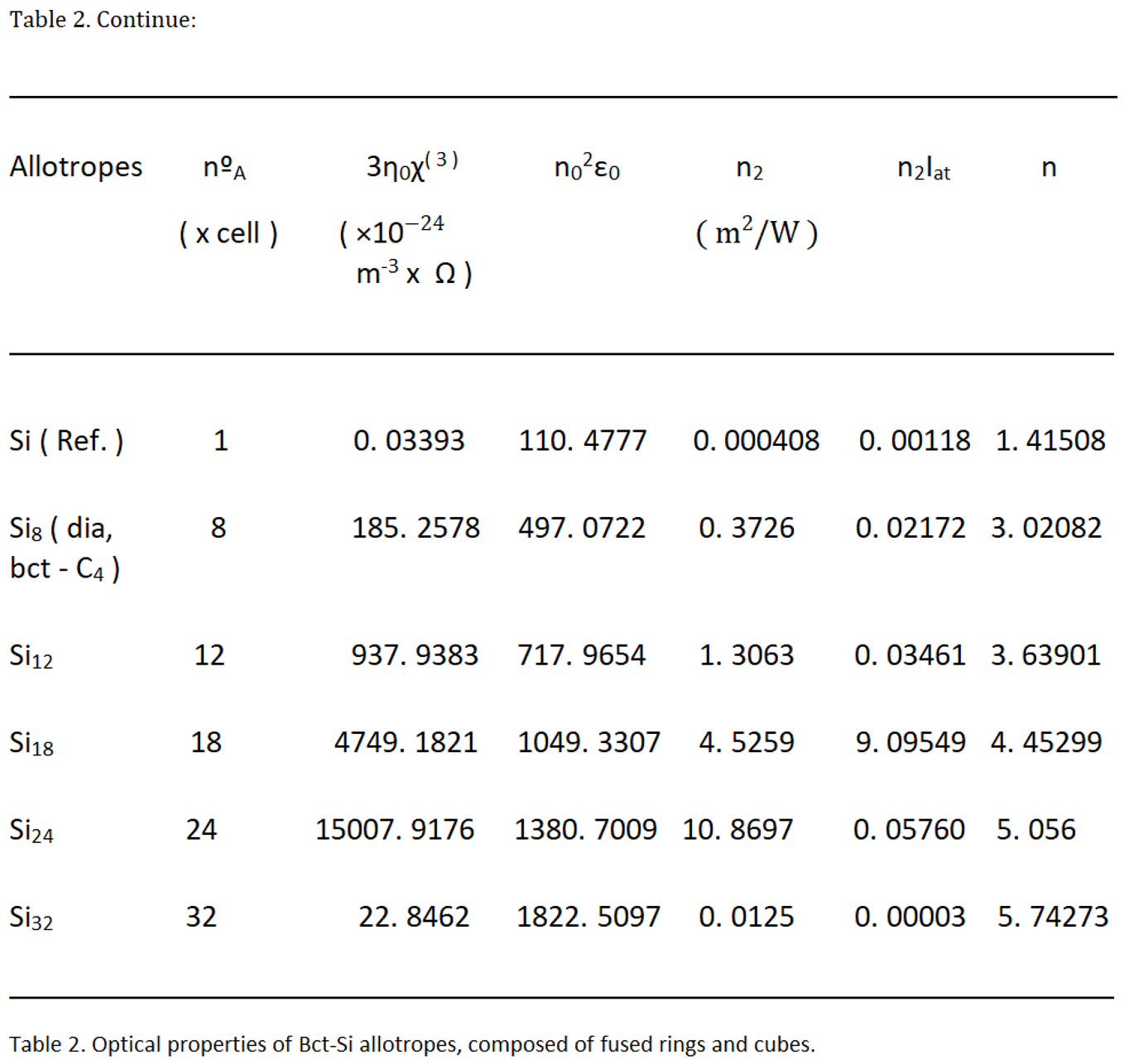
The impedance, the vacuum, has a value of η0 = 377 ohms (Ω ). That will allow us to calculate the refractive index, of second order, n2. This nonlinear refractive index, n2, relates the vacuum impedance, η0 , with the third-order susceptibility χ(3), with a directly proportional relationship, between them; and with the linear refractive index, n0, in this case inversely proportional, with respect to the first two.
Light intensity, at the atomic level, is related to the amplitude of the electric field, atomic; with equation [
15].
Also called Kerr's optical coefficient. Finally, we can calculate the optical Kerr effect, using the equation [
16]:
Conclusions
New theoretical variants of bct, (tetraphonal allotropes, centered on the body), derived from flat square rings and cbos, of Si4 and Si8, fused or not, have been obtained; as in
Figure 3.
As we have seen, in electronic properties, the energy gap, or band gap, increases proportionally with the number of atoms that make up a unit cell. The value closest to a diamente-type structure, and to a bct - C
4 , will be the allotrope bct - Si
12, with an E
g of 4, 5276 eV, From there, this energy gap increases from 6, 7914 eV, of the bct - Si
18; up to 12, 0736 eV, of the bct - Si
32 (
Figure 7 ).
Electronic mobilities, on the other hand, are inversely proportional to the number of atoms present in a unit cell; since a greater presence of atoms would hinder or slow down the flock of electrons through a crystal lattice; and a smaller number would facilitate the flow of these electrons. Thus, the lowest values will be for allotropes. Thus, the lowest values will be for the allotropes bct - Si32 and bct - Si24. And those closest to bct - C4, the variants bct - Si12 and bct - Si18; As expected.
As for the optical properties, the values are increasing, proportionally to the number of nanotomes that make up a unit cell. Those with the lowest value in the Kerr index, n, correspond to the theoretical allotropes, bct - Si12 and bct - Si18, whit, 3, 63901 and 4, 45299, respectively; Greater than 3, 02082 of the diamente and bct - C4 forms, with 8 atoms per unit cell. This figure reaches higher values in allotropes formed by a greater number of silicon atoms, such as bct - Si24, with 5, 056, and bct - Si32, with 5, 74273; which could be interesting or attractive, in this regard, for possible applications in nonlinear optics.
References
- Bct-C4: a viable sp3-carbon allotrope. Koichiro Umemoto, Renata M. Wentzcovitch, Susumu Saito, and Takashi Miyake.
- Electronic and optical properties of novel carbon allotropes. Zhanyu Wang, F. Dong, B. Shen, R.J. Zhang, Y.X. Zheng, L.Y. Chen, S.Y. Wang, C.Z. Wang, K.M. Ho, Yuan-Jia Fan, Bih-Yaw Jin, Wan-Sheng Su. El Sevier. Carbon. Volume 101, May 2016, Pages 77-85.
- General principles to high-throughput constructing two-dimensional carbon allotropes. Qing Xie(谢庆), Lei Wang(王磊), Jiangxu Li(李江旭), Ronghan Li(李荣汉), and Xing-Qiu Chen(陈星秋). Chin. Phys. B Vol.29, No. 3 (2020) 037306. Page 5.
- First-principles investigation in the Raman and infrared spectra of sp3 carbon allotropes. Ying Bai, Xiaoyan Zhao, Tongwei Li, Zhenlong Lv, Shijie Lv, Han Han, Yanfeng Yin, Hui Wang. Carbon. Volume 78, November 2014, Pages 70-78. El Sevier.
- Tight-Binding Simulation of Cubane C8H8, Methylcubane C9H10 and Cubane-Based Nanostructures. M. M. Maslov. Pages 127-132 | Published online: 03 Dec 2010. Taylor & Francis.
- Kittel, Introduction to Solid State Physics, 7th Ed. Chapter 6 Free Electron Fermi Gas.
- vol-1-tipler-mosca-solucionario. Chapter 38.Solids and the Theory of Conduction. Page 2786.
- Combined theoretical and experimental characterizations of semiconductors for photoelectrocatalytic applications. Tangui Le Bahers and Kazuhiro Takanabe. ( Pág. 34 ).
- Analysis of Electron Mobility in Some " Problematic Materials " from Magnetoresistance E ect at High Magnetic Fields. V.V. Shchennikov, A.E. Kar'kin, , S.V. Ovsyannikov and N.V. Morozova.
- Elemental Radii. Atomic and Ionic Radii of Elements 1–96. MartinRahm,Roald Hoffmann and N. W. Ashcroft. ChemPubSoc Europe ( Chemistry a European Journal ).
- Revisiting Bohr’s Theory via a Relationship between Magnetic Constant and Bohr Radius of Any Element. Ikechukwu Iloh Udema. Asian Journal of Physical and Chemical Sciences. Published 22 May 2018. https://journalajopacs.com/index.php/AJOPACS/article/view/33/65.
- Refractive Index of Dense Materials. https://www.feynmanlectures.caltech.edu/II_32.html.
- POLARIZABILITY APPROACH, STRUCTURE, AND NONLINEAR OPTICAL CHARACTERISTICS OF TELLURITE GLASSES IN THE V205 - BaO - TeO2 SYSTEM. Tina Tasheva, Vesselin Dimitrov. Journal of Chemical Technology and Metallurgy, 56, 5, 2021, 945-954.
- Physics in the Modern World, Jerry Marion · 2012, Academic Press. 6. 5 The Electrical Force, page 133.
- Surface Microscopy with Low Energy Electrons.De Ernst Bauer · 2014 -- 2. 2 Photoemision ( Pag. 43 ).
-
https://www.igntu.ac.in/eContent/IGNTU-eContent-521473741283-M.Sc-Physics--2-IshwarPrasadSahu-AdvancedOpticsMPHYT-C204-III.pdf.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).